Submitted:
04 April 2024
Posted:
07 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Materials
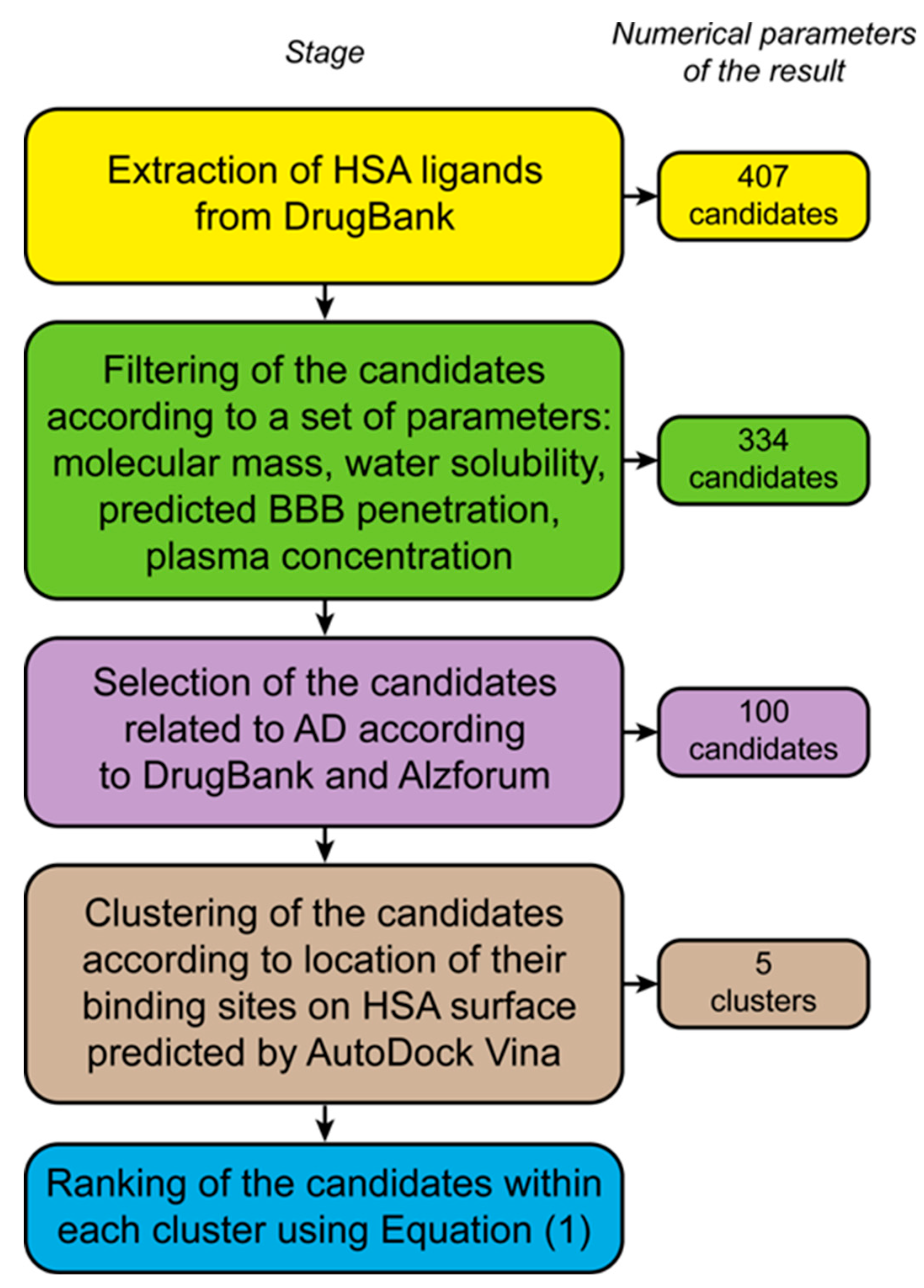
2.2. Bioinformatic Selection and Structural Analysis of the Therapeutic Low-Molecular-Weight HSA Ligands Associated with AD
- water solubility of the ligand (Experimental Water Solubility / Calculated Water Solubility, field “Properties” of DrugBank) should exceed 1 μM to ensure the possibility of efficient HSA loading with the ligand;
- blood-brain barrier (BBB) penetration of the ligand (the field “Predicted ADMET Features” of DrugBank) should exceed 50% to ensure its efficient transfer from the bloodstream into the brain;
- plasma ligand concentration (manually collected from Pubmed (https://pubmed.ncbi.nlm.nih.gov, accessed on 14 March 2024) and DrugBank (field “Absorption”) should exceed 0.5 nM, which corresponds to the total plasma Aβ40 concentration [20].
2.3. Preparation of Recombinant Aβ
2.4. Solubility of HSA Ligands
2.5. Surface Plasmon Resonance Studies
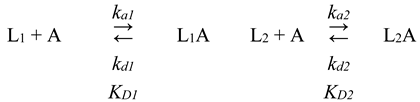
3. Results
3.1. Bioinformatic Selection of the Therapeutic Low-Molecular-Weight HSA Ligands Associated with AD
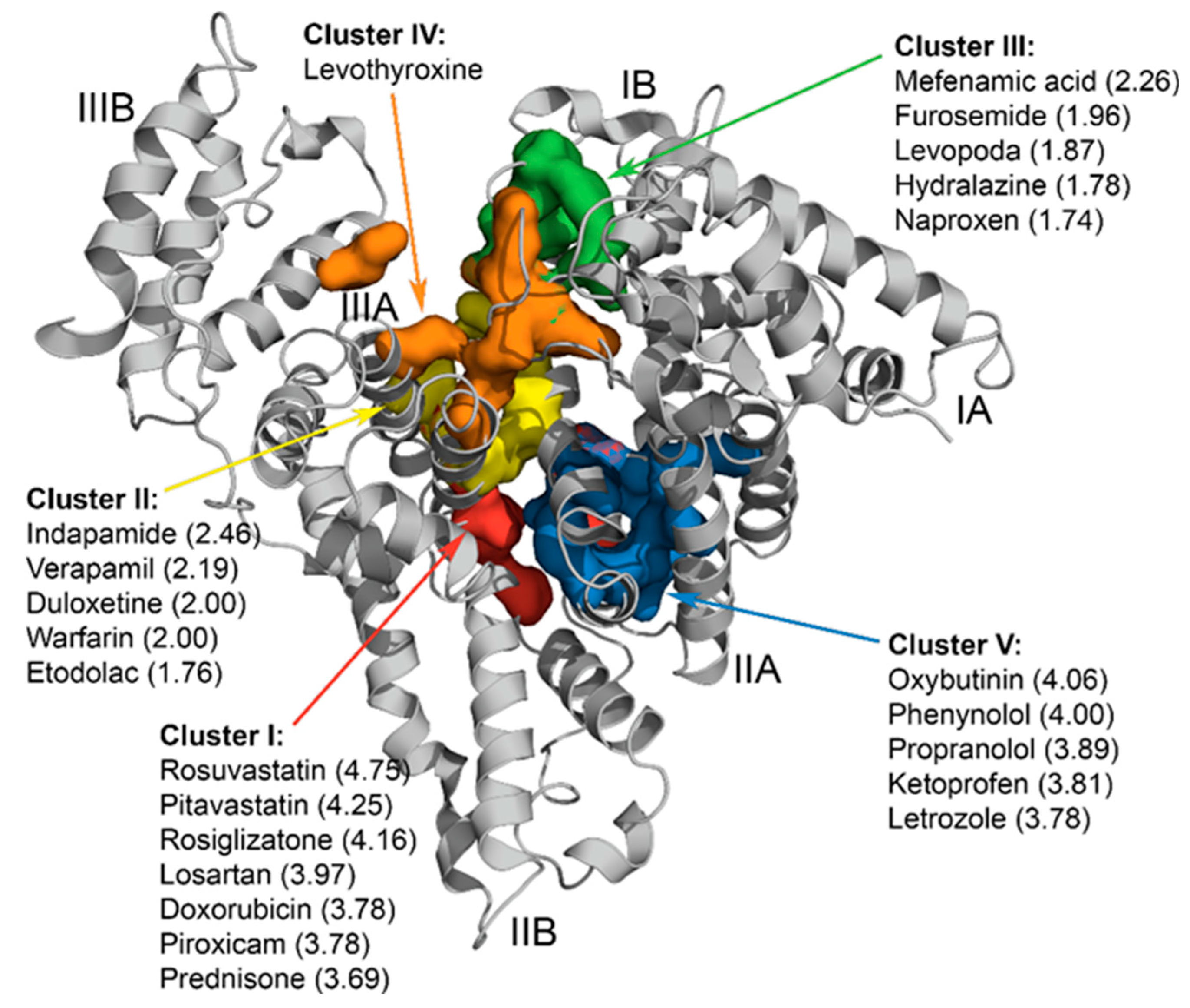
3.2. Classification and Ranking of the Selected LMWLs According to the Expected Location of Their Binding Sites on HSA
- Cluster I: P447 (faa = 84.4%), R222 (78.1%), K444 (62.5%), D451 (59.4 %), E292 (46.9%);
- Cluster II: Y452 (faa = 40.5%), K436 (32.4%), K195 (32.4%), A191 (27.0%) and K432 (27.0%);
- Cluster III: R117 (faa = 39.0%), E141 (34.8%), R145 (30.4%), E86, P118 (26%) and Y140 (26%);
- Cluster IV: R145, E425, N109, Q459, H146, R114, K525, R186;
- Cluster V: E292 (faa = 55.6%), R257 (47.2%), K199 (44.4%), R218 (41.7%), L219 (38.9%).
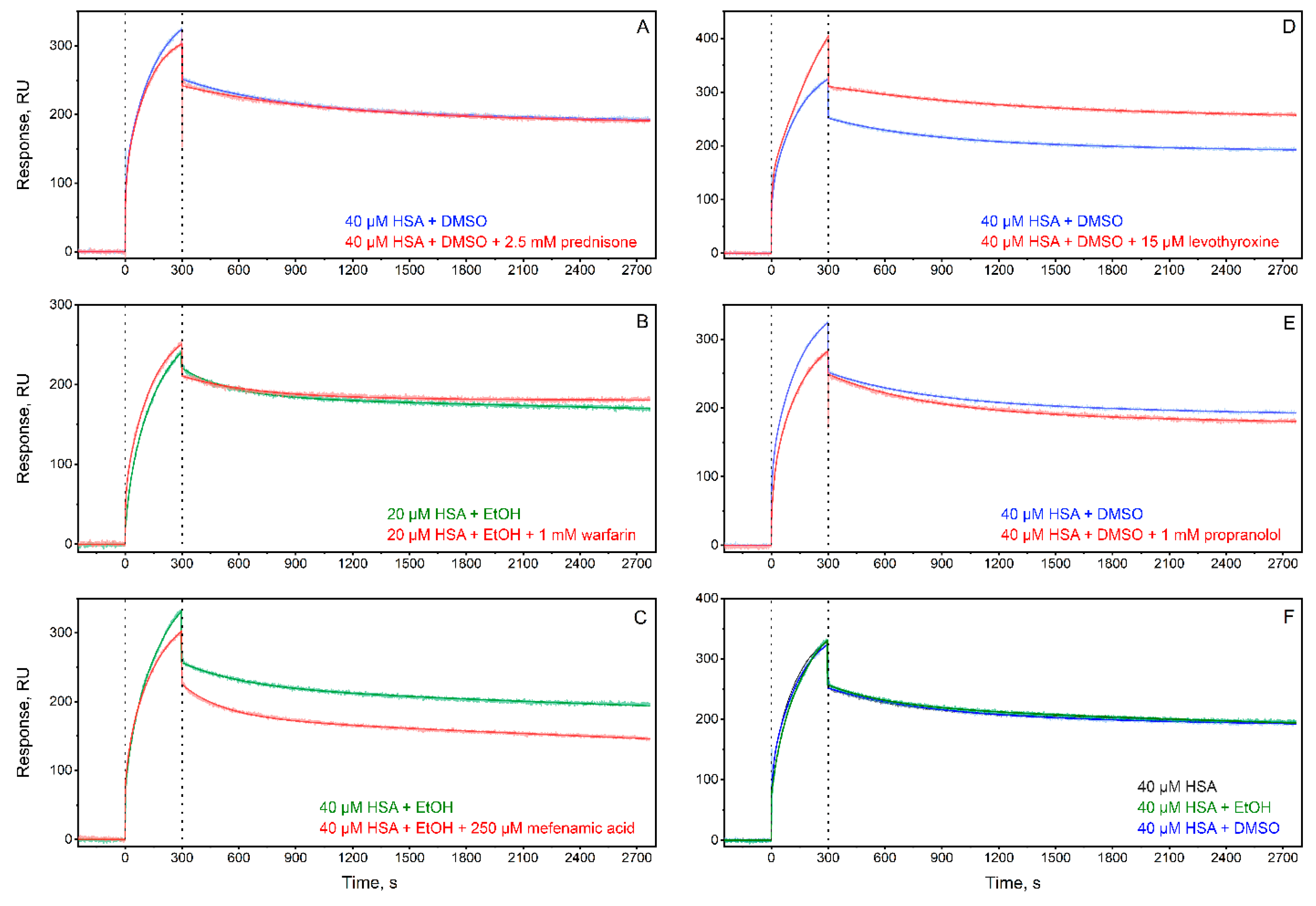
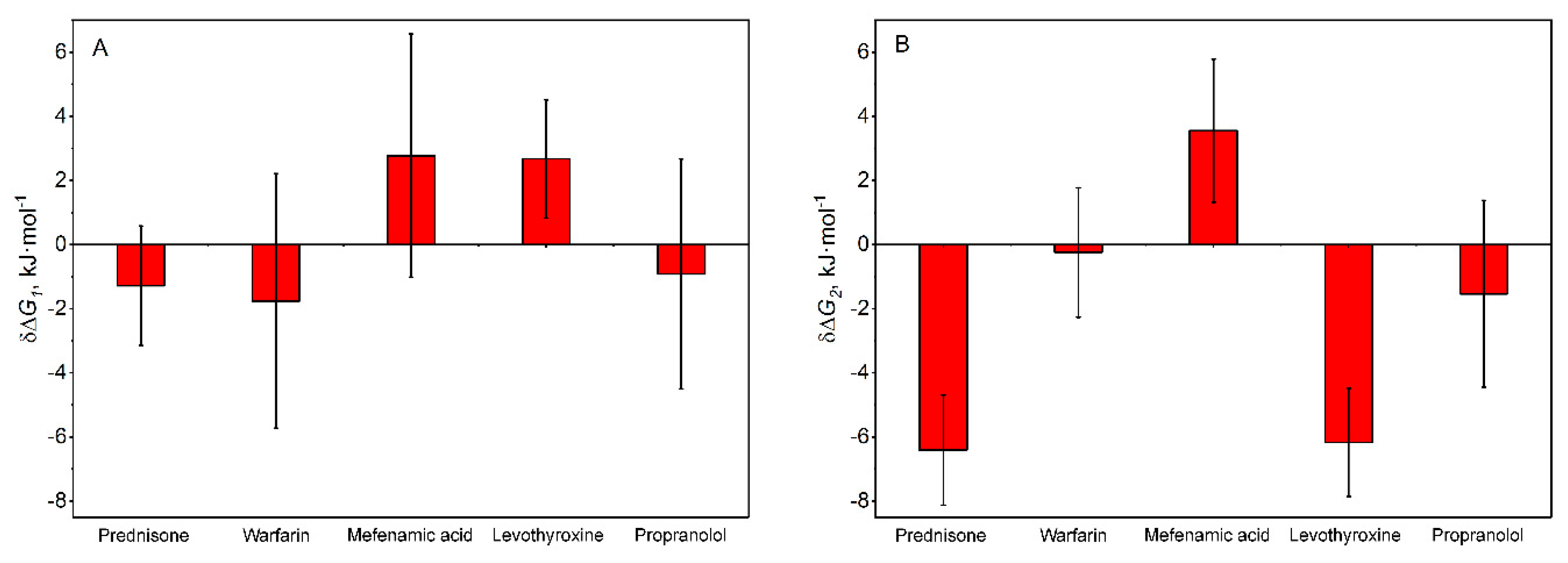
3.3. Experimental Validation of the Ability of the Top-Ranked AD-Related LMWLs to Affect HSA Affinity for Aβ40
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, B.; Carballal, S.; Turell, L.; Radi, R. Formation and Reactions of Sulfenic Acid in Human Serum Albumin. Methods Enzymol 2010, 473, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Ezra, A.; Rabinovich-Nikitin, I.; Rabinovich-Toidman, P.; Solomon, B. Multifunctional Effects of Human Serum Albumin Toward Neuroprotection in Alzheimer Disease. In Neuroprotection in Alzheimer’s Disease; Elsevier, 2017; pp. 217–238. [Google Scholar]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural Basis of the Drug-Binding Specificity of Human Serum Albumin. J Mol Biol 2005, 353, 38–52. [Google Scholar] [CrossRef]
- Carter, D.C.; He, X.M. Structure of Human Serum Albumin. Science 1990, 249, 302–303. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic Structure and Chemistry of Human Serum Albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, S.; Feroz, S.R. Serum Albumin: Clinical Significance of Drug Binding and Development as Drug Delivery Vehicle. Adv Protein Chem Struct Biol 2021, 123, 193–218. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Ghuman, J.; Komatsu, T.; Tsuchida, E.; Curry, S. Crystal Structural Analysis of Human Serum Albumin Complexed with Hemin and Fatty Acid. BMC Struct Biol 2003, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, A.; Schwanz, H.A.; Spencer, D.J.; Bhasin, S.; Hamilton, J.A.; Jayaram, B.; Goldman, A.L.; Krishna, M.; Krishnan, M.; Shah, A.; et al. Allosterically Coupled Multisite Binding of Testosterone to Human Serum Albumin. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- van der Vusse, G.J. Albumin as Fatty Acid Transporter. Drug Metab Pharmacokinet 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Zaragoza, F. Non-Covalent Albumin Ligands in FDA-Approved Therapeutic Peptides and Proteins. J Med Chem 2023, 66, 3656–3663. [Google Scholar] [CrossRef]
- van Witteloostuijn, S.B.; Pedersen, S.L.; Jensen, K.J. Half-Life Extension of Biopharmaceuticals Using Chemical Methods: Alternatives to PEGylation. ChemMedChem 2016, 11, 2474–2495. [Google Scholar] [CrossRef]
- Bech, E.M.; Pedersen, S.L.; Jensen, K.J. Chemical Strategies for Half-Life Extension of Biopharmaceuticals: Lipidation and Its Alternatives. ACS Med Chem Lett 2018, 9, 577–580. [Google Scholar] [CrossRef]
- Biere, A.L.; Ostaszewski, B.; Stimson, E.R.; Hyman, B.T.; Maggio, J.E.; Selkoe, D.J. Amyloid β-Peptide Is Transported on Lipoproteins and Albumin in Human Plasma. Journal of Biological Chemistry 1996, 271, 32916–32922. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Kokjohn, T.A.; Kalback, W.; Luehrs, D.; Galasko, D.R.; Chevallier, N.; Koo, E.H.; Emmerling, M.R.; Roher, A.E. Amyloid-Beta Peptides Interact with Plasma Proteins and Erythrocytes: Implications for Their Quantitation in Plasma. Biochem Biophys Res Commun 2000, 268, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. β-Amyloid: The Key Peptide in the Pathogenesis of Alzheimer’s Disease. Front Pharmacol 2015, 6, 221. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Algamal, M.; Milojevic, J.; Jafari, N.; Zhang, W.; Melacini, G. Mapping the Interactions between the Alzheimer’s Aβ-Peptide and Human Serum Albumin beyond Domain Resolution. Biophys J 2013, 105, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Gonzalez, M.; Gasparovic, C. Albumin Exchange in Alzheimer’s Disease: Might CSF Be an Alternative Route to Plasma? Front Neurol 2019, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Boada, M.; Ortiz, P.; Anaya, F.; Hernández, I.; Muñoz, J.; Núñez, L.; Olazarán, J.; Roca, I.; Cuberas, G.; Tárraga, L.; et al. Amyloid-Targeted Therapeutics in Alzheimer’s Disease: Use of Human Albumin in Plasma Exchange as a Novel Approach for Abeta Mobilization. Drug News Perspect 2009, 22, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Stanyon, H.F.; Viles, J.H. Human Serum Albumin Can Regulate Amyloid-β Peptide Fiber Growth in the Brain Interstitium: Implications for Alzheimer Disease. J Biol Chem 2012, 287, 28163–28168. [Google Scholar] [CrossRef]
- Costa, M.; Ortiz, A.M.; Jorquera, J.I. Therapeutic Albumin Binding to Remove Amyloid-β. J Alzheimers Dis 2012, 29, 159–170. [Google Scholar] [CrossRef]
- Boada, M.; López, O.; Núñez, L.; Szczepiorkowski, Z.M.; Torres, M.; Grifols, C.; Páez, A. Plasma Exchange for Alzheimer’s Disease Management by Albumin Replacement (AMBAR) Trial: Study Design and Progress. Alzheimers Dement (N Y) 2019, 5, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Boada, M.; Anaya, F.; Ortiz, P.; Olazarán, J.; Shua-Haim, J.R.; Obisesan, T.O.; Hernández, I.; Muñoz, J.; Buendia, M.; Alegret, M.; et al. Efficacy and Safety of Plasma Exchange with 5% Albumin to Modify Cerebrospinal Fluid and Plasma Amyloid-β Concentrations and Cognition Outcomes in Alzheimer’s Disease Patients: A Multicenter, Randomized, Controlled Clinical Trial. J Alzheimers Dis 2017, 56, 129–143. [Google Scholar] [CrossRef]
- Prajapati, K.D.; Sharma, S.S.; Roy, N. Current Perspectives on Potential Role of Albumin in Neuroprotection. Rev Neurosci 2011, 22, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-M.; Byun, K.; Cho, K.; Kim, J.Y.; Yoo, J.S.; Kim, D.; Paek, S.H.; Kim, S.U.; Simpson, R.J.; Lee, B. Human Microglial Cells Synthesize Albumin in Brain. PLoS One 2008, 3, e2829. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.M.; Kozlowski, P.B. Evidence for Blood-Brain Barrier Changes in Senile Dementia of the Alzheimer Type (SDAT). Ann N Y Acad Sci 1982, 396, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, M.H.; Kaplan, A.A. Therapeutic Plasma Exchange: Complications and Management. Am J Kidney Dis 1994, 23, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Szczeklik, W.; Wawrzycka, K.; Włudarczyk, A.; Sega, A.; Nowak, I.; Seczyńska, B.; Fajfer, I.; Zając, K.; Królikowski, W.; Kózka, M. Complications in Patients Treated with Plasmapheresis in the Intensive Care Unit. Anaesthesiol Intensive Ther 2013, 45, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.M.; Nair, R.C.; Rock, G. Complications of Plasma Exchange. Transfusion (Paris) 1989, 29, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Litus, E.A.; Kazakov, A.S.; Sokolov, A.S.; Nemashkalova, E.L.; Galushko, E.I.; Dzhus, U.F.; Marchenkov, V.V.; Galzitskaya, O.V.; Permyakov, E.A.; Permyakov, S.E. The Binding of Monomeric Amyloid β Peptide to Serum Albumin Is Affected by Major Plasma Unsaturated Fatty Acids. Biochem Biophys Res Commun 2019, 510, 248–253. [Google Scholar] [CrossRef]
- Litus, E.A.; Kazakov, A.; Deryusheva, E.; Nemashkalova, E.; Shevelyova, M.; Nazipova, A.; Permyakova, M.; Raznikova, E.; Uversky, V.; Permyakov, S. Serotonin Promotes Serum Albumin Interaction with the Monomeric Amyloid β Peptide. Int J Mol Sci 2021, 22, 5896. [Google Scholar] [CrossRef]
- Litus, E.A.; Kazakov, A.S.; Deryusheva, E.I.; Nemashkalova, E.L.; Shevelyova, M.P.; Machulin, A. V; Nazipova, A.A.; Permyakova, M.E.; Uversky, V.N.; Permyakov, S.E. Ibuprofen Favors Binding of Amyloid-β Peptide to Its Depot, Serum Albumin. Int J Mol Sci 2022, 23, 6168. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and Brain Fatty Acid Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. Journal of Alzheimer’s Disease 2012, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Vlad, S.C.; Miller, D.R.; Kowall, N.W.; Felson, D.T. Protective Effects of NSAIDs on the Development of Alzheimer Disease. Neurology 2008, 70, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H.; et al. Serotonin Signaling Is Associated with Lower Amyloid-β Levels and Plaques in Transgenic Mice and Humans. Proc Natl Acad Sci U S A 2011, 108, 14968–14973. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, A.-M.; Soboleva, T.A.; Jans, D.A.; Board, P.G.; Baker, R.T. An Efficient System for High-Level Expression and Easy Purification of Authentic Recombinant Proteins. Protein Sci 2004, 13, 1331–1339. [Google Scholar] [CrossRef]
- Travis, J.; Pannell, R. Selective Removal of Albumin from Plasma by Affinity Chromatography. Clin Chim Acta 1973, 49, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to Measure and Predict the Molar Absorption Coefficient of a Protein. Protein Sci 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51, D523–D531. [CrossRef] [PubMed]
- Law, V.; Knox, C.; Djoumbou, Y.; Jewison, T.; Guo, A.C.; Liu, Y.; Maciejewski, A.; Arndt, D.; Wilson, M.; Neveu, V.; et al. DrugBank 4.0: Shedding New Light on Drug Metabolism. Nucleic Acids Res 2014, 42, D1091–7. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A Knowledgebase for Drugs, Drug Actions and Drug Targets. Nucleic Acids Res 2008, 36, D901–6. [Google Scholar] [CrossRef]
- Macielag, M.J. Chemical Properties of Antimicrobials and Their Uniqueness. In Antibiotic Discovery and Development; Springer US: Boston, MA, 2012; pp. 793–820. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J Comput Chem 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr D Biol Crystallogr 2002, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein-Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, T. Computing and Visualizing Dynamic Time Warping Alignments in R : The Dtw Package. J Stat Softw 2009, 31. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Grüne, T.; Curry, S. Crystallographic Analysis Reveals Common Modes of Binding of Medium and Long-Chain Fatty Acids to Human Serum Albumin. J Mol Biol 2000, 303, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Amisaki, T. Fatty Acid Binding to Serum Albumin: Molecular Simulation Approaches. Biochim Biophys Acta 2013, 1830, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the General Anesthetics Propofol and Halothane to Human Serum Albumin. High Resolution Crystal Structures. J Biol Chem 2000, 275, 38731–38738. [Google Scholar] [CrossRef] [PubMed]
- Boudinot, F.D.; Jusko, W.J. Plasma Protein Binding Interaction of Prednisone and Prednisolone. J Steroid Biochem 1984, 21, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.G.; Larsen, C.G.; Jakobsen, P.; Brodersen, R. Interaction of Warfarin with Human Serum Albumin. A Stoichiometric Description. Mol Pharmacol 1985, 27, 263–270. [Google Scholar]
- Rahim, S.; Oise Aubry, A.-F. Location of Binding Sites on Immobilized Human Serum Albumin for Some Nonsteroidal Anti-Inflammatory Drugs. J Pharm Sci 1995, 84, 949–952. [Google Scholar] [CrossRef]
- Zsila, F. Subdomain IB Is the Third Major Drug Binding Region of Human Serum Albumin: Toward the Three-Sites Model. Mol Pharm 2013, 10, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.F.; Roth, J.; Robbins, J. The Binding of Thyroxine by Serum Albumin as Measured by Fluorescence Quenching. J Biol Chem 1966, 241, 560–567. [Google Scholar] [CrossRef]
- Housaindokht, M.R.; Rouhbakhsh Zaeri, Z.; Bahrololoom, M.; Chamani, J.; Bozorgmehr, M.R. Investigation of the Behavior of HSA upon Binding to Amlodipine and Propranolol: Spectroscopic and Molecular Modeling Approaches. Spectrochim Acta A Mol Biomol Spectrosc 2012, 85, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat Rev Drug Discov 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Andrich, K.; Bieschke, J. The Effect of (-)-Epigallo-Catechin-(3)-Gallate on Amyloidogenic Proteins Suggests a Common Mechanism. Adv Exp Med Biol 2015, 863, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Nosi, D.; Natalello, A.; Porcari, R.; Ramazzotti, M.; Chiti, F.; Bellotti, V.; Doglia, S.M.; Stefani, M.; Bucciantini, M. The Polyphenol Oleuropein Aglycone Hinders the Growth of Toxic Transthyretin Amyloid Assemblies. J Nutr Biochem 2016, 30, 153–166. [Google Scholar] [CrossRef]
- Rahmani, M.; Negro Álvarez, S.E.; Hernández, E.B. The Potential Use of Tetracyclines in Neurodegenerative Diseases and the Role of Nano-Based Drug Delivery Systems. Eur J Pharm Sci 2022, 175, 106237. [Google Scholar] [CrossRef]
- Ferrié, L.; Figadère, B.; Rose, C.; Tomas-Grau, R.H.; Chehín, R.; Zabala, B.; Michel, P.P.; Raisman-Vozari, R.; Brunel, J.-M. C9-Functionalized Doxycycline Analogs as Drug Candidates to Prevent Pathological α-Synuclein Aggregation and Neuroinflammation in Parkinson’s Disease Degeneration. ChemMedChem 2024, e202300597. [Google Scholar] [CrossRef]
- Tokuda, T.; Oide, T.; Tamaoka, A.; Ishii, K.; Matsuno, S.; Ikeda, S. Prednisolone (30-60 Mg/Day) for Diseases Other than AD Decreases Amyloid Beta-Peptides in CSF. Neurology 2002, 58, 1415–1418. [Google Scholar] [CrossRef]
- Alisky, J.M. Intrathecal Corticosteroids Might Slow Alzheimer’s Disease Progression. Neuropsychiatr Dis Treat 2008, 4, 831–833. [Google Scholar] [CrossRef]
- Barber, M.; Tait, R.C.; Scott, J.; Rumley, A.; Lowe, G.D.O.; Stott, D.J. Dementia in Subjects with Atrial Fibrillation: Hemostatic Function and the Role of Anticoagulation. J Thromb Haemost 2004, 2, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K. Direct Oral Anticoagulants (DOACs) for Therapeutic Targeting of Thrombin, a Key Mediator of Cerebrovascular and Neuronal Dysfunction in Alzheimer’s Disease. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Almqvist, E.G.; Johansson, J.-O.; Mattsson, N.; Hansson, O.; Wallin, A.; Blennow, K.; Zetterberg, H.; Svensson, J. Reduced Cerebrospinal Fluid Level of Thyroxine in Patients with Alzheimer’s Disease. Psychoneuroendocrinology 2013, 38, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Beaman, E.E.; Bonde, A.N.; Larsen, S.M.U.; Ozenne, B.; Lohela, T.J.; Nedergaard, M.; Gíslason, G.H.; Knudsen, G.M.; Holst, S.C. Blood-Brain Barrier Permeable β-Blockers Linked to Lower Risk of Alzheimer’s Disease in Hypertension. Brain 2023, 146, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, V.; Tiburcio-Jimenez, D.; Mendoza-Hernandez, M.A.; Delgado-Enciso, J.; De-Leon-Zaragoza, L.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Lara-Esqueda, A.; Delgado-Enciso, O.G.; et al. Improve Cognitive Impairment Using Mefenamic Acid Non-Steroidal Anti-Inflammatory Therapy: Additional Beneficial Effect Found in a Controlled Clinical Trial for Prostate Cancer Therapy. Am J Transl Res 2021, 13, 4535–4543. [Google Scholar] [PubMed]
- Dobarro, M.; Gerenu, G.; Ramírez, M.J. Propranolol Reduces Cognitive Deficits, Amyloid and Tau Pathology in Alzheimer’s Transgenic Mice. Int J Neuropsychopharmacol 2013, 16, 2245–2257. [Google Scholar] [CrossRef]
- Dobarro, M.; Orejana, L.; Aguirre, N.; Ramírez, M.J. Propranolol Restores Cognitive Deficits and Improves Amyloid and Tau Pathologies in a Senescence-Accelerated Mouse Model. Neuropharmacology 2013, 64, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Kim, H.-S.; Woo, R.-S.; Park, C.H.; Shin, K.-Y.; Lee, J.-P.; Chang, K.-A.; Kim, S.; Suh, Y.-H. Mefenamic Acid Shows Neuroprotective Effects and Improves Cognitive Impairment in in Vitro and in Vivo Alzheimer’s Disease Models. Mol Pharmacol 2006, 69, 76–84. [Google Scholar] [CrossRef]
- Choi, T.S.; Lee, H.J.; Han, J.Y.; Lim, M.H.; Kim, H.I. Molecular Insights into Human Serum Albumin as a Receptor of Amyloid-β in the Extracellular Region. J Am Chem Soc 2017, 139, 15437–15445. [Google Scholar] [CrossRef]
- Aisen, P.S.; Davis, K.L.; Berg, J.D.; Schafer, K.; Campbell, K.; Thomas, R.G.; Weiner, M.F.; Farlow, M.R.; Sano, M.; Grundman, M.; et al. A Randomized Controlled Trial of Prednisone in Alzheimer’s Disease. Alzheimer’s Disease Cooperative Study. Neurology 2000, 54, 588–593. [Google Scholar] [CrossRef]
- Deryusheva, E.; Kazakov, A.; Nemashkalova, E.; Shevelyova, M.; Nazipova, A.; Permyakova, M.; Raznikova, E.; Permyakov, S.; Litus, E. Modulation of Human Serum Albumin Interaction with Amyloid b Peptide by Ibuprofen, Risperidone, Serotonin and Tryptophan. FEBS Open Bio 2022, 12, 230–230. [Google Scholar] [CrossRef]
- Brodaty, H.; Ames, D.; Snowdon, J.; Woodward, M.; Kirwan, J.; Clarnette, R.; Lee, E.; Greenspan, A. Risperidone for Psychosis of Alzheimer’s Disease and Mixed Dementia: Results of a Double-Blind, Placebo-Controlled Trial. Int J Geriatr Psychiatry 2005, 20, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.S.; Kim, Y.H.; Kwon, M.J.; Kim, J.-H.; Min, C.Y.; Yoo, D.M.; Choi, H.G. The Association Between Thyroid Diseases and Alzheimer’s Disease in a National Health Screening Cohort in Korea. Front Endocrinol (Lausanne) 2022, 13, 815063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, C. Multipronged Regulatory Functions of Serum Albumin in Early Stages of Amyloid-β Aggregation. ACS Chem Neurosci 2021, 12, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, J.; Raditsis, A.; Melacini, G. Human Serum Albumin Inhibits Abeta Fibrillization through a “Monomer-Competitor” Mechanism. Biophys J 2009, 97, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, F.; Xu, L.; Jia, L. HSA Targets Multiple Aβ42 Species and Inhibits the Seeding-Mediated Aggregation and Cytotoxicity of Aβ42 Aggregates. RSC Adv 2016, 6, 71165–71175. [Google Scholar] [CrossRef]
- Bode, D.C.; Stanyon, H.F.; Hirani, T.; Baker, M.D.; Nield, J.; Viles, J.H. Serum Albumin’s Protective Inhibition of Amyloid-β Fiber Formation Is Suppressed by Cholesterol, Fatty Acids and Warfarin. J Mol Biol 2018, 430, 919–934. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Da Costa, G.; Cerezo, A.B.; Troncoso, A.M.; Richard, T.; Garcia-Parrilla, M.C. In Vitro Effects of Serotonin, Melatonin, and Other Related Indole Compounds on Amyloid-β Kinetics and Neuroprotection. Mol Nutr Food Res 2018, 62. [Google Scholar] [CrossRef]
| Predicted cluster on HSA molecule | Drug | Discovery date | Drug class | Application area | Equilibrium association constant for the drug-HSA interaction | Calculated occupancy of HSA binding sites for 2.5-40 µM HSA | Drug concentration used for the SPR studies |
|---|---|---|---|---|---|---|---|
| I | Prednisone | 1950 | Corticosteroid | Transplantology; treatment of allergy, inflammation, infection, cancer, endocrine, autoimmune conditions | K = 103 M-1 [50] | 71% | 2.5 mM |
| II | Warfarin | 1945 | Antithrombotic agent | Thromboembolism treatment | K1 = 2×105 M-1 K2 = 5×104 M-1 [51] |
site1: 99-99.5%; site2: 98% | 1 mM |
| III | Mefenamic acid | 1961 | non-steroidal anti-inflammatory agent | Analgesia, treatment of inflammation и fever | K1=4×105 М-1 [52] K2 = 1×105 M-1 [53] |
95-96% | 250 μM |
| IV | Levothyroxine | 1914 | Thyroid hormone | treatment thyroid diseases including hypothyroidism and cancer | K = 105 M-1 (4 sites) [54] | 9-41% | 15 μM |
| V | Propranolol | Early 1960s | Beta blockers | Cardiology, including hypertension and myocardial infarction | K = 104 M-1 (2 sites) [55] | 90-91% | 1 mM |
| Ligand/Additive | ka1×102, M-1s-1 |
kd1×10-4, s-1 |
KD1×10-7, M |
ka2×102, M-1s-1 |
kd2×10-4, s-1 |
KD2×10-6, M |
|---|---|---|---|---|---|---|
| Without ligand + DMSO | 2.7±2.6 | 0.216±0.007 | 0.64±0.48 | 8.9±9.0 | 26±13 | 5.0±3.4 |
| Without ligand + ethanol | 5.6±4.7 | 0.27±0.20 | 1.2±1.6 | 13±8 | 34±11 | 3.2±1.5 |
| Prednisone + DMSO | 2.73±0.96 | 0.11±0.05 | 0.38±0.05 | 36±8 | 13.6±1.2 | 0.38±0.05 |
| Warfarin + ethanol | 7.9±3.7 | 0.50±0.39 | 0.57±0.43 | 24±28 | 32±11 | 2.9±1.9 |
| Mefenamic acid + ethanol | 3.3±1.6 | 0.97±0.56 | 3.6±2.1 | 3.6±3.4 | 31±12 | 13±10 |
| Levothyroxine + DMSO | 0.575±0.011 | 0.109±0.006 | 1.90±0.14 | 27.0±1.6 | 11.20±0.12 | 0.41±0.02 |
| Propranolol + DMSO | 22±31 | 0.204±0.010 | 0.45±0.55 | 25±34 | 18.8±0.2 | 2.7±2.6 |
| Cluster | HSA ligand | Effect of the ligand on HSA affinity for Aβ | Relevance for AD progression |
|---|---|---|---|
| I | prednisone | ↑ † | decline of AD biomarkers in non-AD patients after taking prednisone [61,62]; lack of effect in the treatment of AD patients [72] |
| risperidone | ↓ [73] | reduces psychosis and favors functioning in elderly patients with psychosis of AD and mixed dementia [74] | |
| II | ibuprofen | ↑ [32] | reduces the risk of AD progression [34] |
| III | mefenamic acid | ↓ † | not available |
| IV | levothyroxine | bidirectional effect † | hypothyroidism, thyroiditis and hyperthyroidism are more common among AD patients [75]; lowered levothyroxine level in cerebrospinal fluid of AD patients [65] |
| V | serotonin | ↑ [31] | modulates Aβ level in the central nervous system of AD patients [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





