1. Introduction
Amyotrophic lateral sclerosis (ALS) is described as a destructive neurodegenerative disease with multifarious causes ending with upper and lower selective loss of motor neuron function [
1]. One of the characterizations of ALS disease is the progressive wasting of skeletal muscle due to neuronal deterioration, leading to respiration failure and death in the first five years of diagnosis [
2]. Due to overlapping with similar neurodegenerative disease mimickers, unfortunately, patients are often misdiagnosed with other conditions before ALS is finally identified[
3].
Until now, there has been no certain treatment for ALS disease, so there is an urgent need to find ways to diagnose it sooner to stop the progression of the disease [
4]. One of the reliable biomarkers that critically takes part in the relevance of ALS with the definitive predictive role is microRNAs (miRNAs), which help rapidly identify ALS disease [
5]. MicroRNAs are small noncoding RNAs with an average length of 18 to 25 nucleotides that critically control gene expression at the posttranscriptional level in a se-quence-specific manner[
6]. Due to high accumulations in specific cells and tissues and having an essential role in biological processes, the regulation number of coding genes can contribute to disease diagnosis, particularly in the early stages[
7]. Several studies have confirmed that Cerebra spinal fluid (CSF) and blood miRNAs are potentially useful biomarkers of illnesses of the central nervous system (CNS) [
8].
Several studies have shown the effects of stem cell transplantation, as a new thera-peutic approach for ALS disease, on alteration in clinical and molecular biomarkers[
9]. Many different stem cell types have been used in experimental ALS models. Mesenchymal stem cells (MSCs) have attracted much more attention due to easy accessibility and lack of ethical concerns[
10]. Recently, these cells and their derivatives have been considered good therapeutic strategies for various diseases like diabetes[
11], Parkinson's[
12], and ALS[
13], which can play a role in the inhibition of neural and glial cell apoptosis[
14] due to the secretion of neurotrophic factors [
15], and numerous other immunomodulatory properties[
13].
Respecting the above information, in this research, we examined ALS patients' clinical and molecular data following repeated bone marrow-derived mesenchymal stem cell transplantation during a 3-month follow-up period, aiming to find a new therapeutic strategy by targeting valid prognostic and diagnostic biomarkers in ALS patients.
2. Materials and Methods
2.1. Study Design
This experiment was designed as a single-centre, prospective, open-label, retro-spective trial without a placebo-control group conducted at Pastoor Hospital, Mashhad, Iran, passing all international standards [
16].
2.2. Participants
Fifteen patients entered the study and completed the treatment process. All patients were of Iranian nationality and had forced vital capacity (FVC) > 65% of the predicted average value for gender, body size, and age. The patients were followed three months after the intervention as published previously[
16]. The characterization of inclusion and exclusion properties is as follows:
2.2.1. Inclusion Criteria
Individuals (from both genders) with the age range of 18 to 75 years, signing an in-formed written consent form, established ALS disease based on the EI scoria[
17] approved by a specialist neurologist, having ALS history for at minimum one year.
2.2.2. Exclusion Criteria
If they have an active infection within a week before injections, severe underlying diseases at present or in the previous six months (cancer, myocardial infarction, liver, heart, or kidney dysfunction), failure to fully receive all three injections, simultaneous participation in other clinical trials, Pregnancy, or ventilator dependency
2.3. Bone Marrow Aspiration
The stem cells used for injections were aspirated from the patient's bone marrow under septic conditions and by local anaesthesia. The specialist doctor aspirated approximately 12 ml of samples, and the site of aspiration was checked one day after the procedure to ensure no serious complications.
2.4. Stem Cell Preparations and Characterization
Good manufacturing practice (GMP) conditions were used for the isolation, culture, and analysis of the MSCs. Using a Ficoll density gradient centrifugation technique (Ficoll-Paque Premium; GE Healthcare Bio-Sciences, Uppsala, Sweden), peripheral blood mononuclear cells (PBMCs) were separated from the bone marrow by special centrifugation method and were grown in medium enriched with essential elements (Lonza, Basel, Switzerland) that contains 10% fetal bovine serum (FBS) (Life Technologies, Grand Island, USA) and 1% penicillin-streptomycin (Biochrom, Berlin, Germany) and incubated in a humidified incubator at 37 °C with 5% CO2. The culture media was changed after 24 hr. to remove debris and non-adherent cells. The culture media was also changed twice weekly. Cells were digested and re-plated for expansion to passage 3 when the primary cultures of BM-MSCs reached 80% confluence using 0.125% trypsin-EDTA (Life Technologies, Darmstadt, Germany).
To ensure sterility, the samples were tested with Endotoxin, Reverse transcription-polymerase chain reaction (RT-PCR), and Bactec tests for bacteria, mycoplasma, and fungal or yeast infection. Before transplantation, the cell viability rate was >95% using the Trypan blue staining method. The autologous BM-MSCs products were then released and transported to the place of investigation at a temperature of between 2 and 8 degrees Celsius. Harvesting, processing, and releasing the BM-MSCs were all carried out in a cleanroom (Grade B) that met GMP standards.
Characterization of stem cells was done according to the International Society for Cellular Therapy (ISCT) and based on flow cytometry and the differentiation test of cells into bone and fat. In more detail, Alizarin red S staining (Kia zist, Iran) was used to evaluate the osteogenic differentiation of MSCs. Furthermore, oil red O staining (Kia zist, Iran) was used to evaluate the differentiation ability of MSCs to adipogenic lineage[
16] .
2.5. Procedure
Stem cells were transplanted three times into patients simultaneously intrathecally (IT) (by lumber puncture in the L3-L4 region) and intravenously (IV) routes (1 × MSC/Kg BW). The intervals between injections were one month. For biological assessment, the patient's blood samples were collected three times (1 month before intervention, 1 and 3 months after cell transplantation), and CSF samples were taken three times (months o, 1 and 3 after cell transplantation). A clinical assay using the ALS functional rating scale-revised (ALSFRS) and FVC score was applied three times (1 month before intervention, 1 and 3 months after cell transplantation). All patients also received their routine pharmacotherapy based on their physician's opinion. Investigation of possible systemic and local infections is done through paraclinical tests on two occasions (one week and one day before the intervention), and patients were examined in terms of inflammatory factors and liver profile [
16].
2.6. Stem Cell Transplantation
Stem cells were suspended in 2ml of normal saline for each IT transplantation, which took approximately 2 minutes. For IV transplantation, cells were suspended in 50-60 ml of normal saline, and transplantation time was approximately 30-40 minutes.
2.7. Molecular Assay
For molecular analysis, two types of samples were used: serum samples (collected three times in months -1 (before), 1 and 3) and CSF samples (collected three times in months 0, 1 and 3). In this study, three miRNAs were measured, which include Mir206, Mir 338-3p, and Mir133a-3p. Measurement occurs in three steps that include:
a-miRNAs isolation: Briefly, 200 µl of samples were used for miRNAs isolation based on the kit manufacturer’s protocol (Favor Prep BIOTECH; IRAN).
b-cDNA Synthesis using the kit protocol (Stem cell TECHNOLOGY, IRAN)
c-Amplification uses SYBR GREEN RT-PCR Master Mix (Parstous; Iran). Briefly, specific primers and thermal cycles are as mentioned below (
Table 1 and
Table 2):
It should be noted that the sequence of Reverse primers is exclusive to the manufacturing company (Universal Reverse Primer…100 Reaction …BN-0011.17.5).
2.8. Endpoints
The main endpoint of this trial was to investigate the effectiveness of three doses of injection of autologous BM-MSCs concurrently via IT & IV routes in ALS patients through molecular analysis by measuring specific miRNAs done for efficacy evaluation.
2.9. Statistical Analysis
Statistical analysis was done using the SPSS 23.0 software package (SPSS GmbH Software, Germany). Testing normality was done by using the Shapiro-Wilk test. Test the effect of time For normally distributed data done by Generalized Linear Model (GLM) and Repeated Measure ANOVA (RM ANOVA) analysis. Continuous variables, as non-normally distributed, are described as the median (interquartile range, IQR). The comparison of the medians between two related groups by paired T-test or Wilcoxon signed ranks. For Continuous variables, normally distributed data are illustrative as the mean or as median (for non-normally distributed data) ± SEM (standard error of the mean/median). The statistical differences in the patient's data were compared three times (before the intervention, months 1 and 3) for serum and (months 0, 1, and 3) for CSF samples. P-values less than 0.05 were considered to be a level of significance. The Chi2 test was used also. The statistical graphs were created using the Graph Pad Prism8.1 software program (Graph Pad Software, San Diego, CA, USA).
3. Results
3.1. Patients' Clinical Description and Safety Results
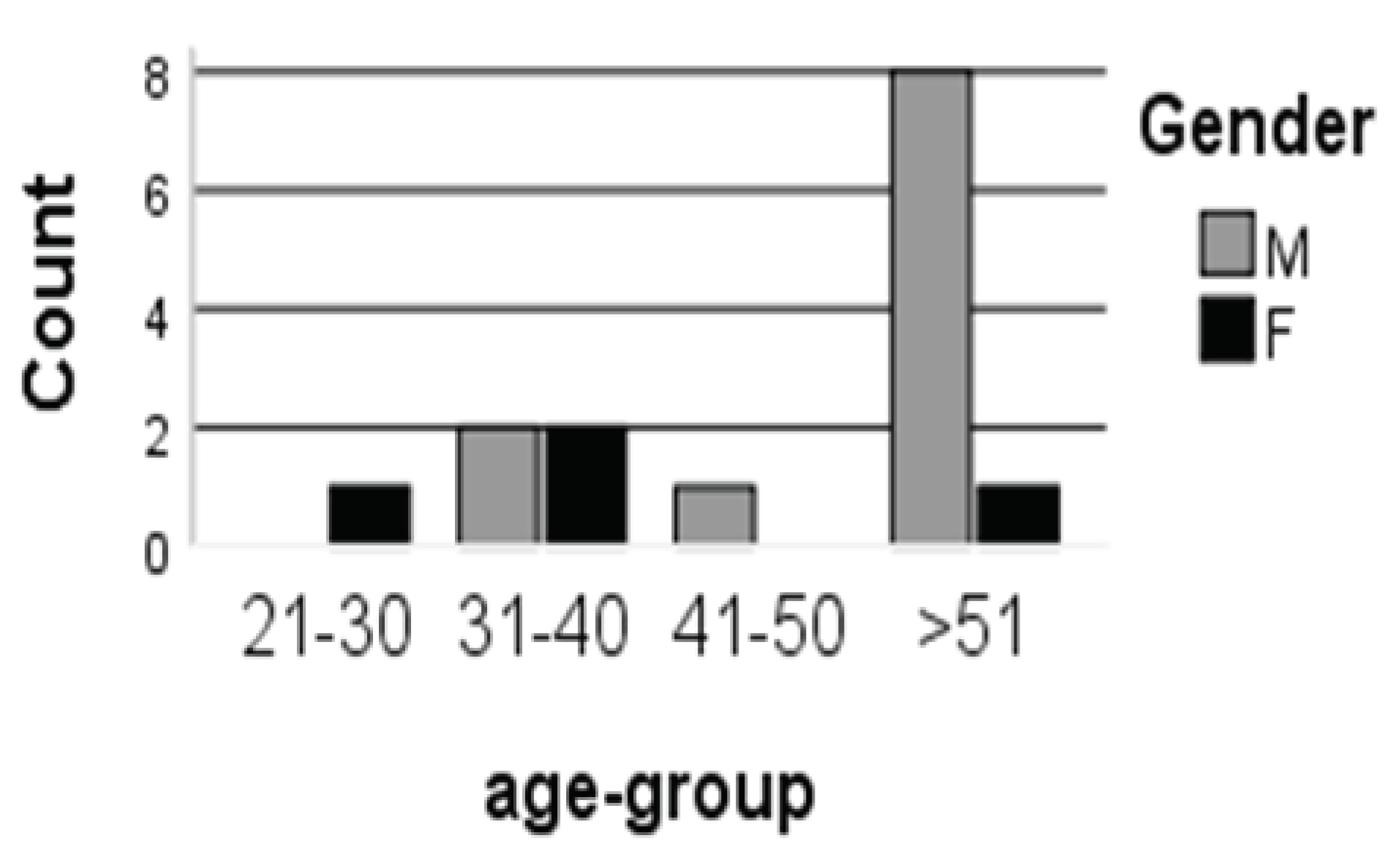
As previously published, 15 patients participated (4 female and 11 male) with an average age of 45.8 years, and a duration of disease 39.9 months. The outcomes showed that MSC transplantation was safe with no serious side consequences in patients [
16]. The highest percentage of patients were male within the age group (>51 years) as 88.9% (n=8) while females accounted as 11.1% (n=1) , and in the case of the age group (31-40 years) represented as 50% (n=2) for male and female, and P.value non-significant (P=0.14) (
Figure 1), Additionally, FVC indicating significant increasing (P-value <0.05), ALSFRS exposed stability (P-value >0.05)[
16].
3.2. microRNA Expression
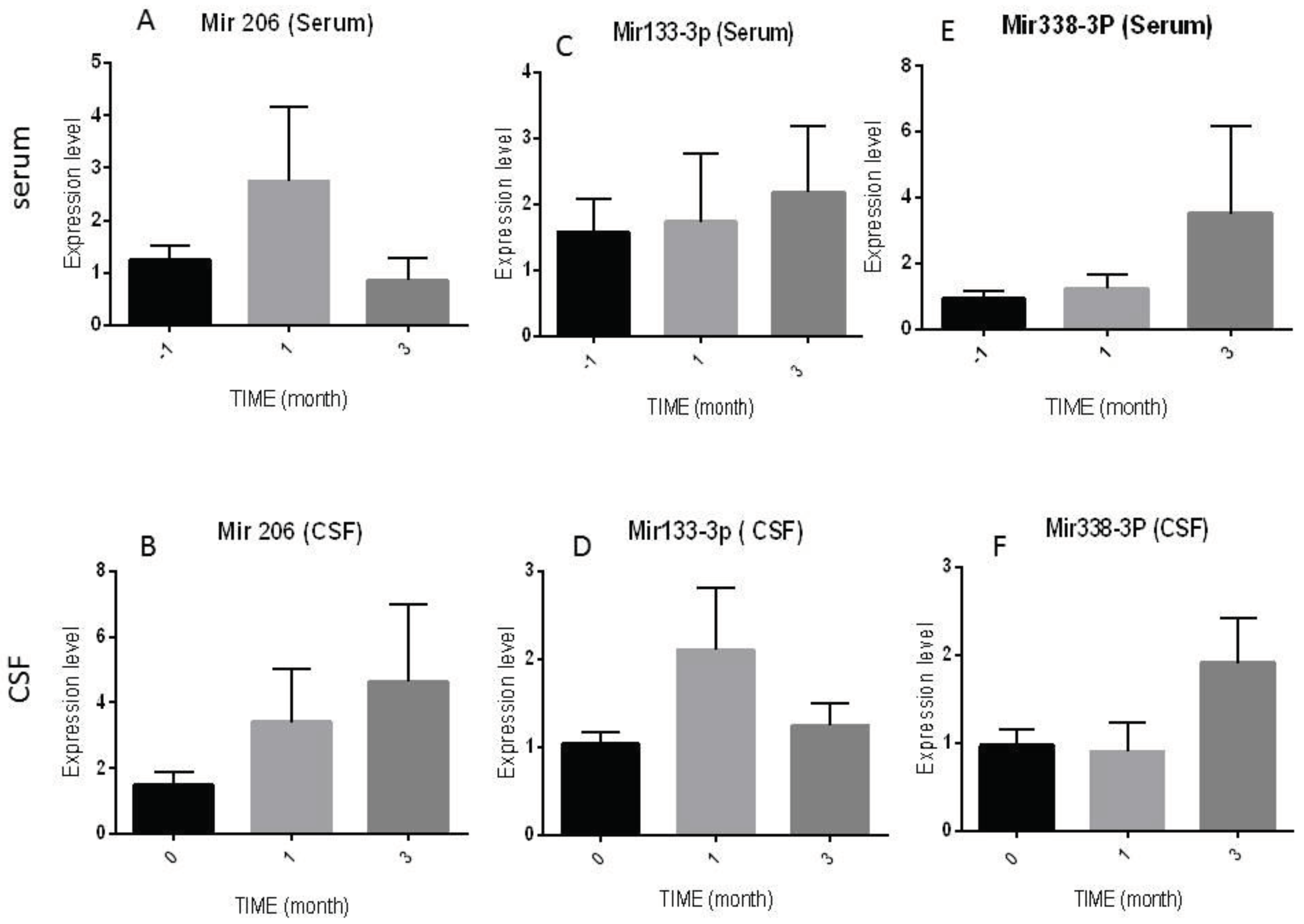
The expression of specific MicroRNAs (mir206, mir133a-3p, and mir338-3p) was measured three times in serum- (before (-1); month 1, and month 3), and three times in CSF- (month 0, month1, and 3) samples of the patients by RT -PCR technique and the results are as follows.
3.2.1. Mir206 Expression Levels
As shown in
Figure 2. A serum Mir 206 showed a non-significant increasing level after one month of injection (2.74±1.41) in comparison with month -1 (1.25±0.25, P=0.14) and month 3 (0.85±0.42, P= 0.08), respectively. However, a slow and insignificant increasing trend was observed in CSF Mir 206 at the end of the study (4.64±2.34, P= 0.33) in comparison with month 1 (1.94±0.57, P= 0.94) and month 3 (3.40±1.63, P=0.66) (
Figure 2B).
3.2.2. Mir 133a-3p Expression Levels
The expressed levels of Mir 133a-3p in serum increased significantly during the follow-up period. In detail, its level was (2.18±1.01) at the end of the 3 months, compared to before injection (1.57±0.49, P=0.53) and month 1 (1.73±1.04, P=0.72) (
Figure 2C).
On the other hand, as shown in (
Figure 2D), the expression levels of this Micro-RNA in the patient's CSF fluctuated between times of intervention, as at the end of the study was (1.28±0.28) in compression with months 0 (1.24 ± 0.26, P= 0.75), and 1 (2.10±0.71, P= 1.00).
3.2.3. Mir 338-3p Expression Levels
Equally in (
Figure 2E), a non-significant difference was noted in serum measurement of Mir 338-3p expression levels in patients before (0.91±0.24) and after one month (1.23±0.43) and three months (3.51 ±2.67, P= 0.20) of stem cell transplantation, respectively. In the CSF, similar results were observed in the constant level of mir338-3p expression in month 3 (1.91±0.52, P=0.59) in comparison with month 0 (0.965 ± 0.269) and month1 (0.985 ± 0. 197, P=0.41) after the intervention (
Figure 2F).
4. Discussion
In this study, we further estimate the effect of administering BM-MSCs in ALS patients by investigating some parameters, including measuring specific miRNA expression. They've found before, that concurrently administering BM-MSCs in ALS patients through IT and IV routes is safe, resulting in no serious adverse events. However, the temporary minor headache was recorded through treatment-related side events throughout the injections, which were eradicated rapidly with complementary therapies. The results also showed preliminary signs of clinical improvement, indicating stability, a non-significant decrease in ALSFRS, and a reciprocally significant increase in FVC values over a 3-month follow-up period[
16].
There are several types of miRNAs; one is myo- miRNAs, which include mir-1,133a, 133b, 206, 208a, and 208b, which are indicators of myogenesis and muscle regeneration[
18]. Several investigators studied these miRNAs due to their high accumulation in specific cells and tissues which can contribute to diagnosing neurodegenerative diseases in their early stages[
19]. In this course, the study of Pegoraro et al. that examined the various miRNAs related to muscle ( Mir-206, Mir-1, Mir-133a, and Mir-133b) in patients with ALS found an increase in the level of these miRNAs in the early stage of ALS disease when compared with cases in disease's end-stages or normal individuals, offering a unique diagnostic marker[
20]. In keeping with our results, the study by Sobuś, A. et al. showed an increased expression of Mir-206 in CSF of ALS patients after cell transplant compared to before cell administration[
21]. Mir-206 performs as a myo-miRNA that shows an essential role in the signalling regulation between skeletal muscle and motor neurons, promoting the formation of new muscle fibres. Down-regulation of Mir-206 in a mouse model led to faster disease deterioration due to skeletal muscle denervation, as published previously[
22]. In a clinical trial by Bartłomiej et al. 2020. manifestation of certain miRNAs in plasma and CSF in ALS patients who underwent cell transplantation before and after seven days showed increased levels of Mir-206 in CSF and not in plasma[
23]. The results of our study in this course align with the studies above, as the repeated transplantation of bone marrow mesenchymal stem cells led to a rise in the expressed levels of Mir-206 in both serum and CSF samples of patients. In more detail, in our study, the increase in the expression of this miRNA in the CSF samples was more than in serum samples. However, this increase was not statistically significant. Still, in the CSF samples, there was a continuously increasing trend, and in the serum samples, there was an increasing trend in the first month, which decreased non-significantly and remained stable during the study follow-up period. This increasing trend showed a direct correlation with improving patients' clinical symptoms and respiratory function which was measured by ALS-FRS and FVC, as mentioned earlier.
Another miRNA that acts as myo-specific is Mir-133, which was noted to increase in ALS and play a crucial role in protecting synapse function as Mir-206 [
24] and can serve as markers to a muscle problem in ALS patients [
25]; the results of an experimental study showed that Mir-133a promotes muscle stem cell proliferation, resulting in improved neuromuscular junction[
26]. Our study interpreted gradually increased for Mir133a-3p in serum, and there was the same for CSF samples overall.
Mir338-3p is another type of miRNA that enrolled in this study, which is a key player in numerous physiological processes and may participate in the pathogenesis of ALS[
27]. Many studies showed the role of mir 338 -3p in oxidative stress, one of the causes that could lead to ALS progression [
28]. The study of De Felice Bruna. et al. noted the increased level of Mir338-3p in different samples of ALS: serum, CSF, and blood leukocyte. This type of mir could be a prominent factor in developing sporadic ALS[
29]. Another study in SOD1G93A mice showed an increased level in the spinal cord of Mir338-3p, which led to affecting decreasing glycogen phosphorylase brain form (PYGB ); this will lead to increase of glycogen in the spinal cord lumber in mice models with ALS[
28]. In our result, this miR increased non-significantly in patients following stem cell transplantation, suggesting protective inhibitory effects of stem cells in rapid disease progression.
There are certain limitations to this study. Due to the small patient numbers in this clinical trial, additional research is required to validate our results through increasing sample size. Furthermore, pre- and post-study monitoring periods and considering control groups will help better discover therapeutic mechanisms.
5. Conclusions
In conclusion, to sum up, the clinical study results displayed that concurrently (IV and IT) repeated transplantation of BM-MSCs in ALS patients is a safe procedure leading to delay in disease progression and improvement in clinical symptoms. Also, the molecular data evaluating specific microRNAs (Mir 206,133a-3p and 338-3p) demonstrated a close connection with clinical improvement and patient respiratory functions. Certainly, more trials with greater sample sizes and elongated follow-up periods are wanted to settle these results.
Author Contributions
Conceptualization of this draft, J.T.A; Z.A.A; original draft writing, Z.A.A; J.T.A.; review and editing, S.S.N; A.R.B; investigation, Z.A.A; J.T.A; data curation, Z.A.A.; N.K.F; M.A.K; G.R. All authors read and agree to published this draft.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was achieved by the rules and morals of the Helsinki Medical Principle of Clinical Practice and under the direction of the Ethics Committee of the Mashhad University of Medical Sciences, Mashhad, Iran. (Reg. No. IR.MUMS.REC.1399.269). Furthermore, this clinical trial was registered with the Iranian Organization for Clinical Trials (ID: IRCT20160809029275N2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that used in this study can be requested from the corre-sponding author.
Acknowledgments
We thank all employees and patients who helped complete this investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic Lateral Sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Ajroud-Driss, S.; Siddique, T. Sporadic and Hereditary Amyotrophic Lateral Sclerosis (ALS). Biochim. Biophys. Acta (BBA)-Molecular Basis Dis. 2015, 1852, 679–684. [Google Scholar] [CrossRef]
- Benatar, M.; Boylan, K.; Jeromin, A.; Rutkove, S.B.; Berry, J.; Atassi, N.; Bruijn, L. ALS Biomarkers for Therapy Development: State of the Field and Future Directions. Muscle Nerve 2016, 53, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Bowser, R.; Bruijn, L.; Dupuis, L.; Ludolph, A.; McGrath, M.; Manfredi, G.; Maragakis, N.; Miller, R.G.; Pullman, S.L. Mechanisms, Models and Biomarkers in Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 19–32. [Google Scholar] [CrossRef]
- Ravnik-Glavač, M.; Glavač, D. Circulating RNAs as Potential Biomarkers in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 1714. [Google Scholar] [CrossRef]
- Quinlan, S.; Kenny, A.; Medina, M.; Engel, T.; Jimenez-Mateos, E.M. MicroRNAs in Neurodegenerative Diseases. Int. Rev. Cell Mol. Biol. 2017, 334, 309–343. [Google Scholar] [CrossRef]
- Gascon, E.; Gao, F.-B. Cause or Effect: Misregulation of MicroRNA Pathways in Neurodegeneration. Front. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Marzocchi, C.; Battistini, S. MicroRNAs as Biomarkers in Amyotrophic Lateral Sclerosis. Cells 2018, 7, 219. [Google Scholar] [CrossRef]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sorarù, G.; Ferrari, D.; Copetti, M.; Muzi, G.; Ricciolini, C.; Carletti, S.; Giorgi, C. Results from Phase I Clinical Trial with Intraspinal Injection of Neural Stem Cells in Amyotrophic Lateral Sclerosis: A Long-Term Outcome. Stem Cells Transl. Med. 2019, 8, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Najafi, P.; Farkhad, N.K.; Torshizi, G.H.; Darban, R.A.; Boroumand, A.R.; Sahab-Negah, S.; Khodadoust, M.A.; Tavakol-Afshari, J. Mesenchymal Stem Cell Therapy in Amyotrophic Lateral Sclerosis (ALS) Patients: A Comprehensive Review of Disease Information and Future Perspectives. Iran. J. Basic Med. Sci. 2023, 26, 872. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, P.; Wang, X.; Dai, G.; Cheng, H.; Zhang, Z.; Hua, R.; Niu, X.; Shi, J.; An, Y. A Preliminary Evaluation of Efficacy and Safety of Wharton’s Jelly Mesenchymal Stem Cell Transplantation in Patients with Type 2 Diabetes Mellitus. Stem Cell Res. Ther. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, N.K.; Kumar, S.K. V; Balaraju, S.; Radhakrishnan, R.C.; Bansal, A.; Dixit, A.; Rao, D.K.; Das, M.; Jan, M.; Gupta, P.K. Open-Labeled Study of Unilateral Autologous Bone-Marrow-Derived Mesenchymal Stem Cell Transplantation in Parkinson’s Disease. Transl. Res. 2010, 155, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Noh, M.; Kwon, M.; Kim, H.Y.; Oh, S.; Park, J.; Kim, H.; Ki, C.; Kim, S.H. Repeated Intrathecal Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis. Ann. Neurol. 2018, 84, 361–373. [Google Scholar] [CrossRef]
- Sykova, E.; Cizkova, D.; Kubinova, S. Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis. Front. cell Dev. Biol. 2021, 9, 695900. [Google Scholar] [CrossRef] [PubMed]
- Zalfa, C.; Rota Nodari, L.; Vacchi, E.; Gelati, M.; Profico, D.; Boido, M.; Binda, E.; De Filippis, L.; Copetti, M.; Garlatti, V. Transplantation of Clinical-Grade Human Neural Stem Cells Reduces Neuroinflammation, Prolongs Survival and Delays Disease Progression in the SOD1 Rats. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tavakol-Afshari, J.; Boroumand, A.R.; Farkhad, N.K.; Moghadam, A.A.; Sahab-Negah, S.; Gorji, A. Safety and Efficacy of Bone Marrow Derived-Mesenchymal Stem Cells Transplantation in Patients with Amyotrophic Lateral Sclerosis. Regen. Ther. 2021, 18, 268–274. [Google Scholar] [CrossRef]
- Hemmatian, H.; Bakker, A.D.; Klein-Nulend, J.; van Lenthe, G.H. Aging, Osteocytes, and Mechanotransduction. Curr. Osteoporos. Rep. 2017, 15, 401–411. [Google Scholar] [CrossRef]
- Giagnorio, E.; Malacarne, C.; Mantegazza, R.; Bonanno, S.; Marcuzzo, S. MyomiRs and Their Multifaceted Regulatory Roles in Muscle Homeostasis and Amyotrophic Lateral Sclerosis. J. Cell Sci. 2021, 134, jcs258349. [Google Scholar] [CrossRef]
- Dube, U.; Del-Aguila, J.L.; Li, Z.; Budde, J.P.; Jiang, S.; Hsu, S.; Ibanez, L.; Fernandez, M.V.; Farias, F.; Norton, J. An Atlas of Cortical Circular RNA Expression in Alzheimer Disease Brains Demonstrates Clinical and Pathological Associations. Nat. Neurosci. 2019, 22, 1903–1912. [Google Scholar] [CrossRef]
- Pegoraro, V.; Merico, A.; Angelini, C. Micro-RNAs in ALS Muscle: Differences in Gender, Age at Onset and Disease Duration. J. Neurol. Sci. 2017, 380, 58–63. [Google Scholar] [CrossRef]
- Sobuś, A.; Baumert, B.; Litwińska, Z.; Gołąb-Janowska, M.; Stępniewski, J.; Kotowski, M.; Pius-Sadowska, E.; Kawa, M.P.; Gródecka-Szwajkiewicz, D.; Peregud-Pogorzelski, J. Safety and Feasibility of Lin-Cells Administration to ALS Patients: A Novel View on Humoral Factors and MiRNA Profiles. Int. J. Mol. Sci. 2018, 19, 1312. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice. Science (80-) 2009, 326, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.; Sobuś, A.; Gołąb-Janowska, M.; Ulańczyk, Z.; Paczkowska, E.; Łuczkowska, K.; Zawiślak, A.; Milczarek, S.; Osękowska, B.; Meller, A. Local and Systemic Humoral Response to Autologous Lineage-Negative Cells Intrathecal Administration in ALS Patients. Int. J. Mol. Sci. 2020, 21, 1070. [Google Scholar] [CrossRef] [PubMed]
- Tasca, E.; Pegoraro, V.; Merico, A.; Angelini, C. Circulating MicroRNAs as Biomarkers of Muscle Differentiation and Atrophy in ALS. Clin. Neuropathol 2016, 35, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Raheja, R.; Regev, K.; Healy, B.C.; Mazzola, M.A.; Beynon, V.; Von Glehn, F.; Paul, A.; Diaz-Cruz, C.; Gholipour, T.; Glanz, B.I. Correlating Serum Micrornas and Clinical Parameters in Amyotrophic Lateral Sclerosis. Muscle Nerve 2018, 58, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, C.; Galbiati, M.; Giagnorio, E.; Cavalcante, P.; Salerno, F.; Andreetta, F.; Cagnoli, C.; Taiana, M.; Nizzardo, M.; Corti, S. Dysregulation of Muscle-Specific MicroRNAs as Common Pathogenic Feature Associated with Muscle Atrophy in ALS, SMA and SBMA: Evidence from Animal Models and Human Patients. Int. J. Mol. Sci. 2021, 22, 5673. [Google Scholar] [CrossRef] [PubMed]
- Daneshafrooz, N.; Joghataei, M.T.; Mehdizadeh, M.; Alavi, A.; Barati, M.; Panahi, B.; Teimourian, S.; Zamani, B. Identification of Let-7f and MiR-338 as Plasma-Based Biomarkers for Sporadic Amyotrophic Lateral Sclerosis Using Meta-Analysis and Empirical Validation. Sci. Rep. 2022, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, Q.; Gu, X.; Chen, Y.; Chen, X.; Cao, B.; Ou, R.; Shang, H. Decreased Glycogenolysis by MiR-338-3p Promotes Regional Glycogen Accumulation within the Spinal Cord of Amyotrophic Lateral Sclerosis Mice. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- De Felice, B.; Annunziata, A.; Fiorentino, G.; Borra, M.; Biffali, E.; Coppola, C.; Cotrufo, R.; Brettschneider, J.; Giordana, M.L.; Dalmay, T. MiR-338-3p Is over-Expressed in Blood, CFS, Serum and Spinal Cord from Sporadic Amyotrophic Lateral Sclerosis Patients. Neurogenetics 2014, 15, 243–253. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).