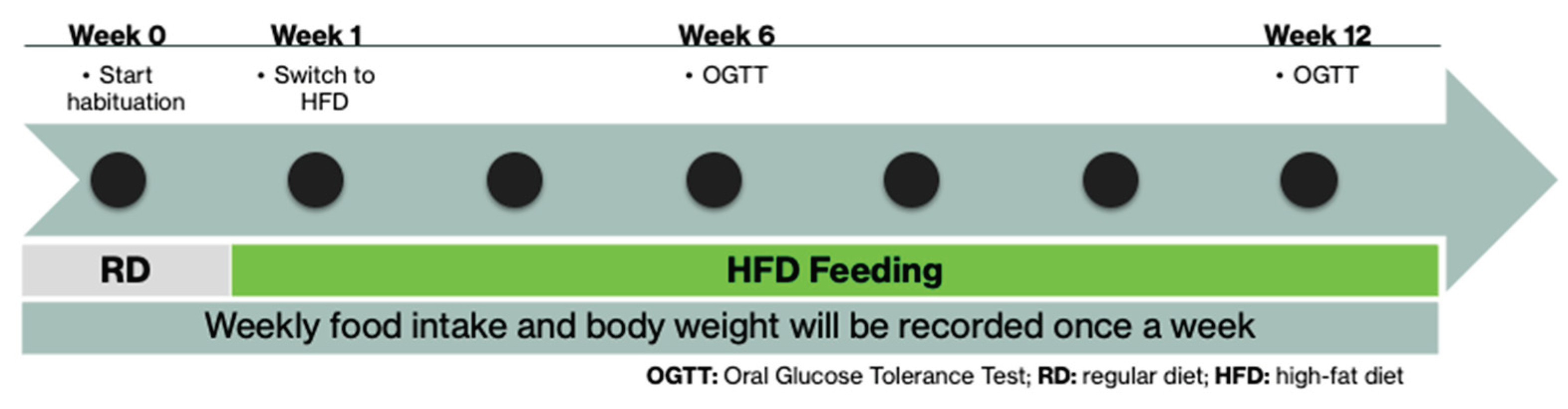
1. Introduction
Despite the growing recognition of the problem, the rates of obesity and obesity-related diseases had nearly tripled since 1975. Obesity is a known complex disease with multifaceted etiology stemming from interactions between genetic, behavioral, physiological, socioeconomic, and environmental factors [
1,
2,
3]. While many factors have been recognized, the consumption of the Western (American) diet or energy-dense foods high in fat, sodium, and sugar, is considered as one of the major contributing factors to the global epidemic [
4,
5,
6,
7].
The endogenous opioid system has been linked to regulate feeding behavior, however, the role of each opioid peptide has not been fully characterized. More specifically, previous studies have shown that the expression of β-END increases after consumption of a highly palatable food, suggesting its role in the natural reward pathway [
8,
9,
10]. Furthermore, Triscari and colleagues have initially shown that an acute intraperitoneal or intracerebroventricular (icv) administration of β-END stimulated food intake while a continuous infusion was ineffective [
11]. Another study has shown that a ventricular administration of β-END produced a dose-dependent and time-dependent increase in food intake and this effect was significantly reduced by naltrexone [
12]. In addition, a single icv injection of β-END significantly increased food intake over a six-hour period while a chronic (4 d) icv infusion did not simulate food consumption or weight gain in mice [
13]. These studies illustrate that administration of β-END stimulates food intake in animal models, however, its long-term implications in feeding behavior are quite different.
For instance, male mice lacking the ability to produce β-END were hyperphagic and obese when compared to their wildtype littermates [
14,
15]. In the same study, the authors observed a sexually dimorphic obesity phenotype, in which differences were only seen in male, but not female, β-END knockout mice. Furthermore, Low and colleagues have suggested that β-END release increases the incentive value of food during appetitive phase; on the other hand, endogenous β-END inhibits food consumption along with other melanocortin peptides [
15]. Furthermore, another study has shown that mice lacking the gene encoding for mu opioid receptor (MOP), β-END’s preferred receptor, displayed decreased motivation but unaltered hedonic processing of food intake [
16] while MOP agonists including [D-Ala
2,
N-MePhe
4, Gly-ol]-enkephalin (DAMGO) have generally shown to stimulate food consumption [
17,
18].
Based on the above findings, it is evident that β-END is involved in food consumption, more specifically exogenous β-END can stimulate and regulate food intake, however, the role of endogenous β-END has not been fully characterized. Therefore, our present study aimed to further investigate the role of endogenous β-END in regulation of body weight and food intake by utilizing β-END deficient mice [91] and their wildtype littermates/controls. In addition, we also examined if there are sex-related differences in this regard given that sexual dimorphism has been observed in body weight [
15] and feeding behaviors [95].
3. Results
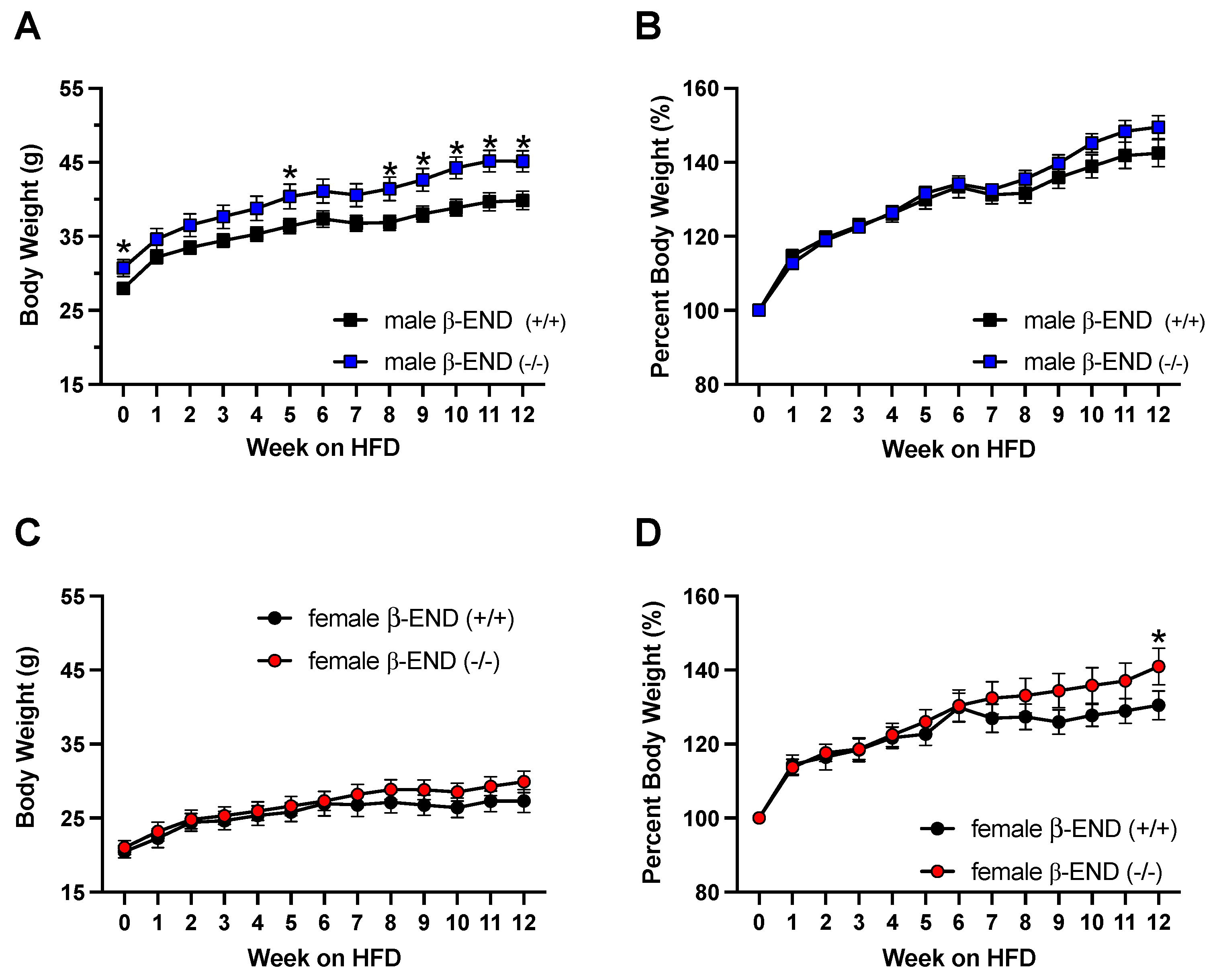
3.1. β-END Deficient Male Mice Had Higher Body Weights Than Their Wildtype Controls, However, This Difference Was Not Observed in Female Mice
Body Weight
Previous studies have shown that male β-END knockout mice became obese given ad libitum access to standard chow diet [
14,
15]. To examine if long-term access to HFD can exacerbate this effect, we exposed β-END wildtype and knockout male mice to HFD for twelve weeks after a week of habituation. We recorded a total of 20 male and 12-13 female mice per genotype for this experiment. We measured and recorded the body weights of each male mouse weekly as shown in
Figure 2A. A two-way ANOVA revealed significant effects of genotype [F (1, 38) = 5.208; P = 0.0282], time [F (3.348, 127.2) = 198.4; P < 0.0001], and a significant interaction between time and genotype [F (12, 456) = 3.588; P < 0.0001].
Post hoc analysis using the Fisher’s LSD test showed a significant difference in raw body weights at weeks 0, 5, 8, 9, 10, 11, and 12 (P = 0.0451, P = 0.0491, P = 0.0192, P = 0.0174, P = 0.0063, P = 0.0064, P = 0.0077;
Figure 2A). Given that the initial raw body weights of β-END deficient mice were significantly higher than wildtype mice (P = 0.0451;
Figure 2A), we normalized the data as shown in
Figure 2B. There were no differences between the percent body weight gain between the mice of the two genotypes [F (1, 38) = 0.6473; P = 0.4261]. Meanwhile, a two-way ANOVA showed significant effects of time [F (2.549, 96.87) = 194.2; P < 0.0001] and a significant interaction between time and genotype [F (12, 456) = 2.516; P = 0.0033].
Unlike male β-END knockout mice, previous studies have shown that female β-END deficient mice were only transiently heavier compared to their wildtype controls [
15]. We also did not observe any body weight changes between female wildtype and knockout mice given ad libitum access to HFD at the start of the experiment (
Figure 2C). Two-way ANOVA revealed a significant effect of time [F (3.311, 76.16) = 61.19; P < 0.0001] but no interaction between time and genotype [F (12, 276) = 1.658; P = 0.0760] nor effects of genotype [F (1, 23) = 0.5003; P = 0.4875]. In addition, the
post hoc test did not reveal any significant differences in raw body weight between mice of the two groups at any time points.
Figure 2D depicts the body weight gain as percent of their original weight in female mice. Two-way ANOVA revealed a significant effect of time [F (12, 276) = 51.68; P < 0.0001] and a significant interaction between time and genotype [F (12, 276) = 2.031; P = 0.0218], but no genotype effects [F (1, 23) = 0.8600; P = 0.3634]. Furthermore,
post hoc analysis using Fisher’s LSD test revealed a significant difference at week 12, in which female knockout mice gained more weight than wildtype mice when fed an HFD (P = 0.0383;
Figure 2D). Overall, our data shows that male mice lacking the ability to produce β-END had increased body weights when compared to their wildtype controls. On the other hand, we did not see this difference in female mice. Furthermore, the percent body weight change was not significant between the mice of the two genotypes at the first 8 weeks. On weeks 8-12, however, it appears that mice lacking β-END gained more weight than their wildtype controls (Figs. 2B and 2D, compare the body weight of mice of the two genotypes on weeks 8-12). Although the normalized that did not show a significant difference between male mice of the two genotypes, the change was significant in female mice on week 12.
3.2. Comparison of HFD Consumption and Caloric Intake in Male and Female Mice
Food and Caloric Intake
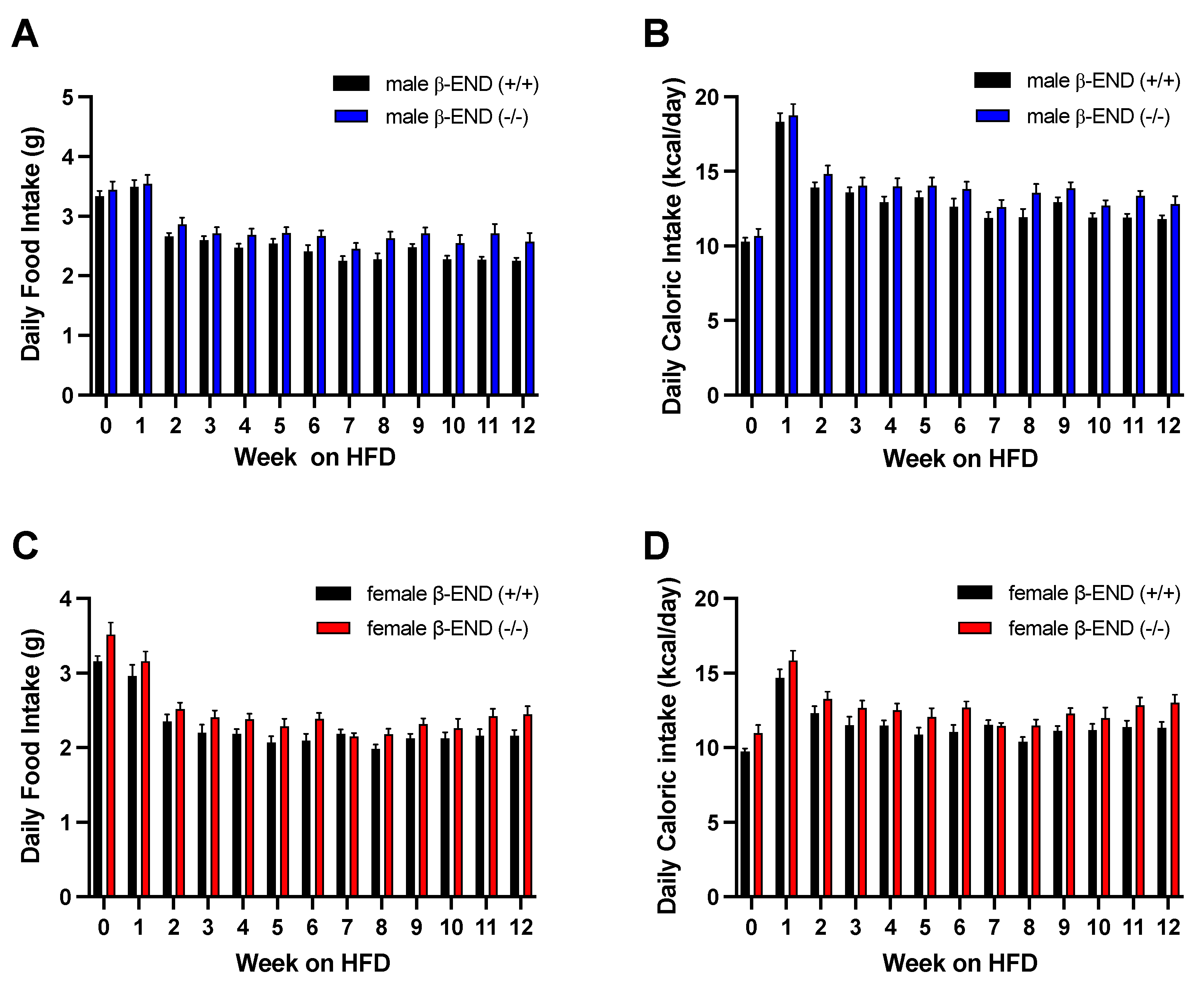
To determine if there was a difference in food and caloric intake between male or female β-END wildtype and knockout mice, we recorded weekly food intake. We also determined the caloric intake by using the following conversions: RD = 3.1 kcal/g, HFD = 5.24 kcal/g. The average daily food intake (g) and caloric intake (kcal) of the male and female β-END wildtype and knockout male mice are shown in
Figure 3. Analysis of food intake in male mice showed significant effects of genotype [F (1, 38) = 5.809; P = 0.0209] and time [F (4.702, 178.7) = 39.44; P < 0.0001] but no time and genotype interaction [F (12, 456) = 0.8134; P = 0.6368] in food intake (
Figure 3A). We also observed significant effects of time [F (4.694, 173.7) = 51.83; P < 0.0001] and genotype [F (1, 37) = 4.492; P = 0.0408] but no interaction between time and genotype [F (12, 444) = 0.5397; P = 0.8887], when analyzing the caloric intake in male mice (
Figure 3B).
Similar to male mice, we also measured the weekly food and caloric intake of female mice lacking β-END and their wildtype controls (
Figure 3C and D). A two-way ANOVA showed significant effects of time [F (4.751, 114.0) = 45.63; P < 0.0001] and genotype [F (1, 24) = 5.751; P = 0.0246] but no interaction between time and genotype [F (12, 288) = 0.7276; P = 0.7242]. Meanwhile, significant effects of time [F (5.447, 125.3) = 18.83; P < 0.0001] and genotype [F (1, 23) = 6.970; P = 0.0146] on daily caloric intake were also observed. While our data did not show significant differences, we observed a trend toward increased food and caloric intake in the knockout male and female mice when compared to their wildtype controls.
3.3. Female Mice Exhibited Overall Increased Daily Caloric Intake per Body Weight When Compared to Male Mice Regardless of Genotype
We also determined the calories consumed per body weight (kcal/g body weight) for both wildtype and knockout mice and determined if sex-related differences existed in this regard (
Figure 4).
Interestingly, female mice of both genotypes showed a higher caloric intake per body weight than their respective male. A three-way ANOVA revealed significant effects of time [F (12, 744) = 63.77; P < 0. 0001] and sex [F (1, 62) = 38.75; P < 0.0001], but no effects of genotype [F (1, 62) = 0.06499; P = 0.7996]. In addition, there was a significant interaction between time and sex [F (12, 744) = 1.826; P = 0.0406] but no other interactions were observed. The post-hoc Fisher’s LSD test showed that female wildtype mice had significantly higher caloric intake per body weights than male wildtype mice (left panel) at weeks 2-12 (P<0.05). Similarly, the β-END knockout female demonstrated higher caloric intake per body weights compared to male knockout mice (right panel). The post-hoc test revealed significant differences at weeks 2-12 (P<0.05). As shown in
Figure 4, there were no differences in caloric intake per body weight at week 0, when female and male mice were maintained on RD (week 0). While we did not observe differences in calories consumed per body weight when comparing the two genotypes (P>0.05), our data suggests that there were differences between male and female mice. Our data suggests that despite smaller body weights observed in female mice, the female mice consumed more food than the male mice, regardless of genotype.
3.4. Differences in Glucose Tolerance in Male and Female β-END Mice Following Six and Twelve Weeks of HFD
Glucose Homeostasis
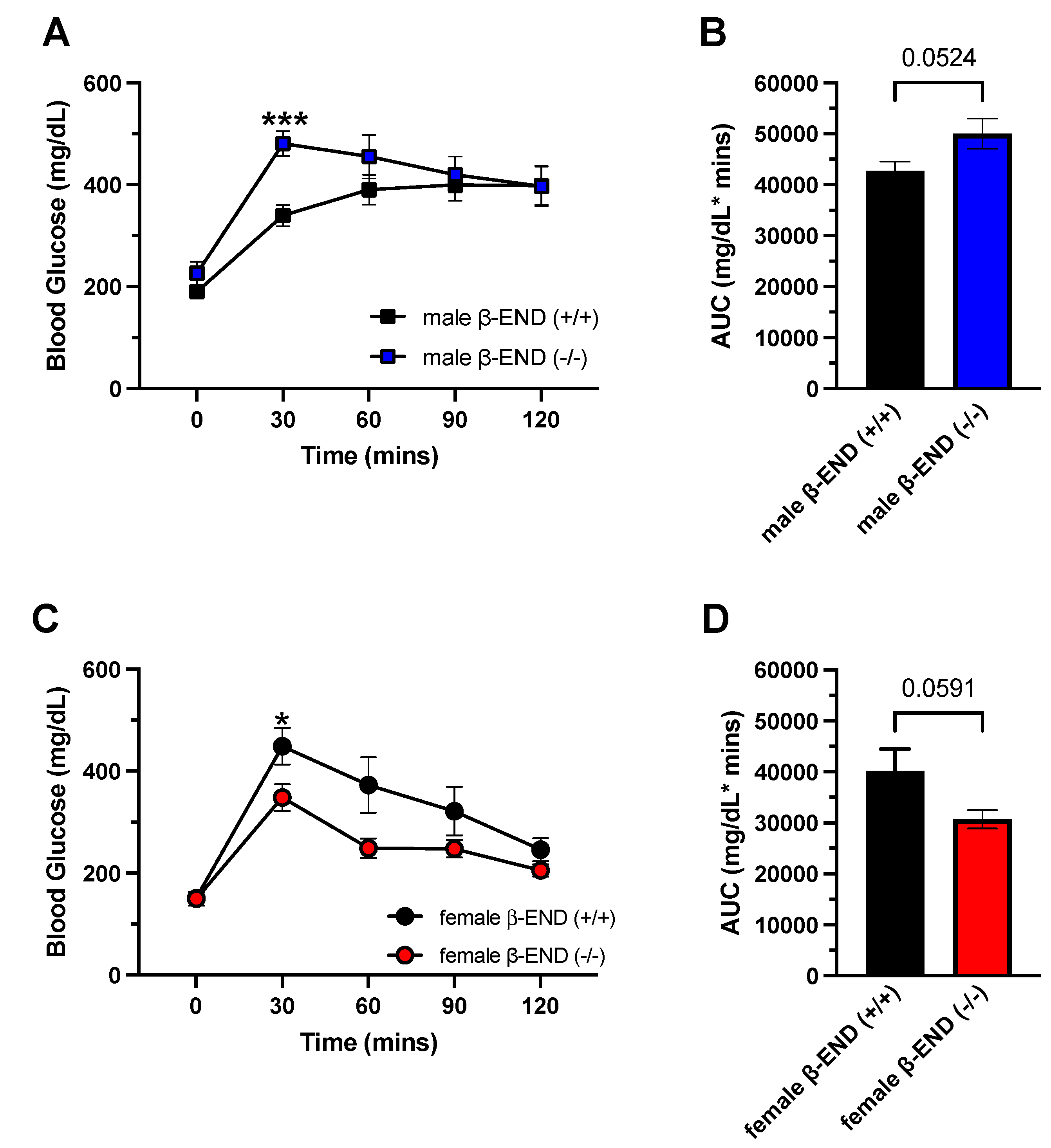
To determine if chronic HFD consumption affected glucose homeostasis, we performed OGTT at weeks 6 (
Figure 5) and 12 (
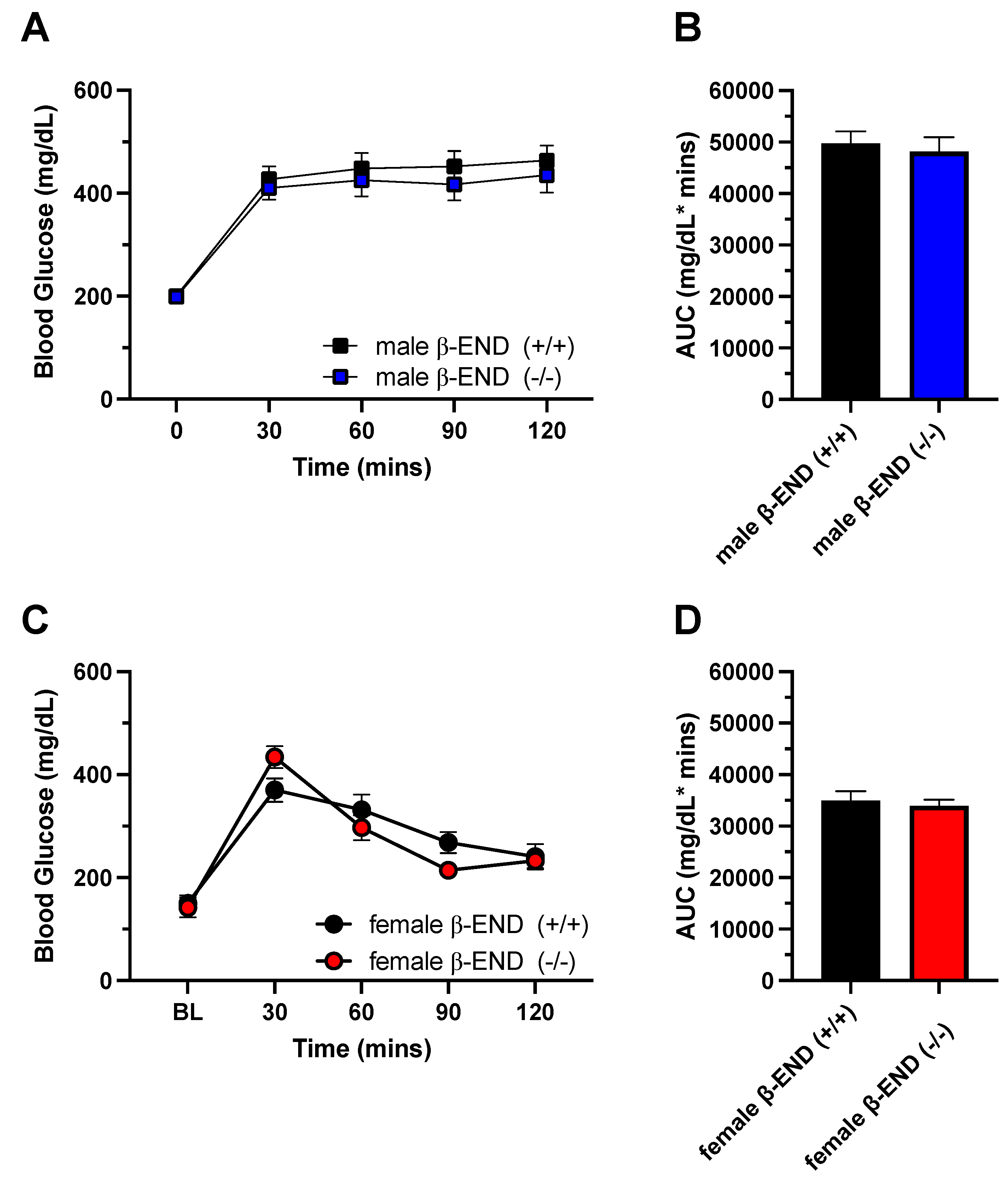
Figure 6) for male and female mice of the two genotypes. The mice were fasted overnight (10 h) and were given 20% glucose solution orally. Blood glucose levels were measured from tail bleeds at baseline (0), and at 30-, 60-, 90-, and 120-mins following sugar administration. After six weeks of HFD consumption in male mice, a two-way ANOVA revealed no significant effect of genotype [F (1, 14) = 3.411; P = 0.0860] and no interaction between time and genotype [F (4, 56) = 2.229; P = 0.0774]. However, we found a significant effect of time [F (2.851, 39.92) = 23.97; P < 0.0001]. Furthermore, we determined the areas under the curve (AUC) using GraphPad Prism (
Figure 5B). Male wildtype and knockout mice had average total AUCs of 42706.9 mg/dL * mins and 50015.6 mg/dL * mins, respectively. Additionally, an unpaired t-test showed a robust trend toward a significant difference (P = 0.0524) between male mice of the two genotypes. On the other hand, female mice exhibited different response to glucose after six weeks of HFD (
Figure 5C). A two-way ANOVA revealed a significant interaction between time and genotype [F (4, 48) = 2.793; P = 0.0365] and a significant effect of time [F (2.319, 27.83) = 41.07; P < 0.0001], but there were no genotype effects [F (1, 12) = 4.253; P = 0.0615]. Furthermore, Fisher’s LSD test showed that female knockout mice had better glucose tolerance than wildtype mice at 30 mins (P = 0.0445;
Figure 5C). Unlike male mice, female wildtype mice had an AUC of 40240.7 mg/dL * mins and knockout mice had 30662.1 mg/dL * mins. An unpaired t-test also revealed a robust trend toward a significant difference between the two genotypes (P = 0.0591).
We also performed oral glucose tolerance test after twelve weeks on HFD to determine if chronic access to HFD affects glucose homeostasis. We followed the same protocol as above. As shown on
Figure 6A and 6B, we observed no differences between male mice of the two genotypes. A two-way ANOVA revealed no significant interaction between time and genotype [F (4, 112) = 0.1934; P = 0.9414]. However, there were significant effects of time [F (3.037, 85.04) = 53.86; P < 0.0001] but not in genotype F (1, 28) = 0.5846; P = 0.4509]. Interestingly, the blood glucose levels remained elevated throughout the entire 2-h test period in male mice of the two genotypes, which is typically observed in naïve mice during this test. Furthermore, male wildtype and knockout mice had average total areas of 49781 and 48184.7 mg/dL * mins, respectively. There was no significant difference (P>0.05) between mice of the two genotypes. Furthermore, we observed no differences in glucose tolerance between female mice of the two genotypes. A two-way ANOVA showed no significant interaction between time and genotype [F (4, 56) = 2.459; P = 0.0559] an no genotype effects [F (1, 14) = 0.3118; P = 0.5854]. On the other hand, there was a significant effect of time [F (2.967, 41.53) = 45.12; P < 0.0001]. In addition, an unpaired t-test revealed no difference (P = 0.6372) between the areas of the curve between mice of the two genotypes (wildtype: 34965 mg/dL * mins and knockout: 33972 mg/dL* mins) of glucose tolerance in female mice (
Figure 6D).
3.5. Differences in Glucose Tolerance in Male and Female β-END Mice after Six and Twelve Weeks of HFD
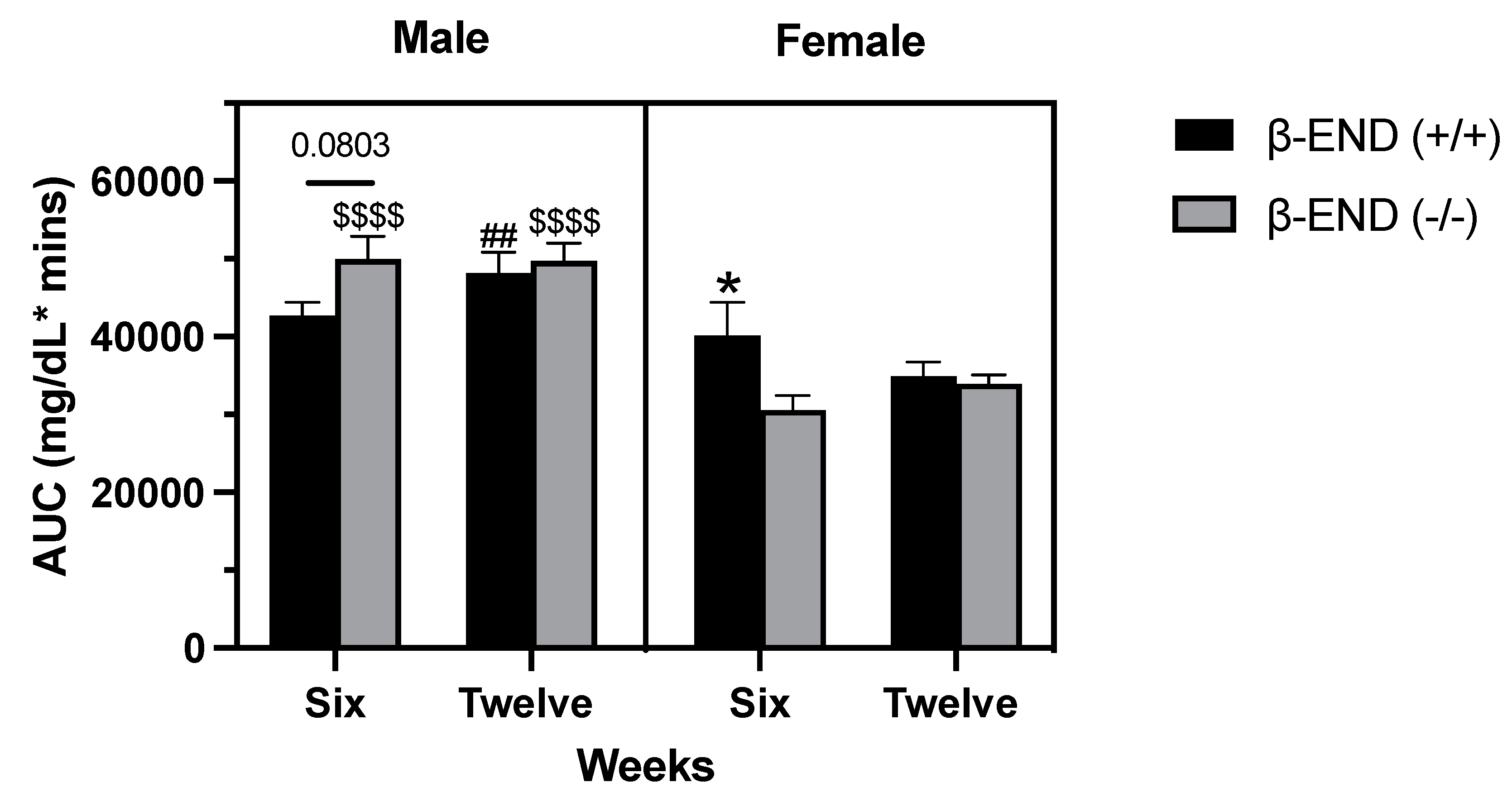
We also determined if the timepoint (week 6 vs. 12) in which OGTT is performed affected the glucose levels in the β-END deficient mice and their wildtype controls. Using the AUCs as the unit, we compared the OGTT results and determined if there were sex-related differences in this regard (
Figure 7).
Using a mixed-effects analysis, we observed a significant interaction between time and genotype [F (1, 26) = 7.369; P= 0.0116) and significant effect of genotype [F (1, 26) = 47.65; P < 0.0001]; however, no other significant interactions or effects were observed. Our data suggests that the AUC was not different between male or female mice of the two genotypes. However, female wildtype mice exhibited higher AUC at week 6 when compared to the female mice lacking β-END (right panel; P = 0.04). Moreover, we also observed a similar trend in the male mice at six weeks, although not significant (P < 0.08; left panel). Our data also demonstrate sex-specific differences in terms of OGTT-AUCs that may be time-dependent. The current findings show that the OGTT-AUCs for the male knockout mice were significantly higher at both weeks 6 (P < 0.0001) and 12 (P < 0.0001) when compared to the female knockout mice. On the other hand, we only observed a significant difference between the male and female wildtype mice at week 12 (P < 0.0013).
4. Discussion
Several studies have shown that the POMC-derived peptide β-END could modulate feeding behavior, but the role of endogenous β-END in glucose and energy homeostasis following a long-term access to HFD is still unknown. Therefore, in the present study, using genetically engineered knockout mice, we determined the role of β-END in food intake and plasma glucose levels when fed chronically an HFD (12 weeks) and assessed if sex-related differences existed in these processes. The main findings of the present study are that deletion of β-END results in increased body weights in male mice, but not in female mice; however, female mice consumed more calories than the male mice despite their smaller body weights. In addition, male and female mice lacking the ability to produce β-END demonstrated different glucose tolerance when compared to their respective wildtype controls, but this was only seen at week 6. Male mice of both genotypes exhibited significantly greater impairment in glucose tolerance test than their female counterparts but only male mice lacking β-END had greater impairment in OGTT on week 6 than their female controls. Together, our results suggest an involvement of β-END in regulating body weight, food consumption, and glucose homeostasis and highlight the potential sex-specific differences involved in these processes.
As previously stated, β-END has been implicated to influence feeding behavior, but its role has not been fully elucidated. While several studies suggest that administration of exogenous β-END increases food intake [
11,
12], other studies show that genetic deletion of the peptide demonstrated opposing effects [
14,
20]. Given that pharmacological studies have only shown acute effects on food intake, there is no prior study assessing the role of endogenous β-END as a regulator of food intake, body weight and glucose homeostasis following long-term access to an HFD. Therefore, we focused on the effects of chronic access to an HFD on energy and glucose homeostasis in male and female mice lacking β-END and their wildtype controls. As expected, we observed an increased in caloric intake when male and female mice were switched to HFD, however, the effects on body weights were sexually dimorphic. Our results show that male β-END deficient mice exhibited increased body when compared to their wildtype controls. Interestingly, β-END knockout male mice displayed higher body weights, especially at later time points, without alteration in feeding when compared to their wildtype controls, at least for specific weeks. These findings were consistent with previous studies demonstrating that male mice were obese given unlimited access to standard chow [
14,
15]. These results suggest that endogenous β-END might have an anorectic effect, in contrast to increased food intake observed in mice given exogenous β-END. However, in this study, we did not include animals exposed to regular diet to determine whether this effect is due to palatability of food or other factors in these mice. Our previous work showed no difference in regular chow intake between mice of the two genotypes [
21].
We included female mice in our study since previous works have mainly focused on male mice. Our present study illustrated that female mice lacking β-END, unlike their male counterparts, did not exhibit higher body weights when compared to their wildtype controls despite the increased caloric intake observed. Interestingly, irrespective of genotype, female mice demonstrated increased caloric intake per body weight. While the role of β-END in food intake in female mice remains unclear, this finding suggests that there might be a sex-specific difference in compensatory energy expenditure in male and female mice. A possible explanation why female mice exhibited smaller body weights when compared to male mice was due to an increased locomotor activity (Stojakovic et al. 2019), but this was not measured in our study. However, there is previous evidence demonstrating that female rodents maintained a higher energy expenditure following short-term HFD-feeding [
22,
23]. Thus, further studies are needed to fully characterize the role of β-END in energy homeostasis, especially its role in energy expenditure and its interaction with sex hormones.
Despite the known affinity of β-END for mu-, kappa-, and delta- opioid receptors, it has been suggested that β-END exerts its effects on feeding behavior by preferentially binding to mu opioid receptors [
12]. Several studies have supported this claim; demonstrating that opioid receptor antagonists, such as naloxone or naltrexone, could reverse the effects of exogenous β-END on feeding behavior [
12,
13,
14,
24]. Although pharmacological studies involving opioid antagonists have suggested that MOPs are involved in modulating the effects of β-END, genetic deletion of this opioid receptor had yielded different outcomes. Consistent with our studies, Wen and colleagues have demonstrated that both male and female mice lacking the MOP-1 on C57BL/6J background had higher body weight and a preferential increase in fat deposition [
25]. On the other hand, MOP-deficient mice were resistant to diet-induced obesity despite a comparable food intake to that of wildtype mice [
26]. In another study, MOP-deficient mice had decreased motivation to eat when compared to wildtype controls [
16]. Thus, it is possible that β-END may act on its own receptor to regulate food intake and body weight. Consistent with this hypothesis, we have previously shown that the rewarding action of acute cocaine is blunted in mice lacking β-END but not in MOP knockout mice [
27].
Opioid peptides have also been linked to regulate glucose homeostasis; however, their role is poorly characterized. In this study, we wanted to determine if β-END played any functional role in regulating plasma glucose levels. While we set out to perform oral glucose tolerance tests after long-term access to HFD, we also included an earlier time point (week 6) to see if we can detect any differences at this time and do not miss any opportunity to observe a difference between mice of the two genotypes. Interestingly, our findings demonstrated that deletion of β-END in female mice improved glucose tolerance compared to their wildtype controls at week 6, despite no differences in body weights; while male mice showed opposite results. This demonstrates that female β-END wildtype mice could not clear glucose as efficiently as the knockouts, despite having similar baseline levels. On the other hand, male mice lacking β-END show signs of impaired glucose tolerance at week 6 compared to male wildtype mice at 30 min as well as against their female mice lacking β-END, as their glucose levels did not return to baseline after a 2-hour period as seen in female mice. Moreover, while we did not observe differences between the knockout and wildtype mice at week 12, our results demonstrate a sexually dimorphic response in glucose tolerance test showing that male mice, regardless of genotype, displayed impaired glucose tolerance. After 12 weeks of exposure to HFD, the differences between the wildtype and knockout mice, regardless of genotype, have diminished. However, male mice showed a more pronounced impaired glucose tolerance test compared to their female counterparts. These findings suggest that β-END may play a transient role in regulating homeostasis—that can only be observed in short-term HFD feeding. These results also reveal male/female differences in glucose tolerance following chronic (12 weeks) access to an HFD but this impairment was observed in the absence of β-END at even earlier time point (6 weeks). Thus, further studies are needed to assess the role of sex hormones and its interaction with β-END in this impairment in future studies.
Although our current study did not measure blood glucose levels of mice fed a regular diet only, a previous work had already shown no differences between the female genotypes under this condition [
14]. We have also conducted OGTT in male and female mice fed a regular diet and found no difference between mice of the two genotypes (Espinosa and Lutfy, data not shown). A study has previously shown that genetic deletion of MOP resulted in an improved glucose tolerance by modulating insulin secretion [
25], consistent with our results in female mice lacking β-END and their wildtype controls. Based on our findings, genetic deletion of β-END might be beneficial as it offers short-term protection from an impaired glucose tolerance, at least in the female mice. On the other hand, pharmacological studies have demonstrated that infusion of β-END could improve insulin resistance in rats [
28]. In addition, the Lai Lab has previously suggested that MOP activation by β-END can increase glucose utilization in peripheral tissues in diabetic rats [
29]. Furthermore, a more recent study has shown that infusion of DAMGO, a MOP agonist, in the nucleus accumbens can enhance glycemic response in rats [
30]. Thus, these findings need to be explored further, either by increasing the sample size or including an insulin tolerance test to observe the correlation between glucose and insulin levels. The enhanced glucose clearance observed in the female β-END knockout mice after six weeks of HFD exposure may suggest an increased insulin release, resulting in an increased glucose uptake and fat storage within these mice. Therefore, determining the underlying cause of increase in body weights of these mice would be of specific interest. For example, measuring fat pads, muscles, liver, and other organs could elucidate the reasons for these changes.
Author Contributions
Conceptualization, K.L. and A.B.M.; methodology, L.T., and A.H.; software, L.T., V.E., and K.L.; validation, L.T.; formal analysis, L.T., V.E. and K.L.; investigation, L.T.; resources, K.L.; data curation, L.T.; writing—original draft preparation, L.T.; writing—review and editing, L.T, V.E., K.L. and A.B.M.; supervision, K.L. and A.B.M.; project administration, K.L.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.