1. Introduction
Plant propagation through in vitro culture has been adopted by commercial propagation companies because it presents some advantages over conventional propagation by soft or hard wood cuttings. It is a weather unaffected propagation system, allows the conservation of the initial plant material sanitary conditions, and exponentially multiplies the selected plant materials every two to four-week-long cycles. However, in vitro propagation systems based on semisolid agar containing media require craft manipulations, under sterile conditions with appropriate tools and expert hand labor. To prevent contamination and yet allow plant material aeration the conventional micropropagation techniques employ glass or plastic containers, flasks or tubes, recycled or disposable, with lids than loosely close the recipients, with differential passive aeration. Caps need to be removed in a laminar flow hood cabinet, before introducing or extracting the cultured plant portions, with the help of forceps and then orientated, divided with the help of scalpels, using a sterile hard surface, and cultured back to fresh medium with sterile forceps. Therefore, the conventional plant in vitro propagation requires a great amount of had labor input.
Temporary immersion system (TIS) bioreactors have been developed during the last decades to favor plantlet or soot development. The best-known commercial ones are RITA
®, its bigger version Matis
® [
4,
5,
6,
7,
8,
9,
10], Plantform
TM [
11,
12,
13,
14,
15,
16,
17], and SETIS
TM [
18,
19,
20]. Besides, there are several other TIS developed for their particular purposes by plant invitro culture laboratories [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31]. With some differential characteristics among them, there are two common properties, which make them similar, and help to differentiate the GreenTray
®. Firstly, the former ones require forceps to introduce or to extract explants in or from the culture recipient. In addition, with the former TIS a supporting surface is needed to divide, with the help of scalpels, the grown explants in smaller pieces. Instead, as it will be described in this charter, GreenTray
® needs no forceps to move the explants in and out of the culture container, and the grown-out part can be rapidly cut with scissors instead of using a scalpel. Consequently, the design of GreenTray
® significantly reduces the labor input required for in vitro propagation, an opens the possibility for further automated manipulations.
Use of the in vitro culture environment-controlled conditions is an asset to select tissues, shoots, or plants with differential levels of tolerance to abiotic or biotic stress agents. Correspondence of the results observed in vitro with those obtained in vivo, in the greenhouse or field selection plots, is a request to adopt the established in vitro protocol for the selection of plant materials and its use in breeding programs. Most protocols established in vitro require the sugar addition to the culture medium, since the growth conditions of the shoots, plantlets or seedlings are heterotrophic. The GreenTray®’s design allows for independent aeration of the culture container, or its enrichment with CO2, making possible to eliminate sugar from the culture medium. This fact, coupled with the current possibility of increasing photosynthetically active radiation, without increasing the temperature, through LED lighting of different spectrums, make possible to carried out in vitro tests under autotrophic conditions, this is, with photosynthetic activity. Consequently, bioassays for tolerance to biotic or abiotic stresses using the GreenTray® will reproduce in vivo conditions in a more reliable manner than with other culture techniques. This article describes the design, operation, and manipulation of plant material in the GreenTray®, and how these allowed the first selection tests to be carried out against abiotic or biotic stress agents for fruit trees, under autotrophic conditions.
2. Materials and Methods
The patented GreenTray
® [
2,
3] design is characterized in the way of accessing the interior of the plant cultivation container. Its design derives from a previously handmade assemblage [
32], and another adaptation to a plastic container [
33]. When opening the cultivation container of the actual GreenTray
®, the lid and the cultivation tray are always joined, and therefore the sprouts or plants in cultivation are oriented with the movement of the lid, with just one hand. In the model presented in this work, the GreenTray
® is adapted to a commercial glass flask, but the GreenTray
® design is applicable to any container or bag, of variable size or shape, as long as the culture surface or tray is attached to the container lid, as for instance the one presented in [
33].
2.1. GreenTray® Parts and Assembly
A commercial cylindrical glass jar with a capacity of 3895 ml, slightly bigger than a US gallon, with a Twist Off 110 lid, has been used as a culture container for the plant materials. Its dimensions (15.9 cm in diameter, 25.45 cm in height) are the basis for building each unit of the GreenTray® bioreactor, and automated modules grouping 7 to 21 bioreactors. Plant materials are contained in this glass flask, while the culture medium is autoclaved and placed in 500 ml ISO GL45 graduated transparent flasks, commonly used in laboratories.
To allow the introduction, cultivation and extraction of plant materials from the culture container, five pieces were designed (
Figure 1), which were manufactured by HP Multi Jet Fusion 3D printing, using PA12 for its manufacture, a material that can withstand sterilization temperatures by autoclaved, necessary for the in vitro cultivation of plants. Each unit was assembled as shown in
Figure 2.
2.2. Autonomous and Automated Controller with Compressed Air and Lightening
A PLC+HMI
TM Touchscreen model “Samba
TM SM35-J-R20” (Unitronics
®, Israel), was programmed to control an air diaphragm compressor and vacuum model “H5P3” (EAD Pumps, Masquefa, Barcelona, Spain), four electro valves, and an external LED lightening. All components were assembled inside an enclosure for low-voltage switchgear and controlgear assemblies, model “Spacial CRN” (Schneider Electric Industries SAS, Rueil Malmaison, France), from which two Legris compressed air pipe clear Polyamide came out, both of 6 and 4 mm of external and internal diameter. A first tube provides compressed air at positive pressure (+5 to +6 psi), and when chosen the same tube exerted a suction or negative pressure (-1 to -1.5 psi). Both air movements arrived to the entrance of the 500 ml ISO GL45. The second tube provides compressed air at positive pressure (+5 to +6 psi) to the entrance of the one gallon cylindrical jar. Both air entrances were protected from contamination with 25-30 mm of diameter air filters 0.22um Teflon-PTFE (Filtros Anoia, S.A., Barcelona, Spain). The assemblage is portable and requires only an AC power plug and socket, becoming a module for 7 GreenTray
® units (
Figure 3).
2.3. Light Source
LED lightening used in the GreenTray® modules are exchangeable between the models of different light spectra produced by Gealed S.L. (Catajorra, Valencia, Spain), namely “Supernova Lima” (Ref. SUP12100LDC), “Alnayr” (Ref. AN-NAYYIR 60S), and “Meissa” (Ref. AL-MAISAN 100S). The last two with a Cool White 5000K sun-like spectrum, which can provide up to 900 uEm-1s-1 PAR at 20cm from the light source. Light intensity, and photoperiod is regulated from the previously described automated controller.
2.4. Plant Materials
Five
Prunus hybrid rootstocks, namely Rootpac
® 20, Rootpac
® R (obtained by Agromillora) [
34], Intensia
® (obtained by IRTA), Garnem
® (obtained by SIA-DGA), and GF667 (obtained by INRA); Adara, a plum rootstock (obtained by EEAD-CSIC); two
Pyrus hybrid rootstocks, namely OHF87
TM (obtained by OSU) and Py170, which is an hybrid between “OH11” [
1] and
Pyrus amygdaliformis, in the last phase of selection in IRTA breeding program, oriented to have tolerance to iron chlorosis and reduced vigor [
35,
36]; three apple varieties, namely Carrandona (M0676), Florina (M0216), and Rozona (M0163) (collection of SERIDA) [
37]; the
Cannabis sativa variety “Ferimon” (obtained by HEMPit), and propagules of
Kalanchoe daigremontiana, were used for the present work.
2.5. Micropropagation in the GreenTray®
2.5.1. Culture Media
Culture in the GreenTray
® was done in liquid Murashige and Skoog (MS) medium, supplemented with 30 g/L sucrose, 5uM 6-Benzylaminopurine (BAP), and the pH adjusted to 4.7 before autoclaving [
38]. The pH before autoclaving was one unit lower than that used in agar containing media, after what was observed in [
39] to prevent outgrowth of endophytes into the culture medium.
2.5.2. Immersion and Aeration Frequencies
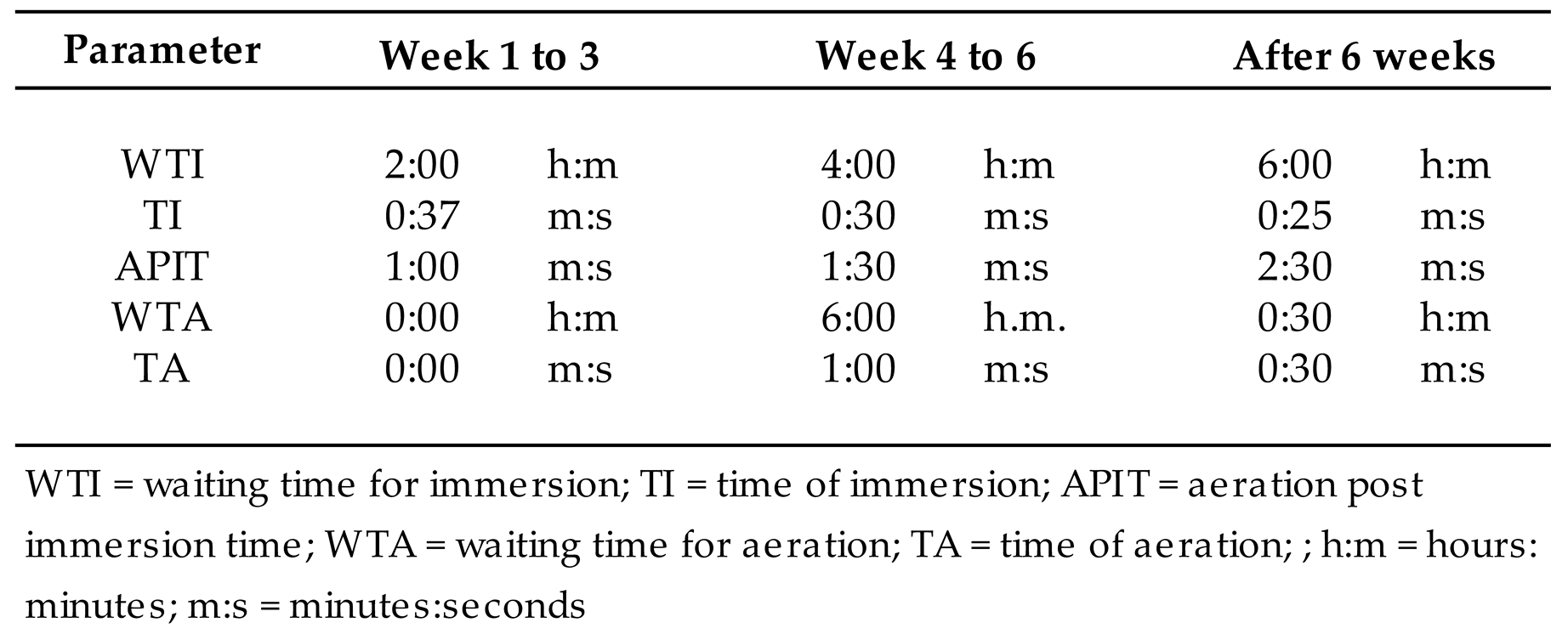
The automated controller allows temporizing the waiting time between explants immersions (WTI), duration of the explants immersion (TI), waiting time between explants aerations (WTA), and duration of the explants aeration (TA). Besides, the TI can last 90 seconds when is a passive movement, driven by gravity, or 30 seconds when is an active movement, forced by air impulsion into the culture jar (35 seconds) or air suction out of the culture medium bottle (30 seconds).
WTI, TI, WTA and TA depend on the plant material capacity to have an optimal grow, avoiding waterlog or waterloos. Such times are adjusted to the plant materials, and for the present work were set up according to what is indicated in
Table 1.
Depending on the plant material volume occupying the tray, 250 to 200 mL of medium is required to immerse the whole culture tray containing the explants.
2.5.3. Establishment of Shoot Cultures in the GreenTray®
Apical and internodal segments derived from in vitro shoot tip cultures in agar-containing mediun were used to establish micropropagation cultures in the GreenTray®, what herein is called 1st Generation (G1). Depending on the shoot explant size, each GreenTray® unit contained between 30 and 40 shoot explants, when the explants were between 1 and 2-cm-long, and up to 80 or 90 explants, when they were 1-cm-long or less.
2.5.4. Sequential Number of Shoot Cuts without Subculture
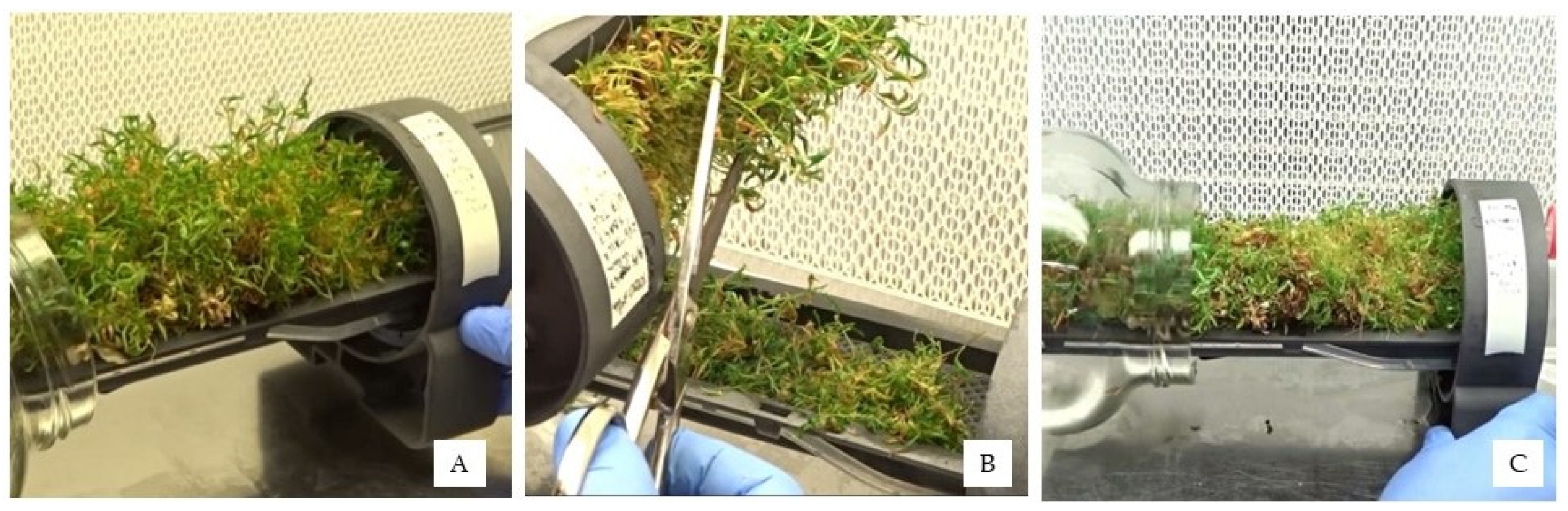
GreenTray
® design allows for extraction and cut of new shoots, grown above the culture tray, followed by the introduction of the remaining basal part back into the culture glass jar (
Figure 4A,C). Once this basis regrows the shoots are cut the same way, for several times, depending on the plant material’s capacity to keep growing. Compared to the conventional in vitro propagation procedures in which shoots are cultured in agar containing medium, the propagation in GreenTray
® needs no subculture of the shoot basis, since they remain in the culture tray, for several cuts, keeping its axillary sprouting capacity, like as a hedge from which new shoots are cut.
2.5.5. Subculture to New GreenTray® and Number of Generations
Shoots produced from the GreenTray
® can either be moved to soil for rooting in an acclimation facility, or placed in another GreenTray
® bioreactor, what herein is called a generation (
Figure 4B) The number of generations is recorded for each plant material.
2.5.6. Plantlet Development under Autotrophic Conditions
Rotted plantlets were moved to GreenTray
®, kept vertical with the help of upper grid and with their roots laid on the culture tray. Culture liquid Murashige and Skoog (MS) medium, with 0 g/L sucrose, air renewal of the culture jar, at 1.5 L/minute, forcing aeration at the regime indicated in
Table 1 for the 6 weeks of culture, and light intensity 400 uEm-1s-1 PAR at 20cm from the LED light, were the conditions to promote autotrophic growth.
2.6. Assays to Abiotic and Biotic Stress Agents
Two
Prunus rootstocks, namely Garnem
® and Rootpac
® 20, and three apple varieties, namely Carrandona (M0676), Florina (M0216) and Rozona (M0163), were established in vitro, multiplied, and rooted in agar containing medium following the protocol described in [
38]. The plantlets were transferred to a GreenTray
® and cultured under autotrophic conditions during the assays to characterize plant materials ability to stand abiotic (high pH, low Ca2+ concentration) or biotic stress agents (inoculation with fire blight).
2.6.1. Abiotic Stress Conditions: Rhizosphere pH and Ca2+ on Plantlet Growth
To determine the differential tolerance of the two Prunus rootstocks to high pHs and Ca2+ availability, as determined by the shoot fresh weights, 15 plantlets per rootstock were cultured eight pH x Ca2+ combinations. Liquid culture Murashige and Skoog (MS) medium adjusted before autoclaving to pH 5.7 (standard) or pH 7.7 (alkaline), were used to test for tolerance to high pHs. In both pH conditions, the standard Ca2+ concentration in the MS medium (3.0 mM CaCl2.2H20) was compared with 1/2 (1.5 mM), 1/3 (1 mM), and 1/4 (0.75 mM) Ca2+ concentration in the rhizosphere. Two weeks after culture in the GreenTray®, the shoot length (cm) of each plantlet was measured.
2.6.2. Abiotic Stress Conditions: In Vitro Atmospheric Humidity on Ca2+ Uptake
Liquid culture media adjusted to pH 5.7, before autoclaving, and with Ca2+ at the MS standard concentration (3.0 mM CaCl2.2H20), were used to determine the differential ability of the two
Prunus rootstocks to uptake and transport Ca2+ from the rhizosphere to the roots and shoots. Since leaf transpiration is determinant for Ca2+ uptake and mobilization to the aerial part, a high aeration regime in the GreenTray
® was set for this assay, as described in [
40].
2.6.3. Biotic Stress Conditions: Tolerance to Fire Blight
Inoculum of fire blight (
Erwinia amylovora) was prepared as described in [
37]. After three days of adaptation to the GreenTray
® under autotrophic conditions, 15 plantlets of each variety were inoculated with the bacteria with a pair of scissors (
Figure 5), with two cuts in opposite sites perpendicular to the mid rip in the upper first expanded leaf. The level of affection of each plantlet was measured 10 days post inoculation to determine the differential tolerance of both varieties to the bacteria, following the same levels of affectation described in [
37]. Briefly, susceptibility was recorded 10 days post-inoculation by dividing necrosis progression by shoot length. Necrosis progression was measured using the scale: 0—no visible symptoms; 0.5—necrosis only affected veins of the inoculated leaf; 1—necrosis reached the petiole of the inoculated leaf; and 1 + necrosis length—necrosis reached the stem.
2.7. Statistical Analysis
The experiment was set up as a completely randomized design (CRD), and the data was analysed by a one-way ANOVA. Statistical significance was judged at the level P < 0.05. When the analysis was statistically significant, Tukey-Kramer HSD test was used for separation of means. Data analysis was performed using JMP® software (version 16.0.0, SAS Institute Inc., Cary, NC).
3. Results
3.1. Micropropagation
3.1.1. Shoot Development
Optimal shoot development from internodal shoot explants cultured in the GreenTray
®, includes multiplication by axillary branching, shoot elongation and leaf development without vitrification (
Figure 6). This was depended on the plant material, as well as on the frequencies of immersion and aeration, the composition of the culture medium and the light intensity. Following the methodology described herein, a good development has been accomplished for the almond x peach (
Prunus dulcis x P. persica) hybrid rootstocks Intensia
®, Garnem
®, and GF677 (
Figure 6 A-C), as well as for the plum hybrid (
P. besseyi x P. cerasifera) rootstock Rootpac
®20, and the plum x almond hybrid (
P. cerasifera x P. dulcis) rootstock Rootpac
®R (
Figure 6 D and E), along with the plum selection (
P. cerasifera) rootstock Adara.
Other plant materials that have shown good shoot elongation and leaf development, but yet required more research on adjusting the culture media and immersion frequencies are the pear (
Pyrus communis) rootstock OHF 87
TM (
Figure 6F) and the pear rootstock Py170.
3.1.2. Extraction and Division of Shoots without Forceps
With all plant materials it was easy to extract all shoots and cut the new growth wih the use of a pair of scissors, returning the shoot bases back to the culture flask of the GreenTray
®, as shown in
Figure 4.
3.1.3. Sequential Shoot Cuts without Subculture and Multiplication Rates
All plant materials used herein had de same multiplication rates in the GreenTray® than in flasks with agar-containing medium. However, with the utilized culture media and protocols of immersion and aeration, the diverse plant materials tested had different capacities to sprout after being cut. The most productive was the Rootpac® R which was able to stand 7 cuts, in a period of 19 week of culture, producing 650 shoots from 25 initial explants, which equals to 93 shoots for each cut, with an overall multiplication rate of 26, or a multiplication rate of 4 for each 3-week-long culture period, equivalent to the subculture time with agar-containing medium flasks.
Rootpac®20, GF677 and Intensia® regrew for 2 consecutive cuts. The Cannabis sativa variety “Ferimon” was also able to regrow for 2 consecutive cuts, while Garnem®, Adara, OHF 87TM, and Py170 could not sprout after a cut. More work is required to develop better culture media and conditions in order favor the growth after being cut.
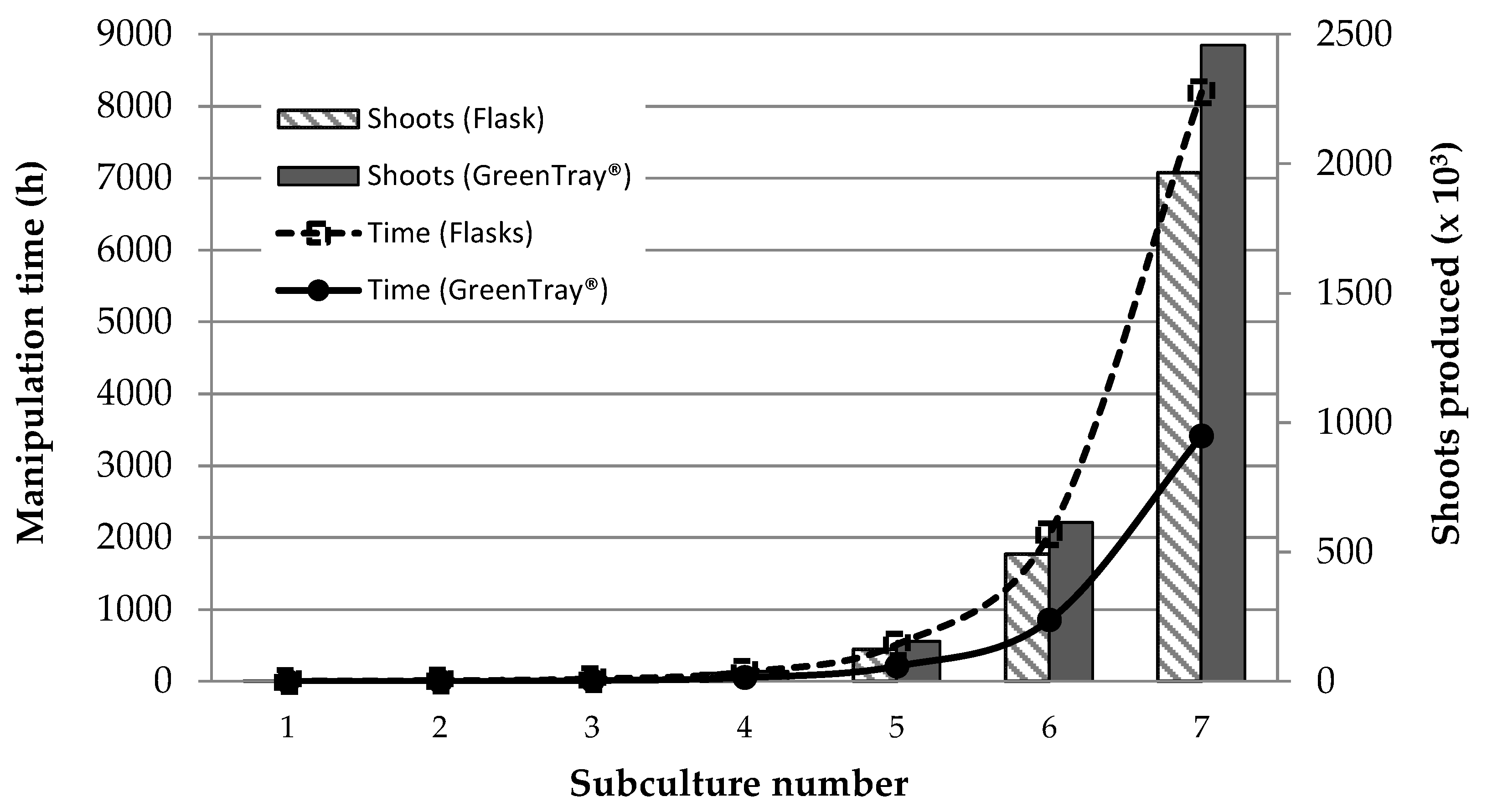
3.1.4. Hand Labor Reduction and Produced Shoots during Micropropagation
As shown in
Figure 7 using the GreenTray
® system there is a reduction in hand labor, accumulated along the subculture time, for the manipulation required to extract the shoots from the flask, cutting them in a sterile surface, and culturing them back into a new flask. When using the GreenTray
® to establish a new generation (
Figure 4B), equivalent to a new subculture in semisolid medium, the time needed is reduced a 65% compared to the required with semisolid medium.
When additional shoot cuts were made from one GreenTray
®, while only one cut is possible from each flask with agar-containing medium, the time saved in the procedure was reduced, but instead the number of shoots produced with the same number of subcultures increased in the GreenTray
®. As shown in
Figure 7, with a second shoot cut of the Roopac
® R, the number of shoots produced in the 7th subculture with the GreenTray
® approaches 2500x10
3, a 20% more than with the conventional semisolid containing flasks.
3.1.5. Number of Generations in the GreenTray®, without Establishing from Agar Cutures
Up to 6 generations of the rootstock Rootpac
® R were initiated, from one GreenTray
® to another, without having to start from cultures maintained in agar-containing medium. The procedure was done as indicated in
Figure 4B, and there were no contaminations in either the GreenTray
® source of shoots or the newly established GreenTray
®.
With other plant materials the number of generations was smaller. For Rootpac® 20, the maximum number of generations was 4; while for Intensia®, Garnem®, GF667, Adara, OHF87TM and Py170 it was possible to establish only 1 generation.
3.1.6. Autotrophic and Long-Term Cultures
Under the conditions stated to promote autotrophic growth, excellent root and shoot development were obtained in the GreenTray
® with rooted shoots of Rootpac
®20, and Garnem
®, used for abiotic stress assays [
40].
The three apple varieties, Carrandona, Florina, and Rozona, doubled their size after two weeks of culture and before inoculation with the biotic stress agent (
Figure 8).
Kalanchoe daigremontiana propagules collected from leaves of a mature plant, established in the GreenTray
®, developed and produced new propagules in their leaves, which were spontaneously dropped on the culture tray, and became new mature plants, in a cycle process that has been repeated for four times in a year-long period, without needing any manipulation inside the culture flask (
Figure 9). Observe the propagules at the edges of the mature leaves and the small plants growing on the surface of the culture tray, in which the white yellowish film observed is not a contamination but as a result of the natural degradation of the previous generation of plants.
3.2. Assays to Abiotic and Biotic Stress Agents
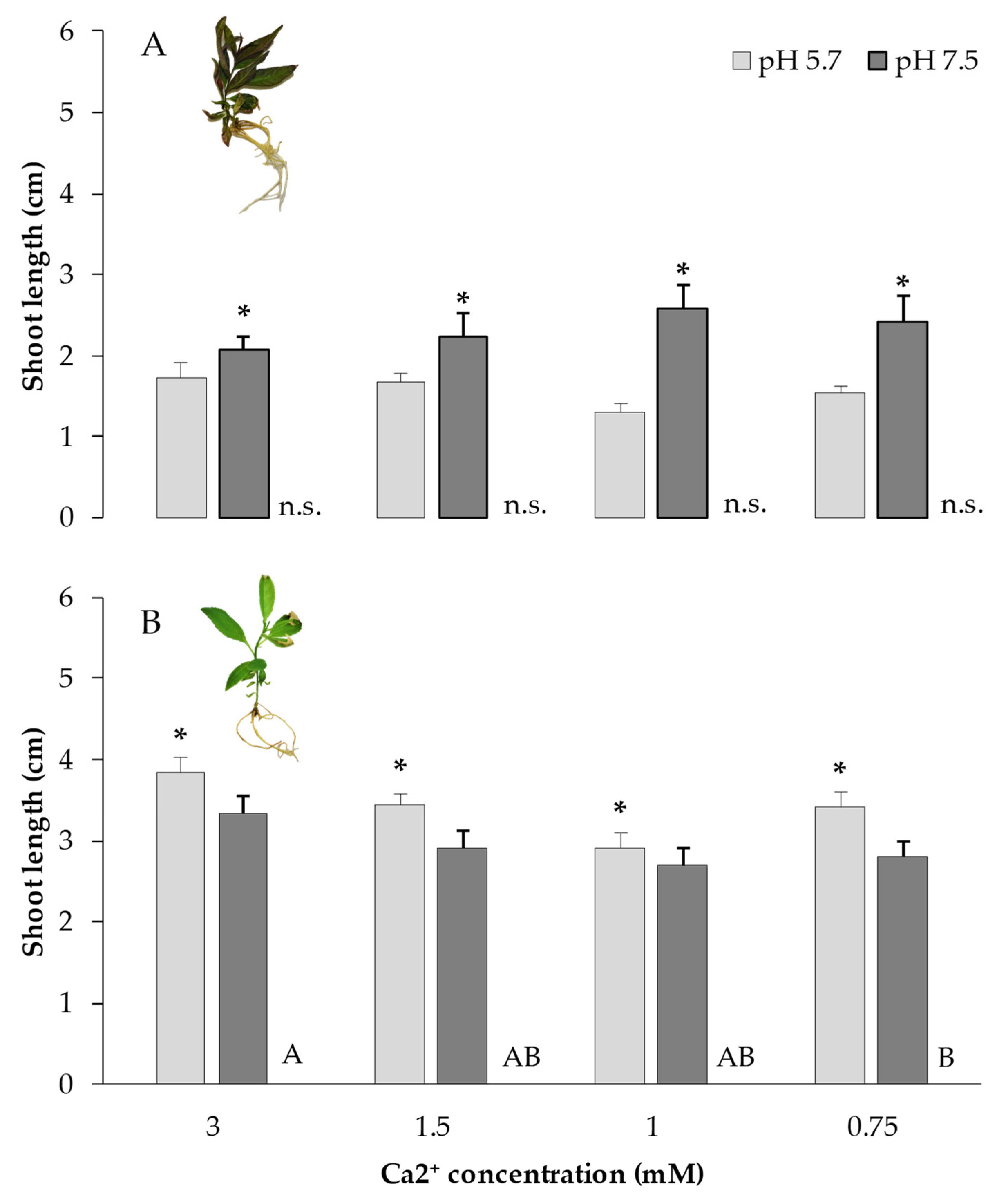
3.2.1. Rhizosphere pH and Ca2+ on Plantlet Growth
Both
Prunus rootstocks behaved differently to the high pH and lower Ca2+ availability in the liquid culture medium. Garnem
® (
Figure 10A), an almond x peach hybrid, showed a significant (p < 0.001) better shoot growth at basic pH 7.5 than at the conventional culture pH 5.7, without interaction with the Ca2+ concentration (p = 0.100) and without effect of lower Ca2+ availability (p = 0.977). Instead, the plum hybrid Rootpac
® 20 (
Figure 10B) was negatively affected on its shoot growth when plantlets were depleted of Ca2+ (p=0.001) to 0.75mM, a 25% of the habitual 3mM present in the medium, and also when the pH was alkaline (7.5) compared to the conventional pH 5.7 (p=0.001).
3.2.2. Atmospheric humidity on Ca2+ Uptake
As described in [
40], GreenTray
® established to 1 minute of TA and 15 minutes of WTA, resulted in lowering the relative humidity in the culture flask to 60% during aeration and recovered afterwards to 80% during the WTA. Instead, the non-aerated GreenTray
® had a constant 100% relative humidity.
Under these experimental conditions in the GreenTray
®, the shoot Cadff (Calcium derived from the fertilizer) was significantly (p=0.003) higher under aerated (47%) than non-aerated environment (37%) [
40]. What means that aeration induced Ca2+ uptake at roots and transported to the shoots. More interestingly, rootstock Rootpac
® 20, sensible to lower Ca2+ availability in the rhizosphere (
Figure 10) presented a significantly higher (p=0.0173) shoot Cadff (46%) than Garnem
® (38%), a rootstock less affected by Ca2+ depletion [
40].
3.2.3. Tolerance to Fire Blight
After 3 days of culture in the GreenTray
®, apple plantlets inoculated as shown in
Figure 5, the first symptoms of infection in the leaf blade were visible through the culture flask glass. At 7 and 10d of culture, necrosis reached the leaf mid rib (
Figure 12A), and shoot (
Figure 12B) of the sensible apple variety Florina.
Ten days post inoculation the different levels of susceptibility to
Erwinia amylovora, of the three apple varieties tested, Carrandona, Florina and Rozona, was evident (
Figure 13), being in this order the increasing level of susceptibility to fire blight. Shoot and root development were clearly better for Carrandona and Florina (
Figure 13A,B) than for Rozona (
Figure 13C), which showed evident growth reduction, wilting, or shoot and root necrosis.
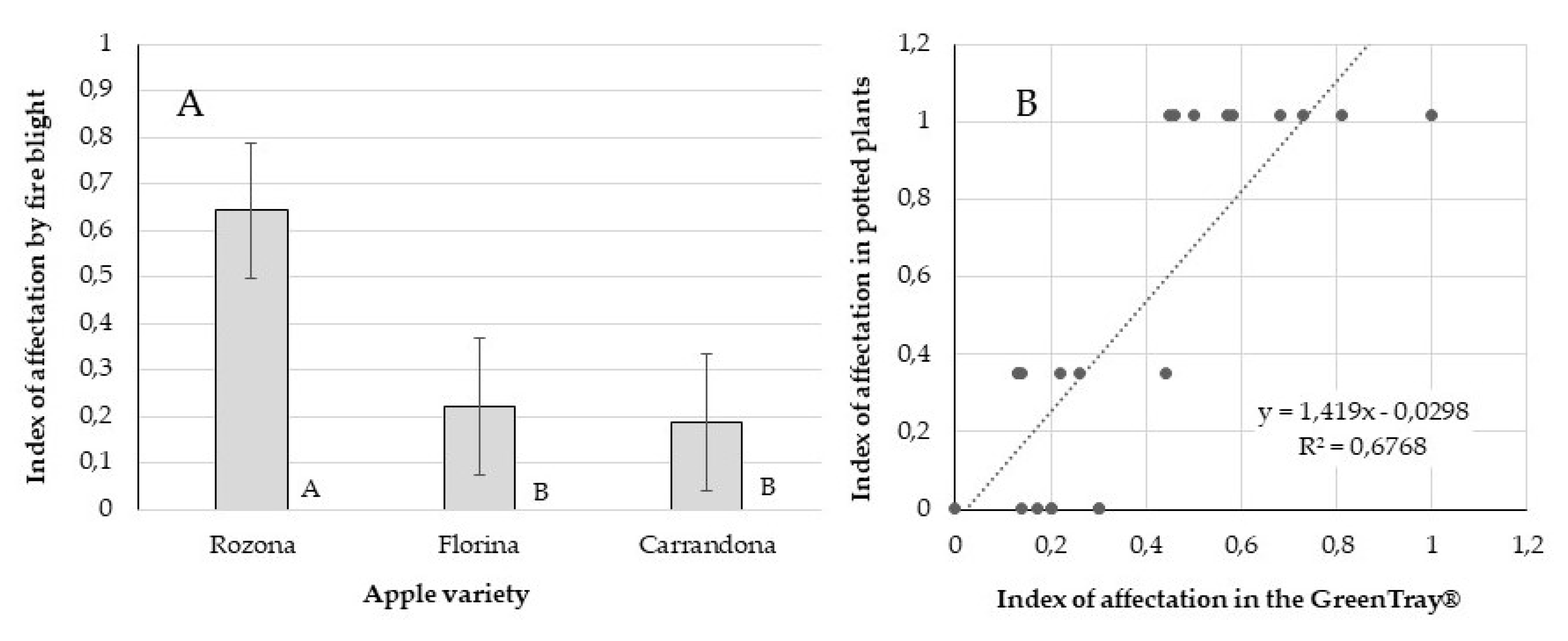
Values of affection obtained in the GreenTray
® (
Figure 14A) showed a significant (p<0.001) difference among the three apple varieties. A significantly (p=0.050) higher tolerance to fire blight was observed for Carrandona and Florina, than Rozona. With a level of R
2 = 0.68 (
Figure 14B) the level of susceptibility to fire blight observed in the GreenTray
® was significantly (P<0.0001) and linearly correlated with the level of tolerance observed in potted plants grown in soil inside a climatic growth chamber of a BSL3 facility [
37].
4. Discussion
Extraction of shoots with forceps form the in vitro culture containers, as well as from TIS bioreactors, arranging them on sterile surfaces in order to divide them with the help of surgical scalpels, followed by their introduction into new culture containers, process known as performing a subculture in the conventional in vitro culture process, is time consuming and represents great part of the hand labor expense dedicated to the perform most of the plant in vitro culture processes. The components of the actual GreenTray
® TIS bioreactor described herein, which is a further evolution of the ones presented in the past [
32,
33], make it significantly improved and versatile compared to other commercial TIS bioreactors, such as RITA
®, Matis
®, Plantform
TM, SETIS
TM [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20], or the TIS developed by several plant invitro culture laboratories oriented to solve particular propagation objectives for diverse plant species [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31].
GreenTray opens the opportunity to automate the in vitro clonal plant propagation processes, because of its new way of disposition of the plant material, extraction from the culture vessel, easy manipulation for reorientation, shoot cut, and reintroduction for a further growth of the shoot basis. These facts represent a significant reduction of the time required for the conventional subcultures, implying a reduction in hand labor, and an increase in the number of shoots produced per container, which reduce the cost per plant produced and therefore facilitates the application of micropropagation to more plant species.
Although an excellent shoot development and sequential shoots cuts were obtained for Rootpac® R, and were also good Rootpac® 20, GF677, Intensia®, and Ferimon, further research is needed to improve the culture media, frequencies or immersion and aeration for Garnem®, Adara, OHF 87TM, and Py170. Indeed, this observation is applicable to all plant species for which a great amount of experience has been accumulated during the last decades of work with semisolid culture media, compared to TIS culture systems.
As reported in previous works [
41,
42,
43,
44,
45,
46] autotrophic in vitro culture is facilitated by aeration in the culture vessel and increasing the PAR radiation without stressing the plant materials. GreenTray
® design is particularly focused in improving the aeration through two mechanisms, suction from the glass bottle containing medium or impulsion of air in the glass culture jar. The PAR radiation has been significantly increased with LED lights, with a variable and high range of intensities, paired with low heating effects. Aeration of the culture flask induces transpiration in the plantlet’s leaves; therefore, water exits through the open stomas, CO2 diffuses in, what in return favors photosynthesis [
41].
Rooted plantlets of the Prunus rootstocks Rootpac®20 and Garnem®, or the apple varieties, Carrandona, Florina, and Rozona, grew in the GreenTray® without sucrose in the culture medium, during the biotic and abiotic stress agent assays that lasted several weeks. As an example of a long term in vitro autotrophic culture, the Kalanchoe daigremontiana especies, demonstrated that the autotrophic culture conditions in the GreenTray® are feasible for at least one year long.
GreenTray
®’s design allows to access shoots or plantlets moving sideways the container’s lid and attached tray. With this simple movement all plants are polled out of the culture recipient, disposed in front of the observer, so it can easily be treated, inoculated, phenotyped, sampled, or pictured. By polling the holding racks, plantlet roots can be observed, sampled or pictured the same way as the aerial part of the plants. Since the container holding the culture medium is independent from the cultured plantlets, it can be aliquoted, supplemented, inoculated, and observed at any time of the culture [
39,
42,
43]. These characteristics make of the GreenTray
® TIS bioreactor an ideal tool for experimenting with biotic and abiotic stresses agents, better than the other commercial TIS bioreactors, and superior to other containers to perform this type of studies for abiotic [
44,
45,
46,
47], or biotic [
48,
49,
50,
51,
52,
53,
54,
55,
56] stress agents.
The experimental conditions to assay the effects of pH and Ca2+ availability in the rhizosphere were easily established and repeated with the GreenTray
® design. The differential capacities of the
Prunus rootstocks Garnem
® and Rootpac
®20 to grow under alkaline and Ca2+ depletion, reported herein, were in accordance with what is known from agronomic trials in calcareous soils [
57,
58]. Therefore, GreenTray
® could be useful for many nutritional studies, including characterization and selection of plant materials [
36].
Forcing a reduction of the atmospheric humidity in the GreenTray
®’s culture vessel, even though is periodic and recovered rapidly through evaporation of wet surfaces and transpiration through the plantlets leaves stomas, implies a lower water potential which induced a higher transpiration of the plantlet’s leaves, and a higher water uptake at the roots, reinforcing the Ca2+ transport to the shoots [
41]. Under these conditions the rootstock Rootpac
® 20 presented a significantly higher shoot Ca2+ uptake than Garnem
® [
40]. All mechanisms of ventilation inside bioreactor, and the GreenTray
® is especially suited for it, increases the water potential in the atmospheric culture recipient and this favors water and nutrient root uptake and translocation to the leaves.
The in vitro assays for tolerance to
Erwinia amylovora have been approached always under heterotrophic conditions [
48,
49,
50,
51,
52,
53,
54,
59]. Although the results presented herein are for a reduced number of apple varieties, is the first time the test is done under in vitro autotrophic conditions of the GreenTray
® and the same plant material was tested to the same
Erwinia amylovora strain under the conditions of a confined growth chamber, with grafted potted plants. Since a verily good correlation was found on the susceptibility to fire blight between the in vitro and the in vivo growing it is recommendable to use the GreenTray
® bioreactor to select for tolerance to fire blight within apple progenies derived from crosses directed to obtain apple or pear varieties with higher tolerance to fire blight. Further improvement will be needed on inoculating all plants with the same amount and concentration of the bacterial strain or getting a more uniform plant development before their inoculation.
Consequently, the GreenTray
®, as it is developed, is a good tool to in vitro propagate plants, at a lower cost and higher multiplication rates. Its design favors the in vitro growth of plantlets in autotrophic conditions, like hydroponic cultures, but in a highly control environment, and in a very reduced space requirement. Bioassays performed in the GreenTray
® to characterize the responses of plants, and selection towards biotic and abiotic stress agents, are more reliable than those performed in semisolid medium, because of the autotrophic plant development conditions favored in this TIS bioreactor. Other plant in vitro culture methodologies applied to plant breeding, such as immature embryo rescue, somatic embryo maturation, adventitious regeneration, genetic transformation, physiological characterization, plant-pathogen interactions, and plant growth-promoting microorganism interactions with plants [
43] can be studied under in vitro conditions with the GreenTray
® as a tool [
39,
42,
43].
Author Contributions
Conceptualization, R.D.-S., N.T. and F.C.-C.; methodology, R.D.-S., F.C.-C., E.T., C.S. and N.T.; validation, R.D.-S., F.C.-C., and C.S.; formal analysis, R.D.-S. and F.C.-C.; investigation, R.D.-S., F.C.-C., E.T., J.R., N.T.; resources, R.D.-S., M.C., S.F., E.A., F.C.-C., C.S.; data curation, R.D.-S. and F.C.-C.; writing—original draft preparation, R.D.-S. and F.C.-C. writing—review and editing, R.D.-S. and F.C.-C.; visualization, R.D.-S. and M.C.; supervision, R.D.-S.; project administration, R.D.-S., M.C., E.T., J.R., and N.T.; funding acquisition R.D.-S., M.C., E.T., J.R., and N.T.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The CERCA Programme / Generalitat de Catalunya, IRTA, the AGAUR grant Producte 2019 Knowledge Industry number IU68-017253, the DACAAR grant number DEMO 56 30080 2021 2A, and the “Ministerio de Ciencia e Innovación” grant number PID2019-111583RR-I00.
Acknowledgments
We acknowledge the support given by Carlos R. Mendoza-Morales (Madre Tierra Puebla, Mexico), John Amin (Intech3D), Francisco Sánchez (DICOMOL), Miguel Reyes (ILERFRED), Francisco Contreras (GEALED), Enrique Dapena (SERIDA), Xavier Abad (IRTA-CReSA) and Buenaventura Begue (IRTA Fruitcentre) for their technical support and materials used for the present work.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- M. Simard, J. Michelesi, and A. Masseron, “Pear Rootstock Breeding in France,” in Proc Ist IS Rootstocks – Decid. Fruit, Acta Hort 658, 2004, pp. 535–540. [CrossRef]
- R. Dolcet-Sanjuan and C. Mendoza, “Reactor system for the in vitro culture of plant material, kit to transform a receptacle in an adequate reactor for such system, and method for the in vitro culture of plant material with such reactor system. Patent No. ES2763637B1,” 2018.
- R. Dolcet-Sanjuan and C. R. Mendoza, “Reactor system for in vitro culture of plant material, kit for transforming a receptacle into a reactor suitable for the system and method for in vitro culture of plant material using the reactor system. Priority date: November 29th, 2018,” WIPO PCT WO 2020/109637 A1, 2020.
- H. Etienne et al., “Development of coffee somatic and zygotic embryos to plants differs in the morphological, histochemical and hydration aspects,” Tree Physiol, vol. 33, no. 6, pp. 640–653, Jun. 2013. [CrossRef]
- M. A. Ramírez-Mosqueda and L. G. Iglesias-Andreu, “Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks,” In Vitro Cellular and Developmental Biology - Plant, vol. 52, no. 2, pp. 154–160, Apr. 2016. [CrossRef]
- M. Bayraktar, “Micropropagation of stevia rebaudiana bertoni using RITA® bioreactor,” HortScience, vol. 54, no. 4, pp. 731–725, Apr. 2019. [CrossRef]
- A. Nasri et al., “Large-scale propagation of Myrobolan (Prunus cerasifera) in RITA® bioreactors and ISSR-based assessment of genetic conformity,” Sci Hortic, vol. 245, pp. 144–153, Feb. 2019. [CrossRef]
- A. C. Melviana, R. R. Esyanti, M. Mel, and R. H. Setyobudi, “Biomass enhancement of stevia rebaudiana bertoni shoot culture in temporary immersion system (TIS) RITA® bioreactor optimized in two different immersion periods,” in E3S Web of Conferences, EDP Sciences, Jan. 2021. [CrossRef]
- D. Gago, S. Vilavert, M. Á. Bernal, C. Sánchez, A. Aldrey, and N. Vidal, “The effect of sucrose supplementation on the micropropagation of salix viminalis l. Shoots in semisolid medium and temporary immersion bioreactors,” Forests, vol. 12, no. 10, Oct. 2021. [CrossRef]
- G. Peña-Rojas, R. Carhuaz-Condori, V. Andía-Ayme, V. A. Leon, and O. Herrera-Calderon, “Improved Production of Mashua (Tropaeolum tuberosum) Microtubers MAC-3 Morphotype in Liquid Medium Using Temporary Immersion System (TIS-RITA®),” Agriculture (Switzerland), vol. 12, no. 7, Jul. 2022. [CrossRef]
- C. Benelli and A. De Carlo, “In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (PlantformTM),” 3 Biotech, vol. 8, no. 7, Jul. 2018. [CrossRef]
- E. Skrzypczak-Pietraszek, A. Urbańska, P. Żmudzki, and J. Pietraszek, “Elicitation with methyl jasmonate combined with cultivation in the PlantformTM temporary immersion bioreactor highly increases the accumulation of selected centellosides and phenolics in Centella asiatica (L.) Urban shoot culture,” Eng Life Sci, vol. 19, no. 12, pp. 931–943, Dec. 2019. [CrossRef]
- Y. Aka Kaçar, B. Biçen, Şimşek, D. Dönmez, and M. H. Erol, “Evaluation and comparison of a new type of temporary immersion system (Tis) bioreactors for myrtle (myrtus communis l.),” Appl Ecol Environ Res, vol. 18, no. 1, pp. 1611–1620, 2020. [CrossRef]
- M. Klimek-Szczykutowicz et al., “Precursor-boosted production of metabolites in nasturtium officinale microshoots grown in plantform bioreactors, and antioxidant and antimicrobial activities of biomass extracts,” Molecules, vol. 26, no. 15, Aug. 2021. [CrossRef]
- E. A. Ozudogru, E. Karlik, D. Elazab, and M. Lambardi, “Establishment of Direct Organogenesis Protocol for Arachis hypogaea cv. Virginia in Liquid Medium by Temporary Immersion System (TIS),” Horticulturae, vol. 8, no. 12, Dec. 2022. [CrossRef]
- I. Grzegorczyk-Karolak, P. Staniewska, L. Lebelt, and D. G. Piotrowska, “Optimization of cultivation conditions of Salvia viridis L. shoots in the Plantform bioreactor to increase polyphenol production,” Plant Cell Tissue Organ Cult, vol. 149, no. 1–2, pp. 269–280, May 2022. [CrossRef]
- Ö. Şimşek et al., “In vitro and ex vitro propagation of Turkish myrtles through conventional and plantform bioreactor systems,” PeerJ, vol. 11, 2023. [CrossRef]
- M. A. Ramírez-Mosqueda and J. J. Bello-Bello, “SETISTM bioreactor increases in vitro multiplication and shoot length in vanilla (Vanilla planifolia Jacks. Ex Andrews),” Acta Physiol Plant, vol. 43, no. 4, Apr. 2021. [CrossRef]
- W. Vendrame, J. Xu, and D. G. Beleski, “Micropropagation of Brassavola nodosa (L.) Lindl. using SETISTM bioreactor,” 2022. [CrossRef]
- H. A. Méndez-Hernández et al., “In Vitro Conversion of Coffea spp. Somatic Embryos in SETISTM Bioreactor System,” Plants, vol. 12, no. 17, Sep. 2023. [CrossRef]
- K. Hoon Moon, H. Honda, and T. Kobayashi, “Development of a Bioreactor Suitable for Embryogenic Rice Callus Culture,” J Biosci Bioeng, vol. 87, no. 5, pp. 661–665, 1999. [CrossRef]
- Y. Kim, B. E. Wyslouzil, and P. J. Weathers, “Secondary metabolism of hairy root cultures in bioreactors,” In Vitro Cellular and Developmental Biology - Plant, vol. 38, no. 1. pp. 1–10, 2002. [CrossRef]
- A. K. Hvoslef-Eide, O. A. S. Olsen, R. Lyngved, C. Munster, and P. H. Heyerdahl, “Bioreactor design for propagation of somatic embryos,” in Plant Cell, Tissue and Organ Culture, Jun. 2005, pp. 265–276. [CrossRef]
- S. Berkov, I. Ivanov, V. Georgiev, C. Codina, and A. Pavlov, “Galanthamine biosynthesis in plant in vitro systems,” Engineering in Life Sciences, vol. 14, no. 6. Wiley-VCH Verlag, pp. 643–650, Nov. 01, 2014. [CrossRef]
- V. Georgiev, A. Schumann, A. Pavlov, and T. Bley, “Temporary immersion systems in plant biotechnology,” Engineering in Life Sciences, vol. 14, no. 6. Wiley-VCH Verlag, pp. 607–621, Nov. 01, 2014. [CrossRef]
- D. Wilken, E. Jiménez Gonzalez, A. Gerth, R. Gómez-Kosky, A. Schumann, and D. Claus, “Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp. cv. ‘Grande naine’ AAA),” In Vitro Cellular and Developmental Biology - Plant, vol. 50, no. 5, pp. 582–589, Oct. 2014. [CrossRef]
- S. Godoy et al., “Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality,” In Vitro Cellular and Developmental Biology - Plant, vol. 53, no. 5, pp. 494–504, Oct. 2017. [CrossRef]
- M. A. Ramírez-Mosqueda, L. G. Iglesias-Andreu, E. Favián-Vega, J. A. Teixeira da Silva, O. R. Leyva-Ovalle, and J. Murguía-González, “Morphogenetic stability of variegated Vanilla planifolia Jacks. plants micropropagated in a temporary immersion system (TIB®),” Rendiconti Lincei, vol. 30, no. 3, pp. 603–609, Sep. 2019. [CrossRef]
- R. A. Ayub, A. B. Pereira, J. N. Dos Santos, D. M. da Silva, and I. L. Pessenti, “Sucrose concentration and blueberry plant density in temporary immersion systems (Tis),” Rev Bras Frutic, vol. 43, no. 4, 2021. [CrossRef]
- S. Gautam, N. Solis-Gracia, M. K. Teale, K. Mandadi, J. A. da Silva, and M. I. Vales, “Development of an in vitro Microtuberization and Temporary Immersion Bioreactor System to Evaluate Heat Stress Tolerance in Potatoes (Solanum tuberosum L.),” Front Plant Sci, vol. 12, Aug. 2021. [CrossRef]
- M. Wang et al., “Establishment of adventitious root culture system of Cynanchum wilfordii in air-lift bioreactors for the efficient production of bioactive compounds,” In Vitro Cellular and Developmental Biology - Plant, vol. 59, no. 2, pp. 216–226, Apr. 2023. [CrossRef]
- C. Mendoza and R. Dolcet-Sanjuan, “A temporary immersion system (TIS) bioreactor used for the in vitro propagation of Prunus and Pyrus rootstocks,” in Agrárias, vol. Volume VIII, A. Oliveira and V. Mocellin, Eds., Curitiba-PR Brasil: Editora Artemis, 2022, pp. 110–124. [CrossRef]
- C. Rolando Mendoza Morales, R. Dolcet-Sanjuan, M. Lizeth Orellana Caballero, M. Isidro López Sánchez, M. Belen Vargas Angel, and D. Isaac Rivera Antonio, “Micropropagación de Agave marmorata utilizando un Nuevo Sistema de Inmersión Temporal,” Revista Ciencia, Tecnología y Sociedad, vol. 10, no. 1, p. 2022.
- J. Pinochet, “a Plum-almond Hybrid Rootstock for Replant Situations,” HORTSCIENCE, vol. 45, no. 2, pp. 299–301, 2010.
- E. Claveria et al., In vitro screening for tolerance to iron chlorosis as a reliable selection tool in a pear rootstock breeding program, vol. 935. 2012. [CrossRef]
- C. P. Mora-Córdova et al., “Rhizosphere Acidification as the Main Trait Characterizing the Differential In Vitro Tolerance to Iron Chlorosis in Interspecific Pyrus Hybrids,” Horticulturae, vol. 8, no. 6, Jun. 2022. [CrossRef]
- B. García-Fernández et al., “Susceptibility Evaluation to Fire Blight and Genome-Wide Associations within a Collection of Asturian Apple Accessions,” Plants, vol. 12, no. 23, Dec. 2023. [CrossRef]
- D. Cantabella, N. Teixidó, G. Segarra, R. Torres, M. Casanovas, and R. Dolcet-Sanjuan, “Rhizosphere microorganisms enhance in vitro root and plantlet development of Pyrus and Prunus rootstocks,” Planta, vol. 253, no. 4, Apr. 2021. [CrossRef]
- D. Cantabella, N. Teixidó, C. Solsona, M. Casanovas, R. Torres, and R. Dolcet-Sanjuan, “Acidification of the culture medium as a strategy to control endophytic contaminations in Prunus spp. rootstocks cultured in GreenTray TIS bioreactor,” Sci Hortic, vol. 290, Dec. 2021. [CrossRef]
- F. Carrasco-Cuello, L. Jené, R. Dolcet-Sanjuan, A. Quiñones, J. Rufat, and E. Torres, “Differential response to calcium-labelled (44Ca) uptake and allocation in two peach rootstocks in relation to transpiration under in vitro conditions,” Sci Hortic, vol. 326, Feb. 2024. [CrossRef]
- J. Girona and J. M. Villar, “Aigua i producció d’aliments. Per què els cultius necessiten aigua?,” pp. 59–85, 2021. [CrossRef]
- D. Cantabella, C. R. Mendoza, N. Teixidó, F. Vilaró, R. Torres, and R. Dolcet-Sanjuan, “GreenTray® TIS bioreactor as an effective in vitro culture system for the micropropagation of Prunus spp. rootstocks and analysis of the plant-PGPMs interactions,” Sci Hortic, vol. 291, Jan. 2022. [CrossRef]
- D. Cantabella, R. Dolcet-Sanjuan, and N. Teixidó, “Using plant growth-promoting microorganisms (PGPMs) to improve plant development under in vitro culture conditions,” Planta, vol. 255, no. 6. Springer Science and Business Media Deutschland GmbH, Jun. 01, 2022. [CrossRef]
- D. Hsissou and J. Bouharmont, “In vitro selection and characterization of drought-tolerant plants of durum wheat (Triticum durum Desf),” Agronomie, vol. 2, pp. 65–70, 1994. [CrossRef]
- J. Gopal and K. Iwama, “In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol,” Plant Cell Rep, vol. 26, no. 5, pp. 693–700, May 2007. [CrossRef]
- P. Asthana, M. K. Rai, and U. Jaiswal, “In vitro selection, regeneration and characterization of NaCl-tolerant plants of Sapindus trifoliatus: An important multipurpose tree,” Plant Cell Tissue Organ Cult, vol. 154, no. 2, pp. 227–238, Aug. 2023. [CrossRef]
- M. Sahu, S. Maurya, and Z. Jha, “In vitro selection for drought and salt stress tolerance in rice: An overview,” Plant Physiology Reports, vol. 28, no. 1. Springer, pp. 8–33, Mar. 01, 2023. [CrossRef]
- J. Viseur and T. M. Figueroa, “In vitro co-culture as a tool for the evaluation of fire blight resistance in pears and apples,” Acta Hortic, vol. 217, pp. 273–282, 1987. [CrossRef]
- H. Abdollahi, E. Rugini, M. Ruzzi, and R. Muleo, “In vitro system for studying the interaction between Erwinia amylovora and genotypes of pear,” Plant Cell Tissue Organ Cult, vol. 79, pp. 203–212, 2004. [CrossRef]
- L. Švábová and A. Lebeda, “In vitro selection for improved plant resistance to toxin-producing pathogens,” Journal of Phytopathology, vol. 153, no. 1, pp. 52–64, Jan. 2005. [CrossRef]
- S. Loreti, A. Bosco, A. Gallelli, C. Damiano, M. Tonelli, and E. Caboni, “Factors Affecting In Vitro Evaluation of Resistance to Erwinia amylovora in Pear Genotypes,” in Proc. Xth IS on Pear. Acta Hort. 800, 2008, pp. 885–890. [CrossRef]
- M. Hevesi, A. Végh, M. Tóth, E. Benczur, and L. Palkovics, “Investigating the Virulence of Erwinia amylovora Isolates by Using Apple Tissue Culture and Pear Fruit,” in 12th Int. Workshop on Fire Blight. Acta Hort. 896, 2011, pp. 223–230. [CrossRef]
- J. Sedlak, F. Paprstein, J. Korba, and J. Sillerova, “Development of In Vitro System for Testing of Pome Fruit Resistance to Fire Blight,” in Proc. 12th Int. Workshop on Fire Blight. Acta Hort. 896, 2011, pp. 375–379. [CrossRef]
- F. Paprstein, J. Sedlak, J. Sillerova, and J. Korba, “In Vitro Evaluation of Cultivar Resistance to Fire Blight,” in Proc. XIIIth International Workshop on Fire Blight. Acta Hort. 1056, 2014, pp. 259–262. [CrossRef]
- J. Sedlák, F. Paprštein, J. Korba, and J. Šillerová, “Development of a system for testing apple resistance to erwinia amylovora using in vitro culture techniques,” Plant Protection Science, vol. 51, no. 1, pp. 1–5, 2015. [CrossRef]
- V. Sharma, M. Thakur, and M. Tomar, “In vitro selection of gamma irradiated shoots of ginger (Zingiber officinale Rosc.) against Fusarium oxysporum f.sp. zingiberi and molecular analysis of the resistant plants,” Plant Cell Tissue Organ Cult, vol. 143, no. 2, pp. 319–330, Nov. 2020. [CrossRef]
- S. Jiménez, J. Pinochet, J. Romero, Y. Gogorcena, M. Á. Moreno, and J. L. Espada, “Performance of peach and plum based rootstocks of different vigour on a late peach cultivar in replant and calcareous conditions,” Sci Hortic, vol. 129, no. 1, pp. 58–63, May 2011. [CrossRef]
- J. Ben Yahmed, M. Ghrab, and M. Ben Mimoun, “Eco-physiological evaluation of different scion-rootstock combinations of almond grown in Mediterranean conditions,” Fruits, vol. 71, no. 3, pp. 185–193, 2016. [CrossRef]
- J. Sedlák, F. Paprštein, J. Korba, and J. Šillerová, “Development of a system for testing apple resistance to erwinia amylovora using in vitro culture techniques,” Plant Protection Science, vol. 51, no. 1, pp. 1–5, 2015. [CrossRef]
Figure 1.
3D printed parts of each GreenTray® bioreactor unit.
Figure 1.
3D printed parts of each GreenTray® bioreactor unit.
Figure 2.
Assembled GreenTray®, sterilized and ready for the cultivation of plants.
Figure 2.
Assembled GreenTray®, sterilized and ready for the cultivation of plants.
Figure 3.
Portable, autonomous module for 7 GreenTray® units.
Figure 3.
Portable, autonomous module for 7 GreenTray® units.
Figure 4.
Propagation of Rootpac® 20 in the GreenTray®: (A) culture tray extraction from the glass flask; (B) orientation of the culture tray to facilitate shoot cut, for the next GreenTray® generation; (C) introduction of the culture tray holding the shoot basis into the culture glass jar.
Figure 4.
Propagation of Rootpac® 20 in the GreenTray®: (A) culture tray extraction from the glass flask; (B) orientation of the culture tray to facilitate shoot cut, for the next GreenTray® generation; (C) introduction of the culture tray holding the shoot basis into the culture glass jar.
Figure 5.
Inoculation of apple leaves with fire blight, grown in the GreenTray®.
Figure 5.
Inoculation of apple leaves with fire blight, grown in the GreenTray®.
Figure 6.
Shoot development in the GreenTray® of the Prunus rootstocks (A) Intensia®, (B) Garnem®, (C) GF677, (D) Rootpac® 20, (E) and Rootpac® R, and the Pyrus rootstock (F) OHF 87TM.
Figure 6.
Shoot development in the GreenTray® of the Prunus rootstocks (A) Intensia®, (B) Garnem®, (C) GF677, (D) Rootpac® 20, (E) and Rootpac® R, and the Pyrus rootstock (F) OHF 87TM.
Figure 7.
Time required for manipulation (lines) and number of shoots produced (bars) of Rootpac® R grown in flasks with agar containing medium (segmented) or in GreenTray® (solid) during the first 7 subcultures.
Figure 7.
Time required for manipulation (lines) and number of shoots produced (bars) of Rootpac® R grown in flasks with agar containing medium (segmented) or in GreenTray® (solid) during the first 7 subcultures.
Figure 8.
Plantlets of the apple variety Carrandona, grown under autotrophic conditions for three weeks in the GreenTray®.
Figure 8.
Plantlets of the apple variety Carrandona, grown under autotrophic conditions for three weeks in the GreenTray®.
Figure 9.
Plantlets of Kalanchoe daigremontiana grown in the GreenTray®.for one-year long period under autotrophic conditions.
Figure 9.
Plantlets of Kalanchoe daigremontiana grown in the GreenTray®.for one-year long period under autotrophic conditions.
Figure 10.
Shoot length of (A) Garnem® and (B) Rootpac® 20 plantlets cultured in the GreenTray® at pH 5.7 (light gray) or pH 7.5 (dark gray) as affected by lowering the Ca2+ concentration from 3 to 1.5, 1, and 0.75mM, in the culture medium. Mean values come from the measures of 15 replicates per genotype. Bars represent the standard error. Ca2+ concentrations with different letter are significantly different (Tukey-Kramer HSD, p < 0.05).
Figure 10.
Shoot length of (A) Garnem® and (B) Rootpac® 20 plantlets cultured in the GreenTray® at pH 5.7 (light gray) or pH 7.5 (dark gray) as affected by lowering the Ca2+ concentration from 3 to 1.5, 1, and 0.75mM, in the culture medium. Mean values come from the measures of 15 replicates per genotype. Bars represent the standard error. Ca2+ concentrations with different letter are significantly different (Tukey-Kramer HSD, p < 0.05).
Figure 12.
Symptoms of fire blight on (A) leaves, 7 days post inoculation, and on (B) shoot and leaves, 10 days post infection of the apple variety Florina grown in the GreenTray®.
Figure 12.
Symptoms of fire blight on (A) leaves, 7 days post inoculation, and on (B) shoot and leaves, 10 days post infection of the apple variety Florina grown in the GreenTray®.
Figure 13.
Plantlet development in the GreenTray® of the apple varieties (A) Carrandona, (B) Florina, and (C) Rozona, after 10 days post inoculation with Erwinia amylovora.
Figure 13.
Plantlet development in the GreenTray® of the apple varieties (A) Carrandona, (B) Florina, and (C) Rozona, after 10 days post inoculation with Erwinia amylovora.
Figure 14.
Levels of affectation to Erwinia amylovora of three apple varieties (A) tested for tolerance in the GreenTray®, and (B) their correlation with the known levels of affectation observed with potted plants. Mean values come from the measures of 15 replicates per genotype. Bars represent the standard error. Means with different letter are significantly different (Tukey-Kramer HSD, p < 0.05).
Figure 14.
Levels of affectation to Erwinia amylovora of three apple varieties (A) tested for tolerance in the GreenTray®, and (B) their correlation with the known levels of affectation observed with potted plants. Mean values come from the measures of 15 replicates per genotype. Bars represent the standard error. Means with different letter are significantly different (Tukey-Kramer HSD, p < 0.05).
Table 1.
Time set up of the controller, for 7 GreenTray® units, along the week of culture.
Table 1.
Time set up of the controller, for 7 GreenTray® units, along the week of culture.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).