1. Introduction
Lung cancer today is the second most common malignancy and the leading cause of cancer death for both sexes worldwide [
1]. According to the Surveillance, Epidemiology, and End Results (SEER) database the estimated number of new lung cancer cases for 2023 in the United States is 238,340 with 127,070 deaths [
2]. In the last few years there is a trend toward increase in patients’ survival probably due to progress in early detection and the introduction of new biological and immunological treatments with better patient targeted therapy [
3].
More than 70% of all lung cancer new cases are diagnosed in advance stage, either locally advanced /regional (stage III) or metastatic (stage IV) [
2]. Patients in metastatic stage are referred to definitive oncological treatment which include chemotherapy, radiotherapy and today also biological and immunological therapy for suitable patients [
4,
5]. The stage of locally advanced disease is very heterogenic and according to the 8
th edition of the lung cancer staging system, it is composed of three sub groups (IIIA, IIIB and IIIC). Subgroup IIIA includes patients with tumor up to 5 cm with mediastinal lymph nodes involvement, patients with larger tumor size (> 5 cm) with hilum lymph nodes involvement and patient with tumor size larger than 7 cm without lymph nodes involvement. Subgroup IIIB includes patients with tumor up to 5 cm and contralateral lymph nodes involvement or patients with larger tumor size (> 5 cm) and mediastinal lymph nodes involvement. Subgroup IIIC includes patients with tumor size larger than 5 cm and contralateral lymph nodes involvement [
6].
Treatment recommendations for this complex stage is debatable. According to the National Comprehensive Cancer Network (NCCN) guidelines, patients in stage IIIA group (T1-2 N2, T3 N1, T4 N0-1) with resectable disease should be evaluated for surgical resection after induction of systemic oncological therapy with or without radiotherapy and with no apparent tumor progression during neoadjuvant therapy [
4]. For higher stage of locally advance disease (IIIB, IIIC with the exception of selected T3 noninvasive N2 positive cases) the recommendation is for definitive oncological treatment.
Several databases estimate that 20-35% of lung cancer patients are diagnosed at stage III [
7]. A recent study from Spain demonstrated, based on the thoracic tumor registry that 28.4% of lung cancer patients presented with stage III of which 15.8% with stage IIIA, 11.6% with stage IIIB and 1% with stage IIIC [
8]. Survival data of this lung cancer stage is problematic due to the heterogenicity of this group of patients. The SEER database analyzed 4,564 cases of unresectable stage III lung cancer (2009-2014) and found that the overall survival was 14.8 months with radio-chemotherapy and 13.2 months with chemotherapy. An overall 5-year survival rate of 34.5% was calculated for regional lung cancer patients (2009-2015) [
2,
9]. Patients with resectable locally advanced lung cancer disease seems to have better overall survival rates reaching as high as 27-34 months [
10]. Other factors that are associated with improved survival are young age, Caucasians race, female gender, Adenocarcinoma subtype and good performance status [
7]. Nevertheless, data of epidemiological and survival aspects of this stage is lacking due to small cohort studies and short duration of follow-up.
This study retrospectively evaluated all aspects of Carmel medical center experience with this complex group of patients. The work of the center's multidisciplinary committee (comprised of specialized medical professionals e.g. lung oncologist, pulmonologist, thoracic surgeon, and radiotherapist) and the decision making in real life are explored, including in-house and referred cases, which don’t always align with the best recommendations for diagnostic or follow-up strategy. The current study focus is on surgical patients who represent a very unique and small subgroup of the heterogenic stage III lung cancer population.
2. Patients and Methods
This study retrospectively evaluated all cases of locally advanced lung cancer that were assessed in Carmel medical center by a multidisciplinary lung cancer committee ( comprised of physicians specializing in medical oncology, thoracic surgery, pulmonology, radiotherapy, radiology, pathology, nuclear medicine) and were referred to surgical intervention after neoadjuvant oncological treatment from July 2013 to September 2020. The committee evaluated in-house cases as well as referral cases from other oncological institutions. The study was approved by theInstitutional Review Board, study No. 0118-21-CMC, 2021. Consent of patients has been waived due to the retrospective nature of the study.
All patients had undergone Positron Emission Tomography-Computed Tomography (PET-CT) as part of their evaluation and in 21 patients, invasive mediastinal investigation using Endobronchial Ultrasound (EBUS) was performed as part of their clinical staging. All cases were operated in Carmel medical center cardiothoracic surgical department.
Descriptive analyses assessing demographic and oncological data as well as treatment patterns was retrieved from medical records files for all patients. Disease diagnosis and clinical staging was reviewed according to the 8
th edition of the TNM lung cancer staging system [
6]. Neoadjuvant oncological treatment and post treatment evaluation was analyzed. Detailed surgical data was examined including post-surgical pathological staging. Patient
's follow-up and survival data including overall survival, defined as the duration from surgery to death, was calculated. The Kaplan–Meier method was used to estimate overall survival.
3. Results
3.1. The study population
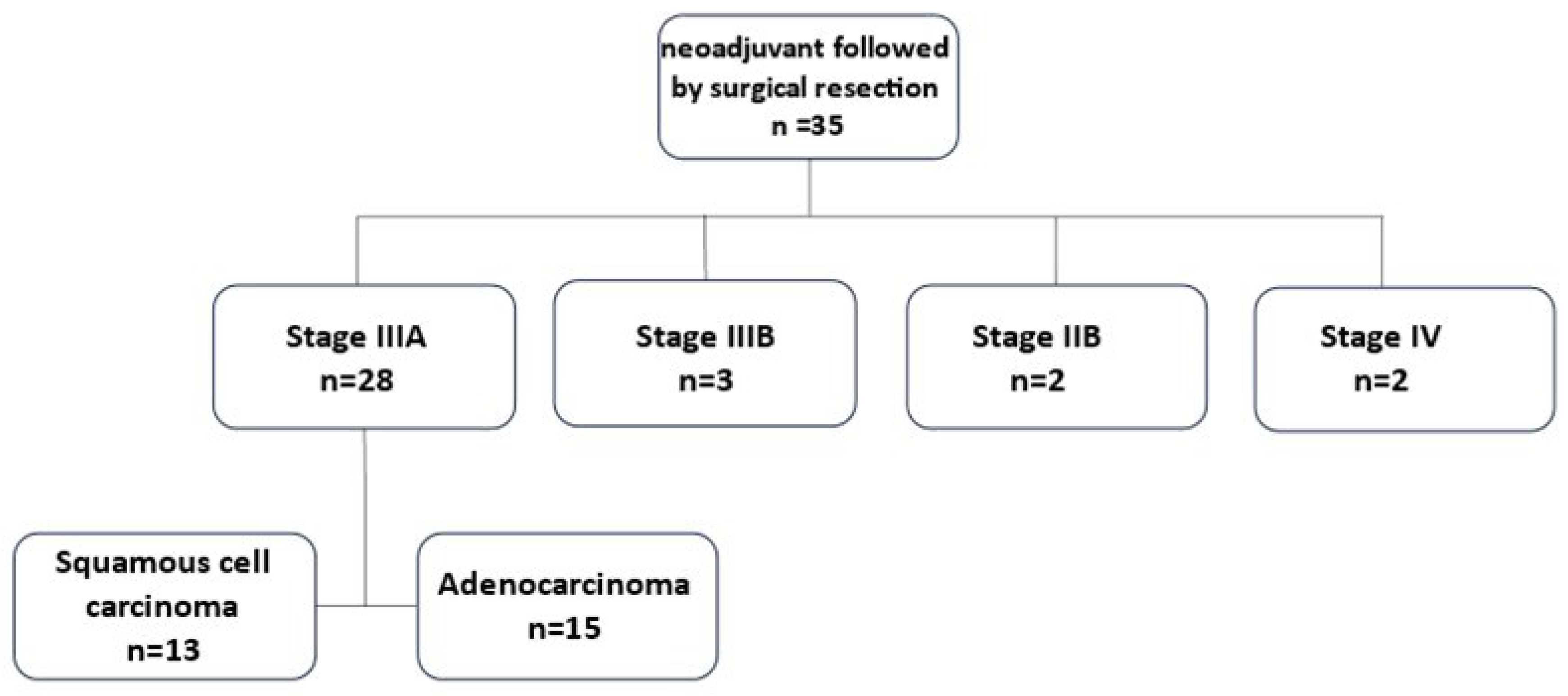
From July 2013 to September 2020, thirty-five patients diagnosed with locally advanced Non-Small Cell Lung Cancer (NSCLC) received neoadjuvant oncological treatment followed by surgical resection in Carmel medical center (
Figure 1).
Twenty-eight patients were assessed as clinical stage IIIA. Fifteen (53.6%) of them had adenocarcinoma and thirteen (46.4%) squamous cell carcinoma. The NCCN guidelines advocate for surgery as part of a multimodality treatment up to stage IIIA, therefore it was decided to focus on this group of patients.
The other seven patients include three patients assessed as clinical stage IIIB due to tumor size over 7 cm (T4) and metastatic lymph nodes in one mediastinal station (N2). In these three specific cases the multidisciplinary committee decided upon surgical intervention due to good response to neoadjuvant oncological treatment and very limited mediastinal disease. The patients were found to be without evidence of disease at follow-up from 3.5 to 7 years.
Two patients evaluated as clinical stage IIB. One patient had Pancoast tumor with chest wall invasion to the second right rib and the other had limited small cell lung cancer. In those two uncommon cases the multidisciplinary team support neoadjuvant oncological treatment prior to surgical intervention. 57 months after the surgical intervention of the patient with Pancoast tumor, no evidence of disease was detected. The patient with small cell lung cancer had spleen metastasis 6 months after anatomical lung resection (lobectomy) that was treated with radiation. 54 months from the last intervention the patient was found to be without evidence for lung cancer recurrence.
Two patients were evaluated as clinical stage IV. Those cases were determined as oligometastatic disease with single brain metastasis each. Overall survival in these cases was 3 months and 36 months after surgery.
The study cohort of lung cancer clinical stage IIIA patients included twenty-two (78.6%) male and six (21.4%) female with an average age of 64.6 years at surgery (range 47-82 years) (
Table 1). Clinical staging was assessed according to the 8th edition of the TNM lung cancer staging system [
6].
3.1.1. The T Component
Mean tumor size was 3.9 cm with a median of 3.6 cm (range 0.8-7.5 cm).
3.1.2. The N Component
In two patients no lymph node involvement was found (N0). In four patients only ipsilateral intra-parenchymal or hilum lymph nodes involvement was found (N1). In 22 patients, mediastinal lymph node involvement was found (N2). A more detailed analysis of the mediastinal lymph node involvement group showed that 20 patients had only single lymph node station involvement and only 2 patients had multiple station involvement. N1+N2 lymph node involvement was observed in 13 patients. In the other 9 patients with N2 disease no N1 involvement was detected.
3.2. Treatment
3.2.1. Neoadjuvant Treatment
The neoadjuvant oncological treatment included chemotherapy with two drug combination for all patients. The most common combination was Carboplatin and Taxol which was administered to 20 patients. Radiotherapy was given for 24 patients, 23 patients received a total dose of 60Gy and one patient received 72Gy. In 3 patients, preoperative treatment with immunotherapy, either Durvalumab or Pembrolizumab, was added.
All patients, but one, were evaluated using PET-CT after completion of neoadjuvant therapy. The only patient that didn’t have a PET-CT scan had CT Angiography and invasive mediastinal investigation using EBUS. In all cases but one, post neoadjuvant disease regression was noted, either in tumor size or in tumor avidity. In one case the disease was stable without progression.
The median time from neoadjuvant treatment to surgery was 67.5 days (range 40-230 days).
3.2.2. Surgical Treatment
In terms of surgical approach 16 patients had video assisted thoracoscopic surgery (VATS) and 10 patients had open thoracotomy. In 2 cases the minimal invasive approach had to be converted into an open approach due to massive adhesions in the lung hilum. One conversion was elective and the second was necessary due to bleeding from the pulmonary artery. No other major intraoperative incidents were noted.
25 lobectomies and 3 pneumonectomies were performed. In 2 of the lobectomies, the surgery was extended, in one case a segmental resection of the lung from the neighboring lobe was added; and a chest wall segment was added in the other case.
25 patients had uneventful post-surgical course. Post-surgical complications were noted in 3 patients and included a septic shock, a broncho-pleural fistula, arterial fibrillation and lung atelectasis requiring bronchoscopy. The 30-day mortality was nil.
3.3. Pathological Staging
The post-surgery pathological staging demonstrated one case of disease progression, eight cases of stable disease and 19 cases of disease regression. Importantly, in 7 (25%) cases pathological complete response was achieved. In these cases, neoadjuvant chemoradiotherapy was administered to 6 patients and chemoimmunotherapy to one case.
Table 2.
Post-surgery pathological staging of the study participants (n=28).
Table 2.
Post-surgery pathological staging of the study participants (n=28).
| Pathological staging |
Total |
| Pathological Complete response, n1 (%) |
7 (25%) |
| Local disease stage I, n (%) |
8 (29%) |
| Stage IA1, n
|
2 |
| Stage IA2, n
|
4 |
| Stage IA3, n
|
1 |
| Stage IB, n
|
1 |
| Local disease stage II, n (%) |
4 (14%) |
| Stage IIA, n
|
2 |
| Stage IIB, n
|
2 |
| Locally advanced disease stage III, n (%) |
8 (29%) |
| Stage IIA, n
|
8 |
| Stage IIB, n
|
0 |
| Metastatic disease stage IV, n (%) |
1 (3%) |
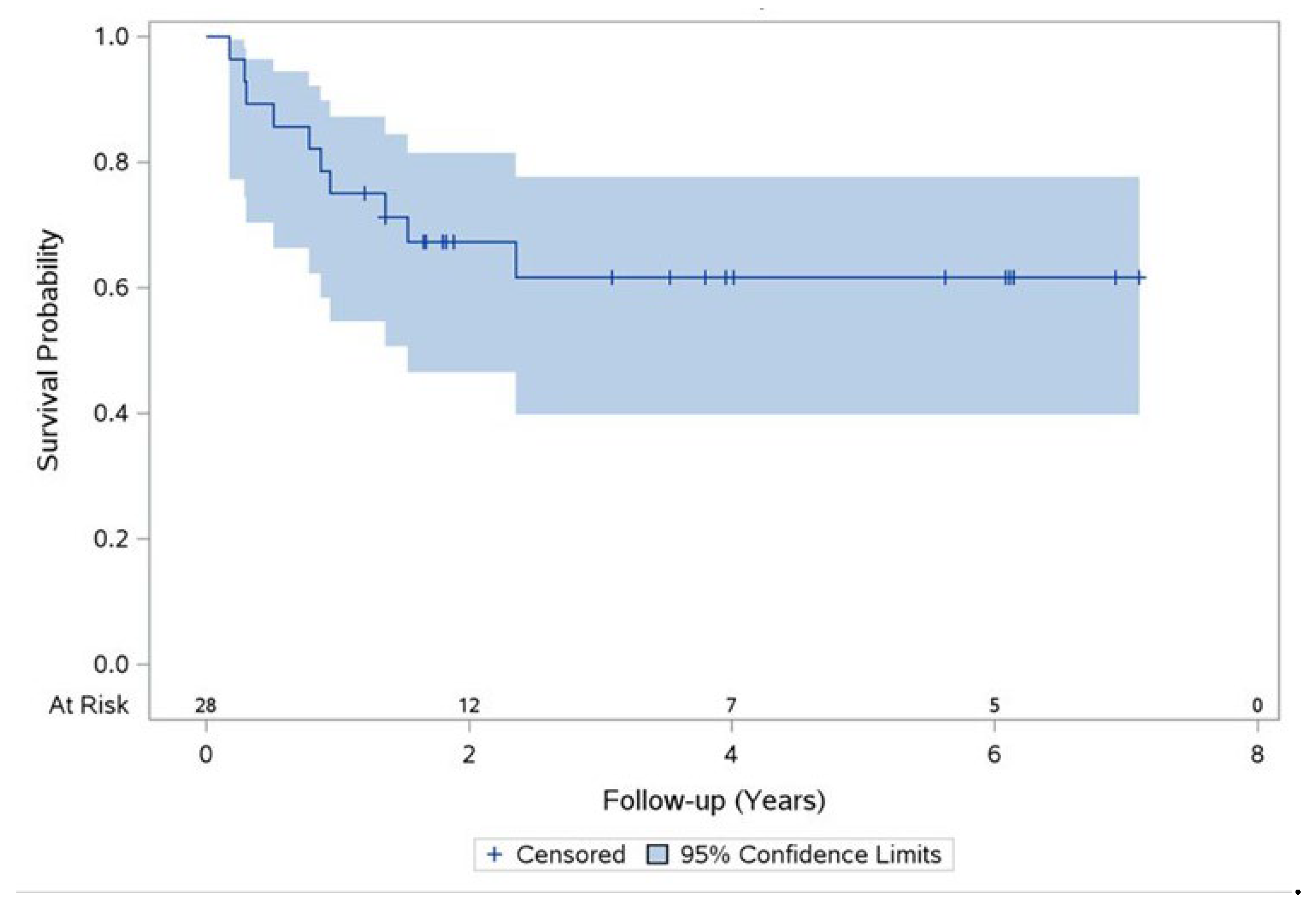
Ten cases of mortality from any cause during the study follow-up were documented. The 2-years overall survival rate was 67% and the 5-years overall survival rate was 62% (
Figure 2).
Disease recurrence was documented in 10 (36%) patients out of which seven patients had distant disease recurrence and three patients had combination of locoregional and distant recurrence.
4. Discussion
The aim of this study was to evaluate the real-life results of multi-modality treatment approach to locally advanced lung cancer patients. Although the combination of neoadjuvant therapy followed by surgery for stage IIIA is recommended in the NCCN guidelines for suitable patients [
4] with documented increase in survival [
10] it is not the routine strategy for the majority of stage III lung cancer patients. Literature review indicate that most patients in this stage (IIIA) will be referred to oncological treatment only and surgery will be abandoned [
9,
11,
12]. Clinical studies that evaluate multi-modality treatment for locally advanced lung cancer are relatively few. The small number of patients with stage IIIA treated with multimodality therapy including surgery is probably low due to several reasons. Some could be patient related reasons such as low performance status, inability to endure surgery due to medical or mental condition. Other reasons could reflect the patient’s physician preference of oncological treatment. The necessity for a cooperative multidisciplinary team that evaluates the patient status before and during all stages of treatment is a demanding requirement, which is needed for this kind of patient-oriented treatment in today’s medicine. The role of the multidisciplinary committee is especially important in light of the rapid changes and developments taking place in lung cancer treatment these days.
The end point result of this study demonstrated relatively high overall survival in two and five years after the combined oncological-surgical treatment concluded. This result reflects a suitable selection of patients for this line of multimodality treatment. An early indication for this good outcome was seen in the pathological complete response rate (25%) after surgery. This factor that is growingly used today as a surrogate endpoint for survival is as high as seen in recently published studies of modern chemo-immuno combination systemic therapies for operable lung cancer patients [
13].
The surgical results can attest for the feasibility of surgery after chemoradiation treatment as reported in other studies [
14,
15]. The ability to perform surgery in most cases with minimal invasive approach and low conversion rate, combined with R0 pathological resection in all cases and low complication rate, represents the important value of a dedicated oncological thoracic surgical team. Although the present study cohort includes a small number of pneumonectomies, complications were not encountered in this subgroup [
16].
The local disease control as represented in the described disease recurrence patterns (7 cases of distant recurrence versus 3 cases of locoregional and distant recurrence) is an indication for the overall good local control achieved in this multimodality treatment approach.
This study has an inherent limitation due to its selection bias of patients, small sample size and inconsistent treatment regimens which are the result of retrospective data collection of relatively highly selective group of patients. Yet, it is important to show the good results of this combined treatment approach for suitable patients.
5. Conclusions
It can be concluded that in this real-life small number highly selective patients’ study there is a benefit for the combination of oncological neoadjuvant chemoradiation followed by surgery treatment for resectable locally advanced lung cancer patients.
Author Contributions
Conceptualization, D.L.F. and A.A.; methodology, D.L.F. and A.A.; software, D.L.F. and A.A.; validation, D.L.F. and A.A.; formal analysis, D.L.F. and A.A..; investigation, D.L.F. and A.A.; resources, D.L.F. and A.A.; data curation, D.L.F. and A.A.; writing—original draft preparation, D.L.F. and A.A.; writing—review and editing, DL.F. and A.A.; visualization, D.L.F. and A.A.; supervision, D.L.F.; project administration, D.L.F. and A.A.; funding acquisition, D.L.F., A.A., B.C., M.L., S.K.R., S.S., E.S., and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Carmel Medical Center (protocol code 0118-21-CMC).
Informed Consent Statement
Patient consent was waived due to retrospective nature of the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. April 2023. Available online: http://seer.cancer.gov/ (accessed on 4 April 2024).
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.L.; Mariotto, A.B.; Lowy, D.R.; Feur, E.J. al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–6492. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer Guidelines in Oncology. Version 3.2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 4 April 2024).
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S.Jr.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Casal-Mouriño, A.; Ruano-Ravina, A.; Lorenzo-González, M.; Rodríguez-Martínez, Á.; Giraldo-Osorio, A.; Varela-Lema, L.; Pereiro-Brea, T.; Barros-Dios, J.M.; Valdés-Cuadrado, L.; Pérez-Ríos, M. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl. Lung Cancer Res. 1037. [Google Scholar]
- Provencio, M.; Carcereny, E.; Rodríguez-Abreu, D.; López-Castro, R.; Guirado, M.; Camps, C.; Bosch- Barrera, J.; García-Campelo, R.; Ortega-Granados, A.L.; González-Larriba, J.L.; Casal-Rubio, J.; Domine, M.; Massutí, B.; Sala, M.Á.; Bernabé, R.; Oramas, J.; Del Barco, E. Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study). Transl. Lung Cancer Res. 2019, 8, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Bobbili, P.; Ryan, K.; Duh, M.S.; Dua, A.; Fernandes, A.W.; Pavilack, M.; Gomez, J.E. Treatment patterns and overall survival among patients with unresectable, stage III non-small-cell lung cancer. Future Oncol. 2019, 15, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Vinod, S.K.; Wai, E.; Alexander, C.; Tyldesley, S.; Murray, N. Stage III Non-Small-Cell Lung Cancer Population-Based Patterns of Treatment in British Columbia, Canada. J. Thorac Oncol. 2012, 7, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Urvay, S.E.; Yucel, B.; Erdis, E.; Turan, N. Prognostic Factors in Stage III Non-Small-Cell Lung Cancer Patients. Asian Pac. J. Cancer Prev. 2016, 17, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Santana-Davila, R.; Devisetty, K.; Szabo, A.; Sparapani, R.; Arce-Lara, C.; Gore, E.M.; Moran, A.; Williams, C.D.; Kelley, M.J.; Whittle, J. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J. Clin. Oncol. 2015, 33, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Shyr, Y.; Sepesi, B.; Forde, P.M. Preoperative and Postoperative Systemic Therapy for Operable Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 20, 40:546-555. [CrossRef]
- Suh, J.W.; Park, S.Y.; Lee, C.Y.; Lee, J.G.; Kim, D.J.; Paik, H.C.; Chung, K.Y. Feasibility and Surgical Outcomes of Video-Assisted Thoracoscopic Pulmonary Resection in Patients with Advanced-stage Lung Cancer after Neoadjuvant Chemoradiotherapy. Thorac. Cancer 2019, 10, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Romero Roman, A.; Campo-Canaveral de la Cruz, J.; Macia, I.; Escobar Campuzano, I.; Figueroa Almanzar, S.; Delgado Roel, M.; Galvez Munoz, C.; Garcia Fontan, E.M.; Muguruza Trueba, I.; Romero Vielva, L.; et al. Outcomes of Surgical Resection after Neoadjuvant Chemoimmunotherapy in Locally Advanced Stage IIIA Non-Small-Cell Lung Cancer. Eur. J. Cardiothorac. Surg. 2021, 60, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Rocco, G.; Szanto, Z.; Thoomas, P.; Falcoz, P.E. Morbidity and Mortality of Lobectomy or Pneumonectomy after Neoadjuvant Treatment: An Analysis from the ESTS Database. Eur. J. Cardiothorac Surg. 2019, 57, 740–746. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).