Submitted:
05 April 2024
Posted:
08 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ehrlichia and Its Morphology
3. Tick Biological Cycle
4. Epidemiology of Canine Ehrlichiosis
5. Pathogenesis
6. Immune Response
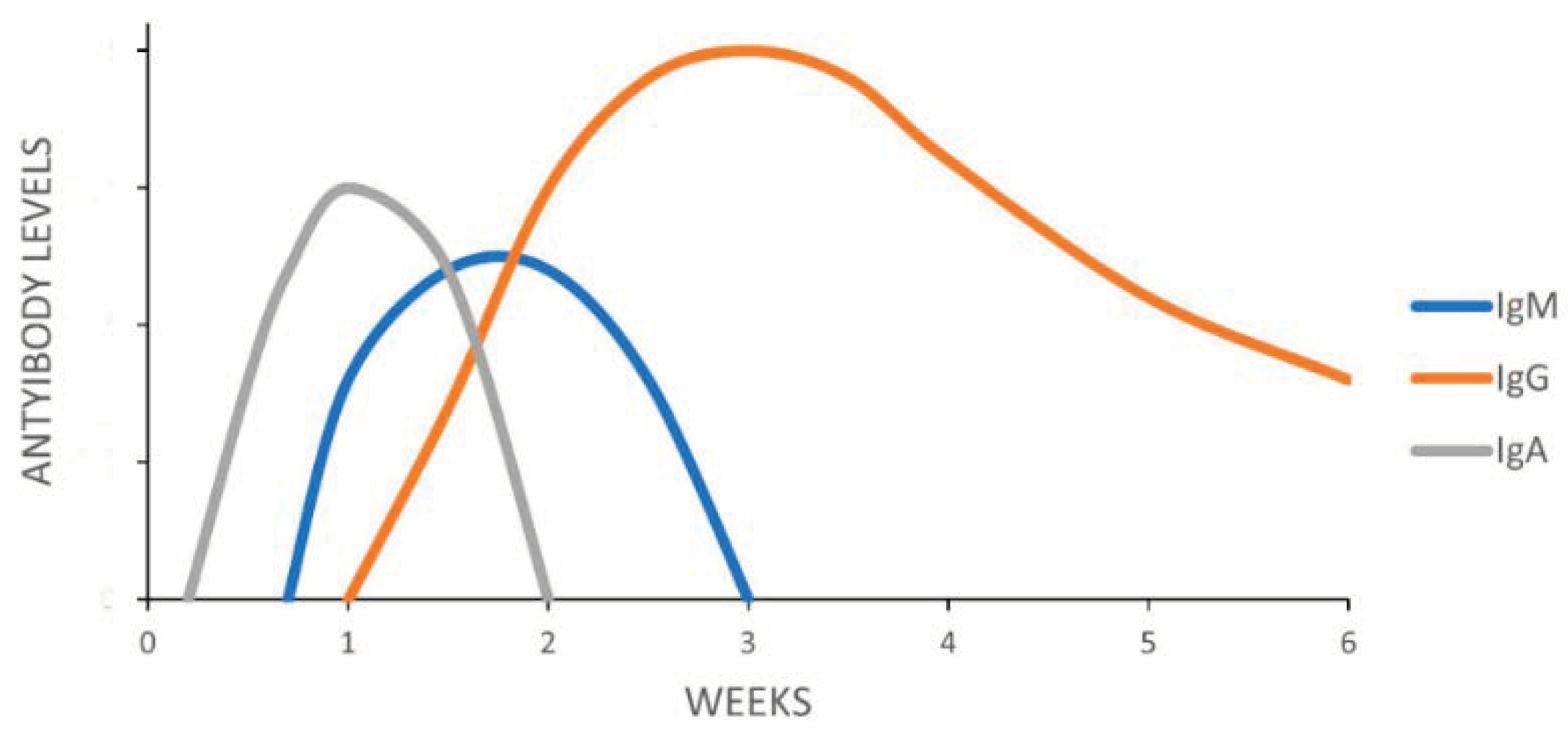
7. Clinical Signs
8. Diagnosis
9. Future Outlook and Recent Advances
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and 'HGE agent' as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.B.; Walker, D.H. Ehrlichioses: An Important One Health Opportunity. Vet Sci 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Sanches, G.S.; Villar, M.; Couto, J.; Ferrolho, J.; Fernández de Mera, I.G.; André, M.R.; Barros-Battesti, D.M.; Machado, R.Z.; Bechara, G.H.; Mateos-Hernández, L.; et al. Comparative proteomic analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) tropical and temperate lineages: Uncovering differences during Ehrlichia canis infection. Front Cell Infect Microbiol 2020, 10, 611113. [Google Scholar] [CrossRef] [PubMed]
- Beall, M.J.; Alleman, A.R.; Breitschwerdt, E.B.; Cohn, L.A.; Couto, C.G.; Dryden, M.W.; Guptill, L.C.; Iazbik, C.; Kania, S.A.; Lathan, P.; et al. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis, and Ehrlichia ewingii in dogs in North America. Parasit Vectors 2012, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Canine vector-borne diseases in Brazil. Parasit Vectors 2008, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.B.; Machado, R.Z.; de Aquino, L.P.; Alessi, A.C.; Costa, M.T. Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Parasitol 2004, 119, 73–86. [Google Scholar] [CrossRef]
- Waner, T.; Harrus, S.; Jongejan, F.; Bark, H.; Keysary, A.; Cornelissen, A.W. Significance of serological testing for ehrlichial diseases in dogs with special emphasis on the diagnosis of canine monocytic ehrlichiosis caused by Ehrlichia canis. Vet Parasitol 2001, 95, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Harrus, S.; Breitschwerdt, E.B. An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet J 2019, 246, 45–53. [Google Scholar] [CrossRef]
- Harrus, S.; Waner, T.; Strauss-Ayali, D.; Bark, H.; Jongejan, F.; Hecht, G.; Baneth, G. Dynamics of IgG1 and IgG2 subclass response in dogs naturally and experimentally infected with Ehrlichia canis. Vet Parasitol 2001, 99, 63–71. [Google Scholar] [CrossRef]
- Maggi, R.G.; Krämer, F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit Vectors 2019, 12, 145. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors 2015, 8, 75. [Google Scholar] [CrossRef]
- Cruz, A.C.; Zweygarth, E.; Ribeiro, M.F.; da Silveira, J.A.; de la Fuente, J.; Grubhoffer, L.; Valdés, J.J.; Passos, L.M. New species of Ehrlichia isolated from Rhipicephalus (Boophilus) microplus shows an ortholog of the E. canis major immunogenic glycoprotein gp36 with a new sequence of tandem repeats. Parasit Vectors 2012, 5, 291. [Google Scholar] [CrossRef]
- Goodman, R.A.; Hawkins, E.C.; Olby, N.J.; Grindem, C.B.; Hegarty, B.; Breitschwerdt, E.B. Molecular identification of Ehrlichia ewingii infection in dogs: 15 cases (1997–2001). J Am Vet Med Assoc 2003, 222, 1102–1107. [Google Scholar] [CrossRef]
- Olano, J.P.; Walker, D.H. Human ehrlichioses. Med Clin North Am 2002, 86, 375–392. [Google Scholar] [CrossRef]
- Popov, V.L.; Yu, X.; Walker, D.H. The 120 kDa outer membrane protein of Ehrlichia chaffeensis: preferential expression on dense-core cells and gene expression in Escherichia coli associated with attachment and entry. Microb Pathog 2000, 28, 71–80. [Google Scholar] [CrossRef]
- Ge, Y.; Rikihisa, Y. Surface-exposed proteins of Ehrlichia chaffeensis. Infect Immun 2007, 75, 3833–3841. [Google Scholar] [CrossRef]
- Dunning Hotopp, J.C.; Lin, M.; Madupu, R.; Crabtree, J.; Angiuoli, S.V.; Eisen, J.A.; Seshadri, R.; Ren, Q.; Wu, M.; Utterback, T.R.; et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet 2006, 2, e21. [Google Scholar] [CrossRef]
- Rikihisa, Y. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet Parasitol 2010, 167, 155–166. [Google Scholar] [CrossRef]
- Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol 2008, 152, 173–185. [Google Scholar] [CrossRef]
- Movilla, R.; García, C.; Siebert, S.; Roura, X. Countrywide serological evaluation of canine prevalence for Anaplasma spp., Borrelia burgdorferi (sensu lato), Dirofilaria immitis and Ehrlichia canis in Mexico. Parasit Vectors 2016, 9, 421. [Google Scholar] [CrossRef]
- Taques, I.; Campos, A.N.S.; Kavasaki, M.L.; de Almeida, S.L.H.; de Aguiar, D.M. Geographic distribution of Ehrlichia canis TRP genotypes in Brazil. Vet Sci 2020, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Bremer, W.G.; Schaefer, J.J.; Wagner, E.R.; Ewing, S.A.; Rikihisa, Y.; Needham, G.R.; Jittapalapong, S.; Moore, D.L.; Stich, R.W. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol 2005, 131, 95–105. [Google Scholar] [CrossRef]
- Diniz, P.; Moura de Aguiar, D. Ehrlichiosis and Anaplasmosis: An update. Vet Clin North Am Small Anim Pract 2022, 52, 1225–1266. [Google Scholar] [CrossRef]
- Judy, L.; David, K.; Peter, K.; Dhaval, S. Canine ehrlichiosis seropositivity and associated factors in Kenya and Tanzania: a retrospective study. BMC Vet Res 2023, 19, 175. [Google Scholar] [CrossRef]
- Miró, G.; Wright, I.; Michael, H.; Burton, W.; Hegarty, E.; Rodón, J.; Buch, J.; Pantchev, N.; von Samson-Himmelstjerna, G. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasit Vectors 2022, 15, 189. [Google Scholar] [CrossRef]
- Ferreira, R.; Cerqueira, A.; Castro, T.; Ferreira, E.; Neves, F.; Barbosa, A.; Macieira, D.; Almosny, N. Genetic diversity of Ehrlichia canis strains from naturally infected dogs in Rio de Janeiro, Brazil. Revista brasileira de parasitologia veterinaria. Braz Jn Vet Parasitol 2014, 23, 301–308. [Google Scholar] [CrossRef]
- Nakaghi, A.C.; Machado, R.Z.; Ferro, J.A.; Labruna, M.B.; Chryssafidis, A.L.; André, M.R.; Baldani, C.D. Sensitivity evaluation of a single-step PCR assay using Ehrlichia canis p28 gene as a target and its application in diagnosis of canine ehrlichiosis. Rev Bras Parasitol Vet 2010, 19, 75–79. [Google Scholar] [CrossRef]
- Aguiar, D.M.; Cavalcante, G.T.; Pinter, A.; Gennari, S.M.; Camargo, L.M.; Labruna, M.B. Prevalence of Ehrlichia canis (Rickettsiales: Anaplasmataceae) in dogs and Rhipicephalus sanguineus (Acari: Ixodidae) ticks from Brazil. J Med Entomol 2007, 44, 126–132. [Google Scholar] [CrossRef]
- Pereira, M.E.; Canei, D.H.; Carvalho, M.R.; Dias Á, F.L.R.; de Almeida, A.; Nakazato, L.; Sousa, V.R.F. Molecular prevalence and factors associated with Ehrlichia canis infection in dogs from the North Pantanal wetland, Brazil. Vet World 2023, 16, 1209–1213. [Google Scholar] [CrossRef]
- Minervino, A.H.H.; Marcili, A.; Moraes-Filho, J.; Lima, J.T.R.; Soares, H.S.; Malheiros, A.F.; Dias, S.R.; Gennari, S.M.; Labruna, M.B. Molecular detection of tick-borne pathogens in dogs from indigenous communities, Amazon, Brazil. Vector Borne Zoonotic Dis 2023, 23, 458–464. [Google Scholar] [CrossRef]
- Fonsêca, A.D.V.; Oliveira, L.M.B.; Jorge, F.R.; Cavalcante, R.O.; Bevilaqua, C.M.L.; Pinto, F.J.M.; Santos, J.; Teixeira, B.M.; Rodrigues, A.; Braz, G.F.; et al. Occurrence of tick-borne pathogens in dogs in a coastal region of the state of Ceará, northeastern Brazil. Rev Bras Parasitol Vet 2022, 31, e021321. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.V.d.F.; Taques, Í.I.G.G.; Miranda, V.C.; Barreto, A.L.G.; Paula, L.G.F.d.; Martins, D.B.; Damasceno, A.D.; Muñoz-Leal, S.; Sevá, A.d.P.; Dantas-Torres, F.; et al. Seroprevalence and hematological abnormalities associated with Ehrlichia canis in dogs referred to a veterinary teaching hospital in central-western Brazil. Ciência Rural 2022, 52, e20201131. [Google Scholar] [CrossRef]
- Pereira, L.S.; Oliveira, P.L.; Barja-Fidalgo, C.; Daffre, S. Production of reactive oxygen species by hemocytes from the cattle tick Boophilus microplus. Exp Parasitol 2001, 99, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Waner, T.; Friedmann-Morvinski, D.; Fishman, Z.; Bark, H.; Harmelin, A. Down-regulation of MHC class II receptors of DH82 cells, following infection with Ehrlichia canis. Vet Immunol Immunopathol 2003, 96, 239–243. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Doyle, C.K.; Zhang, X.; Nethery, K.; Corstvet, R.E.; Walker, D.H.; McBride, J.W. Enzyme-linked immunosorbent assay with conserved immunoreactive glycoproteins gp36 and gp19 has enhanced sensitivity and provides species-specific immunodiagnosis of Ehrlichia canis infection. Clin Vaccine Immunol 2007, 14, 123–128. [Google Scholar] [CrossRef]
- McBride, J.W.; Walker, D.H. Molecular and cellular pathobiology of Ehrlichia infection: targets for new therapeutics and immunomodulation strategies. Expert Rev Mol Med 2011, 13, e3. [Google Scholar] [CrossRef]
- Byerly, C.D.; Patterson, L.L.; McBride, J.W. Ehrlichia TRP effectors: moonlighting, mimicry and infection. Pathog Dis 2021, 79, ftab026. [Google Scholar] [CrossRef]
- Nethery, K.A.; Doyle, C.K.; Zhang, X.; McBride, J.W. Ehrlichia canis gp200 contains dominant species-specific antibody epitopes in terminal acidic domains. Infect Immun 2007, 75, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, T.; Keysary, A.; Baneth, G.; Miyashiro, S.; Strenger, C.; Waner, T.; McBride, J.W. Genetic and antigenic diversities of major immunoreactive proteins in globally distributed Ehrlichia canis strains. Clin Vaccine Immunol 2008, 15, 1080–1088. [Google Scholar] [CrossRef]
- Feng, H.M.; Walker, D.H. Mechanisms of immunity to Ehrlichia muris: a model of monocytotropic ehrlichiosis. Infect Immun 2004, 72, 966–971. [Google Scholar] [CrossRef]
- Castro, M.B.; Szabó, M.P.J.; Aquino, L.; Dagnoni, A.S.; Alessi, A.C.; Costa, M.T.; Nakaghi, A.C.H.; Santi, M.; Calchi, A.C.; André, M.R.; et al. Immunophenotypical and pathological changes in dogs experimentally infected with Ehrlichia canis. Rev Bras Parasitol Vet 2022, 31, e021621. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.W.; Corstvet, R.E.; Gaunt, S.D.; Boudreaux, C.; Guedry, T.; Walker, D.H. Kinetics of antibody response to Ehrlichia canis immunoreactive proteins. Infect Immun 2003, 71, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, M.E.; Ceron, J.J.; Leontides, L.; Siarkou, V.I.; Martinez, S.; Tvarijonaviciute, A.; Koutinas, A.F.; Harrus, S. Serum acute phase proteins as clinical phase indicators and outcome predictors in naturally occurring canine monocytic ehrlichiosis. J Vet Intern Med 2011, 25, 811–817. [Google Scholar] [CrossRef]
- Harrus, S.; Waner, T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J 2011, 187, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Shipov, A.; Klement, E.; Reuveni-Tager, L.; Waner, T.; Harrus, S. Prognostic indicators for canine monocytic ehrlichiosis. Vet Parasitol 2008, 153, 131–138. [Google Scholar] [CrossRef]
- Rodríguez-Alarcón, C.A.; Beristain-Ruiz, D.M.; Olivares-Muñoz, A.; Quezada-Casasola, A.; Pérez-Casio, F.; Álvarez-Martínez, J.A.; Tapia-Alanís, J.; Lira-Amaya, J.J.; Rivera-Barreno, R.; Cera-Hurtado, O.S.; et al. Demonstrating the presence of Ehrlichia canis DNA from different tissues of dogs with suspected subclinical ehrlichiosis. Parasit Vectors 2020, 13, 518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nair, A.D.S.; Alhassan, A.; Jaworski, D.C.; Liu, H.; Trinkl, K.; Hove, P.; Ganta, C.K.; Burkhardt, N.; Munderloh, U.G.; et al. Multiple Ehrlichia chaffeensis genes critical for its persistent infection in a vertebrate host are identified by random mutagenesis coupled with in vivo infection assessment. Infect Immun 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zetina, M.; Adame-Gallegos, J.; Dzul-Rosado, K. Effectivity of diagnostic methods for the detection of human and canine monocytic ehrlichiosis. Rev Chilena Infectol 2019, 36, 650–655. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Koutinas, A.F.; Billinis, C.; Leontides, L.S.; Kontos, V.; Papadopoulos, O.; Rallis, T.; Fytianou, A. Evaluation of cytology in the diagnosis of acute canine monocytic ehrlichiosis (Ehrlichia canis): a comparison between five methods. Vet Microbiol 2003, 91, 197–204. [Google Scholar] [CrossRef]
- Wichianchot, S.; Hongsrichan, N.; Maneeruttanarungroj, C.; Pinlaor, S.; Iamrod, K.; Purisarn, A.; Donthaisong, P.; Karanis, P.; Nimsuphan, B.; Rucksaken, R. A newly developed droplet digital PCR for Ehrlichia canis detection: comparisons to conventional PCR and blood smear techniques. J Vet Med Sci 2022, 84, 831–840. [Google Scholar] [CrossRef]
- Silva, L.; Oliveira, P.; Campos, A.; Silva, V.; Saturnino, K.; Braga, Í.; Aguiar, D.; Ramos, D.; Moreira, C. Misdiagnosis of canine monocytic ehrlichiosis: why do we still risk animal lives? Braz J Vet Res An Sci 2023, 60, e213508. [Google Scholar] [CrossRef]
- Kledmanee, K.; Suwanpakdee, S.; Krajangwong, S.; Chatsiriwech, J.; Suksai, P.; Suwannachat, P.; Sariya, L.; Buddhirongawatr, R.; Charoonrut, P.; Chaichoun, K. Development of multiplex polymerase chain reaction for detection of Ehrlichia canis, Babesia spp and Hepatozoon canis in canine blood. Southeast Asian J Trop Med Public Health 2009, 40, 35–39. [Google Scholar]
- Azhahianambi, P.; Jyothimol, G.; Baranidharan, G.R.; Aravind, M.; Latha, B.R.; Raman, M. Evaluation of multiplex PCR assay for detection of Babesia spp, Ehrlichia canis and Trypanosoma evansi in dogs. Acta Trop 2018, 188, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bilgiç, H.B.; Karagenç, T.; Simuunza, M.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata, Babesia bovis and Anaplasma marginale in cattle. Exp Parasitol 2013, 133, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.U.; Hussain, S.; Song, B.; Ghauri, H.N.; Zeb, J.; Sparagano, O.A. Ehrlichiosis in Dogs: A Comprehensive Review about the Pathogen and Its Vectors with Emphasis on South and East Asian Countries. Vet Sci 2022, 10, 21. [Google Scholar] [CrossRef]
- Little, S.E.; Beall, M.J.; Bowman, D.D.; Chandrashekar, R.; Stamaris, J. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasit Vectors 2014, 7, 257. [Google Scholar] [CrossRef]
- Bélanger, M.; Sorenson, H.L.; France, M.K.; Bowie, M.V.; Barbet, A.F.; Breitschwerdt, E.B.; Alleman, A.R. Comparison of serological detection methods for diagnosis of Ehrlichia canis infections in dogs. J Clin Microbiol 2002, 40, 3506–3508. [Google Scholar] [CrossRef] [PubMed]
- Harrus, S.; Alleman, A.R.; Bark, H.; Mahan, S.M.; Waner, T. Comparison of three enzyme-linked immunosorbant assays with the indirect immunofluorescent antibody test for the diagnosis of canine infection with Ehrlichia canis. Vet Microbiol 2002, 86, 361–368. [Google Scholar] [CrossRef]
- Kaewmongkol, S.; Suwan, E.; Sirinarumitr, T.; Jittapalapong, S.; Fenwick, S.G.; Kaewmongkol, G. Detection of specific IgM and IgG antibodies in acute canine monocytic ehrlichiosis that recognize recombinant gp36 antigens. Heliyon 2020, 6, e04409. [Google Scholar] [CrossRef]
- Haglund, S.; Lager, M.; Gyllemark, P.; Andersson, G.; Ekelund, O.; Sundqvist, M.; Henningsson, A.J. CXCL13 in laboratory diagnosis of Lyme neuroborreliosis-the performance of the recomBead and ReaScan CXCL13 assays in human cerebrospinal fluid samples. Eur J Clin Microbiol Infect Dis 2022, 41, 175–179. [Google Scholar] [CrossRef]
- DeGroat, W.; Abdelhalim, H.; Patel, K.; Mendhe, D.; Zeeshan, S.; Ahmed, Z. Discovering biomarkers associated and predicting cardiovascular disease with high accuracy using a novel nexus of machine learning techniques for precision medicine. Sci Rep 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Magni, R.; Luchini, A.; Liotta, L.; Molestina, R.E. Proteomic analysis reveals pathogen-derived biomarkers of acute babesiosis in erythrocytes, plasma, and urine of infected hamsters. Parasitol Res 2020, 119, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Setubal, J.C.; Almeida, N.F.; Wattam, A.R. Comparative genomics for prokaryotes. Methods Mol Biol 2018, 1704, 55–78. [Google Scholar] [CrossRef]
- Neave, M.J.; Mileto, P.; Joseph, A.; Reid, T.J.; Scott, A.; Williams, D.T.; Keyburn, A.L. Comparative genomic analysis of the first Ehrlichia canis detections in Australia. Ticks Tick Borne Dis 2022, 13, 101909. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Patel, J.G.; Zhang, X.; Walker, D.H.; McBride, J.W. Immunoreactive protein repertoires of Ehrlichia chaffeensis and E. canis reveal the dominance of hypothetical proteins and conformation-dependent antibody epitopes. Infect Immun 2021, 89, e0022421. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




