Submitted:
08 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Modification of Alginate with L-DOPA
2.3. Spectral Characterization of Modified Alginate
2.4. Expression of Laccase on Cell Surface of Saccharomyces cerevisiae
2.5. Cell lysis and Cell Wall Laccase Preparation
2.6. Determination of Enzyme Activity of Whole Cells and Cell Walls with Laccase
2.7. Immobilization of Cell Wall Laccase in L-DOPA-Alginate
2.8. Incubation of Beads in Eosin Y and Photopolimerization
2.9. Determination of Enzyme Activity of Immobilized Cell Wall Laccase
2.10. Determination of Thermal Stability of Immobilized Cell Wall Laccase
2.11. Dye Decolorization
2.12. Reusability
3. Results and Discussion
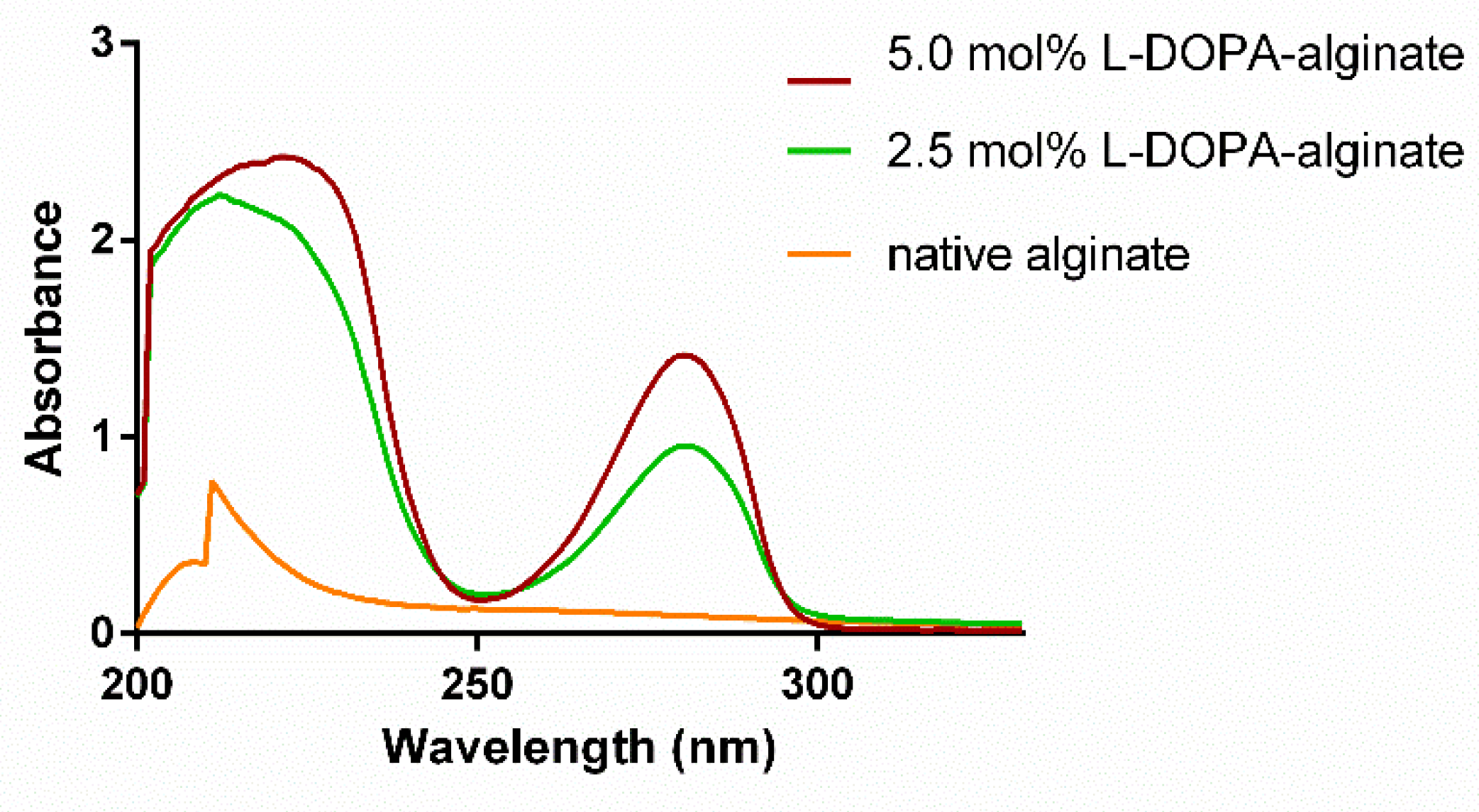
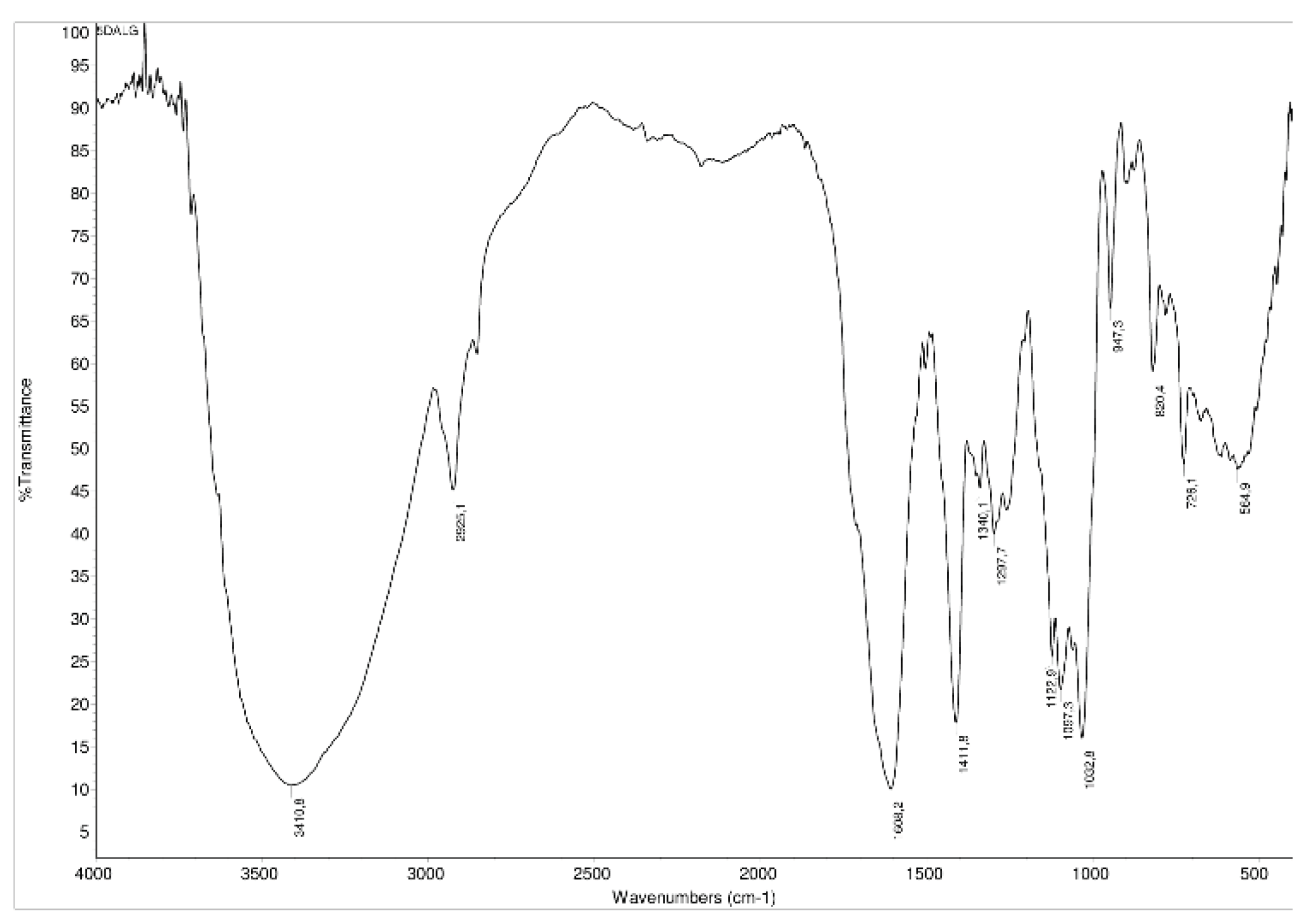
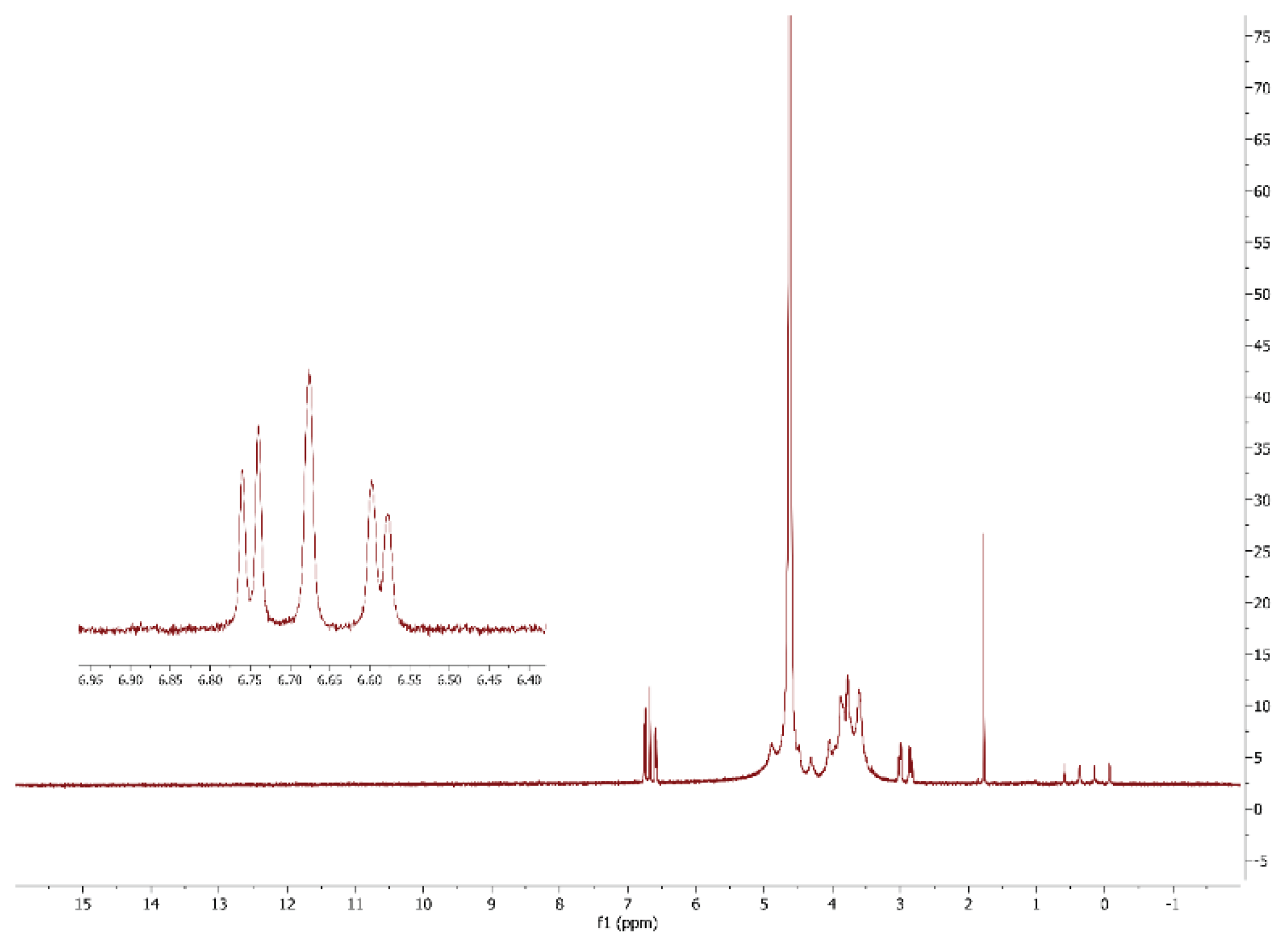
3.1. Modification of Alginate with L-DOPA and Its Characterization
3.2. Yeast Surface Display of Laccase
3.3. Preparation of Cell Wall Laccase
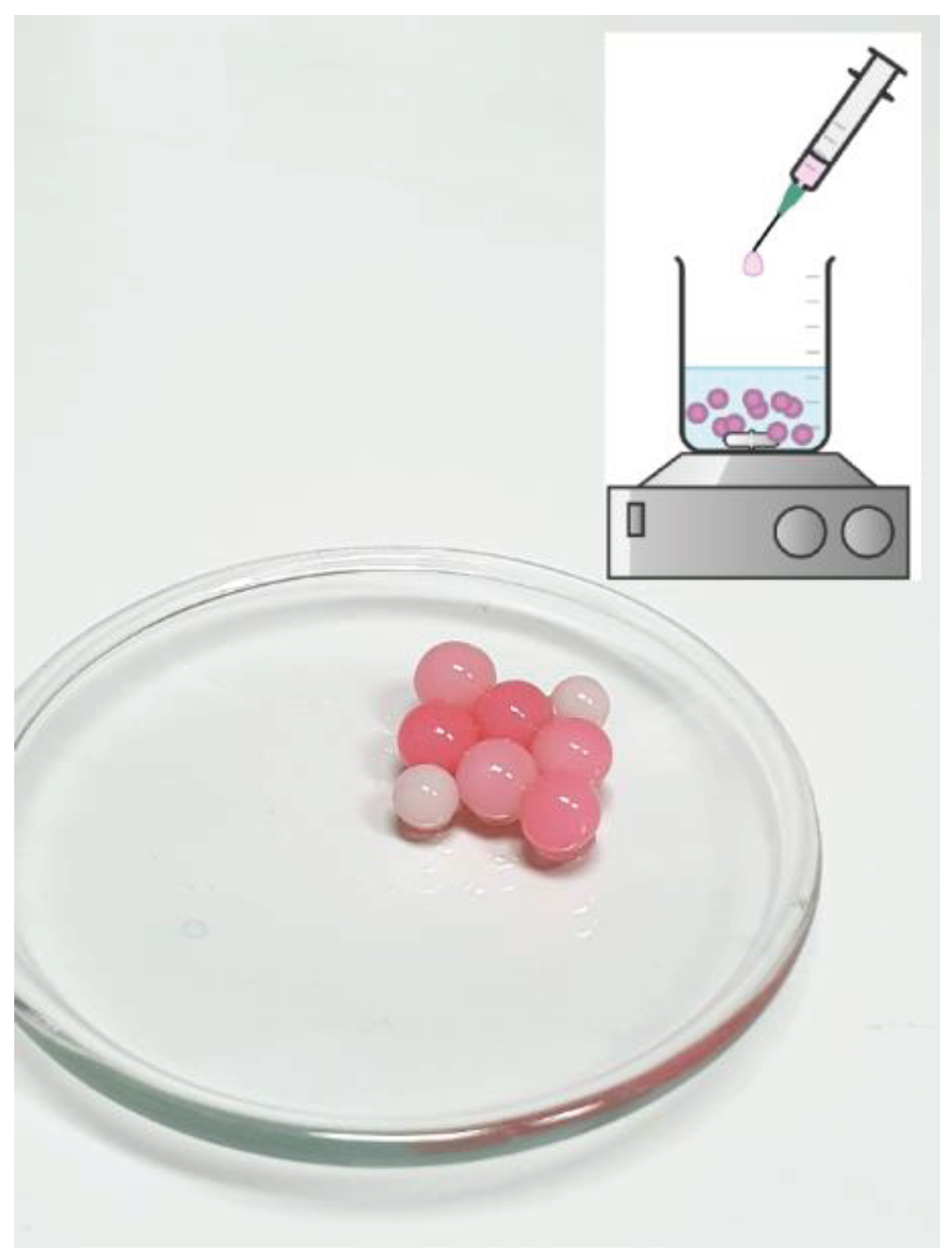
3.4. Immobilization od Cell Wall Laccase in L-DOPA-Alginate
3.5. Photopolimerization of L-DOPA-Alginate Beads with Immobilized Cell Wall Laccase
3.6. Determination of Enzyme Activity of Immobilized Cell Wall Laccase in Different Types of Beads
| 2.5 mol% L-DOPA | 5.0 mol% L-DOPA | |
| Beads without EY | 0.0170 ± 0.0012 | 0.0276 ± 0.0033 |
| Beads with 0.0001% EY | 0.0324 ± 0.0021 | 0.0337 ± 0.0004 |
| Beads with 0.001% EY | 0.0247 ± 0.0045 | 0.0232 ± 0.0031 |
| Incubated beads in 0.01% EY for 5 min. | 0.0288 ± 0.0037 | 0.0322 ± 0.0022 |
| Incubated beads in 0.01% EY for 30 min. | 0.0124 ± 0.013 | 0.0162 ± 0.0017 |
| Incubated beads in 0.005% EY for 5 min. | 0.0207 ± 0.0021 | 0.0294 ± 0.0026 |
| Incubated beads in 0.005% EY for 30 min. | 0.0142 ± 0.0012 | 0.0131 ± 0.0011 |
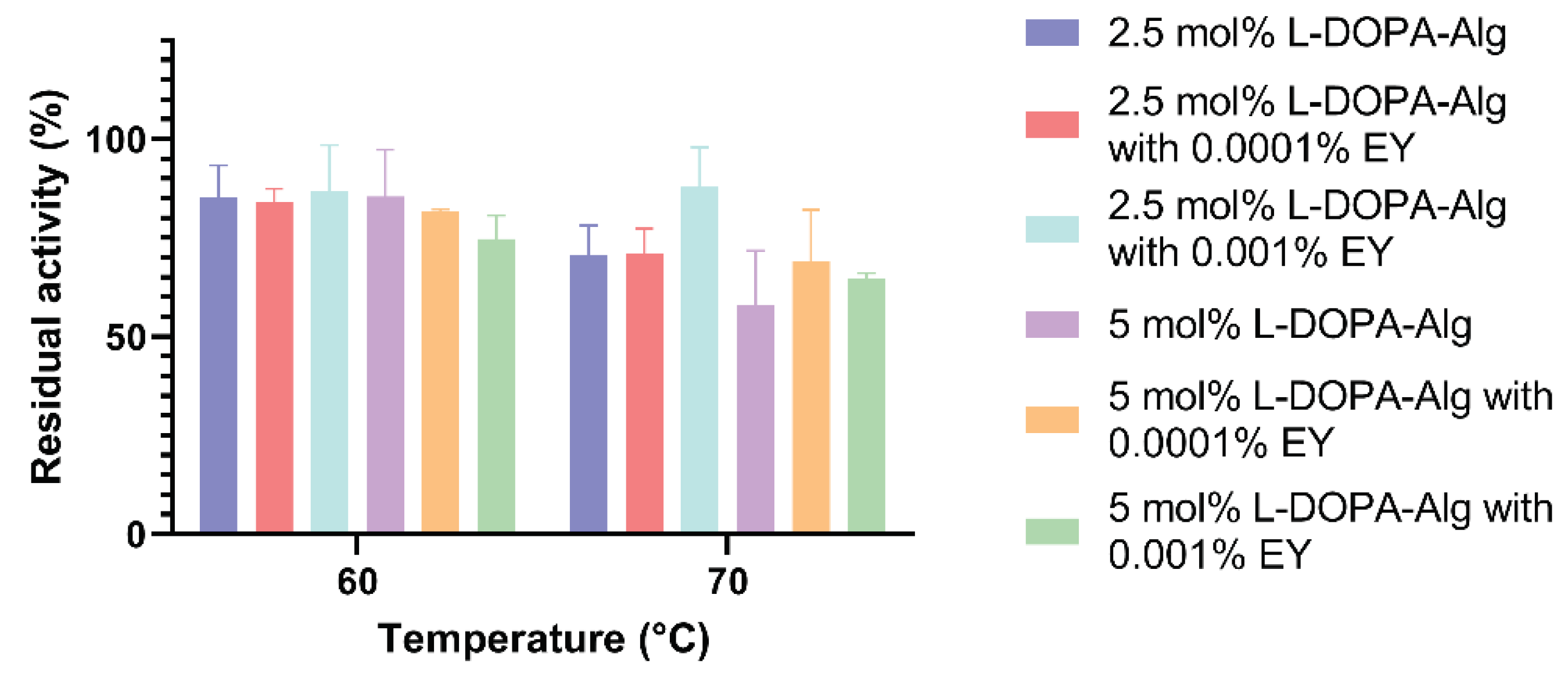
3.7. Determination of Temperature Stability
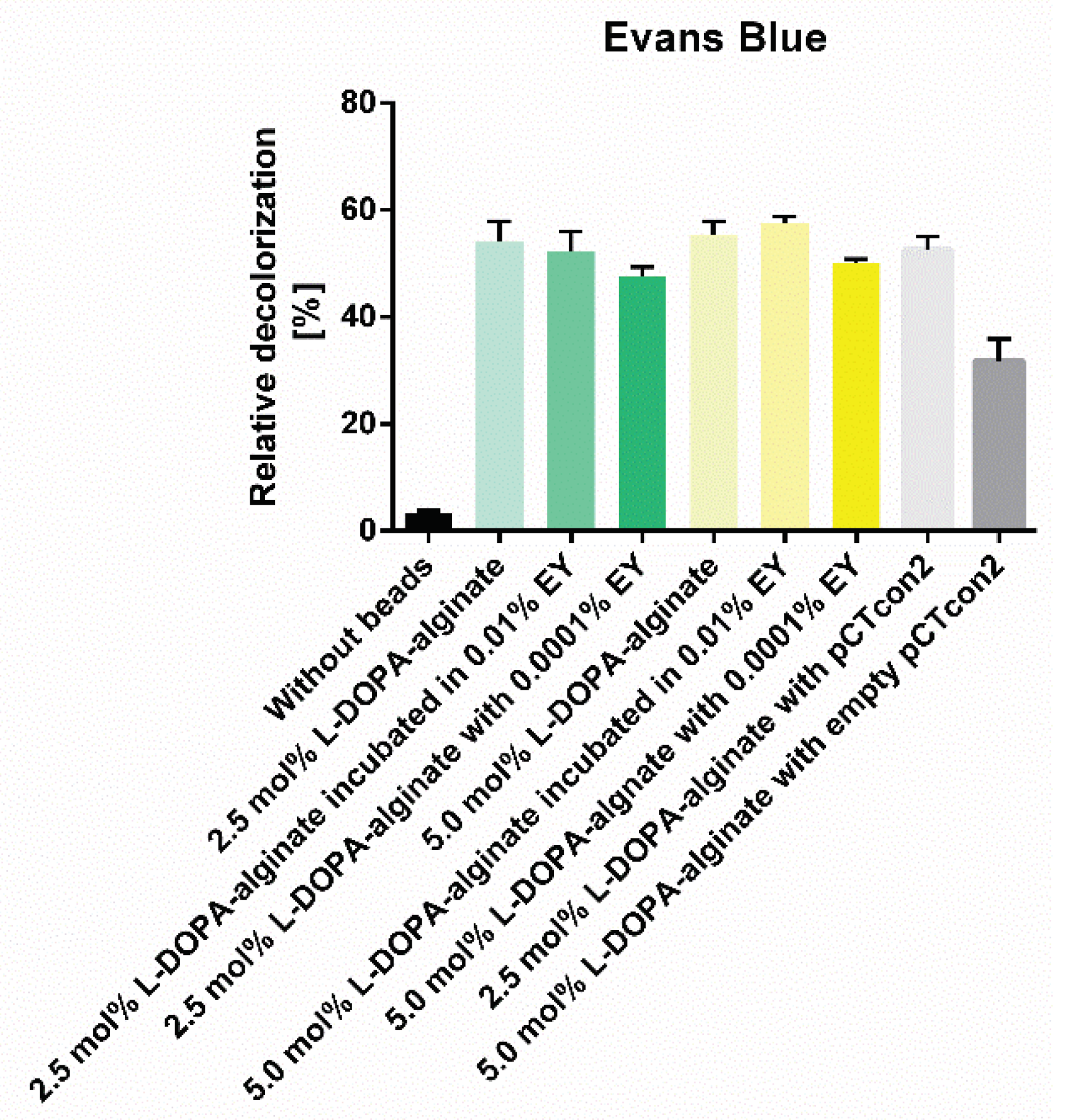
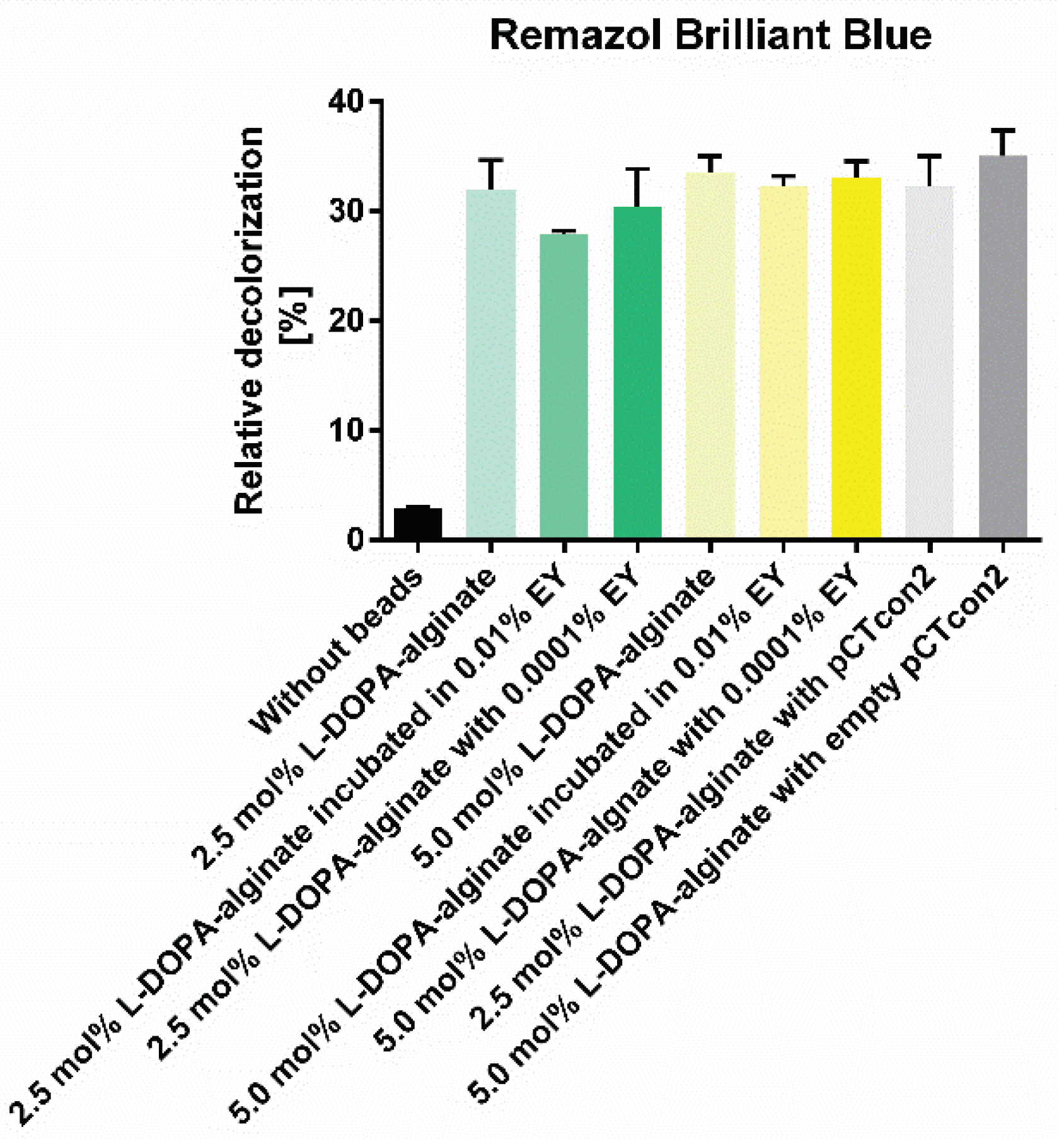
3.8. Decolorization of Dyes
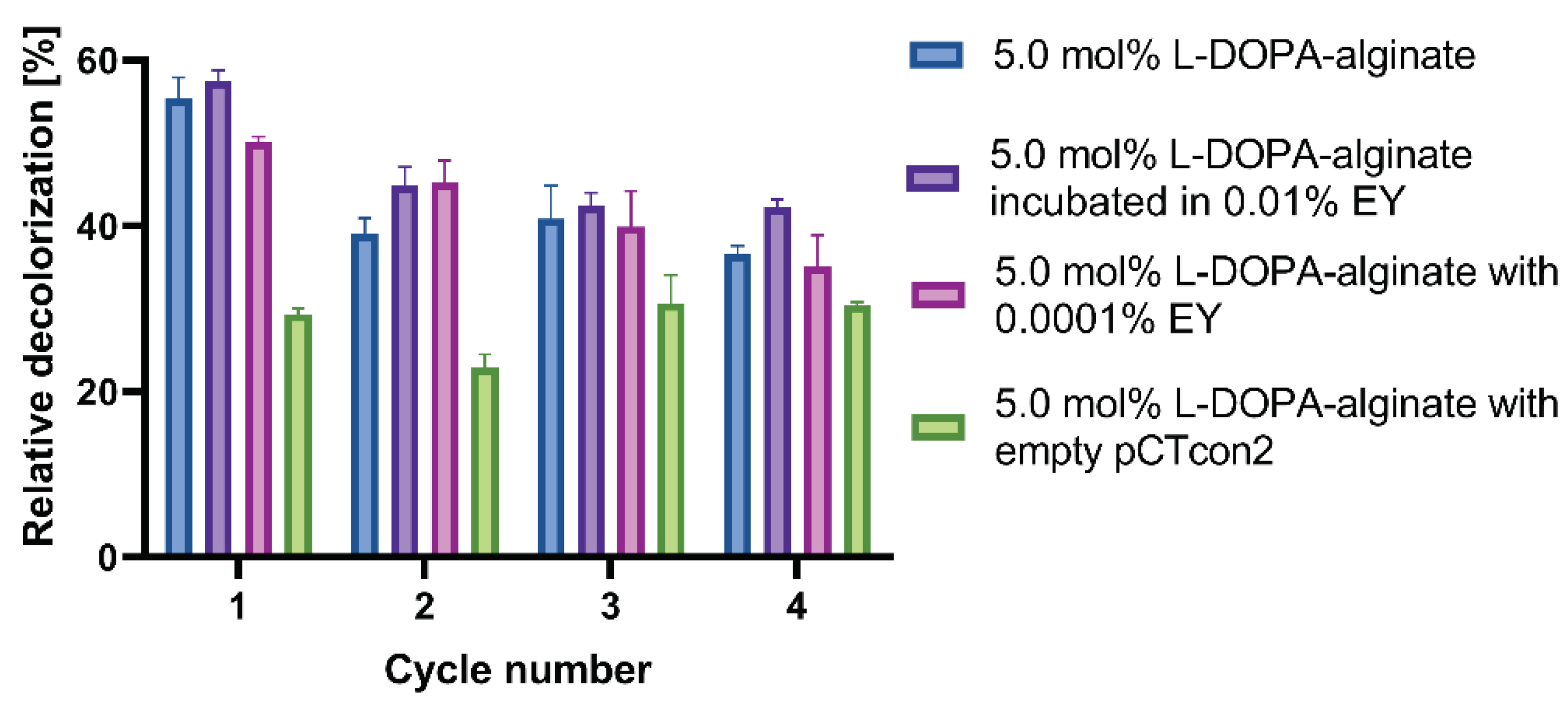
3.9. Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Akshaya, S.; Nathanael, A.J. A Review on Hydrophobically Associated Alginates: Approaches and Applications. ACS Omega 2024, 9, 4246–4262. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydrate Polymers 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Tao, L.; et al. A review on the chemical modification of alginates for food research: Chemical nature, modification methods, product types, and application. Food Hydrocolloids 2024, 147, 109338. [Google Scholar] [CrossRef]
- Hurtado, A.; et al. Alginate: Enhancement Strategies for Advanced Applications. International Journal of Molecular Sciences 2022, 23, 4486. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Kafrani, A.; et al. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Critical Reviews in Food Science and Nutrition 2021, 61, 3160–3196. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; et al. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chemical Reviews 2020, 120, 10950–11027. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; et al. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Scientific Reports 2021, 11, 23276. [Google Scholar] [CrossRef] [PubMed]
- Shopperly, L.K.; et al. Blends of gelatin and hyaluronic acid stratified by stereolithographic bioprinting approximate cartilaginous matrix gradients. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2022, 110, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; et al. Photocrosslinkable and self-healable hydrogels of chitosan and hyaluronic acid. International Journal of Biological Macromolecules 2022, 216, 291–302. [Google Scholar] [CrossRef]
- Moon, S.H.; et al. Photocrosslinkable natural polymers in tissue engineering. Frontiers in Bioengineering and Biotechnology 2023, 11. [Google Scholar] [CrossRef]
- Lu, S.-Y.; et al. Saccharomyces cerevisiae surface display of endolysin LysKB317 for control of bacterial contamination in corn ethanol fermentations. Frontiers in Bioengineering and Biotechnology 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.V.H.; et al. Displaying Lipase B from Candida antarctica in Pichia pastoris Using the Yeast Surface Display Approach: Prospection of a New Anchor and Characterization of the Whole Cell Biocatalyst. PLOS ONE 2015, 10, e0141454. [Google Scholar] [CrossRef] [PubMed]
- Teymennet-Ramírez, K.V.; Martínez-Morales, F.; Trejo-Hernández, M.R. Yeast Surface Display System: Strategies for Improvement and Biotechnological Applications. Frontiers in Bioengineering and Biotechnology 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kondo, A. Cell-surface display of enzymes by the yeast Saccharomyces cerevisiae for synthetic biology. FEMS Yeast Research 2015, 15, 1–9. [Google Scholar] [PubMed]
- Lim, S.; et al. Dual display of proteins on the yeast cell surface simplifies quantification of binding interactions and enzymatic bioconjugation reactions. Biotechnol J 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh-Sarmazdeh, M.; et al. Directed evolution of the metalloproteinase inhibitor TIMP-1 reveals that its N- and C-terminal domains cooperate in matrix metalloproteinase recognition. Journal of Biological Chemistry 2019, 294, 9476–9488. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; et al. Heterologous laccase production and its role in industrial applications. Bioengineered Bugs 2010, 1, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Necochea, R.; et al. Phylogenetic and biochemical characterisation of a recombinant laccase from Trametes versicolor. FEMS Microbiology Letters 2005, 244, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Crnoglavac Popović, M.; Stanišić, M.; Prodanović, R. State of the Art Technologies for High Yield Heterologous Expression and Production of Oxidoreductase Enzymes: Glucose Oxidase, Cellobiose Dehydrogenase, Horseradish Peroxidase, and Laccases in Yeasts P. pastoris and S. cerevisiae. Fermentation 2024, 10, 93. [Google Scholar]
- Popović, N.; et al. Immobilization of yeast cell walls with surface displayed laccase from Streptomyces cyaneus within dopamine-alginate beads for dye decolorization. International Journal of Biological Macromolecules 2021, 181, 1072–1080. [Google Scholar] [CrossRef]
- Kunamneni, A.; et al. Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochemistry 2008, 43, 169–178. [Google Scholar] [CrossRef]
- Lin, S.H.; Peng, C.F. Treatment of textile wastewater by electrochemical method. Water Research 1994, 28, 277–282. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2007, 2, 31–4. [Google Scholar] [CrossRef]
- Eldin, M.M.S.; Mita, D.G. Immobilized Enzymes: Strategies for Overcoming the Substrate Diffusion- Limitation Problem. Current Biotechnology 2014, 3, 207–217. [Google Scholar] [CrossRef]
- Ece, S.; et al. Heterologous expression of a Streptomyces cyaneus laccase for biomass modification applications. AMB Express 2017, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; et al. Functional expression, production, and biochemical characterization of a laccase using yeast surface display technology. Fungal Biology 2016, 120, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Cell Surface Display Fungal Laccase as a Renewable Biocatalyst for Degradation of Persistent Micropollutants Bisphenol A and Sulfamethoxazole. Environmental Science & Technology 2016, 50, 8799–8808. [Google Scholar]
- Bleve, G.; et al. Construction of a Laccase Chimerical Gene: Recombinant Protein Characterization and Gene Expression via Yeast Surface Display. Applied Biochemistry and Biotechnology 2014, 172, 2916–2931. [Google Scholar] [CrossRef]
- Lu, J.Z.; et al. Construction of a Yeast Cell-Surface Display System and Expression of Trametes sp. laccase. Advanced Materials Research 2012, 347-353, 3635–3640. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Wei, N. Biocatalytic properties of cell surface display laccase for degradation of emerging contaminant acetaminophen in water reclamation. 2020, 117, 342–353. [Google Scholar] [CrossRef]
- Nieto, D.; et al. Fundamentals of light-cell-polymer interactions in photo-cross-linking based bioprinting. APL Bioeng 2020, 4, 041502. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; et al. Microfabrication of Photo-Cross-Linked Hyaluronan Hydrogels by Single- and Two-Photon Tyramine Oxidation. Biomacromolecules 2015, 16, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; et al. Visible light cured thiol-vinyl hydrogels with tunable degradation for 3D cell culture. Acta Biomater 2014, 10, 104–14. [Google Scholar] [CrossRef] [PubMed]
- Tincu Iurciuc, C.E.; et al. An Accessible Method to Improve the Stability and Reusability of Porcine Pancreatic α-Amylase via Immobilization in Gellan-Based Hydrogel Particles Obtained by Ionic Cross-Linking with Mg(2+) Ions. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Teerapatsakul, C.; et al. Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. International Biodeterioration & Biodegradation 2017, 120, 52–57. [Google Scholar]
- Bagewadi, Z.K.; Mulla, S.I.; Ninnekar, H.Z. Purification and immobilization of laccase from Trichoderma harzianum strain HZN10 and its application in dye decolorization. Journal of Genetic Engineering and Biotechnology 2017, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Daâssi Dalel, R.-C.S.; Moncef, N.; Tahar, M. Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads. International Biodeterioration & Biodegradation 2014, 90, 71–78. [Google Scholar]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. International Journal of Biological Macromolecules 2016, 86, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, M.; Wang, Y. Immobilization of Laccase by Alginate-Chitosan Microcapsules and its Use in Dye Decolorization. 2007, 159–166. [Google Scholar] [CrossRef]
- Sharma, D.; et al. A novel laccase from newly isolated Cotylidia pannosa and its application in decolorization of synthetic dyes. Biocatalysis and Agricultural Biotechnology 2015, 4, 661–666. [Google Scholar] [CrossRef]
- Khaled, J.M.; et al. Laccase producing bacteria influenced the high decolorization of textile azo dyes with advanced study. Environmental Research 2022, 207, 112211. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-J.; Park, J.-H. Refolding, characterization, and dye decolorization ability of a highly thermostable laccase from Geobacillus sp. JS12. Protein Expression and Purification 2020, 173, 105646. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; et al. Magnetic Metal Organic Framework Immobilized Laccase for Wastewater Decolorization. 2021, 9, 774. [Google Scholar] [CrossRef]
- Chopra, N.K.; Sondhi, S. Cloning, expression and characterization of laccase from Bacillus licheniformis NS2324 in E. coli application in dye decolorization. International Journal of Biological Macromolecules 2022, 206, 1003–1011. [Google Scholar] [CrossRef]
- Zabłocka-Godlewska, E.; Przystaś, W.; Grabińska-Sota, E. Decolourization of Diazo Evans Blue by Two Strains of Pseudomonas fluorescens Isolated from Different Wastewater Treatment Plants. Water, Air, & Soil Pollution 2012, 223, 5259–5266. [Google Scholar]
- Pardo, I.; et al. New colorimetric screening assays for the directed evolution of fungal laccases to improve the conversion of plant biomass. BMC biotechnology 2013, 13, 90–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; et al. Purification and characterization of a novel laccase from Cerrena sp. HYB07 with dye decolorizing ability. PloS one 2014, 9, e110834–e110834. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; et al. Secretory expression and optimization of Bacillus pumilus CotA-laccase mutant GWLF in Pichia pastoris and its mechanism on Evans blue degradation. Process Biochemistry 2019, 78, 33–41. [Google Scholar] [CrossRef]
- Yang, X.; et al. Encapsulated laccase in bimetallic Cu/Zn ZIFs as stable and reusable biocatalyst for decolorization of dye wastewater. International Journal of Biological Macromolecules 2023, 233, 123410. [Google Scholar] [CrossRef]
- Morsy, S.A.G.Z.; et al. Current Development in Decolorization of Synthetic Dyes by Immobilized Laccases. 2020, 11. [Google Scholar]
- Lopez-Barbosa, N.; et al. Congo Red Decolorization Using Textile Filters and Laccase-Based Nanocomposites in Continuous Flow Bioreactors. 2020, 10, 1227. [Google Scholar]
- Silveira, T.R.; et al. An efficient decolorization of methyl orange dye by laccase from Marasmiellus palmivorus immobilized on chitosan-coated magnetic particles. Biocatalysis and Agricultural Biotechnology 2020, 30, 101859. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





