1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds widespread in the environment during the incomplete combustion of organic matter such as wood, coal, oil, and gas. This can occur in various settings, including certain industrial processes
, motor vehicle emissions, wildfires, tobacco smoke, grilled or charbroiled foods (incomplete combustion of fats and juices). It can be also accumulated in soil due to deposition from the atmosphere, runoff from contaminated sites, or improper disposal of industrial waste. Its sediments at the bottom of rivers, lakes, and oceans, where they can persist for long periods. We can found it in aliments fish or plant [
1]. Many PAHs are considered hazardous environmental pollutants due to their persistence in the environment. The exposure to high levels of PAHs has been associated with potential health risks and has been suspected carcinogens such as 7,12-dimethylbenz[α]anthracene (DMBA): a pure carcinogen substance that induce tumors in rodents. Significant exposure to DMBA can cause skin irritations, allergic reactions, respiratory disorders, and other adverse health effects [
2,
3,
4].
To combat the adverse effects caused by everyday exposure to air pollution, which we often live, studies have favored the exploration of natural antioxidants to mitigate the damage from these pollutants that can lead to chronic diseases such as cancer [
4].
Several researches have proven that
Pistacia lentiscus (anacardiaceae) (PL) is characterized by important therapeutic properties [
5,
6]. The famous physician Avicenna mentioned this plant for its hepato-protective effects on his book
Canon of Medicine [
7]. The traditional culture of this plant was inscribed as Intangible Cultural Heritage of Humanity by UNESCO [
8] for its use in folk medicine. It was cited several times in the Chilandar Medical Codex. This plant presents a promising alternative of phyto-therapeutic agents. Some bio-compounds of PL have proven liver protective effects on carbon tetrachloride (CCl4) induced a hepatotoxicity [
9]. Also, it was proven that this plant have an antioxidant effect demonstrated through the retraction of hepatic function after intoxication with sodium arsenite [
10]. It has an important capacity to treat some cancers especially gastric cancers [
11]. Studies have proven that PL biologic activities are strongly correlated to a high content of phenolic compounds [
12,
13].
As far as it could be ascertained, this is the first study investigating PL traditionally extracted fixed oil against DMBA-intoxication. Our study delves to investigate the in vivo antioxidant hepato-protective potential of PL against DMBA-intoxication on metabolic status and oxidative stress disorders in the liver and kidney of C57BL/6 female mice against the resultant steatosis and inflammation in liver, the in vitro anti-cancer effect as well as to assess the phytochemical composition of PL antioxidant phyto-compounds.
2. Materials and Methods
2.1. Plant Material and Fixed Oil Extraction
Pistacia lentiscus L., 1753 (Sapindales; Anacardiaceae) drupes were harvested from the region of Tabarka in December 2020, District of Jendouba, and Northeast Tunisia (Latitude: 36°57’16”N, Longitude: 8°45’29”E, altitude 108 m; annual rainfall 800-600 mm). The collected plants were identifïed and the certifïed specimen was deposited in the Herbarium of the National Research Institute of Rural Engineering Water and Forestry I.N.G.R.E.F-Tunisia under the reference VS1-PL 2009. The landscape in Tabarka region is not polluted with absence of both domestic and industrial pollution. Fixed oil (FOt) was extracted from freshly collected plants using a traditional method used in the region of Tabarka-Tunisia. Firstly, the harvested PL fruits were rinsed and stored. The black drupes (500gr) were grinded using a porcelain mortar. The mixture resulting from the grinding was put to kneading and skimming using a wooden spatula in a heated water bath. After that, the shredded material was put in the manual press to separate the liquid phase of the waste. Then it was put to sedimentation for 24-48h and thus oil was easily recovered. The extracted FOt was stored at 8°C, until analysed.
2.2. Cell Lines
The MDA-MB-231 (ATCC® HTB-26™) and MCF-7(ATCC® HTB-22™) human breast cancer cell lines were provided by Pr. José Luis, Institute of Neuro-physiopathology, University of Aix-Marseille, France.
2.3. Animals
C57BL/6-female mice breastfed aged four weeks were purchased from the Pasteur Institute of Tunis-Tunisia. The handling of the animals was in the respect of the code of practice for the Care and Use of Animals for Scientifïc Purposes and the European Community guidelines-(86/609/EEC). The trial was approved by the Ethical Committee of the National School of Veterinary Medicine of Tunis (approval number: 14/2020/ENMV). Mice were acclimated and housed in polypropylene cages under standard controlled conditions of the animal facility of the National School of Veterinary Medicine-Tunisia: 12/12h light/dark cycle, 20±2°C temperatures, 55%±15% humidity. Food and water were ad-libitum.
2.4. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
Chemometric profïling was performed using a GC-MS system (Thermo Fïsher Scientifïc, Walthan, Massachusetts, USA). The extracts were solubilised in methanol (1% v/v) and 1 μL of each sample was injected in a split mode (ratio 15:1) for 75 min, using Agilent GC7890B gas chromatography instrument coupled with an Agilent MS 240 Ion Trap (Agilent, CA, USA). The separation was accomplished in a HP-5MS capillary column (30 m×250 μm, fïlm thickness: 0.25 μm). Helium (99.99 %) was used as carrier gas, released at a constant flow rate of 1 mL/min. The initial oven temperature started at 40°C, maintained for 2 min, then increased 5°C/min to 250°C, and held constant at this temperature for 20 min. The injector temperature was set at 280°C. The detection was made in full scan mode for 60 min. Mass spectrometry (MS) operating parameters were as follows: ion source temperature: 200°C, interface temperature: 280°C, ionizing electron energy (EI) mode: 70 eV, scan range: 50–1,000 m/z. Interpretation and identifïcation bio-compounds were performed by comparing mass spectra with those referenced in the NIST 05 database (NIST Mass Spectral Database, PC-Version 5.0, 2005 National Institute of Standardization and Technology, Gaithersburg, MD, USA).
2.5. Experimental Design
The research plan obtained ethical clearance from the National School of Veterinary Medicine of Tunis Ethics Committee (Protocol ID Number: 14/2020/ENMV) and was in compliance with directive 2010/63/EU of animal welfare (Articles 26, 30 and 33) [
14]. The experiment was carried out for 28 days in the same conditions for all animals. Mice were divided into 4 groups of 10 animals in each:
Control-group: animals served as control and received equivalent volume of H2O + NaCl (1ml) every day (5/7),
DMBA-group: animals were treated with one dose per week of DMBA (20 mg/kg b.w./week) for 28 days,
DMBA+PL-group: animals received (20 mg/kg b.w.) once a week and daily dose (5/7) of PL FOt (100 mg/kg b.w.) for 28 days,
PL-group: animals received daily dose (5/7) of PL FOt (100 mg/kg b.w.) for 28 days,
Animals were sacrificed by decapitation according to the
American Veterinary Medicine Association Guidelines for the Euthanasia of Animals at the end of the experiment [
15]
2.6. Anthropometric Parameters
During the treatment, weight gain (g), food (g) and water (mL) intake, and blood sugar levels per mouse were taken regularly each two days. Also, blood sugar content was measured every two days through a drop of blood from the codal vein using an Accu-Chek blood meter.
2.7. Plasma Biochemical Parameters
Blood was collected in heparinized tubes for estimation of liver and kidney plasma parameters. A blood centrifugation at 3000 g for 10 min at 4°C was carried out and plasma was harvested.
Lipid profile of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) and liver and Kidney function parameters of aspartate aminotransferase (AST), alanines aminotransferases (ALT), alkaline phosphatase (PAL), C-reactive protein (C-RP) (kit-ELISA), urea, creatinine (Crea) were carried out according to a standard method [
16] using commercial diagnostic kits (BioSystems S.A., Costa Brava 30, 08030 Barcelona, Spain, certified according to ISO 13485 and ISO 9001).
2.8. Preparation of Homogenate and Estimation of Protein
Animals were sacrificed by decapitation then kidney and liver were quickly removed, washed in 0.9% NaCl and blotted on ash-free paper to weights. 0.4g of each tissue were placed in 4mL (50mM) phosphate buffered saline (PBS) solution (pH 7.8), homogenized and centrifuged for 15min at 9000g (4°C). Supernatants were collected and were stored at -80°C for the estimated oxidative stress biomarkers and various enzyme activities below. Total proteins in liver and kidney tissues homogenates were determined according to the biuret method [
17] using serum albumin as standard. Briefly, proteins in kidney and liver supernatants constituted with copper a colorful complex measurable at 546 nm wavelength and compared to the blank. Results were expressed as mg of protein.
2.9. Oxidative Stress Biomarkers
2.9.1. Lipid Peroxidation (TBARS) Assay
The level of malondialdehyde (MDA) in liver and kidney supernatants was determined as an index of lipid peroxidation according to the double heating method (TBARS) [
18]. A BHT-TCA solution (1% BHT dissolved in 20% TCA) was added to supernatant. After centrifuging at 1000g for 5 min at 4°C, the supernatants were mixed with HCl (0.5), TBA-Tris (TBA (120 mM) dissolved in Tris (26 mM)), then heated for 10 min at 80°C. After that the mixture was put directly in ice for cooling to stop the activity of the resulting chromophore. The MDA levels were determined by using an extinction coefficient for the MDA-TBA complex of 1.56 1M
− 1 cm
− 1 and expressed as nmol/mg protein.
2.9.2. Sulfhydryl Groups (-SH) Determination
Sulfhydryl groups concentration (-SH) was performed according to the method Ellman et al. (1959). Briefly, liver and kidney homogenates were each mixed with 100mL of EDTA (20mM; pH 8.2). Mixture was vortexed and absorbance was measured at 412 nm (A1). Then, 100mL of DTNB (10mM) was added to the mixture incubated for 15min and the optic density was measured at 412nm (A2). Results were calculated as (A2-A1-B) *1.57mM where B was the blank. The concentration of the sulfhydryl group was expressed as nmol /mg of protein [
19].
2.9.3. Hydrogen Peroxide (H2O2) Determination
Hydrogen peroxide was measured according to a standard colorimetric technique of Kakinuma et al. (1979), using available kit (BioSystems S.A., Costa Brava 30, 08030 Barcelona, Spain, certified according to ISO 13485 and ISO 9001). Briefly, H2O2 forms a red colored quinoa-eradicate after interaction with 4-amino-antipyrine and phenol. Absorbance was redden at 505 nm and results were deducted from a standard calibration curve and expressed as nmol/mg protein [
20].
2.10. Enzymes Antioxidant Capacity
The determination of enzymatic antioxidant activities was accomplished by detection of the glutathione peroxidase activity (GPx) performed according to the method of Rotruck et
al., (1980). Briefly, 0.2 mL of liver and kidney tissues homogenates were added to 0.2 mL of phosphate buffer (0.1 M, pH7.4), 0.2 mL of GSH (4 mM) and 0 .4 mL of H
2O
2 (5 mM). Then, the reaction mixture was incubated for 1 min at 37°C. Centrifugation was carried out for 5 min at 1500 g, after adding 0.5 mL of TCA (5%) to block the reaction. The supernatant (0.2 ml) was collected and mixed with 0.5 ml of DTNB (10 mM) and phosphate buffer (0.1 M, pH 7.4) [
21]. The glutathione peroxidase activity was measured at 412 nm wavelength and compared to the blank. Results were expressed as UI/mg of protein. The catalase activity CAT was also measured according to a standard method [
22] and expressed as µmol H
2O
2/min/mg of protein. The superoxide dismutase enzyme SOD activity was measured according to a standard method [
22] and expressed as Unity U SOD/mg of protein.
2.11. Histopathological Analysis
Directly after euthanasia, small pieces of liver and kidney were removed, washed with NaCl (0.9%) and were preserved in a buffered neutral formalin solution (10%). After dehydration with ethanol then xylene, samples were finished in paraffin to be cut into 0.2 µm thick sections. Putting on the slides, the sections were deparaffinized, hydrated (with decreasing concentrations of ethanol) to facilitate their staining with hematoxylin and eosin.
2.12. In Vitro Anticancer Effect
MDA-MB-231 and MCF7 cell lines were initially seeded in 96-well culture plates at a concentration of 10
4 cells/well. Following this seeding, the cells were subjected to incubation either alone or in escalating concentrations of PL FOt. After incubation periods of 24 hours or 72 hours, cellular viability and proliferation were evaluated through the utilization of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazolium reduction colorimetric MTT assay, as described by Mosmann in 1983 [
23]. Subsequently, cells were fixed with 3.7% formaldehyde, stained with 0.1% crystal violet, and lysed with SDS. Absorbance readings were taken at 560 nm wavelengths using a Multiskan microplate reader (Lab systems, GmbH). The untreated cells (medium) served as the positive control, and the results were presented as percentages of viable cells compared to the non-treated cells used as controls.
2.13. Statistical Analysis
The data was analysed using GraphPad Prism 8.4.2 Software (La Jolla, CA, USA). Data were determined by one-way analysis of variance (ANOVA), followed by Tukey post hoc test, and was expressed as the mean ± standard error (SEM). All statistical tests were three-tailed and a 𝑃 value of 0.05 or less was considered significant.
4. Discussion
In the present study we investigated the toxicity of DMBAcarcinogen induced oxidative damage in liver tissues on C57BL/6 female mice as well as the hepato-protective potential of PL FOt bio-compounds on the induced oxidative stress.
The PL composition underwent a chromatographic identification. We found that FOt GC-MS analysis has depicted new chemotypes, mainly: 1H-Indole-3-acetic acid, Benzeneacetic acid, methyl ester, Hexadecanoic acid, 13-octodecenoic acid and Bicyclo (2.2.1) heptan-2-one,1. These bio-compounds belong to monoterpenes, fatty acid and ester classes. Previous phytochemical studies carried out on
PL, have proven that FO was rich in alpha-pinene, beta-myrtene, beta-pinene, limonene and beta-karyophylene. FOt is a combination of terpenes where α-pinene (67%) can be the significant compounds. Some combination with myrcene induced can give a potent anticancer effect [
11]. It is also characterized by the presence of flavanol glycosides (flavonoids) (
Table 2 and Figure A1) such as: quercetin, myricetin, luteolin, isoflavone genistein, gallic acid and quinic acid [
24].
The main objective of this research was to evaluate the hepato-protective effect and antioxidant potential of
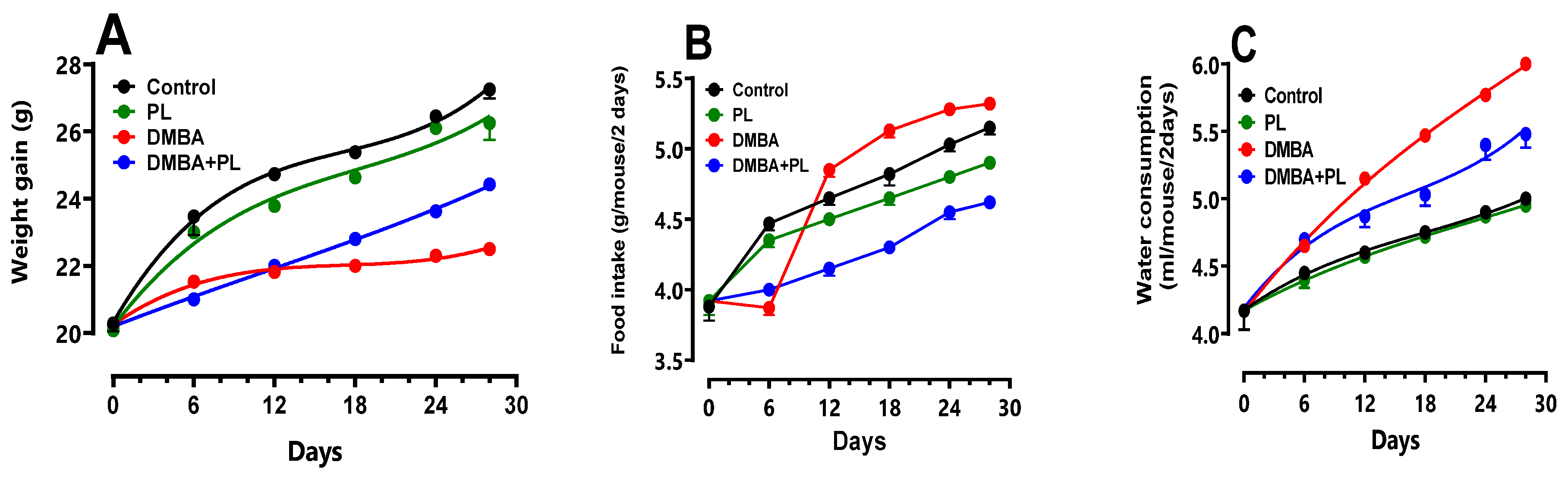
PL vs DMBA-oxidative damage on C57BL/6 female mice. Overall, the DMBA treatment induced an increase in body weight, water intake, blood sugar levels and a decrease in food intake compared to control and co-treated groups (
p<0.05). PL extract has a corrective effect in anthropometric parameters of weight, water intake, food intake and blood sugar levels compared to the DMBA group (
Figure 1.) (
p<0.05).
Our study has proven that DMBA induced an increase in lipid profile, liver function parameters and kidney function parameters (Table. 2.) compared to the control group and
PL group (
p<0.05). While the same parameters have decreased in the co-treated group compared to DMBA group (Table. 2.) (
p<0.05). Some metabolic disorders can be caused by a high-fat diet that can induce oxidative stress in C57BL/6J mice, leading to TG accumulation and hepatic steatosis [
25]. It was proven that oxidative stress induced by an intra-peritoneal hepatotoxic injection in C57BL/6 mice significantly increased the serum hepatic transaminase (ALT, myeloperoxidase), cytokines (TNF-α, IL-6, and IL-17), and lipid peroxidation [
26]. One the other hand, study have proven that different concentrations of
PL FO (0.1%-5%) reduced the cell viability of human fat cells [
27].
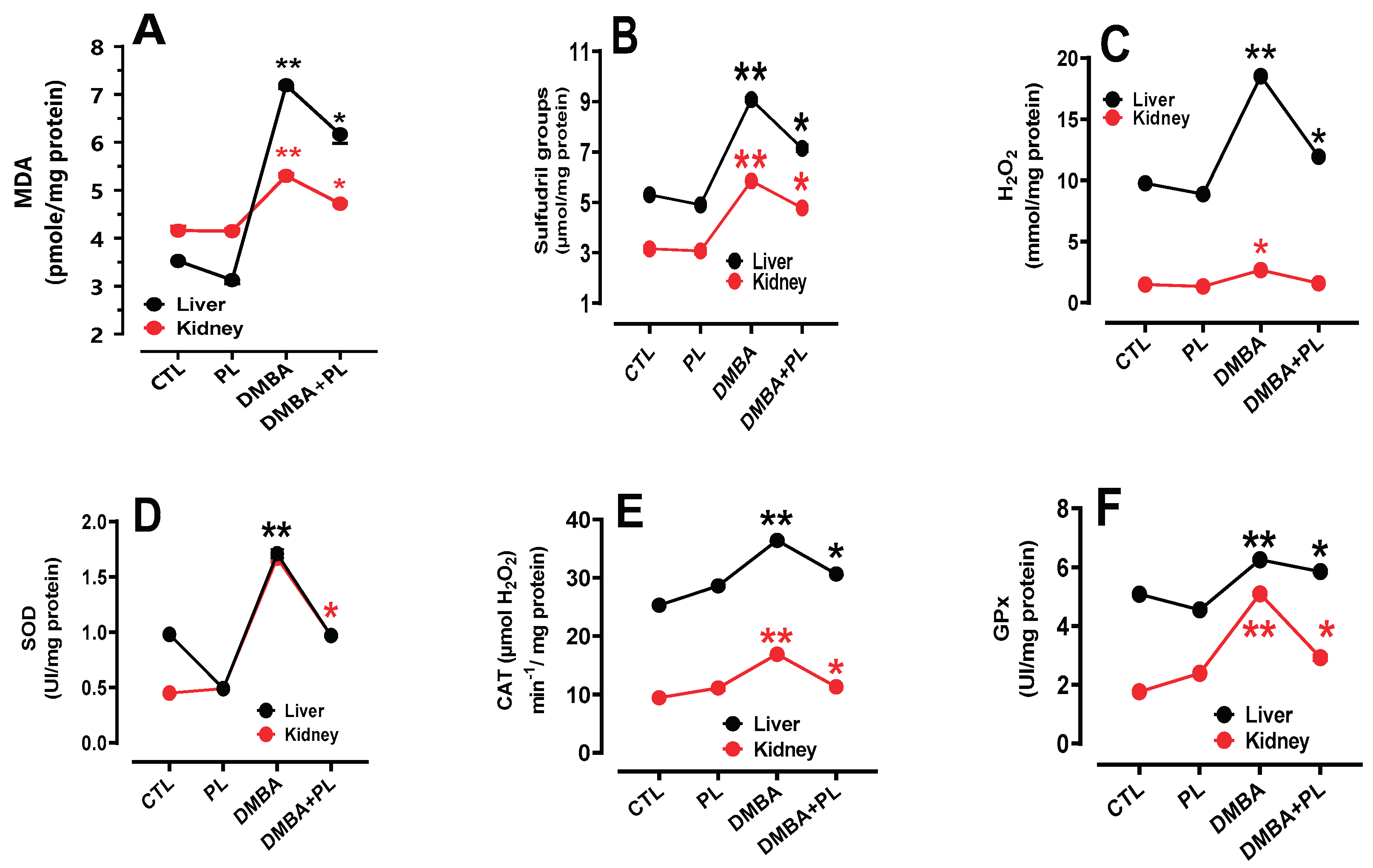
Our result proved that DMBA induced disturbance in oxidative stress biomarkers (MDA, H
2O
2 and -SH) in liver and kidney tissues (
Figure 2a–c) (Figure A2). Our results agree with other research proving that DMBA can cause an increase in ROS production, leading to lipid peroxidation and damage to liver cells. It was proven that DMBA significantly modulates cutaneous lipid peroxidation and induced an exhaustion of total antioxidant capacity that consequently inducing skin cancer [
28]. DMBA may also interfere with the normal antioxidant defense mechanisms and enzymes antioxidant activities (SOD, CAT, GPx) in liver and kidney cells (
Figure 2d–f). Antioxidants are molecules that can neutralize ROS and prevent oxidative damage. However, exposure to DMBA may reduce the levels of antioxidants in liver cells, making them more susceptible to oxidative stress and damage. Moreover, oxidative stress can lead to cellular damage and it is implicated in the development of various diseases, including liver and kidney disorders.
Antioxidants are bio-compounds that help protect cells from oxidative stress and damage caused by ROS, which are highly reactive molecules that can harm cellular components like DNA, proteins, and lipids [
28]. In this context, the most important result drawn from the present study is the corrector effect of
PL to prevent oxidative damages in liver. FOt decrease the lipid profile of
PL group compared to the control and in the cotreated group compared to DMBA group (p<0.05) (Table. 2.). Also, it has a potent antioxidant therapeutic effect that increases the antioxidant enzymes and reduces stress biomarkers in liver and kidney tissues. Our results are in agreement with results showing the antioxidant potential of PL extracts [
9]. There is some promising evidence to suggest that PL may have hepato-protective effects [
7]. Moreover, it has been proven that this plant has a corrective effect on the retraction of hepatic function after sodium arsenite intoxication [
9].
PL has relatively mitigated oxidative damage and can exhibit an increase in hepatic antioxidant enzymes [
10].
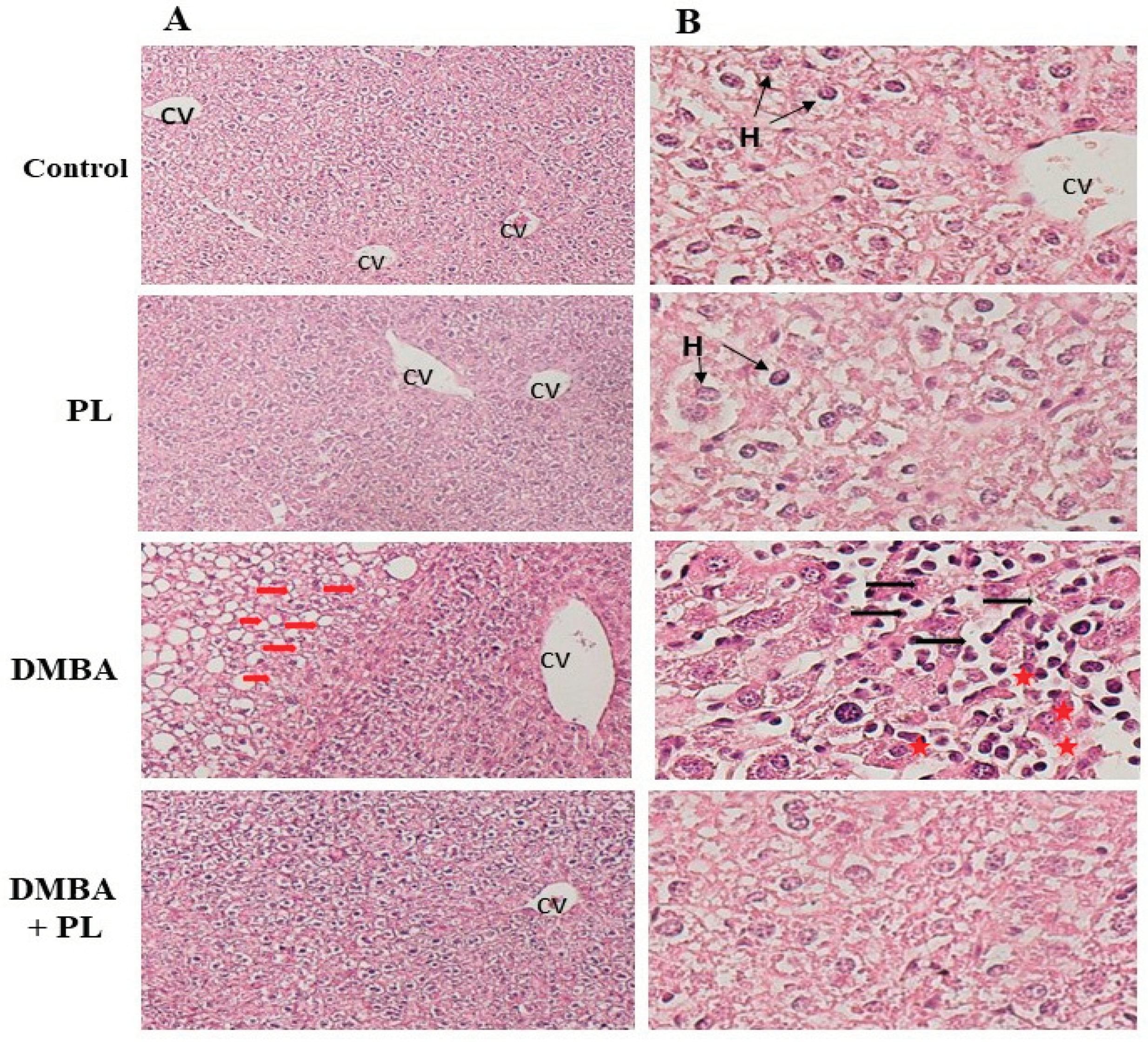
In the present study, our histology data has proven that DMBA treatment induced alteration in hepatocytes cells while no significant funding has been proven in the kidney in the short-term treatment (28 days). Several research have proven that exposure to DMBA led to the activation of an enzyme called sterol regulatory element-binding protein-1c (SREBP-1c), which is known to promote lipid synthesis in liver cells. The activation of SREBP-1c resulted in an increase in the expression of genes involved in fatty acid synthesis and lipid accumulation, leading to the development of steatosis. Some medicinal plant can be a potential hepato-protective agent. Artemisia annua leaf extract attenuates hepatic steatosis and inflammation in high-fat diet-fed mice [
9]. PL induces tumor-suppressing effects against experimental colon cancer [
11,
29]. Thus, these hepato-protective effects were attributed to the presence of bioactive compounds, such as polyphenols and flavonoids, which are known to possess antioxidant properties [
12].
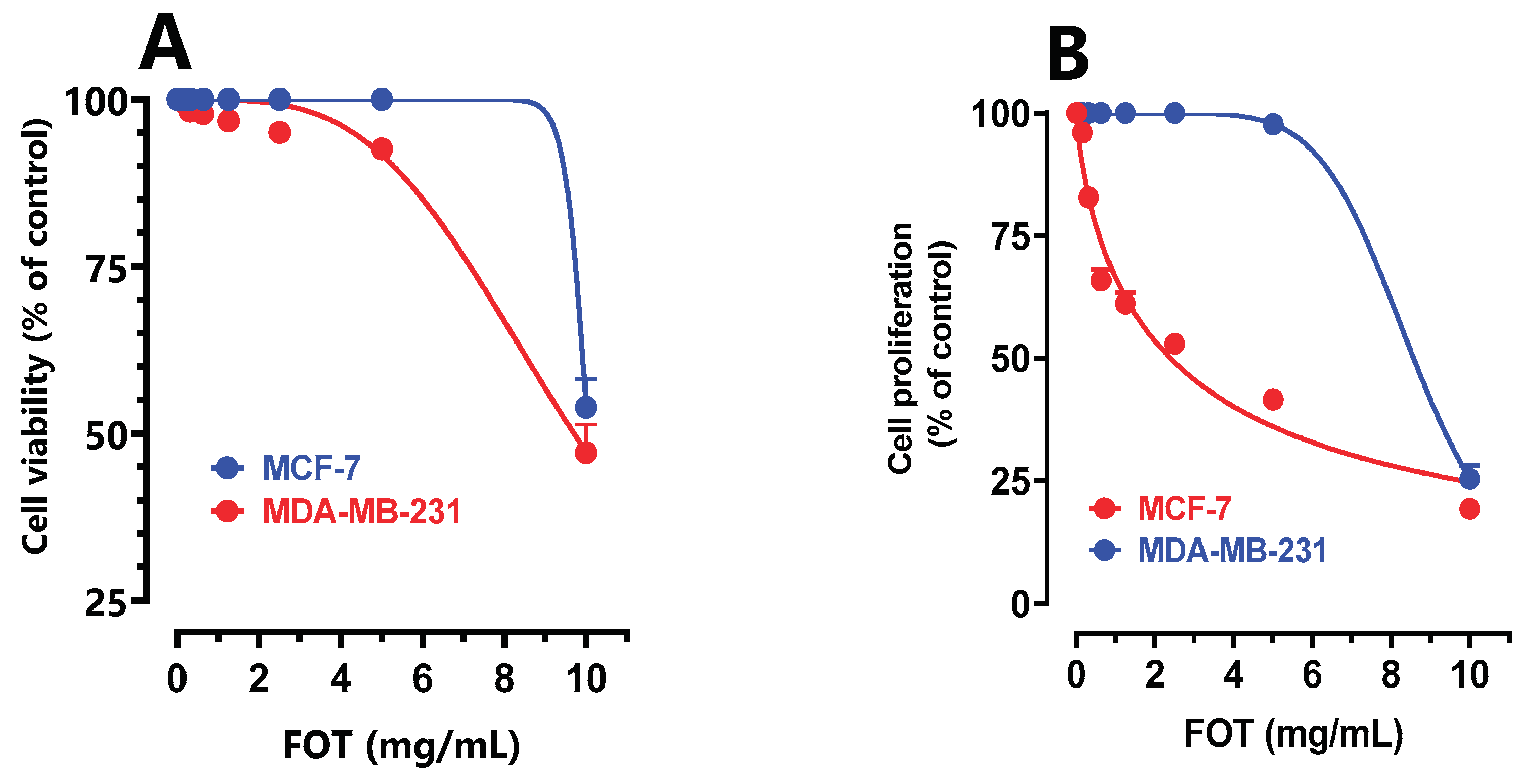
Our findings finding has proven an anti-proliferative potential of PL FOt unveiled in a dose dependent manner (
Figure 5). Our results agree with studies reporting that PL extracts blocks the differentiation of cancerous of 13 types of human tumour/leukaemia cells, CRC HCT116 cell lines and gastric cancer [
11,
29]. These naturally occurring phyto-compounds contribute to various biological activities and health benefits. Phytochemicals with antioxidant effects play a crucial role in maintaining cellular health and reducing the risk of chronic diseases, including cardiovascular diseases, cancer, and neurodegenerative disorders.
It has been proven that oxidative stress results in abnormal circumstances at the cellular and tissues levels, inducing the development and progression of liver cancers [
10]. In fact, oxidative stress is closely linked to the accumulation of fat in liver cells. The excessive accumulation of fat (triglycerides) in hepatocytes can lead to several conditions such as lipid peroxidation, mitochondrial dysfunction, inflammation, DNA damage and antioxidant defenses perturbation, fibrosis [
9] and even liver cancer in rare cases [
10]. In addition, the liver normally has a finely tuned system of pro-inflammatory and anti-inflammatory signals that maintain tissue homeostasis. However, exposure to oxidative stress may disrupt this balance and lead to an excessive or deregulated inflammatory response (
Figure 3). It can stimulate the production of cytokines and chemokines by these immune cells, leading to the recruitment of more immune cells to the liver and the initiation of an inflammatory response that can cause damage to liver cells and contribute to the development of steatosis [
26]. Moreover, fatty liver disease can cause changes in the way liver cells metabolize certain compounds, which can also contribute to the development of liver cancer [
28]. Overall, it may also have other coexisting conditions such as diabetes, obesity, and metabolic syndrome, which are also known to increase the risk of fatty liver disease and liver cancer.
One possible explanation for the link between fatty liver disease and cancer is that chronic inflammation and oxidative stress caused by the accumulation of fat in liver cells can damage DNA and other cellular components, leading to mutations that promote the development of cancer [
9].
There is evidence to suggest that
PL extract may have a protective effect against steatosis. Supplementation diet with
PL reduced the accumulation of fat in the liver [
30] witch improve liver function. One possible mechanism is through its antioxidant properties:
PL is rich in antioxidants, such as polyphenols and flavonoids [
6], which can scavenge free radicals and reduce oxidative stress in liver cells [
10,
13,
26].
PL bio-compounds may have antioxidant and anti-inflammatory properties to inhibit the production of pro-inflammatory cytokines and chemokines, which can reduce inflammation in the liver.
Figure 1.
Anthropometric parameters of body weight gain, water intake, food intake and blood sugar levels of C57/B6 female mice during the DMBA-treatment period and the protective effect of Pistacia lentiscus Animals were pretreated 28 days per oral (p.o), one dose per week of DMBA (20 mg kg, b.w.) alone for the DMBA group and with addition of daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o) in the DMBA + PL group. The PL was treated with daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o). While the control group was treated with (0.9%) NaCl every day (5/7). Values are given as median and (minimum value- maximum value), p<0.05 (ANOVA test).
Figure 1.
Anthropometric parameters of body weight gain, water intake, food intake and blood sugar levels of C57/B6 female mice during the DMBA-treatment period and the protective effect of Pistacia lentiscus Animals were pretreated 28 days per oral (p.o), one dose per week of DMBA (20 mg kg, b.w.) alone for the DMBA group and with addition of daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o) in the DMBA + PL group. The PL was treated with daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o). While the control group was treated with (0.9%) NaCl every day (5/7). Values are given as median and (minimum value- maximum value), p<0.05 (ANOVA test).
Figure 2.
The protective effect of Pistacia lentiscus against the 7,12-dimethylbenz(a)anthracene, inducing oxidative stress and antioxidant enzyme disorders in liver and kidney tissues of C57Bl/6-female mice. a, b, c: oxidative stress disorders in MDA, -SH and H2O2 parameters, respectively. d, e, f: antioxidant activity of SOD, CAT and GPx, respectively. Animals were pretreated 28 days per oral (p.o), one dose per week of DMBA (20 mg kg, b.w.) alone for the DMBA group and with addition of daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o) in the DMBA + PL group. The PL was treated with daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o). Values are given as median and (minimum value- maximum value), **p<0.05: compared to the control group, (ANOVA test) and * p<0.05: Compare to DMBA using non-parametric Kruskal-Wallis’s test.
Figure 2.
The protective effect of Pistacia lentiscus against the 7,12-dimethylbenz(a)anthracene, inducing oxidative stress and antioxidant enzyme disorders in liver and kidney tissues of C57Bl/6-female mice. a, b, c: oxidative stress disorders in MDA, -SH and H2O2 parameters, respectively. d, e, f: antioxidant activity of SOD, CAT and GPx, respectively. Animals were pretreated 28 days per oral (p.o), one dose per week of DMBA (20 mg kg, b.w.) alone for the DMBA group and with addition of daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o) in the DMBA + PL group. The PL was treated with daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o). Values are given as median and (minimum value- maximum value), **p<0.05: compared to the control group, (ANOVA test) and * p<0.05: Compare to DMBA using non-parametric Kruskal-Wallis’s test.
Figure 3.
Pistacia lentiscus protective effect against the 7,12-dimethylbenz(a)anthracene induced histo-pathologic alteration in liver and kidney tissues of C57/B6 female mice. A: The liver (H&E) Microscopic observation (X10 on the left), B: The liver Microscopic observation (X40 on the between), (n= 10/group) Animals were pretreated 28 days per oral (p.o), one dose per week of DMBA (20 mg kg, b.w.) alone for the DMBA group and with addition of daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o) in the DMBA + PL group. The PL was treated with daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o). H: hepatocytes injury, *: dilated sinusoids, PS: the portal space. Black arrows: accumulation of inflammatory and red cells.
Figure 3.
Pistacia lentiscus protective effect against the 7,12-dimethylbenz(a)anthracene induced histo-pathologic alteration in liver and kidney tissues of C57/B6 female mice. A: The liver (H&E) Microscopic observation (X10 on the left), B: The liver Microscopic observation (X40 on the between), (n= 10/group) Animals were pretreated 28 days per oral (p.o), one dose per week of DMBA (20 mg kg, b.w.) alone for the DMBA group and with addition of daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o) in the DMBA + PL group. The PL was treated with daily dose (5/7) of fixed oil of PL (100 mg kg, b.w. p.o). H: hepatocytes injury, *: dilated sinusoids, PS: the portal space. Black arrows: accumulation of inflammatory and red cells.
Figure 4.
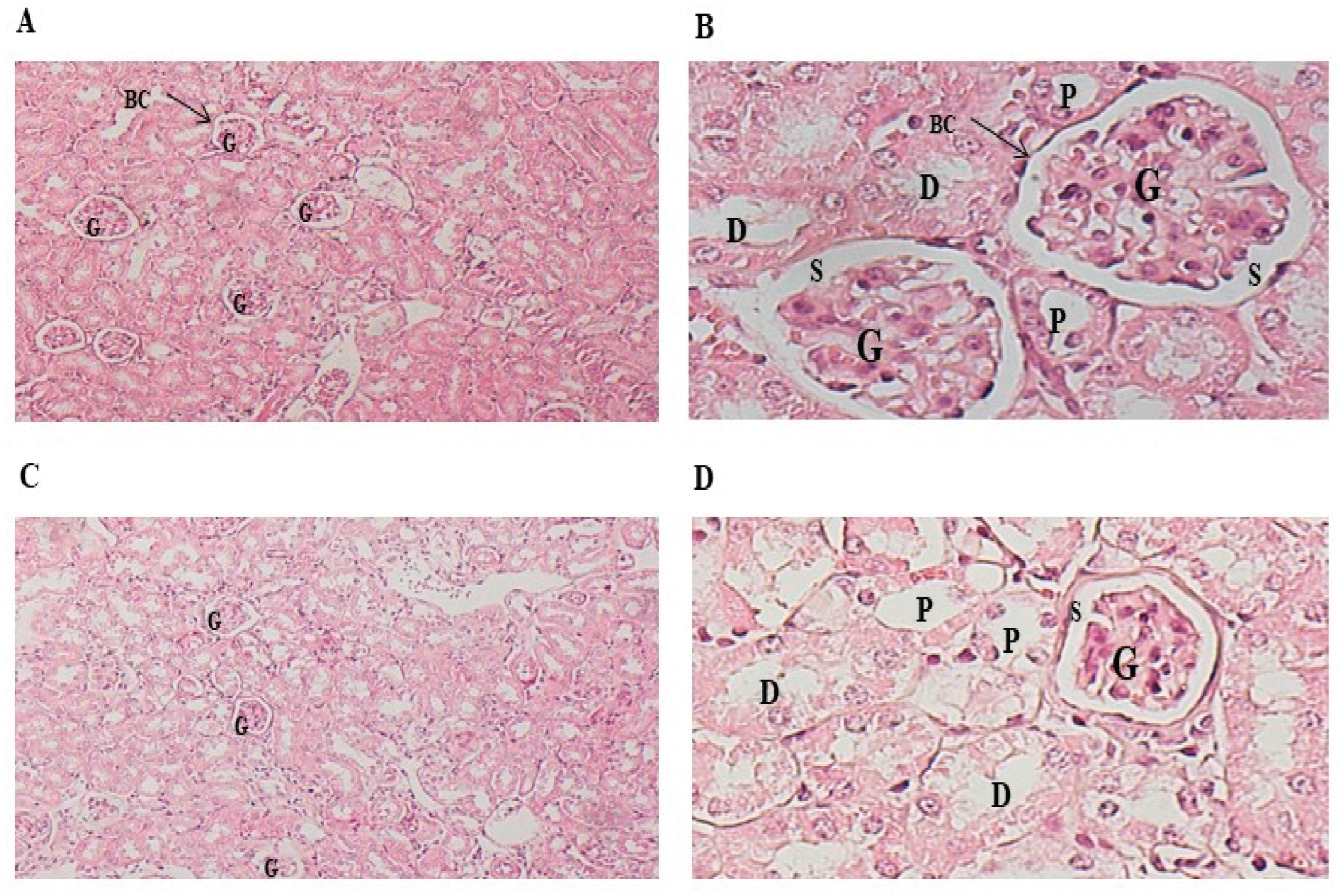
Protective effect of Pistacia lentiscus traditionally extracted fixed oil on DMBA induced histo-pathologic alteration in kidney tissues of C57/B6 female mice. Microscopic observation (X40) in the kidney (H&E) of A: the control group, B: PL group treated with fixed oil of PL 100 mg/kg bw daily dose (5/7), C: DMBA group treated with 20 mg/kg b.w dose per week for 28 days and D: DMBA+ PL group treated with fixed oil of PL at 100 mg/kg bw daily dose (5/7) dose and DMBA at 20 mg/kg bw dose per week for 28 days. BC: Bowman capsule, S: Bowman space, G: a glomerulus, P: The proximal tubule, S: Bowman space, D: distal tubules.
Figure 4.
Protective effect of Pistacia lentiscus traditionally extracted fixed oil on DMBA induced histo-pathologic alteration in kidney tissues of C57/B6 female mice. Microscopic observation (X40) in the kidney (H&E) of A: the control group, B: PL group treated with fixed oil of PL 100 mg/kg bw daily dose (5/7), C: DMBA group treated with 20 mg/kg b.w dose per week for 28 days and D: DMBA+ PL group treated with fixed oil of PL at 100 mg/kg bw daily dose (5/7) dose and DMBA at 20 mg/kg bw dose per week for 28 days. BC: Bowman capsule, S: Bowman space, G: a glomerulus, P: The proximal tubule, S: Bowman space, D: distal tubules.
Figure 5.
Effect of Pistacia lentiscus FOT extracted fixed oil on MCF-7 and MDA-MB cell viability (A) and cell proliferation (B).
Figure 5.
Effect of Pistacia lentiscus FOT extracted fixed oil on MCF-7 and MDA-MB cell viability (A) and cell proliferation (B).
Table 2.
Biochemical parameters of lipid profile, liver and kidney function and blood glucose content.
Table 2.
Biochemical parameters of lipid profile, liver and kidney function and blood glucose content.
| |
Lipid profile |
|
Liver function |
|
kidney function |
| |
TC (g/L) |
TG (g/L) |
HDL (g/L) |
LDL (g/L) |
|
ALT (UI/I) |
AST (UI/I) |
PAL (UI/I) |
C-RP (µg/dL) |
|
Crea (mg/L) |
Urea (g/L) |
| Group control |
1 ± 0.2 |
1.9 ± 0.1 |
1.1 ± 0.2 |
0.9 ± 0.1 |
|
98 ± 52 |
196 ± 72 |
212 ± 38 |
0.9 ± 0.6 |
|
4.9 ± 0.9 |
0.6 ± 0.1 |
| Group P.L |
0.9 ± 0.03 |
1.8 ± 0.5 |
1 ± 0.03 |
0.8 ± 0.05 |
|
96 ± 62 |
186 ± 82 |
204± 50 |
0.82 ± 0.7 |
|
4.9 ± 0.5 |
0.6 ± 0.05 |
| Group DMBA |
1.5 ± 0.2a
|
2.2 ± 0.9a
|
1.5 ± 0.2a
|
1.2 ± 0.09a
|
|
130 ± 88 a
|
230 ± 51 a
|
249 ± 65 a
|
1.5 ± 0.7 a
|
|
6.2 ± 0.5a
|
0.9 ± 0.09a
|
| Group DMBA+ P.L |
1.2 ± 0.1b |
2 ± 0.8b |
1.2 ± 0.1b |
1 ± 0.1b |
|
158 ± 73 b |
213 ± 62b |
223 ± 51b |
1.1 ± 0.3 b |
|
5.9 ± 0.3b |
0.7 ± 0.1b |