Submitted:
03 April 2024
Posted:
04 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
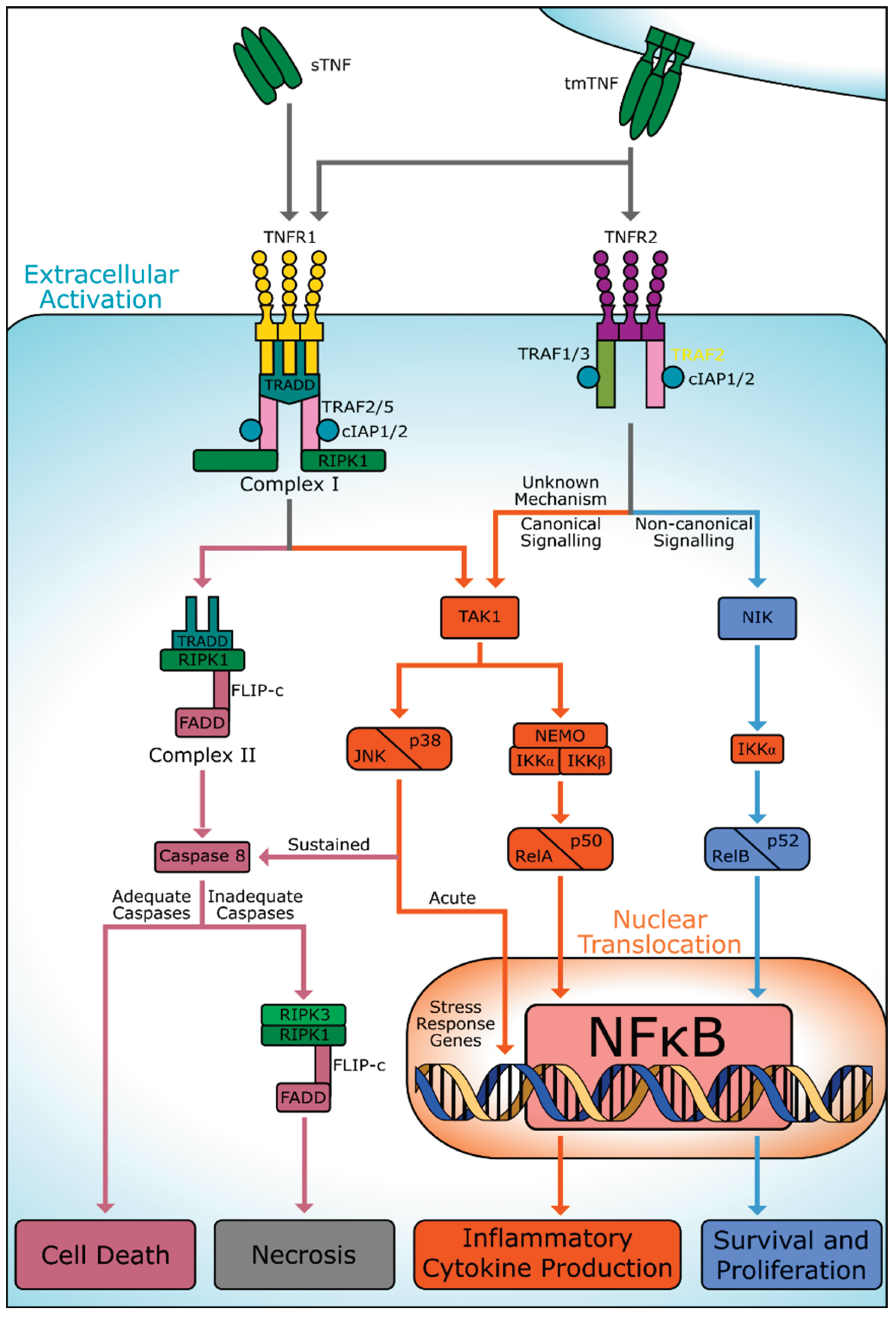
3. TNF Receptors
3.1. TNFR1
3.2. TNFR2
4. Inflammatory Response to Stroke
4.1. sTNFRs and Stroke
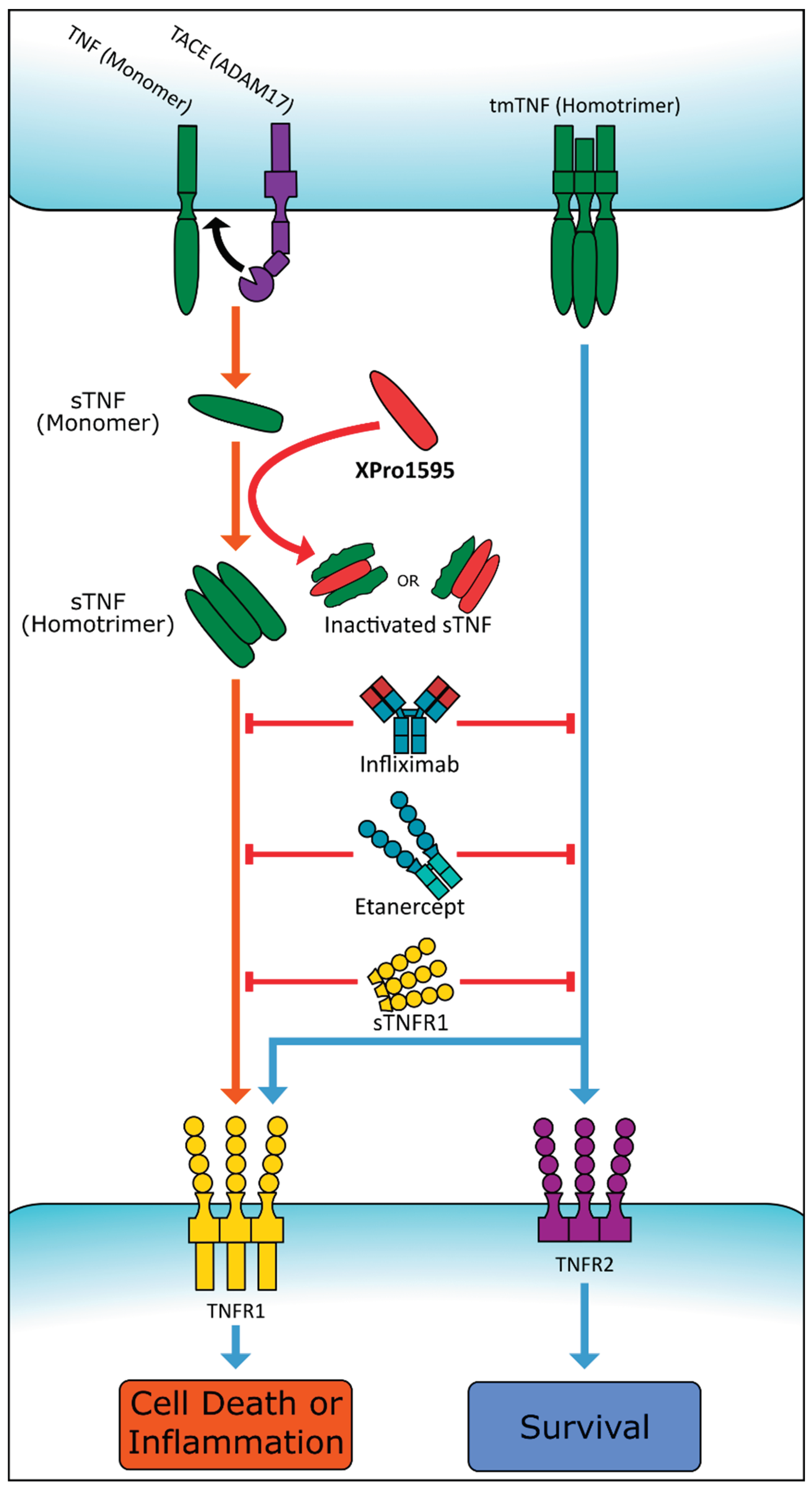
5. TNF as a Therapeutic Target
5.1. Proposed Second-Generation Therapies
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Gregory AP, Dendrou CA, Attfield KE; et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature. 2012, 488, 508. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia LA, Goeddel D V. Two TNF receptors. Immunol Today. 1992, 13, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Gough P, Myles IA. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Published online, 1975. [CrossRef]
- Chan FKM, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A Domain in TNF Receptors That Mediates Ligand-Independent Receptor Assembly and Signaling. Science (1979). 2000, 288, 2351–2354. [Google Scholar] [CrossRef]
- Ruiz A, Palacios Y, Garcia I, Chavez-Galan L, Nieuwenhuizen NE. Molecular Sciences Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Molecular Sciences Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Published online 2021. [CrossRef]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003, 114, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008, 133, 693–703. [Google Scholar] [CrossRef]
- Li J, McQuade T, Siemer AB; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012, 150, 339–350. [Google Scholar] [CrossRef]
- Green LA, Njoku V, Mund J; et al. Endogenous Transmembrane TNF-Alpha Protects Against Premature Senescence in Endothelial Colony Forming Cells. Circ Res. 2016, 118, 1512–1524. [Google Scholar] [CrossRef]
- Rauert H, Wicovsky A, Müller N; et al. Membrane Tumor Necrosis Factor (TNF) Induces p100 Processing via TNF Receptor-2 (TNFR2). J Biol Chem. 2010, 285, 7394. [Google Scholar] [CrossRef]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel ULM. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004, 279, 32869–32881. [Google Scholar] [CrossRef] [PubMed]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JPY. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001, 4, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Emmerich CH, Ordureau A, Strickson S; et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A. 2013, 110, 15247–15252. [Google Scholar] [CrossRef] [PubMed]
- George MG, Fischer L, Koroshetz W; et al. CDC Grand Rounds: Public Health Strategies to Prevent and Treat Strokes. MMWR Morb Mortal Wkly Rep. 2019, 66, 479–481. [Google Scholar] [CrossRef]
- Shatri G, Senst B. Acute Stroke. StatPearls. Published online August 17, 2023. Accessed February 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK535369/.
- Onwuekwe I, Ezeala-Adikaibe B. Ischemic Stroke and Neuroprotection. Ann Med Health Sci Res. 2012, 2, 186. [Google Scholar] [CrossRef] [PubMed]
- Chen AQ, Fang Z, Chen XL; et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. [CrossRef]
- Joshi I, Andrew RD. Imaging anoxic depolarization during ischemia-like conditions in the mouse hemi-brain slice. J Neurophysiol. 2001, 85, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012, 32, 1677–1698. [Google Scholar] [CrossRef] [PubMed]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008; 5. [CrossRef]
- Lambertsen KL, Meldgaard M, Ladeby R, Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005, 25, 119–135. [Google Scholar] [CrossRef]
- Hill JK, Gunion-Rinker L, Kulhanek D; et al. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res. 1999, 820, 45–54. [Google Scholar] [CrossRef]
- Meschia JF, Brott T. Ischaemic stroke. Eur J Neurol. 2018, 25, 35–40. [Google Scholar] [CrossRef]
- Creed Pettigrew L, Kindy MS, Scheff S; et al. Focal cerebral ischemia in the TNFalpha-transgenic rat. Published online, 2008. [CrossRef]
- 26. Maddahi A, Kruse LS, Chen QW, Edvinsson L. The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat. J Neuroinflammation. 2011; 8. [CrossRef]
- 27. Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. Published online. [CrossRef]
- Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998, 95, 570–575. [Google Scholar] [CrossRef] [PubMed]
- King MD, Alleyne CH, Dhandapani KM. TNF-alpha receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrhage in mice. Neurosci Lett. 2013, 542, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Murakami Y, Saito K, Hara A; et al. Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem. 2005, 93, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Ma L, Jiang Y, Dong YN, Gao J, Du B, Liu DW. Anti-TNF-alpha antibody attenuates subarachnoid hemorrhage-induced apoptosis in the hypothalamus by inhibiting the activation of Erk. Neuropsychiatr Dis Treat. 2018, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Belarbi K, Jopson T, Tweedie D; et al. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012; 9. [CrossRef]
- Low PC, Manzanero S, Mohannak N; et al. PI3Kδ inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model. Nature Communications 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Barone FC, Arvin B, White RF; et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997, 28, 1233–1244. [Google Scholar] [CrossRef]
- Nawashiro H, Martin D, Hallenbeck JM. Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res. 1997, 778, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sumbria RK, Boado RJ, Pardridge WM. Combination stroke therapy in the mouse with blood-brain barrier penetrating IgG-GDNF and IgG-TNF decoy receptor fusion proteins. Brain Res. 2013, 1507, 91–96. [Google Scholar] [CrossRef]
- Li J, Zhang J, Zhang Y; et al. TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis. Cell Death Dis. 2019; 10. [CrossRef]
- Aoki T, Fukuda M, Nishimura M, Nozaki K, Narumiya S. Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation. Acta Neuropathol Commun. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. Journal of Cerebral Blood Flow and Metabolism. 1998, 18, 1283–1287. [Google Scholar] [CrossRef]
- Bartsch JW, Wildeboer D, Koller G; et al. Tumor necrosis factor-alpha (TNF-alpha) regulates shedding of TNF-alpha receptor 1 by the metalloprotease-disintegrin ADAM8: Evidence for a protease-regulated feedback loop in neuroprotection. J Neurosci. 2010, 30, 12210–12218. [Google Scholar] [CrossRef]
- Yli-Karjanmaa M, Clausen BH, Degn M; et al. Topical administration of a soluble TNF inhibitor reduces infarct volume after focal cerebral ischemia in mice. Front Genet. 2019, 10, 781. [Google Scholar] [CrossRef]
- Nawashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab. 1997, 17, 229–232. [Google Scholar] [CrossRef]
- Zhang YY, Huang NN, Zhao YX; et al. Elevated Tumor Necrosis Factor-a-induced Protein 8-like 2 mRNA from Peripheral Blood Mononuclear Cells in Patients with Acute Ischemic Stroke. Int J Med Sci. 2018, 15, 1713. [Google Scholar] [CrossRef] [PubMed]
- Emsley HCA, Smith CJ, Gavin CM; et al. Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC Neurol. 2007, 7, 1–12. [Google Scholar] [CrossRef]
- Tufekci KU, Vurgun U, Yigitaslan O; et al. Follow-up Analysis of Serum TNF-Related Apoptosis-Inducing Ligand Protein and mRNA Expression in Peripheral Blood Mononuclear Cells from Patients with Ischemic Stroke. Front Neurol. 2018; 9. [CrossRef]
- Cui G, Wang H, Li R; et al. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. Published online 2012. Accessed February 23, 2024. http://www.jneuroinflammation.com/content/9/1/235.
- 47. Lasek-Bal A, Jedrzejowska-Szypulka H, Student S; et al. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J Physiol Pharmacol. 2019; 70. [CrossRef]
- Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgärde F, Ohlsson K. Leukocyte Activation Detected by Increased Plasma Levels of Inflammatory Mediators in Patients With Ischemic Cerebrovascular Diseases. Stroke. 1996, 27, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Svensson EH, Söderholm M, Abul-Kasim K, Engström G. Tumor Necrosis Factor Receptor 1 and 2 Are Associated With Risk of Intracerebral Hemorrhage. Stroke. 2017, 48, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Christensen H, Boysen G, Christensen E, Johannesen HH, Bendtzen K. Plasma cytokines in acute stroke. J Stroke Cerebrovasc Dis. 2002, 11, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Fragata I, Bustamante A, Penalba A; et al. Venous and arterial TNF-R1 predicts outcome and complications in acute subarachnoid hemorrhage. Neurocrit Care. 2019, 31, 107–115. [Google Scholar] [CrossRef]
- De Torres R, Mancha F, Bustamante A, Canhao P, Fragata I, Montaner J. Usefulness of TNFR1 as biomarker of intracranial aneurysm in patients with spontaneous subarachnoid hemorrhage. Future Sci OA. 2020, 6. [Google Scholar] [CrossRef]
- Witkowska AM, Borawska MH, Socha K, Kochanowicz J, Mariak Z, Konopka M. TNF-α and sICAM-1 in intracranial aneurismal rupture. Arch Immunol Ther Exp (Warsz). 2009, 57, 137. [Google Scholar] [CrossRef] [PubMed]
- Beeftink MMA, Ruigrok YM, Rinkel GJE, Van Den Bergh WM. Relation of serum TNF-α and TNF-α genotype with delayed cerebral ischemia and outcome in subarachnoid hemorrhage. Neurocrit Care. 2011, 15, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Lv S yin, Wu Q, Liu J peng; et al. Levels of Interleukin-1β, Interleukin-18, and Tumor Necrosis Factor-α in Cerebrospinal Fluid of Aneurysmal Subarachnoid Hemorrhage Patients May Be Predictors of Early Brain Injury and Clinical Prognosis. World Neurosurg. 2018, 111, e362–e373. [Google Scholar] [CrossRef] [PubMed]
- Hanafy KA, Grobelny B, Fernandez L; et al. Brain interstitial fluid TNF-α after subarachnoid hemorrhage. J Neurol Sci. 2010, 291, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wu W, Guan Y, Zhao G; et al. Elevated IL-6 and TNF-α Levels in Cerebrospinal Fluid of Subarachnoid Hemorrhage Patients. Mol Neurobiol. 2016, 53, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Fragata I, Bustamante A, Penalba A; et al. TNF-R1 Correlates with Cerebral Perfusion and Acute Ischemia Following Subarachnoid Hemorrhage. Neurocrit Care. 2020, 33, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Botran R, Crespo FA, Sun X. Soluble cytokine receptors in biological therapy. Expert Opin Biol Ther. 2002, 2, 585–605. [Google Scholar] [CrossRef]
- Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: The agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta Mol Cell Res. 2002, 1592, 251–263. [Google Scholar] [CrossRef]
- Mohler,’ KM, Torrance DS, Smith CA; et al. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. The Journal of Immunology. 1993, 151, 1548–1561. [Google Scholar] [CrossRef]
- Olsson I, Gatanaga T, Gullberg U, Lantz M, Granger GA. Tumour necrosis factor (TNF) binding proteins (soluble TNF receptor forms) with possible roles in inflammation and malignancy. Eur Cytokine Netw. 1993, 4, 169–180. [Google Scholar]
- Dawson DA, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. 1996, 218, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Lantz M, Malik S, Slevin ML, Olsson I. Infusion of tumor necrosis factor (TNF) causes an increase in circulating TNF-binding protein in humans. Cytokine. 1990, 2, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Engelmann H, Aderka D, Rubinstein M, Rotman D, Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. Journal of Biological Chemistry. 1989, 264, 11974–11980. [Google Scholar] [CrossRef]
- Seckinger P, Isaaz S, Dayer JM. Purification and biologic characterization of a specific tumor necrosis factor α inhibitor. Journal of Biological Chemistry. 1989, 264, 11966–11973. [Google Scholar] [CrossRef]
- Famenini S, Sako EY, Butler DC, Wu JJ. Etanercept. Moderate to Severe Psoriasis: Fourth Edition. Published online July 24, 2023, 191–198. [CrossRef]
- Chio CC, Chang CH, Wang CC; et al. Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-α. BMC Neurosci. 2013, 14. [Google Scholar] [CrossRef]
- Chio CC, Lin JW, Chang MW; et al. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem. 2010, 115, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat. 2004, 15, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Remicade (Infliximab) : Gastroenterology Nursing. Accessed February 23, 2024. https://journals.lww.com/gastroenterologynursing/citation/1998/11000/remicade__infliximab_.8.aspx.
- Zhou Y, Fan R, Botchway BOA, Zhang Y, Liu X. Infliximab Can Improve Traumatic Brain Injury by Suppressing the Tumor Necrosis Factor Alpha Pathway. Mol Neurobiol. 2021, 58, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Pergel A, Tümkaya L, Çolakoğlu MK; et al. Effects of infliximab against carbon tetrachloride-induced intestinal injury via lipid peroxidation and apoptosis. Hum Exp Toxicol. 2019, 38, 1275–1282. [Google Scholar] [CrossRef]
- Li J, Zhang Z, Wu X, Zhou J, Meng D, Zhu P. Risk of Adverse Events After Anti-TNF Treatment for Inflammatory Rheumatological Disease. A Meta-Analysis. Front Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Karamita M, Barnum C, Möbius W; et al. Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia. JCI Insight. 2017, 2. [Google Scholar] [CrossRef]
- Larson K, Damon M, Randhi R, Nixon-Lee N, Dixon KJ. Selective inhibition of soluble TNF using XPro1595 improves hippocampal pathology to promote improved neurological recovery following traumatic brain injury in mice. CNS Neurol Disord Drug Targets. 2022, 22, 1378–1390. [Google Scholar] [CrossRef]
- Steed PM, Tansey MG, Zalevsky J; et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003, 301, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Fischer R, Kontermann RE, Pfizenmaier K. Selective Targeting of TNF Receptors as a Novel Therapeutic Approach. Front Cell Dev Biol. 2020, 8. [Google Scholar] [CrossRef]
- MacPherson KP, Sompol P, Kannarkat GT; et al. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol Dis. 2017, 102, 81–95. [Google Scholar] [CrossRef] [PubMed]
- INmune Bio Announces First Patient Dosed in Phase 2 XPro1595 Trial for Treatment of Neuroinflammation as a Cause of Alzheimer’s disease. Accessed February 23, 2024. https://www.inmunebio.com/index.php/newsroom/2022-news/inmune-bio-announces-first-patient-dosed-in-phase-2-xpro1595-trial-for-treatment-of-neuroinflammation-as-a-cause-of-alzheimers-disease?highlight=WyJ4cHJvMTU5NSJd.
- Buttgereit F, Aelion J, Rojkovich B; et al. Efficacy and Safety of ABBV-3373, a Novel Anti-Tumor Necrosis Factor Glucocorticoid Receptor Modulator Antibody-Drug Conjugate, in Adults with Moderate-to-Severe Rheumatoid Arthritis Despite Methotrexate Therapy: A Randomized, Double-Blind, Active-Controlled Proof-of-Concept Phase IIa Trial. Arthritis Rheumatol. 2023, 75, 879–889. [Google Scholar] [CrossRef]
- Randhi R, Damon M, Dixon KJ. Selective inhibition of soluble TNF using XPro1595 relieves pain and attenuates cerulein-induced pathology in mice. BMC Gastroenterol. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Winsauer C, Kruglov AA, Chashchina AA, Drutskaya MS, Nedospasov SA. Cellular sources of pathogenic and protective TNF and experimental strategies based on utilization of TNF humanized mice. Cytokine Growth Factor Rev. 2014, 25, 115–123. [Google Scholar] [CrossRef]
- Gogoleva VS, Atretkhany KSN, Dygay AP, Yurakova TR, Drutskaya MS, Nedospasov SA. Current Perspectives on the Role of TNF in Hematopoiesis Using Mice With Humanization of TNF/LT System. Front Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Atretkhany KSN, Mufazalov IA, Dunst J; et al. Intrinsic TNFR2 signaling in T regulatory cells provides protection in CNS autoimmunity. Proc Natl Acad Sci U S A. 2018, 115, 13051–13056. [Google Scholar] [CrossRef]
- Williams SK, Fairless R, Maier O; et al. Anti-TNFR1 targeting in humanized mice ameliorates disease in a model of multiple sclerosis. Sci Rep. 2018, 8. [Google Scholar] [CrossRef]
- Dong Y, Fischer R, Naudé PJW; et al. Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc Natl Acad Sci U S A. 2016, 113, 12304–12309. [Google Scholar] [CrossRef] [PubMed]
| Reference | Age, Weight, Species Tested, Population Size, Sex Predominance, Duration of Study | Disease Studied & Model | Drugs/Treatments | Anatomical Outcomes | Functional Outcomes | |
|---|---|---|---|---|---|---|
| Chen et al. (2019)[18] Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke |
250-300gSprague-Dawley ratsn=ND100% M7d-duration | tMCAO (2h): intraluminal vascular occlusion Mice sacrifice: 1-7d post-ischemia |
Microglial cells transfected with 10nM siRNA for 24h. Infliximab (anti-TNF) administered by tail vein injection (5mg/kg) at 1d, 2d, and 3d post-reperfusion. 7d survival. | Levels of TNF post-tMCAO consistent with double staining results of rat brain tissue sections.TNF ELISA analysis shows decreased microglia-secreted TNF after oxygen-glucose deprivation/reoxygenation (n=6; p<.001 vs. control group p<.0001). Infliximab dramatically reduced microglia migration. | Neurological severity score results revealed that multiple doses of infliximab improved neurological function 3d after tMCAO (p<.05). | |
| King et al. (2013)[27] TNF-alpha receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrhage in mice |
8-10wkCD-1 micen=32100% M72h-duration | ICH (collagenase model): 0.5mm diameter hole drilled over parietal cortex. 26-gauge Hamilton syringe, loaded with 0.04U of bacterial type IV collagenase in 0.5μL saline lowered 3mm into left striatum. Syringe depressed at 450nL/min. | R-7050 (6–18mg/kg) administered via IP route at the time of injury or up to 2h post-ICH. | R-7050 maintains BBB integrity post-ICH. increased Evans blue brain tissue in sham-operated mice from 12.2±1.5μg to 47.2±5.8μg at 24h post-ICH (p<.01 vs. sham). Decreased Evans blue brain tissue extravasation in R-7050 (6mg/kg) to 28.7±5.9μg and 30.3±1.9μg when administered at 0.5h or 2h post-ICH, respectively (p<.05 and p<.01 vs. ICH). TNF elevated in ICH rats. | R-7050 resulted in protective effect during first 3d post-ICH, as compared to placebo, with complete decrease in neurological deficits (p<.05). | |
| Murakami et al. (2005)[28] Increases in tumor necrosis factor-alpha following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus |
10-15wk WT C57BL/6J mice n=ND100% M7d-duration |
Transient Global ischemia: bilateral common carotid arteries occluded by aneurysm clips (75g pressure) for 20min. Mice sacrificed 12h post-ischemia. |
ND | Hippocampal TNF mRNA levels increased (sham: 0.16±0.064 vs. ischemia: 0.459±0.096; p<.01) 3h after recirculation from endogenous brain cells, then decreased to sham-operated levels by 24h. Increase of TNF mRNA again at 36h (sham: 0.160±0.064 vs. ischemia: 0.760±0.092; p<.01). Up-regulated TNF protein level in the hippocampus only at 6h post-ischemia (sham: 0.946±0.150 vs. ischemia: 6.534±2.646; p<.05). | ND | |
| Ma et al. (2018)[29] Anti-TNF-alpha antibody attenuates subarachnoid hemorrhage-induced apoptosis in the hypothalamus by inhibiting the activation of Erk |
250-350gWistar Ratsn=153100% M7d-duration | SAH: fresh non-heparinized blood (0.3mL) from femoral artery injected into cisterna magna at 0.05 mL/min. | U0126 (dissolved in PBS; 250 ng/μL, 10μL per rat) microinfused into left lateral cerebral ventricle 30min before SAH at 0.5μL/min. | U0126 injection blocked SAH-induced TNF increase (p=.024).U0126 micro infusion decreased mRNA levels in hypothalamus (p=.001).Apoptotic and anti-apoptotic gene expression levels increase post-SAH. U0126 decreased Erk phosphorylation. | Decreased anxiety-like behaviors. PBS-injected SAH rats showed decreased total traveled distance compared to control group at 2d and 7d post-SAH. U0126 SAH rats spent less time in the center of the open field both at 2d (p=.003) and 7d (p=.026) post-surgery. | |
| Pettigrew et al. (2008)[25] Focal cerebral ischemia in the TNF alpha-transgenic rat | 250-350gSprague-Dawley, carried the TNF genen=ND100% M7d-duration | Focal cerebral ischemia: Zea Longa suture-occlusion of MCA for 1h. Cortical perfusion measured by LDF. Reperfusion at 3 or 24h. |
ND | TNF-Tg rats had greater infarct volume than non-Tg control at 24h (p≤.05) and 7d (p≤.01).TNF-Tg rats had decreased cortical perfusion within 10min of MCAO (p≤.05). Neural cellular apoptosis increased in transgenic animals with elevated caspase-3 activity (p≤.05) and DNA fragmentation (p≤.001) at 24h. | TNF showed a dose-dependent adverse effect on motor function. | |
| Belarbi et al. (2012)[30] TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation |
3moF344 rats (Charles River Labs) n=28100% M43d-duration | Chronic neuroinflammation via LPS-induced sustained microglia activation. | Artificial cerebrospinal fluid (aCSF; n=11) or lipopolysaccharide (LPS; n=17) loaded into an osmotic minipump at 0.25μl/h; 28d delivery into the fourth brain ventricle. | LPS-vehicle rats had increased TNF mRNA levels (129.97±9.09%; p<.05) and increased TNFR2 expression (124.91±6.25%; p<.05) when compared to aCSF-vehicle rats.DT treatment returned TNF mRNA to control levels (102.18±8.90%; p<.05 vs. LPS-vehicle). | Neuroinflammation decreased novel place recognition, spatial learning, and memory function, but not novel object recognition (aCSF-vehicle: p=.0016; aCSF-DT: p=.0124; LPS-vehicle: p<.0001; LPS-DT: p=.0094).DT treatment restored cognitive function in LPS-infused rats. All treatment groups had similar swim velocity (p=.6878) and similar escape latency to locate the platform (p=.7775). | |
| Low et al. (2014)[31] PI3Kδ inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model | 12-22wkp110d kinase-dead PI3Kδ mice, C57BL/6n=ND100% M20wk-duration | tMCAO (1h): internal carotid artery occluded with a clipReperfusion: 24h to 72h | 40 mg/kg CAL-101 by 25mL infusion into the femoral vein, 15min before ischemia/reperfusion treatment, or 3h and 6h after reperfusion. | PI3Kδ controls intracellular TNF trafficking in macrophages and is prospective target to limit neuroinflammation. p110δ mice had decreased infarct volume than tMCAO WT (p<.005). PI3Kδ inhibition protects from ischemia. Brain PI3Kδ drives ischemia-induced leukocyte infiltration. | p110 mice had decreased brain damage and neurological deficit at 72h post-ischemia/reperfusion than WT mice. | |
| TNFR1 | Barone et al. (1997)[32] Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury | 280-340gSpontaneously Hypertensive Rat (SHR) n=ND100% M3d-duration | IS: induced by permanent or tMCAO for 80m or 160m Reperfusion: 24h |
TNF (2.5 or 25pmol) 24h before pMCAO or tMCAO.mAb (60pmol) or sTNFR1 (0.7nmol) administered ICV 30m before and after tMCAO at 3h and 6h. | Infarct size increase seen in injections of:- 2.5pmol TNF 24h pre-pMCAO (p<.05)- 25pmol TNF (p<.01) (greater than 2.5pmol TNF pre-pMCAO)- tMCAO (80 and 160min) 2.5pmol TNF (p<.05).Reduced focal ischemic injury seen in treatment before and during 24h of focal ischemia, with repeated ICV mAb or sTNFR1 (p<.05). | Neurological deficits not decreased by blocking TNF. |
| Nawashiro et al. (1997)[33] Neuroprotective effects of TNF binding protein in focal cerebral ischemia | 22-26gBALB/Cn=67100% M2wk-duration | Permanent MCAO: anesthetized mice dura and arachnoid opened and left MCAO performed by electrocoagulation. | Treatment groups topically given 3mg/kg TNF-BP or vehicle immediately or 60m post MCAO. | All treatments resulted in reduced infarct volumes, with TNF-BP treatment most potent (p<.001). Significant neuroprotection when TNF-BP administered 60m post-MCAO. In mice treated with TNF-BP, 3/7 showed no DNA fragmentation. | ND | |
| Sumbria et al. (2013)[34] Combination stroke therapy in the mouse with blood-brain barrier penetrating IgG-GDNF and IgG-TNF decoy receptor fusion proteins | 25-33gC57Bl/6J micen=38100% M7d-duration | tMCAO (1h): electrocoagulation of ECA branchesReperfusion: 23h or 7d | After 1h MCAO, 100μl total volume of either saline, rGDNF (170μg/kg), GDNF (1mg/kg), or GDNF and TNFR (1mg/kg each) intravenously injected via tail vein at 45min post arterial occlusion, after 1h MCAO. | Combined GDNF and TNFR fusion protein treatment reduced hemispheric, cortical, and subcortical stroke volumes. Decreased hemispheric, cortical, and subcortical stroke volume present at 7d post-injury. | ND | |
| TNFR2 | Li et al. (2019)[35] TRAF2 protects against cerebral ischemia-induced brain injury by suppressing necroptosis | 25-30gICR micen=86100% M48h-duration | tMCAO (1h): Ligation carried out 1cm to internal and external cervical vascular branch. Reperfusion at 24h. |
2 injections of negative control shRNA lentivirus or TRAF2 shRNA lentivirus (1μl 5×108 TU/ml) injected into ipsilateral striatum of the mice 2wk before MCAO. | TRAF2 levels increased in the ipsilateral cortex 24h post-reperfusion.TRAF2 knockdown increased infarct volumes, cell death, and neuroinflammation. Post-ischemic induction of TRAF2 protected microglia and neurons against necroptotic cell death. | ND |
| TNF, TNFR1, TNFR2 | Aoki et al. (2014)[36] Critical role of TNF-alpha-TNFR1 signaling in intracranial aneurysm formation | 7wkSprague-DawleyTNFR1−/− micen=122100% M5mo-duration | SAH by intracranial aneurysm ligation of the left carotid artery and systemic hypertension induced by salt overloading and left renal artery ligation. | ND | Intracranial artery TNF increase in rats with advanced stage IA 3mo post-aneurysm.Increased TACE activity compared to control rats. suggesting TNF production up- TNFR1-deficient mice had decreased IA when compared to WT mice (p=.012). | ND |
| Maddahi et al. (2011)[37] The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat | MCAO: 300-350g SAH: 300-350gWistar-Hanover rats n=24100% M48h-duration |
MCAO: Blood flow in the right MCA blocked by an intraluminal occluderSAH: suture to perforate ICA. Protein expression evaluated after 48h. |
ND | MCAO: TNF, TNF-R1, and TNF-R2 protein expression in increased compared to control group, P< .05, <.01, and <.05, respectively. SAH: TNF, TNF-R1, and TNF-R2 protein expression increased compared to control, P<.05, <.01, and <.05, respectively. |
ND | |
| Gary et al. (1998)[38] Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor | 3mo25-30gC57BL/6n=129100% M24h-duration | tMCAO (1h): Middle cerebral artery blocked by rounded nylon thread.Reperfusion: 24h | Kainic acid (.3µg in 0.5 µL) injected into the dorsal hippocampus. | WT and p75−/− (TNFR2) mice had similar infarct sizes. p55−/− (TNFR1) and p55/p75−/−mice had increased infarct sizes when compared to WT and p75−/− mice. | No change in brain structure or behavioral test performance in p55/p75 −/− mice when compared to WT. | |
| sTNFR1 and sTNFR2 | Bartsch et al. (2010)[39] Tumor necrosis factor-alpha (TNF-alpha) regulates shedding of TNF-alpha receptor 1 by the metalloprotease-disintegrin ADAM8: evidence for a protease-regulated feedback loop in neuroprotection | wr/+ Adam8+/+wr/+ Adam8−/−wr/wr Adam8+/−wr/wr Adam8−/−n=15055d-duration | Motor Neuron Disease (CNS) | NA | 7 to 10 times increased expression of ADAM8 mRNA after administration of 100U/ml TNF. Administration of ≥500U/ml of TNF resulted in decline in ADAM8 mRNA levels. Concentration of sTNFR1 significantly increased in standard mice, but not in homozygous ADAM8 deficient. | ADAM8-deficient Wobbler mice showed more dramatic decline in the forelimb force at P18 and complete loss of force at approximately day 24 after birth. |
| Yli-Karjanmaa et al. (2019)[40] Topical administration of a soluble TNF inhibitor reduces infarct volume after focal cerebral ischemia in mice |
7-8wkC57BL/6 n=56100% MND | MCAO by electrocoagulation | Topical (2.5 mg/ml for 3d) or ICV (1.25mg) at 1μl/h 30m post-pMCAO with saline, XPro1595, or etanercept post-pMCAO. Topical XPro1595 concentration in brain tissue 1d post-pMCAO: 630,300±160,000pg/mg. ICV XPro1595 concentration: 69,400±51,300pg/mg. | Topical XPro1595 decreased infarct volume at 1d and 3d post-pMCAO. TNF mRNA expression increased 1d and 3d post-pMCAO in mice treated topically with XPro1595 when compared to saline and etanercept mice. Topical etanercept showed no effect. | No difference in grip strength test for ICV Etanercept and ICV XPro1595. | |
| Nawashiro et al. (1997)[41] Inhibition of tumor necrosis factor and amelioration of brain infarction in mice | 22-26gBALB/C micen=38100% M24h-duration | MCAO by electrocoagulation | sTNFR1 linked to polyethylene glycol (TNF-BP) immediately after MCAO, 0.3 or 3mg/kg body weight of TNF-BP (1μl or 10μl) applied topically. |
26% reduction in brain damage volume in animals that received 3mg/kg of TNF-BP, 10% in those given 0.3mg/kg of TNF-BP, 15% in those injected with 3mg/kg of TNF-BP IP, and 20% in animals administered 3mg/kg of TNF-BP IV. | NA |
| Reference | Mean Age, Population size, Sex, Duration, Location | Exclusion Criteria | Anatomical Outcomes | Functional Outcomes | |
|---|---|---|---|---|---|
| IS | Zhang et al. (2018)[42] Elevated tumor necrosis factor-a-induced protein 8-like 2 mRNA from peripheral blood mononuclear cells in patients with acute ischemic stroke |
IS: 68.00yr n=182 49.4% M Ctrl: 64.50yr n=40 42.5% M 8mo-duration Shandong, China |
Patients with TIA, greater than 24h from stroke onset, hemorrhage stroke, severe infections, or malignant tumors. | mRNA levels of TNF in peripheral blood mononuclear cells elevated compared controls [3.74 (2.40-5.48) vs 2.16 (1.68-3.69); p<.001]. TNF mRNA median levels lower in survivals than non-survivals [TNF, 3.42 (2.20-4.86) vs 5.67 (4.67-7.72); p<.001]. | After 3mo follow-up, 33 AIS patients had died. |
| Emsley et al. (2007)[43] Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production |
IS: 69.6±13.0yr n=36 67% M Ctrl: 68.7±12.6yr n=36 67% M 12mo-duration Salford, England |
Patients with decreased symptoms since onset, an indeterminant onset of symptoms, or evidence of active malignancy. | Plasma sTNFR1 concentration was correlated with infarct volume in the first week (r=.62, p=.001), 3mo (r=.59, p<.001), and 1yr (r=.57, p=.001). | 14 AIS patients died by 12mo. Causes of death: index stroke: 8 recurrent stroke:1 pulmonary embolism: 1 left ventricular failure secondary to myocardial infarction: 1 sepsis: 3 Barthel Index (BI) for Activities of Daily Living (ADL) at 3mo (p=.001) and 1yr (p<.001). |
|
| Tufekci et al. (2018)[44] Follow-up analysis of serum TNF-related apoptosis-inducing ligand protein and mRNA expression in peripheral blood mononuclear cells from patients with ischemic stroke |
IS: 67.35yr n=95 58.6% M Ctrl: 69.7yr n=95 39.4% M 6mo-duration Izmir, Turkey |
Inclusion: Patients admitted to hospital within 24h of stroke onset with no history of previous acute ischemic stroke. | Lower TRAIL levels of stroke patients than healthy control (p<.0001). Serum TRAIL levels were increased 1mo after stroke onset (p=.002). Stroke patients had higher TRAIL mRNA expression as compared to the controls (p<.0001). | ND | |
| Cui et al. (2012)[45] Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke |
IS: n=961 Ctrl: n=821 ND 5yr-duration Wuhan, China |
Non-Chinese Han populations. | Higher serum TNF levels in IS than in control (SMD=2.33, 95%CI=1.85-2.81). | ND | |
| Lasek-Bal et al. (2019)[46] The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis |
73.11±11.48yr n=138 46.04% M 30d-duration University of Silesia |
No history of a previous stroke, admission greater than 24h after symptoms, pre-stroke status on mRS less than 1 point. | Patients with higher NIHSS scores showed higher TNF concentrations p=.00218. Median NIHSS score of all patients is 3. Data suggests TNF has an unfavorable effect on stroke infarct volume. | Patients with good functional status on 30d, despite poor initial status, had higher TNF levels. Median mRS score is 2. Influence of TNF on neurological improvement compared to other cytokines (p<.04). High TNF concentration on the first day of stroke correlates with severe status. |
|
| Elneihoum et al. (1996)[47] Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases |
IS: 70yr n=72 59.7% M TIA: 69yr n=48 54.2% M Ctrl: 68yr n=35 57.1% M ND Malmo, Sweden |
Patients with ICH, renal deficit, acute infection, or vascular ischemia. | Increased TNFR1 levels in stroke group than in control (3.1 μg/L vs. 2.1 μg/L, p<.04). Increased TNF in 18% of patient group and 17% of control group. Correlation between TNFR1 and TNF plasma levels (p<.0006). |
ND | |
| ICH | Svensson et al. (2017)[48] Tumor necrosis factor receptor 1 and 2 are associated with risk of intracerebral hemorrhage |
ICH: 62yr n=220 48% M Ctrl: 62yr n=244 49% M 48mo-duration Malmö, Sweden |
Missing plasma samples, laboratory errors, or missing blood pressure information. | Patients who developed ICH during follow-up had higher concentrations of TNFR1/2 (TNFR1: OR, 2.28; 95% CI p=.006; TNFR2: OR, 1.77; p=.008). | TNFR1 and TNFR2 associated with increased fatal ICH (TNFR1: OR, 4.42; TNFR2: OR, 2.90) and with poor mRS results. |
| Christensen et al. (2002)[49] Plasma cytokines in acute stroke |
ICH: 74yr n=17 54% M CI: n=162 3mo-duration Copenhagen, Denmark |
Within 24h of stroke onset. | Positive correlation between sTNFR1&2 levels and age (p<.001). Plasma levels in pg/mL of cytokines and soluble cytokine receptors on inclusion/3mo: sTNFR1 =1.306/1.469 (p=.044) sTNFR2 =2.307/2.805 (p<.001). Higher levels of sTNFR1&2 correlated to an unfavorable outcome at 3mo. |
Increased levels of sTNFR1 and sTNFR2 correlated to bladder cancer, colon cancer, disseminated terminal cancer without identified primary cancer, and urinary tract infection in treatment at 3mo. 3mo fatality rate was 11.2%. | |
| SAH | Fragata et al. (2019)[50] Venous and arterial TNF-R1 predicts outcome and complications in acute subarachnoid hemorrhage |
56.7±16.1yr n=58 39.7% M 6mo-duration ND |
Inclusion: Patients admitted in the first 72h of SAH symptoms. Exclusion: Patients in very poor clinical condition. |
Correlation of arterial and venous levels of TNFR1 (p<.001). No association of TNFR1 with DCI. Cut-off for arterial TNFR1 of 1523.7pg/mL had 75% sensitivity/66% specificity for the prediction of hydrocephalus. | Patients with high venous TNFR1 are correlated with poor outcomes in SAH by GCS and Fisher scales (OR 8.74; p=.018). |
| de Torres et al. (2019)[51] Usefulness of TNFR1 as biomarker of intracranial aneurysm in patients with spontaneous subarachnoid hemorrhage |
56.7±16.1yr n=58 37.9% M 6mo-duration Seville, Spain |
Older than 18yr, within the first 72h of acute SAH, imaging studies performed within the first 72h of SAH. | Venous TNFR1 levels greater than 1658pg/ml had 46.3% sensitivity/94.1% specificity for aneurysms and is independent predictor for its presence [OR=12.03 (1.13-128.16); p=.039]. | Higher clinical and radiological severity in the aneurysm group, both on the HH grade (3 vs 1; p=.022) and on the Fisher scale (4 vs 3; p=.019). | |
| Witkowska et al. (2009)[52] TNF-alpha and sICAM-1 in intracranial aneurismal rupture |
SAH: 56yr n=27 51.8% M Ctrl: 55yr n=17 72h-duration Poland |
Diagnosed with intracranial aneurismal after 72h of ER arrival. | Concentrations of TNF in patients with SAH were 12.42 ± 9.70 pg/ml, and 11.29 ± 8.80 pg/ml in control. Increased TNF levels in the CSF observed 4-10d after SAH. | All GCS scores between 11 and 15. 74% of patients presented with HH grades 0–1. 81% of patients presented with GOS scores between 3 and 5. | |
| Beeftink et al. (2011)[53] Relation of serum TNF-α and TNF-α genotype with delayed cerebral ischemia and outcome in subarachnoid hemorrhage |
TNF mutation: 57yr n=31 26% M Ctrl: 57yr n=67 30% M 3mo-duration Utrecht, The Netherlands |
ND | High-serum TNF levels during days 0–12 showed an adjusted HR of 0.6 for DCI. Non-WT TNF genotype individuals exhibited decreased serum TNF levels with an HR of 0.4 for DCI. | Lower risk of DCI for patients with higher values of TNF. Patients with the variant allele tended to have a lower serum TNF. Patients with good outcomes and control patients had low TNF levels. | |
| Lv et al. (2018)[54] Levels of interleukin-1β, interleukin-18, and tumor necrosis factor-α in cerebrospinal fluid of aneurysmal subarachnoid hemorrhage patients may be predictors of early brain injury and clinical prognosis |
55yr n=81 42% M 6mo-duration Nanjing, China |
Patients who had previously experienced central nervous system diseases or are on aspirin/anti-inflammatory analgesics. | TNF in the CSF of aSAH patients with WFNS grades 4 and 5 were higher than those of patients with WFNS grades 1 and 3 (p<.0001). TNF in the CSF of aSAH patients with Fisher grades 3 and 4 were higher than those of patients with Fisher grades 1 and 2 (p<.01). | TNF (1-3d, r=.4982; 4-6d, r=.5470, and 7-9d, r=.4757) was correlated with mRS (p<.001). Patients with a poor outcome had higher TNF levels than those with good outcomes. | |
| Hanafy et al. (2010)[55] Brain interstitial fluid TNF-alpha after subarachnoid hemorrhage |
48yr n=14 29% M 72h-duration New York, NY |
Patients without cerebral micro dialysis or at least 24h of recoverable data. | Brain interstitial fluid glucose was associated with TNF (p=.018). Aneurysm size was a strong predictor of the TNF response (p<.001). | ND | |
| Wu et al. (2016)[56] Elevated IL-6 and TNF-α levels in cerebrospinal fluid of subarachnoid hemorrhage patients |
SAH: 57.9yr n=57 57.9% M Ctrl: 59.2yr n=65 53.8% M 48h-duration Jilin Univ, China |
Patients with infection, heart disease, malignant tumor, and hematonosis. |
CSF TNF levels of SAH patients and controls were 49.68±7.02 and 12.47±2.15pg/mL, respectively (p<.001). CSF TNF levels in Caucasian SAH patients were higher than corresponding controls (p<.001). | Higher TNF levels in CSF of SAH patients (HH 1-4) compared to healthy controls (p<.05). HH grade 2 SAH patients showed higher TNF levels in CSF compared to HH grade 1 (p=.008). | |
| Fragata et al. (2020)[57] TNF-R1 correlates with cerebral perfusion and acute ischemia following subarachnoid hemorrhage |
54.6yr n=41 48.8% M 18mo-duration Lisbon, Portugal |
Patients with renal insufficiency, unknown time of SAH onset, pregnant women, or in poor clinical condition (GCS 3). Hospital admission 72h after. |
Higher arterial TNF-R1 levels correlated with trends of lower cerebral blood flow (r = -0.262, p = 0.069) and cerebral blood volume (r = -0.24, p = 0.096). TNFR1 levels independent predictors of poor outcomes. |
ND |
| XPro1595 | Etanercept | sTNFR | |
|---|---|---|---|
| MW (kDa) | 7-20 | 105 | 75 |
| Half-life (h) | ~9 | 68 | 2 |
| Ligands | sTNF monomer/dimer | sTNF trimer, TNF | |
| IC50 (ng/mL) | 12.5±4.0 | 10.8±1.8 | 3500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





