Submitted:
03 April 2024
Posted:
04 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanism of Cell Death Induction by GGA in Hepatocellular Carcinoma (HCC) Cells
2.1. Timeline of intracellular Events Related to Cell Death in Human Hepatocellular Carcinoma (HCC)-Derived Cell Lines Induced by GGA
- 15 minutes: The earliest observed phenomena upon adding 10 µM GGA to the culture medium include three main events. Firstly, increased production of superoxide in mitochondria is observed using the MitoSox fluorescence staining technique [14]. Secondly, the splicing of XBP1 mRNA in the endoplasmic reticulum (ER) is detected using the RT-qPCR technique [15,16]. Thirdly, an increase in LC3β-II protein, a marker of autophagosomes, is observed using western blotting [14]. Although these events are observed simultaneously in different cellular organelles (mitochondria, ER, and autophagosomes), it is unlikely that these events occur independently and simultaneously. Since suppressing the increased production of superoxide by GGA with antioxidants does not suppress XBP1 mRNA splicing [16], it appears that at least the increased production of superoxide does not trigger XBP1 mRNA splicing.
- 20 minutes: Monitoring the cytosolic Ca2+ concentration using Fluo-4 AM as a probe reveals a transient peak 16-20 minutes after GGA treatment, which quickly declines ([16]; https://doi.org/10.1042/BSR20194118; see Supplementary Movie S1). This peak is observed, albeit slightly delayed, even when experiments are conducted in a medium without Ca2+, suggesting leakage of Ca2+ from intracellular Ca2+ storage sites such as the ER.
- 30 minutes: Introduction of GFP-LC3 expression vector into HuH-7 cells followed by GGA treatment results in the appearance of green, fluorescent autophagosomes 30 min later [14], consistent with the detection of LC3β-II protein 15 min earlier. An increase in Beclin-1 (BECN1) and Sequestosome 1 (p62/SQSTM1), which are involved in promoting autophagosome formation and cargo protein for autophagosomes, respectively, is also detected [14]. Meanwhile, although there is no change in the transcription level of the Cyclin D1 (CCND1) gene, its translation is significantly suppressed [17].
- 1 hour: Loss of mitochondrial membrane potential (ΔΨm) is observed 1 h after GGA treatment when stained with Rhodamine 123 [18] and 2 h after GGA treatment when stained with MitoTracker® Red CMXRos [14]. Regardless of the staining method, the loss of Δψm is observed after the increased superoxide production in mitochondria. Active caspase-4 (CASP4) is detected by western blotting and observed up to 5 h after GGA addition but disappears thereafter [16]. Concurrently with the detection of active CASP4, the N-terminal fragment of Gasdermin D (GSDMD) is detected, with its amount peaking at 3 h and then decreasing, reaching its maximum at 8 h after GGA treatment when cell death is observed.
- 2 hours: A transient increase in lysophosphatidylcholine containing PA and palmitoleic acid (lysoPC [16:0]; lysoPC [16:1]) is observed. In contrast, a significant increase in lysoPC (lysoPC [20:4]) and lysophosphatidylethanolamine (lysoPE [20:4]) containing arachidonic acid is observed, and then these lysophospholipids (lysoPLs) remain at high levels and continue to increase gradually until 24 h [19].
- 3 hours: Immunofluorescence staining of GSDMD reveals signals mainly in the nucleus in control cells, but in GGA-treated cells, the signals are also detected in the plasma membrane [16]. Immunofluorescence staining of NF-κB, one of the inflammatory transcription factors, shows that its signal is observed granularly in the cytoplasm in control cells, but most of it translocates into the nucleus after GGA treatment [16]. Morphological changes in HuH-7 cells are first observed, with loss of cell adhesion and contraction of cells away from each other. Rod-shaped protrusions or blebs emerge from each contracted cell into the intercellular space ([16]; https://doi.org/10.1042/BSR20194118; see Supplementary Movie S1).
- 6 hours: The transient peak in cytosolic Ca2+ concentration observed 20 min after GGA treatment reappears 6 hours later. When experiments are conducted in a medium without Ca2+, this peak is completely absent, suggesting that it is due to Ca2+ influx into the cytosol from the medium through a ruptured cell membrane by GSDMD translocated to the membrane. Intracellular levels of NLRP3 mRNA and IL1B mRNA increase. Blebs protruding from contracted cells disappear, and spherical balloons appear, gradually increasing in size [16].
- 8 hours: Significant activation of CASP1 is observed by enzyme activity measurement. Detection of the N-terminal fragment of GSDMD also peaks. Leakage of lactate dehydrogenase (LDH) into the medium is observed. The balloons that emerge from contracted cells become larger than the cell diameter [16]. (For chronological morphological changes, [16]; https://doi.org/10.1042/BSR20194118; see Supplementary movie S1)
2.2. Inhibition of GGA-Induced Cell Death by Various Inhibitors
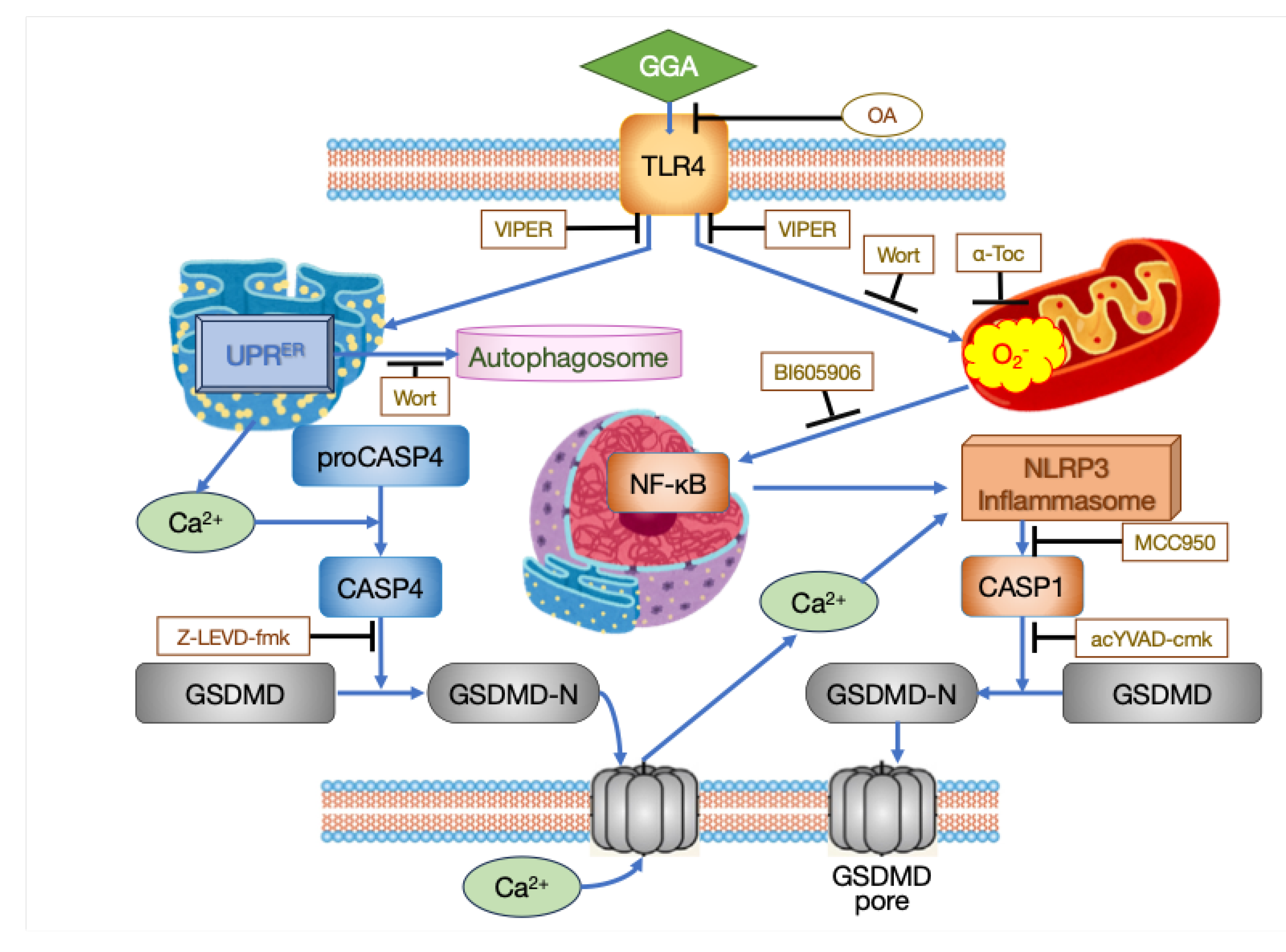
- CASP Inhibitory Peptides: Induction of cell death in HuH-7 cells by GGA is completely inhibited by co-treatment with an active site inhibitor peptide of CASP1 (ac-YVAD-cmk). Co-treatment with an active site inhibitor peptide of CASP3 (ac-DEVD-CHO) delays GGA-induced cell death by several hours but does not inhibit it [18]. Co-treatment with an active site inhibitor peptide of CASP4 (Z-LEVD-fmk) inhibits GGA-induced activation of CASP1 [16].
- TLR4 Inhibitors: Pretreatment of HuH-7 cells with TLR4 (Toll-like receptor 4) siRNA completely inhibits GGA-induced cell death. Co-treatment with VIPER, a peptide that specifically inhibits signaling from TLR4, completely inhibits GGA-induced cell death as well as other processes induced by GGA, such as increased superoxide production in mitochondria, splicing of XBP1 mRNA, nuclear translocation of the cytosolic NF-κB, upregulation of TLR2 and NLRP3 mRNAs, and activation of CASP1 [16].
- Lipotoxicity Inhibitor: Co-treatment with oleic acid (OA), known to inhibit PA-induced lipotoxicity, completely inhibits GGA-induced cell death in HuH-7 cells. Additionally, OA co-treatment inhibits various processes induced by GGA, including splicing of XBP1 mRNA, upregulation of TLR2 mRNA and DDIT3 (CHOP) mRNA, increase in LC3β-II levels, accumulation of autophagosomes, superoxide hyperproduction in mitochondria, translocation of cytoplasmic NF-κB to the nucleus, and activation of CASP1 [16]. The inhibitory effect of OA on GGA is observed only when co-treated simultaneously; treatment with OA before GGA treatment does not exhibit any inhibitory effect [15], suggesting that the inhibitory point of action of OA on GGA is extracellular.
- Antioxidant: Pretreatment with α-tocopherol, a lipid-soluble antioxidant vitamin, dose-dependently inhibits GGA-induced cell death [18]. Co-treatment with α-tocopherol also inhibits other processes induced by GGA, such as dissipation of ΔΨm, activation of CASP1, upregulation of NLRP3 mRNA, translocation of cytoplasmic NF-κB to the nucleus, and increased superoxide production in mitochondria [15,16]. However, co-treatment with α-tocopherol does not inhibit activation of unfolded protein response in the ER (UPRER: splicing of XBP1 mRNA and upregulation of DDIT3 mRNA) by GGA [16].
- Kinase Inhibitors: Co-treatment with wortmannin, a non-specific inhibitor of PI3 kinase, inhibits GGA-induced increase in superoxide production in mitochondria and the appearance of green, fluorescent puncta in GGA-treated GFP-LC3 transfected HuH-7 cells [14]. Each co-treatment with BAY 11 7082, an inhibitor of NF-κB activation, or BI605906, an IKKβ inhibitor, inhibits the translocation of cytoplasmic NF-κB to the nucleus induced by GGA [16].
2.3. The Mechanism of GGA-Induced Cell Death
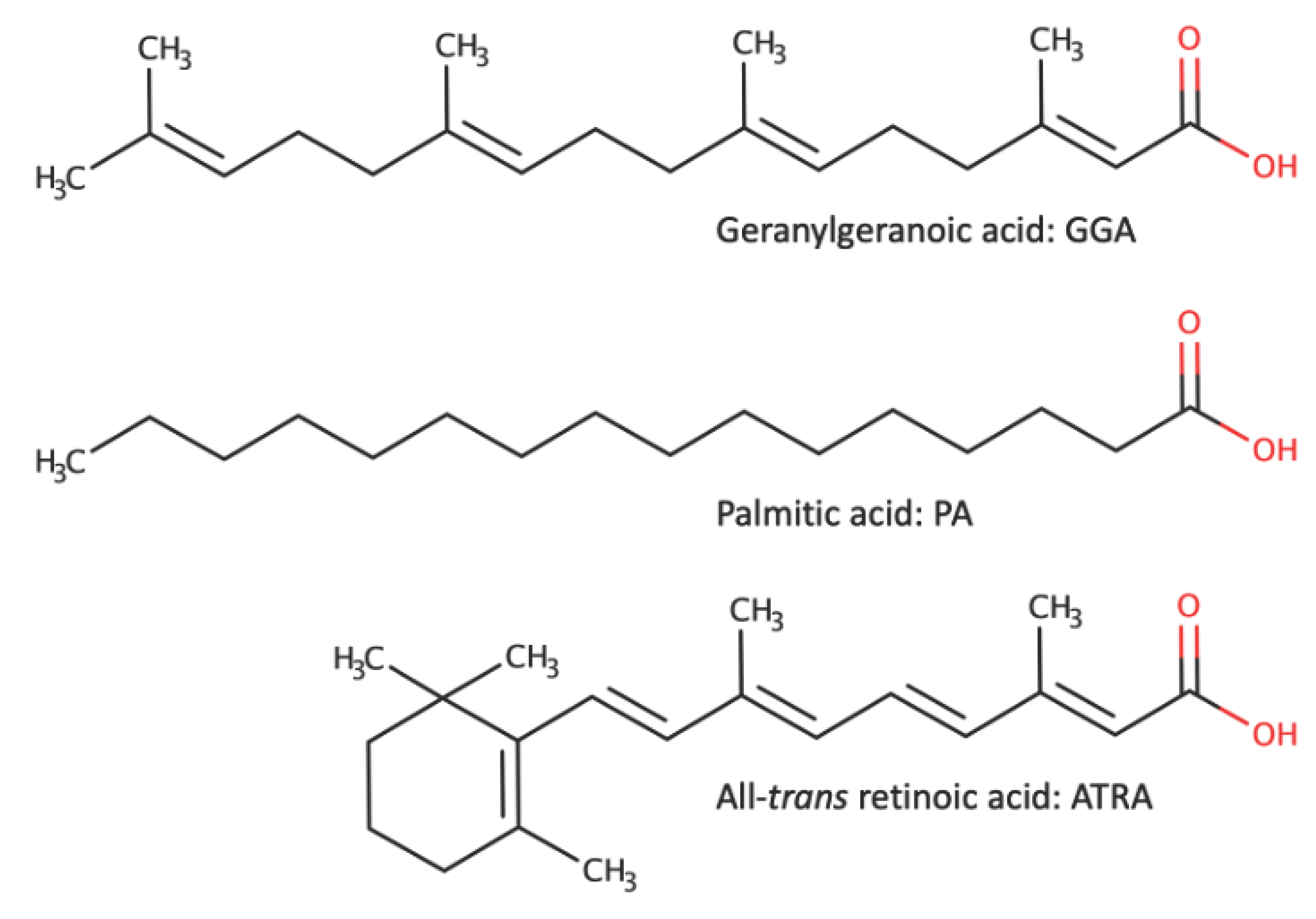
3. GGA-Induced Cell Death in Hepatocellular Carcinoma (HCC) Cells - A Comparison with Palmitic Acid (PA)-Induced Cell Death and All-trans Retinoic Acid (ATRA)-Induced Cell Death
3.1. Effective Concentrations and Observation Time for Inducing Cell Death (Table 1)
3.2. Mode of Regulated Cell Death
3.3. Action on plasma Membrane and intracellular Organelles
- Plasma Membrane: When fatty acids like GGA, PA, and ATRA are added to the medium, they are expected to encounter the plasma membrane. Both PA and GGA are believed to induce cell death via signaling mediated by the cell surface receptor TLR4, which is localized on the plasma membrane [16,51]. However, few reports are suggesting that these lipids directly act as ligands for TLR4. While computational simulations and isothermal titration calorimetry have demonstrated the docking of five PA molecules with the hydrophobic pocket of TLR4/MD-2, experimental evidence confirming PA as a direct ligand for TLR4 is lacking [52].
- Mitochondria: The effects on mitochondrial morphology and function are relatively similar among GGA, PA, and ATRA. Adding GGA to cultured HCC-derived cell lines leads to mitochondrial accumulation around the nucleus and fragmentation within 2 h [14]. Similarly, mitochondrial fragmentation is observed within 2 h of PA treatment [54]. ATRA treatment results in decreased mitochondrial number and average volume, as well as disappearance of mitochondrial cristae after 48 h [49]. Additionally, loss of ΔΨm is observed after 1 h of GGA (10 µM) treatment [18], 24-96 h of ATRA (1 µM) treatment [55], and 24 h of PA (200 µM) treatment [46], indicating mitochondrial permeability transition (mPT) (Table 1). Furthermore, hyperproduction of superoxide in mitochondria is confirmed after treatment with GGA (10µM, 15 min) [14], PA (500 µM, 3-24 h) [46,56], or ATRA (10 µM, 48 h) [49]. Free fatty acids like PA are proposed to induce superoxide production by directly binding to complexes I and III of the respiratory chain, inhibiting electron transfer and leading to increased superoxide production [57]. Although the signaling from TLR4 to ECSIT resulting in an impaired electron transport chain in mitochondria has been proposed for GGA (as mentioned earlier), the mechanism of increased superoxide production by ATRA remains unclear in the literature.
- Endoplasmic reticulum (ER): UPRER is a common response of HCC cells to treatment with these three fatty acids. Treatment with GGA (10-20 µM, 15-30 min) [15,16], PA (400-500 µM, 8-24 h) [44,56,58], or ATRA (10-20 µM, 1-8 h) [15] results in splicing of XBP1 mRNA, translocation of XBP1 protein to the nucleus, and increased expression of DDIT3 (CHOP) mRNA. Induction of UPRER by ATRA has also been observed in mouse embryonal carcinoma cell line P19, not limited to HCC cells [59]. Membrane phospholipids derived from PA are believed to affect the fluidity of the ER membrane and induce UPRER through the dimerization of IRE1 [60]. Since effective concentrations of GGA and ATRA are in the order of 10 µM and induce relatively rapid responses, they are likely mediated through signaling, although detailed mechanisms require further investigation.
- Nucleus: Translocation of NF-κB to the nucleus due to increased production of reactive oxygen species (ROS) is a well-known phenomenon [61]. Indeed, treatment with GGA induces translocation of NF-κB to the nucleus, which can be inhibited by co-treatment with the antioxidant α-tocopherol, or suppression of increased superoxide production in the mitochondria [16]. Additionally, GGA treatment promotes rapid translocation of the cytoplasmic p53 to the nucleus and increases expression of the PUMA gene, one of the p53 target genes [62]. Like GGA, PA also induces nuclear NF-κB activity in HepG2 [63,64] and HepaRG cells [64].
- Cytosol: An increase in cytosolic Ca2+ concentration is another common response of HCC cells to treatment with these three fatty acids (Table 1). However, the effective concentrations and time of occurrence of this effect differ. Treatment with 10 µM GGA leads to two increases in cytosolic Ca2+ concentration at 20 min and 6 h [16], while PA requires concentrations of 500-1000 µM, with an increase observed 6 h after treatment [46]. Similarly, ATRA requires 24-48 h after treatment to observe an increase in cytosolic Ca2+ concentration, despite effective concentrations being in the order of 10 µM [65]. The mechanism of increased cytosolic Ca2+ concentration by PA has been most extensively analyzed. PA enhances mitochondrial ROS (mtROS) production, which releases Ca2+ from lysosomes, resulting in increased cytosolic Ca2+ concentration and leading to mPT and cell death [46].
- Inflammatory extracellular vesicles: When hepatocytes and HCC cells are treated with PA, they secrete inflammatory extracellular vesicles [66,67]. It is believed that lysoPC, one of the intracellular metabolites of PA, is directly involved in the release of inflammatory extracellular vesicles [68]. It has been reported that one-tenth to one-twentieth lysoPC induces cell death in 24 h in primary human hepatocytes to the same extent as cell death by PA [69]. Considering that lysoPC increases upon GGA treatment of HCC cells, it is hypothesized that GGA treatment may lead to the release of inflammatory extracellular vesicles. In contrast, since lysoPC is reduced upon ATRA treatment, the release of extracellular vesicles is unlikely after ATRA treatment [70].
3.4. Role of Mitochondrial ROS Hyperproduction, UPRER, and Autophagy in GGA-Induced Cell Death
| GGA | PA | ATRA | |
|---|---|---|---|
| Cytotoxicity |
|
|
|
| Mitochondrial morphology |
- a fragmentation of mitochondria (unpublished, Shidoji). |
|
- reduced mitochondrial volume (Sun 2022) [49]. - reduced or eliminated mitochondrial cristae (Sun 2022) [49]. ✧1 µM ATRA did not alter the mitochondrial mass for 24-72 h in the acute-promyelocytic-leukemia (APL)-derived NB4 cell line (Gianni 2022) [55]. |
| Mitochondrial membrane potential (ΔΨm) |
- a dissipation of MitoTracker Red fluorescence in 2 h (Okamoto 2011) [14]. |
|
✧1 µM ATRA reduced Mito-Tracker Red/Green fluorescence in NB4 cells for 24-96 h (Gianni 2022) [55]. |
| Mitochondrial ROS production |
- MitoSox Red fluorescence of hepatoma cells in 2 h, which was prevented with either 50 µM oleic acid (OA), 100 µM α-tocopherol or VIPER (Yabuta 2020) [16]. |
|
- lipid peroxides (C11-BODIPY fluorescence) (Sun 2022) [49]. |
| ER stress response (UPRER) |
- splicing of XBP1 mRNA in 15 min (Iwao 2015) [15], which was prevented by cotreatment with either 50 µM OA or VIPER, but not with 100 µM α-tocopherol (Yabuta 2020) [16]. - accumulation of XBP1 protein in the nucleus in 8 h (Iwao 2015) [15]. - upregulation of DDIT3 (CHOP) mRNA in 8 h (Iwao 2015), which was prevented by cotreatment with 50 µM OA or VIPER, but not with 100 µM α-tocopherol (Yabuta 2020) [16]. |
- upregulation of DDIT3 (CHOP) protein in 16 h (Qi 2015) [58]. - phosphorylation of IRE1α in 8 h (Qi 2015) [58].
|
|
| Pyroptosis |
- upregulation of TLR2 expression in 3 h, which was prevented with 50 µM OA or VIPER (Yabuta 2020) [16]. - upregulation of NLRP3 expression in 3 h, which was prevented with 50 µM OA, 100 µM α-tocopherol or VIPER (Yabuta 2020) [16]. - appearance of GSDMD-N terminal fragment in 1 h (Yabuta 2020) [16]. - localization of GSDMD to the plasma membrane in 3 h, which was prevented by co-treatment with VIPER (Yabuta 2020) [16].
|
|
|
| Cytoplasmic Ca2+ |
|
|
|
| LysoPLs |
- the most rapid (2 h) upregulation of lysoPC (C20:4) and lysophosphatidylethanolamine (C20:4) (Shidoji 2021) [19]. |
|
✧Liver lysoPC (16:1 or 18:1) was decreased by oral administration (10 mg/kg, 1 wk) of a synthetic ligand (AM580) for RAR to mice and increased by oral administration (30 mg/kg, 1 wk) of a synthetic ligand for RXR (LG268) to female C57BL6 mice (Weiss 2011) [70]. |
| Autophagy |
- time-dependent accumulation of LC3β-II and p62/SQSTM (Okamoto 2011) [14], which was not prevented by co-treatment with 4μ8C (IRE1 Inhibitor III) that inhibits XBP1 mRNA splicing (Iwao 2015) [15]. - upregulation of ATG4B and BECN1 in 30 min (Okamoto 2011) [14]. - nuclear translocation of cytoplasmic p53 in 3 h (Iwao 2014) [62]. |
|
|
| CASP3 |
|
|
|
4. GGA as an Anti-Oncometabolite
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujimaki, Y. Formation of Gastric Carcinoma in Albino Rats Fed on Deficient Diets. J Cancer Res 1926, 10, 469–477. [Google Scholar] [CrossRef]
- Abels, J.C.; Gorham, A.T.; Pack, G.T.; Rhoads, C.P. Metabolic Studies in Patients with Cancer of the Gastro-Intestinal Tract. I. Plasma Vitamin A Levels in Patients with Malignant Neoplastic Disease, Particularly of the Gastro-Intestinal Tract 1. J Clin Invest 1941, 20, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Saffiotti, U.; Montesano, R.; Sellakumar, A.R.; Borg, S.A. Experimental Cancer of the Lung. Inhibition by Vitamin A of the Induction of Tracheobronchial Squamous Metaplasia and Squamous Cell Tumors. Cancer 1967, 20, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.K.; Sporn, M.B. Recent Advances in Chemoprevention of Cancer. Science (1979) 1997, 278, 1073–1077. [Google Scholar] [CrossRef]
- Hansen, L.A.; Sigman, C.C.; Andreola, F.; Ross, S.A.; Kelloff, G.J.; De Luca, L.M. Retinoids in Chemoprevention and Differentiation Therapy. Carcinogenesis 2000, 21, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Moriwaki, H.; Ninomiya, M.; Adachi, S.; Saito, A.; Takasaki, K.T.; Tanaka, T.; Tsurumi, K.; Okuno, M.; Tomita, E.; et al. Prevention of Second Primary Tumors by an Acyclic Retinoid, Polyprenoic Acid, in Patients with Hepatocellular Carcinoma. Hepatoma Prevention Study Group. N. Engl. J. Med. 1996, 334, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Moriwaki, H.; Omori, M. In Vitro Binding Affinity of Novel Synthetic Polyprenoids (Polyprenoic Acids) to Cellular Retinoid-Binding Proteins. Jpn J Cancer Res 1981, 72, 974–977. [Google Scholar]
- Araki, H.; Shidoji, Y.; Yamada, Y.; Moriwaki, H.; Muto, Y. Retinoid Agonist Activities of Synthetic Geranylgeranoic Acid Derivatives. Biochem Biophys Res Commun 1995, 209, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shidoji, Y. Geranylgeranoic Acid, a Bioactive and Endogenous Fatty Acid in Mammals: A Review. J Lipid Res 2023, 64, 100396. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the Gut-Liver Axis in NASH Pathogenesis. J Hepatol 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Yu, S.J.; Lee, J.H.; Kim, H.Y.; Kim, Y.J. Reduction of Oxidative Stress Attenuates Lipoapoptosis Exacerbated by Hypoxia in Human Hepatocytes. Int J Mol Sci 2015, 16, 3323–3334. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, S.; Kitajima, Y.; Higure, K.; Kimura, N.; Maeda, S.; Yamada, K.; Ito, K.; Tanaka, T.; Kai, K.; Noshiro, H. A HIF-1α Inhibitor Combined with Palmitic Acid and L-Carnitine Treatment Can Prevent the Fat Metabolic Reprogramming under Hypoxia and Induce Apoptosis in Hepatocellular Carcinoma Cells. Cancer Metab 2023, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Sakane, C.; Shidoji, Y. Reversible Upregulation of Tropomyosin-Related Kinase Receptor B by Geranylgeranoic Acid in Human Neuroblastoma SH-SY5Y Cells. J Neurooncol 2011, 104, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Sakimoto, Y.; Imai, K.; Senoo, H.; Shidoji, Y. Induction of an Incomplete Autophagic Response by Cancer-Preventive Geranylgeranoic Acid (GGA) in a Human Hepatoma-Derived Cell Line. Biochem J 2011, 440, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Iwao, C.; Shidoji, Y. Polyunsaturated Branched-Chain Fatty Acid Geranylgeranoic Acid Induces Unfolded Protein Response in Human Hepatoma Cells. PLoS One 2015, 10, e0132761. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, S.; Shidoji, Y. TLR4-Mediated Pyroptosis in Human Hepatoma-Derived HuH-7 Cells Induced by a Branched-Chain Polyunsaturated Fatty Acid, Geranylgeranoic Acid. Biosci Rep 2020, 40, BSR20194118. [Google Scholar] [CrossRef] [PubMed]
- Shimonishi, S.; Muraguchi, T.; Mitake, M.; Sakane, C.; Okamoto, K.; Shidoji, Y. Rapid Downregulation of Cyclin D1 Induced by Geranylgeranoic Acid in Human Hepatoma Cells. Nutr Cancer 2012, 64, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Shidoji, Y.; Nakamura, N.; Moriwaki, H.; Muto, Y. Rapid Loss in the Mitochondrial Membrane Potential during Geranylgeranoic Acid-Induced Apoptosis. Biochem Biophys Res Commun 1997, 230, 58–63. [Google Scholar] [CrossRef]
- Shidoji, Y.; Iwao, C. A Rapid Increase in Lysophospholipids after Geranylgeranoic Acid Treatment in Human Hepatoma-Derived HuH-7 Cells Revealed by Metabolomics Analysis. Biochem Biophys Rep 2021, 28, 101176. [Google Scholar] [CrossRef]
- Smith, J.A.; Turner, M.J.; Delay, M.L.; Klenk, E.I.; Sowders, D.P.; Colbert, R.A. Endoplasmic Reticulum Stress and the Unfolded Protein Response Are Linked to Synergistic IFN-β Induction via X-Box Binding Protein 1. Eur J Immunol 2008, 38, 1194–1203. [Google Scholar] [CrossRef]
- Martinon, F.; Chen, X.; Lee, A.H.; Glimcher, L.H. TLR Activation of the Transcription Factor XBP1 Regulates Innate Immune Responses in Macrophages. Nat Immunol 2010, 11, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Savic, S.; Ouboussad, L.; Dickie, L.J.; Geiler, J.; Wong, C.; Doody, G.M.; Churchman, S.M.; Ponchel, F.; Emery, P.; Cook, G.P.; et al. TLR Dependent XBP-1 Activation Induces an Autocrine Loop in Rheumatoid Arthritis Synoviocytes. J Autoimmun 2014, 50, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Boukeileh, S.; Darawshi, O.; Shmuel, M.; Mahameed, M.; Wilhelm, T.; Dipta, P.; Forno, F.; Praveen, B.; Huber, M.; Levi-Schaffer, F.; et al. Endoplasmic Reticulum Homeostasis Regulates TLR4 Expression and Signaling in Mast Cells. Int J Mol Sci 2022, 23, 11826. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of Caspase-4 in Endoplasmic Reticulum Stress-Induced Apoptosis and Aβ-Induced Cell Death. Journal of Cell Biology 2004, 165, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yukioka, F.; Matsuzaki, S.; Kawamoto, K.; Koyama, Y.; Hitomi, J.; Katayama, T.; Tohyama, M. Presenilin-1 Mutation Activates the Signaling Pathway of Caspase-4 in Endoplasmic Reticulum Stress-Induced Apoptosis. Neurochem Int 2008, 52, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.M.; Elner, S.G.; Elner, V.M. Dual Involvement of Caspase-4 in Inflammatory and ER Stress-Induced Apoptotic Responses in Human Retinal Pigment Epithelial Cells. Invest Ophthalmol Vis Sci 2009, 50, 6006–6014. [Google Scholar] [CrossRef] [PubMed]
- Silva Barcelos, E.C.; Rompietti, C.; Adamo, F.M.; Dorillo, E.; De Falco, F.; Del Papa, B.; Baldoni, S.; Nogarotto, M.; Esposito, A.; Capoccia, S.; et al. NOTCH1-Mutated Chronic Lymphocytic Leukemia Displays High Endoplasmic Reticulum Stress Response with Druggable Potential. Front Oncol 2023, 13, 1218989. [Google Scholar] [CrossRef] [PubMed]
- Correia da Silva, D.; Valentão, P.; Pereira, D.M. A Survey of Naturally Occurring Molecules as New Endoplasmic Reticulum Stress Activators with Selective Anticancer Activity. Cancers (Basel) 2022, 15, 293. [Google Scholar] [CrossRef]
- Chukai, Y.; Ito, G.; Konno, M.; Sakata, Y.; Ozaki, T. Mitochondrial Calpain-5 Truncates Caspase-4 during Endoplasmic Reticulum Stress. Biochem Biophys Res Commun 2022, 608, 156–162. [Google Scholar] [CrossRef]
- Brewer, J.W.; Diehl, J.A. PERK Mediates Cell-Cycle Exit during the Mammalian Unfolded Protein Response. Proc Nat Acad Sci, USA 2000, 97, 12625–12630. [Google Scholar] [CrossRef]
- Vogel, R.O.; Janssen, R.J.R.J.; Van Den Brand, M.A.M.; Dieteren, C.E.J.; Verkaart, S.; Koopman, W.J.H.; Willems, P.H.G.M.; Pluk, W.; Van Den Heuvel, L.P.W.J.; Smeitink, J.A.M.; et al. Cytosolic Signaling Protein Ecsit Also Localizes to Mitochondria Where It Interacts with Chaperone NDUFAF1 and Functions in Complex I Assembly. Genes Dev 2007, 21, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, F.R.G.; Lepelley, A.; Seeley, J.J.; Hayden, M.S.; Ghosh, S. An Essential Role for ECSIT in Mitochondrial Complex I Assembly and Mitophagy in Macrophages. Cell Rep 2018, 22, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- McGregor, L.; Acajjaoui, S.; Desfosses, A.; Saïdi, M.; Bacia-Verloop, M.; Schwarz, J.J.; Juyoux, P.; von Velsen, J.; Bowler, M.W.; McCarthy, A.A.; et al. The Assembly of the Mitochondrial Complex I Assembly Complex Uncovers a Redox Pathway Coordination. Nat Commun 2023, 14, 8248. [Google Scholar] [CrossRef] [PubMed]
- Silwal, P.; Kim, J.K.; Kim, Y.J.; Jo, E.K. Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation during Infection. Front Immunol 2020, 11, 1649. [Google Scholar] [CrossRef] [PubMed]
- Horng, T. Calcium Signaling and Mitochondrial Destabilization in the Triggering of the NLRP3 Inflammasome. Trends Immunol 2014, 35, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, M.; Yangzhong, X.; Zhang, X.; Zu, A.; Hou, Y.; Li, L.; Sun, S. Pyroptosis in Inflammation-Related Respiratory Disease. J Physiol Biochem 2022, 78, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, J.; Tang, Y.; Lu, B. The Noncanonical Inflammasome-Induced Pyroptosis and Septic Shock. Semin Immunol. 2023, 70, 101844. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-L.; Wu, W.-S.; Tyan, Y.-S.; Chou, C.-K. Retinoic Acid-Induced Apoptosis Is Prevented by Serum Albumin and Enhanced by Lipiodol in Human Hepatoma Hep3B Cells. Cancer Letter 1998, 129, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hu, C.; Xu, L.; Cui, J.; Tao, L.; Gong, M.; Wang, Y.; He, Y.; He, T.; Bi, Y. All-Trans-Retinoic Acid Inhibits the Malignant Behaviors of Hepatocarcinoma Cells by Regulating Autophagy. Am J Transl Res 2020, 12, 6793–6810. [Google Scholar]
- Nakamura, N.; Shidoji, Y.; Yamada, Y.; Hatakeyama, H.; Moriwaki, H.; Muto, Y. Induction of Apoptosis by Acyclic Retinoid in the Human Hepatoma-Derived Cell Line, HuH-7. Bioche. Biphys. Res. Commun. 1995, 270, 382–388. [Google Scholar] [CrossRef]
- Lin, L.-M.; Li, B.-X.; Xiao, J.-B.; Lin, D.-H.; Yang ELSEVIER, B.-F.; Yang, B.-F. Synergistic Effect of All-Trans-Retinoic Acid and Arsenic Trioxide on Growth Inhibition and Apoptosis in Human Hepatoma, Breast Cancer, and Lung Cancer Cells in Vitro. World J Gastroenterol 2005, 11, 5633–5637. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, G.; Ding, C.L.; Zhao, L.J.; Zhao, P.; Ren, H.; Qi, Z.T. All-Trans Retinoic Acid Protects Hepatocellular Carcinoma Cells against Serum-Starvation-Induced Cell Death by Upregulating Collagen 8A2. FEBS Journal 2013, 280, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, X.; Liu, Z.; Chen, X.; Li, L.; Luo, R.; Liu, X.; Zhang, J.; Liu, J.; Lu, Y.; et al. Mesenchymal Stromal Cells Protect Hepatocytes from Lipotoxicity through Alleviation of Endoplasmic Reticulum Stress by Restoring SERCA Activity. J Cell Mol Med 2021, 25, 2976–2993. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Oleic Acid Ameliorates Palmitic Acid Induced Hepatocellular Lipotoxicity by Inhibition of ER Stress and Pyroptosis. Nutr Metab (Lond) 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Verma, R.; Kumar, C.; Kumar, B.; Dey, A.K.; Surjit, M.; Mylavarapu, S.V.S.; Maiti, T.K. Proteomic Analysis Reveals USP7 as a Novel Regulator of Palmitic Acid-Induced Hepatocellular Carcinoma Cell Death. Cell Death Dis 2022, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Hwang, Y.; Hur, K.Y.; Lee, M.S. Lysosomal Ca2+ as a Mediator of Palmitate-Induced Lipotoxicity. Cell Death Discov 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Kanki, K.; Akechi, Y.; Ueda, C.; Tsuchiya, H.; Shimizu, H.; Ishijima, N.; Toriguchi, K.; Hatano, E.; Endo, K.; Hirooka, Y.; et al. Biological and Clinical Implications of Retinoic Acid-Responsive Genes in Human Hepatocellular Carcinoma Cells. J Hepatol 2013, 59, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, R.; Shi, Y.; Chen, D.; Weng, H.; Ding, H.; Zhang, C. Intracellular Alpha-Fetoprotein Interferes with All-Trans Retinoic Acid Induced ATG7 Expression and Autophagy in Hepatocellular Carcinoma Cells. Sci Rep 2021, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, Y.; Tong, J.; Liu, D.; Zhang, H.; He, T.; Bi, Y. All-Trans Retinoic Acid Inhibits the Malignant Behaviors of Hepatocarcinoma Cells by Regulating Ferroptosis. Genes Dis 2022, 9, 1742–1756. [Google Scholar] [CrossRef]
- Jakaria, M.; Belaidi, A.A.; Bush, A.I.; Ayton, S. Vitamin A Metabolites Inhibit Ferroptosis. Biomedicine and Pharmacotherapy 2023, 164. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Pernes, G.; Conway, J.R.W.; et al. Evidence That TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab 2018, 27, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, D.A.; Zhang, K.; Hung, C.; Glasgow, S.; Aruni, A.W.; Unternaehrer, J.; Payne, K.J.; Langridge, W.H.R.; De Leon, M. Palmitic Acid Is a Toll-like Receptor 4 Ligand That Induces Human Dendritic Cell Secretion of IL-1β. PLoS One 2017, 12, e0176793. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Lipid Bilayer Stress in Obesity-Linked Inflammatory and Metabolic Disorders. Biochem Pharmacol 2018, 153, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Eynaudi, A.; Díaz-Castro, F.; Bórquez, J.C.; Bravo-Sagua, R.; Parra, V.; Troncoso, R. Differential Effects of Oleic and Palmitic Acids on Lipid Droplet-Mitochondria Interaction in the Hepatic Cell Line HepG2. Front Nutr 2021, 8, 775382. [Google Scholar] [CrossRef] [PubMed]
- Gianni, M.; Goracci, L.; Schlaefli, A.; Di Veroli, A.; Kurosaki, M.; Guarrera, L.; Bolis, M.; Foglia, M.; Lupi, M.; Tschan, M.P.; et al. Role of Cardiolipins, Mitochondria, and Autophagy in the Differentiation Process Activated by All-Trans Retinoic Acid in Acute Promyelocytic Leukemia. Cell Death Dis 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chen, H.-W.; Lo, C.-W.; Wang, Y.-R.; Li, C.-C.; Liu, K.-L.; Lii, C.-K. Luteolin Ameliorates Palmitate-Induced Lipotoxicity in Hepatocytes by Mediating Endoplasmic Reticulum Stress and Autophagy. Food and Chemical Toxicology 2023, 171, 113554. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Fatty Acids as Modulators of the Cellular Production of Reactive Oxygen Species. Free Radic Biol Med 2008, 45, 231–241. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, W.; Chen, J.; Dai, L.; Kaczorowski, D.; Gao, X.; Xia, P. Sphingosine Kinase 1 Protects Hepatocytes from Lipotoxicity via Down-Regulation of IRE1α Protein Expression. Journal of Biological Chemistry 2015, 290, 23282–23290. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Mimori, S.; Okuma, Y.; Kawada, K. Sel1l May Contributes to the Determinants of Neuronal Lineage and Neuronal Maturation Regardless of Hrd1 via Atf6-Sel1l Signaling Affiliations. Neurochem. Res. 2023, 48, 263–272. [Google Scholar] [CrossRef]
- Kitai, Y.; Ariyama, H.; Kono, N.; Oikawa, D.; Iwawaki, T.; Arai, H. Membrane Lipid Saturation Activates IRE1α without Inducing Clustering. Genes to Cells 2013, 18, 798–809. [Google Scholar] [CrossRef]
- Gloire, G.; Piette, J. Redox Regulation of Nuclear Post-Translational Modifications during NF-ΚB Activation. Antioxid Redox Signal 2009, 11, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Iwao, C.; Shidoji, Y. Induction of Nuclear Translocation of Mutant Cytoplasmic P53 by Geranylgeranoic Acid in a Human Hepatoma Cell Line. Sci Rep 2014, 4, 4419. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Barve, S.; Barve, S.S.; Amancherla, K.; Gobejishvili, L.; Hill, D.; Cave, M.; Hote, P.; McClain, C.J. Palmitic Acid Induces Production of Proinflammatory Cytokine Interleukin-8 from Hepatocytes. Hepatology 2007, 46, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.C.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 Signaling in Obese NAFLD. Am J Physiol Gastrointest Liver Physiol 2015, 309, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ye, C.; Liu, F.; Wang, W. All-Trans Retinoic Acid and Arsenic Trioxide Induce Apoptosis and Modulate Intracellular Concentrations of Calcium in Hepatocellular Carcinoma Cells. Journal of Chemotherapy 2014, 26, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrahim, S.H.; Krishnan, A.; Verma, V.K.; Bronk, S.F.; Werneburg, N.W.; Charlton, M.R.; Shah, V.H.; Malhi, H.; Gores, G.J. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles from Hepatocytes. Gastroenterology 2016, 150, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Cobbs, A.; Chen, X.; Zhang, Y.; George, J.; Huang, M. bo; Bond, V.; Thompson, W.; Zhao, X. Saturated Fatty Acid Stimulates Production of Extracellular Vesicles by Renal Tubular Epithelial Cells. Mol Cell Biochem 2019, 458, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrabim, S.H.; Gores, G.J.; Malhi, H. Lipotoxicity: Many Roads to Cell Dysfunction and Cell Death Lipotoxic Lethal and Sublethal Stress Signaling in Hepatocytes: Relevance to NASH Pathogenesis. J Lipid Res 2016, 57, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Park, S.Y.; Shinzawa, K.; Kim, S.; Kun, W.C.; Lee, J.H.; Choon, H.K.; Lee, K.W.; Lee, J.H.; Cheol, K.P.; et al. Lysophosphatidylcholine as a Death Effector in the Lipoapoptosis of Hepatocytes. J Lipid Res 2008, 49, 84–97. [Google Scholar] [CrossRef]
- Weiss, K.; Mihály, J.; Liebisch, G.; Marosvölgyi, T.; Schmitz, G.; Decsi, T.; Rühl, R. Effect of Synthetic Ligands of PPAR α, β/δ, γ, RAR, RXR and LXR on the Fatty Acid Composition of Phospholipids in Mice. Lipids 2011, 46, 1013–1020. [Google Scholar] [CrossRef]
- Frietze, K.K.; Brown, A.M.; Das, D.; Franks, R.G.; Cunningham, J.L.; Hayward, M.; Nickels, J.T. Lipotoxicity Reduces DDX58/Rig-1 Expression and Activity Leading to Impaired Autophagy and Cell Death. Autophagy 2022, 18, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Takahama, M.; Akira, S.; Saitoh, T. Autophagy Limits Activation of the Inflammasomes. Immunol Rev 2018, 281, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.T.; Soh, N.J.H.; Chang, H.C.; Yu, V.C. P62/SQSTM1 in Liver Diseases: The Usual Suspect with Multifarious Identities. FEBS Journal 2023, 290, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, K.; Gajewska, M. Fatty Acids as Potent Modulators of Autophagy Activity in White Adipose Tissue. Biomolecules 2023, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cáceres, M.P.; Toledo-Valenzuela, L.; Díaz-Castro, F.; Ávalos, Y.; Burgos, P.; Narro, C.; Peña-Oyarzun, D.; Espinoza-Caicedo, J.; Cifuentes-Araneda, F.; Navarro-Aguad, F.; et al. Palmitic Acid Reduces the Autophagic Flux and Insulin Sensitivity through the Activation of the Free Fatty Acid Receptor 1 (FFAR1) in the Hypothalamic Neuronal Cell Line N43/5. Front Endocrinol (Lausanne) 2019, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Schilling, J.D. Lysosomes Integrate Metabolic-Inflammatory Cross-Talk in Primary Macrophage Inflammasome Activation. Journal of Biological Chemistry 2014, 289, 9158–9171. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Kasahara, T.; Takahashi, M.; Karasawa, T.; Kawashima, A.; Usui-Kawanishi, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Shirasuna, K.; et al. Saturated Fatty Acids Undergo Intracellular Crystallization and Activate the NLRP3 Inflammasome in Macrophages. Arterioscler Thromb Vasc Biol 2018, 38, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, J.; Cui, Q.; Jiang, T.; Xie, X.; Du, X.; Zhao, Z.; Lai, B.; Xiao, L.; Wang, N. CCN1/Integrin A5β1 Instigates Free Fatty Acid-Induced Hepatocyte Lipid Accumulation and Pyroptosis through NLRP3 Inflammasome Activation. Nutrients 2022, 14, 3871. [Google Scholar] [CrossRef]
- Kakisaka, K.; Cazanave, S.C.; Fingas, C.D.; Guicciardi, M.E.; Bronk, S.F.; Werneburg, N.W.; Mott, J.L.; Gores, G.J. Mechanisms of Lysophosphatidylcholine-Induced Hepatocyte Lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 2012, 302, 77–84. [Google Scholar] [CrossRef]
- Chen, H.; Ma, J.; Liu, J.; Dou, L.; Shen, T.; Zuo, H.; Xu, F.; Zhao, L.; Tang, W.; Man, Y.; et al. Lysophosphatidylcholine Disrupts Cell Adhesion and Induces Anoikis in Hepatocytes. FEBS Lett 2022, 596, 510–525. [Google Scholar] [CrossRef]
- Jorgensen, I.; Miao, E.A. Pyroptotic Cell Death Defends against Intracellular Pathogens. Immunol Rev 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, M.; Yang, F.; Liu, G.; Liu, J.; Zhao, W.; Ma, S.; Duan, Z. Programmed Cell Death and Lipid Metabolism of Macrophages in NAFLD. Front Immunol 2023, 14, 1118449. [Google Scholar] [CrossRef]
- Bourne, C.M.; Taabazuing, C.Y. Harnessing Pyroptosis for Cancer Immunotherapy. Cells 2024, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.L.; Morales, A.L.; Savard, C.; Farrell, G.C.; Ioannou, G.N. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatol Commun 2022, 6, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Shidoji, Y.; Tabata, Y. Unequivocal Evidence for Endogenous Geranylgeranoic Acid Biosynthesized from Mevalonate in Mammalian Cells. J Lipid Res 2019, 60, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Omori, M.; Shidoji, Y. Age-Dependent Decrease in Hepatic Geranylgeranoic Acid Content in C3H/HeN Mice and Its Oral Supplementation Prevents Spontaneous Hepatoma. Metabolites 2021, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Mitsuoka, T. Effect of Dietary Phenobarbital on Spontaneous Hepatic Tumorigenesis in Germfree C3H/He Male Mice. Cancer Lett 1988, 39, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Shidoji, Y.; Moriwaki, H. Inhibition of Spontaneous Hepatocarcinogenesis by 4,5-Didehydrogeranylgeranoic Acid: Effects of Small-Dose and Infrequent Administration. International Journal of Translational Medicine 2023, 3, 487–495. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C. Oncometabolites in Cancer: Current Understanding and Challenges. Cancer Res 2021, 81, 2820–2823. [Google Scholar] [CrossRef]
- Yamada, Y.; Shidoji, Y.; Fukutomi, Y.; Ishikawa, T.; Kaneko, T.; Nakagama, H.; Imawari, M.; Moriwaki, H.; Muto, Y. Positive and Negative Regulations of Albumin Gene Expression by Retinoids in Human Hepatoma Cell Lines. Mol Carcinog 1994, 10, 151–158. [Google Scholar] [CrossRef]
- Brown, G. Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells. Int J Mol Sci 2023, 24, 2373. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, S.P.; Arvanitakis, K.; Stergiou, I.E.; Vallilas, C.; Sougioultzis, S.; Germanidis, G.; Theocharis, S. Interplay of Extracellular Vesicles and TLR4 Signaling in Hepatocellular Carcinoma Pathophysiology and Therapeutics. Pharmaceutics 2023, 15, 2460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





