Submitted:
05 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Clinical Isolates
2.2. MALDI-TOF MS Analysis
2.3. Interpretation and Analysis of the Results
3. Results
3.1. Bacterial and Yeast Identification Using PICKME and Wooden Toothpicks
3.2. Bacterial and Yeast Identification Using VITEK MS PRIME and ASTA MicroIDSys Elite
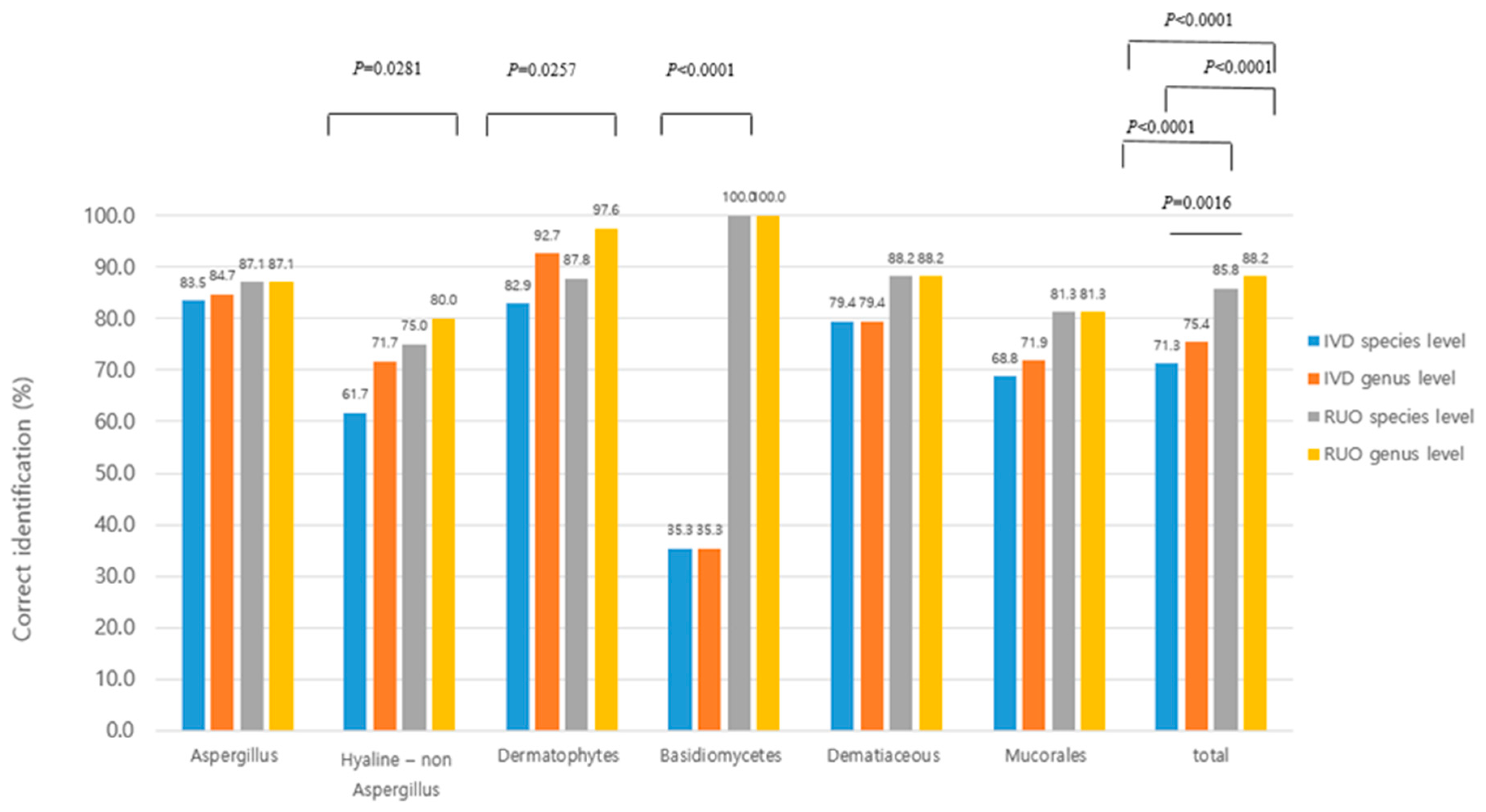
3.3. Identification of Filamentous Fungi Using the IVD and RUO Databases
3.4. Identification Failure of Filamentous Fungi
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, A.; Ringer, M.; Kotroczó, Z.; Mohácsi-Farkas, C.; Kocsis, T. The Current Level of MALDI-TOF MS Applications in the Detection of Microorganisms: A Short Review of Benefits and Limitations. Microbiol. Res. 2023, 14, 80–90. [Google Scholar] [CrossRef]
- Lee, H.; Oh, J.; Sung, G.H.; Koo, J.; Lee, M.H.; Lee, H.J.; Cho, S.I.; Choi, J.S.; Park, Y.J.; Shin, J.H.; et al. Multilaboratory Evaluation of the MALDI-TOF Mass Spectrometry System, MicroIDSys Elite, for the Identification of Medically Important Filamentous Fungi. Mycopathologia 2021, 186, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Grohs, P.; Remaud, E.; Lath, C.; Vuong, K.; Parolini, M.L.; Dannaoui, E.; Podglajen, I. Comparison of the New VITEK MS PRIME System with the Matrix-Assisted Laser Desorption Ionization Biotyper Microflex LT for the Identification of Microorganisms. Ann Lab Med 2023, 43, 574–584. [Google Scholar] [CrossRef]
- Wilkendorf, L.S.; Bowles, E.; Buil, J.B.; van der Lee, H.A.L.; Posteraro, B.; Sanguinetti, M.; Verweij, P.E. Update on Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Identification of Filamentous Fungi. J Clin Microbiol 2020, 58. [Google Scholar] [CrossRef]
- Wei, L.; Shao, J.; Song, Y.; Wan, Z.; Yao, L.; Wang, H.; Yu, J. Performance of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Scedosporium, Acremonium-Like, Scopulariopsis, and Microascus Species. Front Microbiol 2022, 13, 841286. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, M.; Padovani, M.; Fiorentini, S.; Caruso, A.; Yamamura, D.; Gaskin, M.; Jissam, A.; Gonzalez-Lopez, J.J.; Larrosa, M.N.; Pumarola, T.; et al. A side-by-side comparison of the performance and time-and-motion data of VITEK MS. Eur J Clin Microbiol Infect Dis 2022, 41, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Hodille, E.; Prudhomme, C.; Dumitrescu, O.; Benito, Y.; Dauwalder, O.; Lina, G. Rapid, Easy, and Reliable Identification of Nocardia sp. by MALDI-TOF Mass Spectrometry, VITEK((R))-MS IVD V3.2 Database, Using Direct Deposit. Int. J. Mol. Sci. 2023; 24. [Google Scholar] [CrossRef]
- Perrot N, Dauwalder O, Paris, C.; et al. New innovative tool for easy colony picking and sample preparation for MALDI-TOF. Poster presented at: ASM Microbe 2019; June 20-24, 2019; San Francisco, CA.
- Petti, C.A. Interpretive Criteria for Identification of Bacteria and Fungi by Targeted DNA sequencing, 2nd ed.CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Robert, M.G.; Cornet, M.; Hennebique, A.; Rasamoelina, T.; Caspar, Y.; Ponderand, L.; Bidart, M.; Durand, H.; Jacquet, M.; Garnaud, C.; et al. MALDI-TOF MS in a Medical Mycology Laboratory: On Stage and Backstage. Microorganisms 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.C.; Gabriel, F.; Riat, A.; Cassagne, C.; Bourgeois, N.; Huguenin, A.; Chauvin, P.; De Geyter, D.; Bexkens, M.; Rubio, E.; et al. Optimization of MALDI-ToF mass spectrometry for yeast identification: A multicenter study. Med Mycol 2020, 58, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.Y.; Park, Y.J.; Lee, J.; Suk, H.S.; Ha, S.I.; Shin, J.S.; Park, K.G.; Kim, Y. Comparison of the ASTA MicroIDSys and VITEK MS matrix-assisted laser desorption/ionization time-of-flight mass spectrometry systems for identification of clinical bacteria and yeasts. J Infect Chemother 2020, 26, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Thelen, P.; Graeber, S.; Schmidt, E.; Hamprecht, A. A side-by-side comparison of the new VITEK MS PRIME and the MALDI Biotyper sirius in the clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis 2023, 42, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Suzuki, Y.; Abe, N.; Ui, K.; Suzuki, K.; Yamashita, T.; Sakaguchi, A.; Suzuki, S.; Masuo, K.; Nakano, A.; et al. Comparison of MALDI-TOF mass spectrometry and rpoB gene sequencing for the identification of clinical isolates of Aeromonas spp. Heliyon 2022, 8, e11585. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; You, Z.; Zheng, Q.; Tang, J.; Ran, Y.; Huang, G. Biological and genomic analyses of a clinical isolate of Yarrowia galli from China. Curr Genet 2020, 66, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Goker, T.; Asik, R.Z.; Yilmaz, M.B.; Celik, I.; Tekiner, A. Sphingomonas Paucimobilis: A Rare Infectious Agent Found in Cerebrospinal Fluid. J Korean Neurosurg Soc 2017, 60, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, H.; Sarmis, A.; Tigen, E.; Soyletir, G.; Mulazimoglu, L. Delftia acidovorans: A rare pathogen in immunocompetent and immunocompromised patients. Can J Infect Dis Med Microbiol 2015, 26, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin Microbiol Rev 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, S.H.; Lee, D.; Lee, S.Y.; Chun, S.; Lee, J.H.; Won, E.J.; Choi, H.J.; Choi, H.W.; Kee, S.J.; et al. Performance Evaluation of VITEK MS for the Identification of a Wide Spectrum of Clinically Relevant Filamentous Fungi Using a Korean Collection. Ann Lab Med 2021, 41, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Zvezdanova, M.E.; Escribano, P.; Guinea, J.; Munoz, P.; Rodriguez-Temporal, D.; Rodriguez-Sanchez, B. Evaluation of the Vitek Ms system for the identification of filamentous fungi. Med Mycol 2022, 60. [Google Scholar] [CrossRef] [PubMed]
| No. of isolates |
VITEK MS PRIME -wooden toothpick, n (%) | VITEK MS PRIME - PICKME, n (%) | ASTA MicroIDSys Elite, n (%) | Concordance rate, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correct ID (species level) |
Incomplete ID (genus level) |
No ID |
Correct ID (species level) |
Incomplete ID (genus level) |
No ID |
Correct ID (species level) |
Incomplete ID (genus level) |
No ID |
Wooden toothpick and PICK ME | Wooden toothpick and ASTA | PICK ME and ASTA | ||
| Gram positive cocci | 91 | 90 (98.9) | 1 (1.1) | 89 (97.8) | 1 (1.1) | 87 (95.6) | 1 (1.1) | 3 (including 2 misID)* (3.3) |
89 (97.8) | 86 (94.5) | 85 (93.4) | ||
| Gram positive bacilli | 22 | 18 (81.8) | 1 (4.5) | 3 (13.6) | 16 (72.7) | 1 (4.5) | 5 (22.7) | 21 (95.5) | 1 (4.5) | 16 (72.7) | 18 (81.8) | 16 (72.7) | |
| Gram negative cocci | 15 | 15 (100) | 15 (100) | 13 (86.7) | 1 (6.7) | 1 (6.7) | 15 (100) | 13 (86.7) | 13 (86.7) | ||||
| Gram negative bacilli | 124 | 111 (89.5) | 2 (1.6) | 11 (8.9) | 111 (89.5) | 2 (1.6) | 11 (8.9) | 103 (83.1) | 11 (8.9) | 10 (8.1) | 110 (88.7) | 101 (81.5) | 100 (80.6) |
| Anaerobes | 16 | 14 (87.5) | 2 (12.5) | 14 (87.5) | 2 (12.5) | 14 (87.5) | 2 (12.5) | 14 (87.5) | 14 (87.5) | 14 (87.5) | |||
| Yeast | 52 | 46 (88.5) | 6 (11.5) | 44 (84.6) | 8 (15.4) | 44 (84.6) | 8 (15.4) | 42 (80.8) | 41 (78.8) | 39 (75.0) | |||
| Total | 320 | 294 (91.9) | 3 (0.9) | 23 (7.2) | 289 (90.3) | 27(8.4%) | 282 (88.1) | 13 (4.1) | 25 (7.8) | 286 (89.4) | 273 (85.3) | 267 (83.4) | |
| Discordant isolates | VITEK MS PRIME – wooden toothpick | VITEK MS PRIME– PICKME | ASTA MicroIDSys Elite | Re-tests | |||
|---|---|---|---|---|---|---|---|
| result | score | result | score | result | score | ||
| Identified only in wooden toothpick (n=8) | |||||||
| Aeromonas caviae | Aeromonas caviae | 99.9 | No identification | Aeromonas caviae | 233 | ||
| Corynebacterium jeikeium | Corynebacterium jeikeium | 99.9 | No identification | Corynebacterium jeikeium | 178 | ||
| Corynebacterium jeikeium | Corynebacterium jeikeium | 99.9 | No identification | Corynebacterium jeikeium | 178 | ||
| Streptococcus anginosus | Streptococcus anginosus | 99.9 | No identification | Streptococcus anginosus | 211 | ||
| Candida albicans | Candida albicans | 95.3 | No identification | Candida albicans | 139 | ||
| Candida albicans | Candida albicans | 99.9 | No identification | Candida albicans | 224 | Correct ID in PICKME | |
| Candida ciferrii | Candida ciferrii | 99.9 | No identification | No identification | Correct ID in PICKME | ||
| Candida parapsilosis | Candida parapsilosis | 99.9 | No identification | Candida parapsilosis | 141 | Correct ID in PICKME | |
| Identified only in PICKME (n=3) | |||||||
| Acinetobacter nosocomialis | No identification | Acinetobacter nosocomialis | 90.7 | No identification | Correct ID in wooden toothpick in VITEK | ||
| Candida ciferrii | No identification | Candida ciferrii | 99.9 | No identification | Correct ID in wooden toothpick in VITEK | ||
| Candida tropicalis | No identification | Candida tropicalis | 99.9 | Candida tropicalis | 214 | Correct ID in wooden toothpick in VITEK | |
| Identified only in genus level (n=4) | |||||||
| Acinetobacter proteolyticus | No identification | Acinetobacter gyllenbergii | 99.6 | No identification | |||
| Aeromonas dhakensis | Aeromonas veronii/sobria | 50/50 | Aeromonas veronii/sobria | 50/50 | No identification | ||
| Paenibacillus polymyxa | Paenibacillus peoriae | 99.9 | Paenibacillus peoriae | 99.9 | No identification | ||
| Pseudomonas granadensis | Pseudomonas fluorescens | 99.7 | No identification | No identification | |||
| Misidentification (n=19) | |||||||
| Acinetobacter seifertii | No identification | No identification | Acinetobacter calcoaceticus | 137 | |||
| Aerococcus viridans | No identification | No identification | Aerococcus viridans | 160 | |||
| Aeromonas dhakensis | No identification | No identification | Aeromonas sp | 195 | |||
| Aeromonas dhakensis | No identification | No identification | Aeromonas sp | 205 | |||
| Bacteroides thetaiotaomicron | No identification | No identification | No identification | Correct ID in ASTA | |||
| Comamonas acidovorans | No identification | No identification | Comamonas acidovorans | 154 | |||
| Corynebacterium afermentans | No identification | No identification | Corynebacterium afermentans | 169 | |||
| Corynebacterium afermentans | No identification | No identification | Corynebacterium afermentans | 167 | |||
| Eikenella corrodens | No identification | No identification | Invalid Identification | ||||
| Fusobacterium nucleatum | No identification | No identification | No identification | ||||
| Gardnerella vaginalis | No identification | No identification | Invalid Identification | ||||
| Pasteurella multocida | No identification | No identification | Pasteurella multocida | 141 | |||
| Shewanella algae | No identification | No identification | No identification | Correct ID in wooden toothpick & PICKME in VITEK | |||
| Spingomonas paucimobilis | No identification | No identification | Invalid Identification | ||||
| Stenotrophomonas maltophilia | No identification | No identification | Invalid Identification | Correct ID in ASTA | |||
| Trichrosporon faecale | No identification | No identification | Invalid Identification | ||||
| Yarrowia galli | No identification | No identification | Invalid Identification | ||||
| Candida dubliniensis | No identification | No identification | Candida dubliniensis | 153 | Correct ID in wooden toothpick & PICKME in VITEK | ||
| Candida tropicalis | No identification | No identification | Candida tropicalis | 153 | |||
| IVD DB | RUO DB | ||||||
|---|---|---|---|---|---|---|---|
| Identification by DNA sequencing | No. of isolates | Species level ID (%) | Genus or complex level ID (%) | no ID (%) | Species level ID (%) | Genus or complex level ID (%) | no ID (%) |
| Hyaline – Aspergillus species | |||||||
| Aspergillus aculeatus | 2 | 2 | 2 | ||||
| Aspergillus calidoustus | 2 | 2 | |||||
| Aspergillus chevalieri | 5 | 5 | 5 | ||||
| Aspergillus flavus | 7 | 7 | |||||
| Aspergillus fumigatus | 7 | 7 | |||||
| Aspergillus japonicus | 1 | 1 | 1 | ||||
| Aspergillus lentulus | 12 | 12 | |||||
| Aspergillus nidulans | 7 | 6 | 1 | 1 | |||
| Aspergillus niger | 8 | 8 | |||||
| Aspergillus ochraceus | 1 | 1 | |||||
| Aspergillus sydowii | 14 | 13 | 1 | 1 | |||
| Aspergillus tamarii | 2 | 1 | 1 | 1 | |||
| Aspergillus terrei | 8 | 8 | |||||
| Aspergillus tubingensis | 6 | 6 | |||||
| Aspergillus versicolor | 3 | 3 | 3* | ||||
| Total | 85 | 71 | 1 | 13 | 3 | 11 | |
| Hyaline – non Aspergillus species | |||||||
| Acremonium sclerotigenum | 1 | 1 | |||||
| Beauveria bassiana | 1 | 1 | |||||
| Fusarium dimerum | 1 | 1 | |||||
| Fusarium equiseti | 2 | 1 | 1 | 1 | 1 | ||
| Fusarium oxysporum | 2 | 2 | |||||
| Fusarium solani | 4 | 4 | |||||
| Fusarium verticillioides | 4 | 1 | 3 | 2 | 1 | ||
| Paecilomyces formosus | 4 | 4 | 4 | ||||
| Paecilomyces variotii (yssochlamysspectabilis) | 2 | 2 | |||||
| Penicillium chrysogenum | 3 | 3 | |||||
| Penicillium citrinum | 9 | 8 | 1 | 1 | |||
| Penicillium crustosum | 1 | 1 | 1 | ||||
| Penicillium expansum | 1 | 1 | 1 | ||||
| Penicillium glabrum | 2 | 2 | |||||
| Penicillium janthinellum | 2 | 2 | 1 | 1 | |||
| Penicillium oxalicum | 4 | 4 | 3 | 1 | |||
| Penicillium toxicarium | 1 | 1 | 1 | ||||
| Pseudallescheria boydii | 1 | 1 | |||||
| Purpureocillium lilacinum | 8 | 8 | |||||
| Rasamsonia argillacea | 3 | 3 | |||||
| Talaromyces marneffei | 1 | 1 | 1 | ||||
| Talaromyces pinophilus | 2 | 2 | 2 | ||||
| Talaromyces purpureogenus | 1 | 1 | 1 | ||||
| Total | 60 | 37 | 6 | 17 | 8 | 3 | 12 |
| Dermatophytes | |||||||
| Epidermophyton floccosum | 1 | 1 | |||||
| Microsporum canis | 10 | 9 | 1 | 1 | |||
| Nannizzia gypsea | 1 | 1 | 1* | ||||
| Trichoderma longibrachiatum | 2 | 2 | |||||
| Trichophyton erinacei | 1 | 1 | |||||
| Trichophyton interdigitale | 3 | 2 | 1 | 1 | |||
| Trichophyton mentagrophytes | 3 | 3 | 3 | ||||
| Trichophyton rubrum | 17 | 16 | 1 | 1 | |||
| Trichophyton tonsurans | 3 | 3 | |||||
| Total | 41 | 34 | 4 | 3 | 2 | 4 | 1 |
| Basidiomycetes | |||||||
| Bjerkandera adusta | 1 | 1 | |||||
| Coprinellus radians | 4 | 4 | 4 | ||||
| Irpex lacteus | 11 | 11 | |||||
| Schizophyllum commune | 18 | 18 | 18 | ||||
| Total | 34 | 12 | 22 | 22 | |||
| Dematiaceous | |||||||
| Alternaria alternata | 11 | 11 | |||||
| Cladosporium cladosporioides | 6 | 6 | |||||
| Cladosporium halotolerans | 2 | 2 | 2 | ||||
| Colletotrichum gloeosporioides | 1 | 1 | 1 | ||||
| Exophiala xenobiotica | 2 | 2 | 2* | ||||
| Neoscytalidium dimidiatum | 1 | 1 | 1 | ||||
| Scedosporium apiospermum | 10 | 10 | |||||
| Scopulariopsis brevicaulis | 1 | 1 | 1 | ||||
| Total | 34 | 27 | 7 | 3 | 4 | ||
| Mucorales | |||||||
| Cunninghamella bertholletiae | 3 | 3 | 3 | ||||
| Lichtheimia corymbifera | 3 | 3 | |||||
| Mucor circinelloides | 5 | 5 | |||||
| Mucor fragilis | 1 | 1 | 1 | ||||
| Mucor irregularis | 1 | 1 | 1 | ||||
| Mucor velutinosus | 1 | 1 | |||||
| Rhizomucor miehei | 1 | 1 | 1 | ||||
| Rhizomucor pusillus | 4 | 4 | 4 | ||||
| Rhizopus microsporus | 3 | 3 | |||||
| Rhizopus oryzae | 10 | 10 | |||||
| Total | 32 | 22 | 1 | 9 | 4 | 6 | |
| Others | |||||||
| Eutypella scoparia | 3 | 3 | |||||
| Total molds | 289 | 206 | 12 | 71 | 42 | 7 | 34 |
| Isolates included in IVD database | No. of isolates | |
| Aspergillus species | Aspergillus versicolor | 3 |
| Dematiaceous | Exophiala xenobiotica | 2 |
| Dermatophytes | Nannizzia gypsea | 1 |
| Isolates included in only RUO database | ||
| Aspergillus species | Aspergillus aculeatus | 2 |
| Aspergillus chevalieri | 5 | |
| Aspergillus japonicus | 1 | |
| Hyaline – non Aspergillus | Fusarium equiseti | 1 |
| Paecilomyces formosus | 4 | |
| Penicillium janthinellum | 1 | |
| Penicillium oxalicum | 1 | |
| Penicillium toxicarium | 1 | |
| Talaromyces marneffei | 1 | |
| Talaromyces pinophilus | 2 | |
| Talaromyces purpureogenus | 1 | |
| Dematiaceous | Colletotrichum gloeosporioides | 1 |
| Neoscytalidium dimidiatum | 1 | |
| Mucorales | Cunninghamella bertholletiae | 3 |
| Mucor fragilis | 1 | |
| Mucor irregularis | 1 | |
| Rhizomucor miehei | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





