Submitted:
31 March 2024
Posted:
02 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Oxidative Potential of Particulate Matter
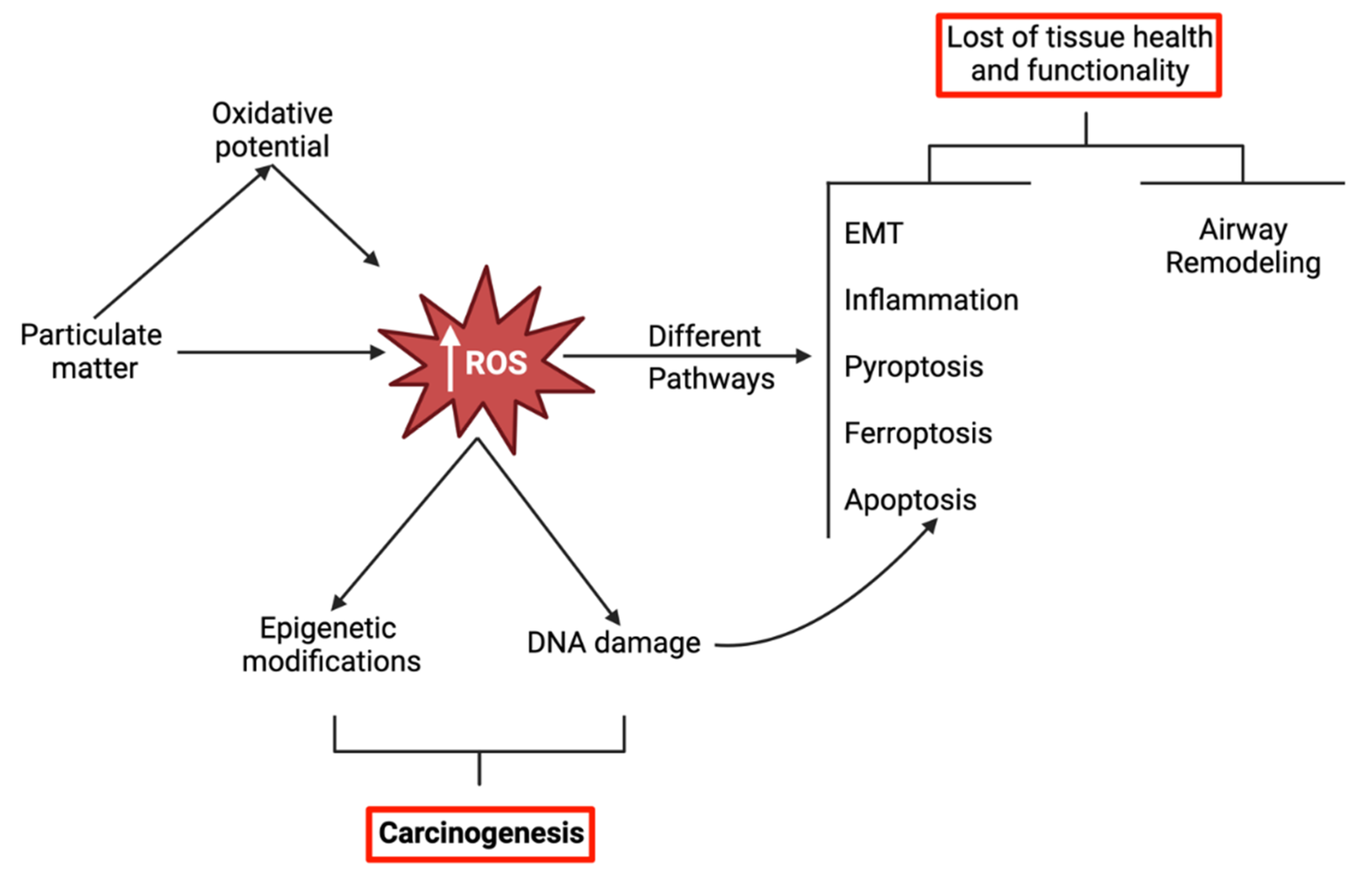
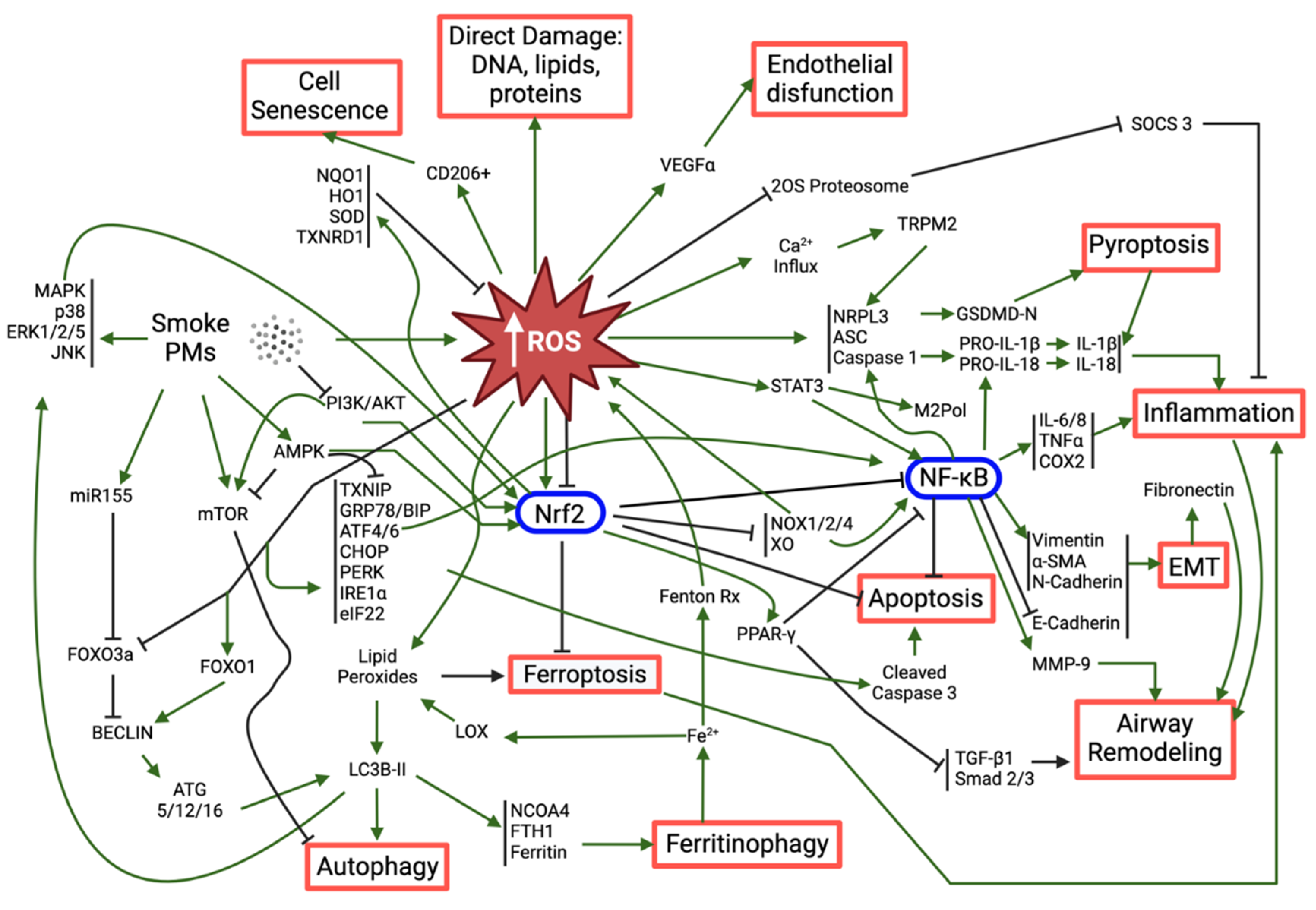
3. Mechanisms of PM-Induced Oxidative Stress-Related Respiratory Toxicity
3.1. Nrf2: the Master Regulator of Antioxidant Response
3.2. Activation of the Inflammatory Response and Its Consequences
3.3. Other Relevant Mechanisms Triggered by PM-Induced Oxidative Stress
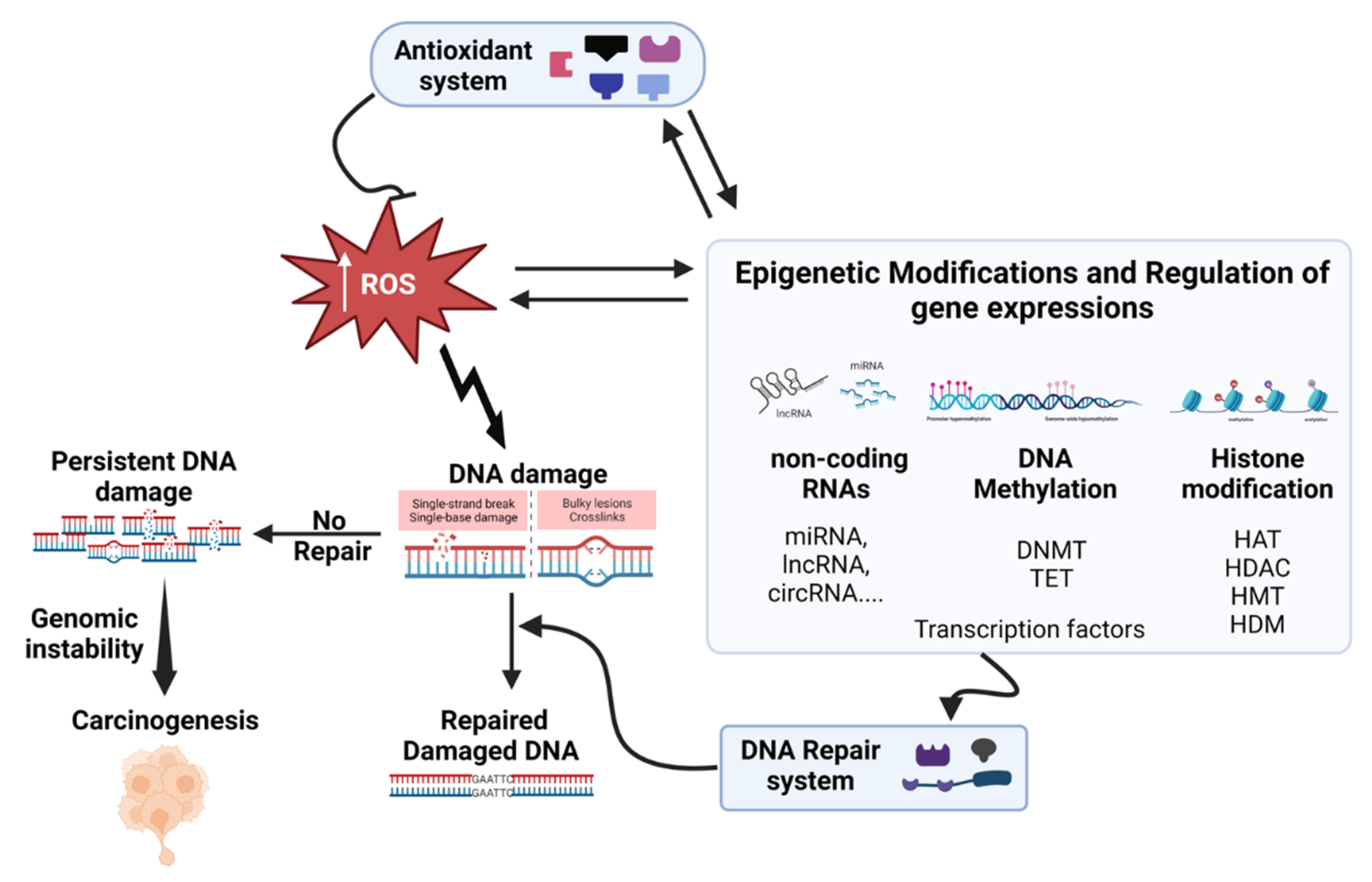
4. Oxidative Stress and DNA Damage-Repair
5. Oxidative Stress and Carcinogenicity
6. Oxidative Stress and Epigenetics Interplay
7. Compounds with Antioxidant Activity against PM-Induced Oxidative Stress
8. Perspectives and Conclusions
Supplementary Materials
Acknowledgements
References
- Albino AP, Huang X, Jorgensen ED, et al. Induction of DNA Double-Strand Breaks in A549 and Normal Human Pulmonary Epithelial Cells by Cigarette Smoke Is Mediated by Free Radicals. Int J Oncol 2006;28(6):1491–1505.
- Badran G, Verdin A, Grare C, et al. Toxicological Appraisal of the Chemical Fractions of Ambient Fine (PM2.5-0.3) and Quasi-Ultrafine (PM0.3) Particles in Human Bronchial Epithelial BEAS-2B Cells. Environmental Pollution 2020;263. [CrossRef]
- Bagam P, Kaur G, Singh DP, et al. In Vitro Study of the Role of FOXO Transcription Factors in Regulating Cigarette Smoke Extract-Induced Autophagy. Cell Biol Toxicol 2021;37(4):531–553. [CrossRef]
- Barzgar F, Sadeghi-Mohammadi S, Aftabi Y, et al. Oxidative Stress Indices Induced by Industrial and Urban PM2.5-Bound Metals in A549 Cells. Science of the Total Environment 2023;877. [CrossRef]
- Baskara I, Kerbrat S, Dagouassat M, et al. Cigarette Smoking Induces Human CCR6+Th17 Lymphocytes Senescence and VEGF-A Secretion. Sci Rep 2020;10(1). [CrossRef]
- Bhat A V, Hora S, Pal A, et al. Stressing the (Epi)Genome: Dealing with Reactive Oxygen Species in Cancer. Antioxid Redox Signal 2018;29(13):1273–1292. [CrossRef]
- Bongaerts E, Mamia K, Rooda I, et al. Ambient Black Carbon Particles in Human Ovarian Tissue and Follicular Fluid. Environ Int 2023;179:108141. [CrossRef]
- Cadet J, Davies KJA, Medeiros MH, et al. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic Biol Med 2017;107:13–34. [CrossRef]
- Calas A, Uzu G, Kelly FJ, et al. Comparison between Five Acellular Oxidative Potential Measurement Assays Performed with Detailed Chemistry on PM≪Sub≫10≪/Sub≫ Samples from the City of Chamonix (France). Atmos Chem Phys 2018;18(11):7863–7875. [CrossRef]
- Calderón-Garcidueñas L, Torres-Jardón R, Greenough GP, et al. Sleep Matters: Neurodegeneration Spectrum Heterogeneity, Combustion and Friction Ultrafine Particles, Industrial Nanoparticle Pollution, and Sleep Disorders—Denial Is Not an Option. Front Neurol 2023;14. [CrossRef]
- Campbell SJ, Utinger B, Lienhard DM, et al. Development of a Physiologically Relevant Online Chemical Assay To Quantify Aerosol Oxidative Potential. Anal Chem 2019;91(20):13088–13095. [CrossRef]
- Campbell SJ, Wolfer K, Utinger B, et al. Atmospheric Conditions and Composition That Influence PM≪Sub≫2.5≪/Sub≫ Oxidative Potential in Beijing, China. Atmos Chem Phys 2021a;21(7):5549–5573. [CrossRef]
- Campbell SJ, Wolfer K, Utinger B, et al. Atmospheric Conditions and Composition That Influence PM≪Sub≫2.5≪/Sub≫ Oxidative Potential in Beijing, China. Atmos Chem Phys 2021b;21(7):5549–5573. [CrossRef]
- Cao X, Padoan S, Binder S, et al. A Comparative Study of Persistent DNA Oxidation and Chromosomal Instability Induced in Vitro by Oxidizers and Reference Airborne Particles. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2022;874–875:503446. [CrossRef]
- Chen YC, Chuang TY, Liu CW, et al. Particulate Matters Increase Epithelial-Mesenchymal Transition and Lung Fibrosis through the ETS-1/NF-ΚB-Dependent Pathway in Lung Epithelial Cells. Part Fibre Toxicol 2020;17(1). [CrossRef]
- Cho C-C, Hsieh W-Y, Tsai C-H, et al. In Vitro and In Vivo Experimental Studies of PM2.5 on Disease Progression. Int J Environ Res Public Health 2018;15(7):1380. [CrossRef]
- Costabile F, Gualtieri M, Rinaldi M, et al. Exposure to Urban Nanoparticles at Low PM$$_1$$ Concentrations as a Source of Oxidative Stress and Inflammation. Sci Rep 2023;13(1):18616. [CrossRef]
- Coyle JP, Derk RC, Kornberg TG, et al. Carbon Nanotube Filler Enhances Incinerated Thermoplastics-Induced Cytotoxicity and Metabolic Disruption in Vitro. Part Fibre Toxicol 2020;17(1). [CrossRef]
- Crobeddu B, Aragao-Santiago L, Bui L-C, et al. Oxidative Potential of Particulate Matter 2.5 as Predictive Indicator of Cellular Stress. Environmental Pollution 2017;230:125–133. [CrossRef]
- Crobeddu B, Baudrimont I, Deweirdt J, et al. Lung Antioxidant Depletion: A Predictive Indicator of Cellular Stress Induced by Ambient Fine Particles. Environ Sci Technol 2020;54(4):2360–2369. [CrossRef]
- Cui J, Zhao W, Xu X, et al. DNA Polymerase Beta Is Involved in the Protection against the Cytotoxicity and Genotoxicity of Cigarette Smoke. Environ Toxicol Pharmacol 2012;34(2):370–380. [CrossRef]
- Cui X, Zhang Y, Lu Y, et al. ROS and Endoplasmic Reticulum Stress in Pulmonary Disease. Front Pharmacol 2022;13. [CrossRef]
- Danielsen PH, Møller P, Jensen KA, et al. Oxidative Stress, DNA Damage, and Inflammation Induced by Ambient Air and Wood Smoke Particulate Matter in Human A549 and THP-1 Cell Lines. Chem Res Toxicol 2011;24(2):168–184. [CrossRef]
- Du H, Sun J, Chen Z, et al. Cigarette Smoke-Induced Failure of Apoptosis Resulting in Enhanced Neoplastic Transformation in Human Bronchial Epithelial Cells. J Toxicol Environ Health A 2012;75(12):707–720. [CrossRef]
- Eiguren-Fernandez A, Kreisberg N and Hering S. An Online Monitor of the Oxidative Capacity of Aerosols (o-MOCA). Atmos Meas Tech 2017;10(2):633–644. [CrossRef]
- Fernando P, Piao MJ, Zhen AX, et al. Extract of Cornus Officinalis Protects Keratinocytes from Particulate Matter-Induced Oxidative Stress. Int J Med Sci 2020;17(1):63–70. [CrossRef]
- Figliuzzi M, Tironi M, Longaretti L, et al. Copper-Dependent Biological Effects of Particulate Matter Produced by Brake Systems on Lung Alveolar Cells. Arch Toxicol 2020;94(9):2965–2979. [CrossRef]
- Fu Y, Li B, Yun J, et al. LncRNA SOX2-OT CeRNA Network Enhances the Malignancy of Long-Term PM(2.5)-Exposed Human Bronchial Epithelia. Ecotoxicol Environ Saf 2021;217:112242. [CrossRef]
- Gao Y, Huang X, Lin H, et al. Adipose Mesenchymal Stem Cell-Derived Antioxidative Extracellular Vesicles Exhibit Anti-Oxidative Stress and Immunomodulatory Effects under PM2.5 Exposure. Toxicology 2021;447. [CrossRef]
- Gao ZX, Song XL, Li SS, et al. Assessment of DNA Damage and Cell Senescence in Corneal Epithelial Cells Exposed to Airborne Particulate Matter (PM2.5) Collected in Guangzhou, China. Invest Ophthalmol Vis Sci 2016;57(7):3093–3102. [CrossRef]
- Gilmour PS, Brown DM, Lindsay TG, et al. Adverse Health Effects of PM10 Particles: Involvement of Iron in Generation of Hydroxyl Radical. Occup Environ Med 1996;53(12):817–822. [CrossRef]
- Gilmour PS, Rahman I, Donaldson K, et al. Histone Acetylation Regulates Epithelial IL-8 Release Mediated by Oxidative Stress from Environmental Particles. Am J Physiol Lung Cell Mol Physiol 2003;284(3):L533-40. [CrossRef]
- Goshua A, Akdis CA and Nadeau KC. World Health Organization Global Air Quality Guideline Recommendations: Executive Summary. Allergy 2022;77(7):1955–1960. [CrossRef]
- Guan M, Tang S, Chang H, et al. Development of Alveolar-Capillary-Exchange (ACE) Chip and Its Application for Assessment of PM2.5-Induced Toxicity. Ecotoxicol Environ Saf 2021;223. [CrossRef]
- Guerra e Oliveira T, Trancoso IA, Lorençoni MF, et al. Toxicological Effects of Air Settled Particles from the Vitoria Metropolitan Area Mediated by Oxidative Stress, pro-Inflammatory Mediators and NFΚB Pathway. Environ Res 2022;204. [CrossRef]
- Guillaumet-Adkins A, Yañez Y, Peris-Diaz MD, et al. Epigenetics and Oxidative Stress in Aging. Oxid Med Cell Longev 2017;2017:9175806. [CrossRef]
- Haggadone MD, Mancuso P and Peters-Golden M. Oxidative Inactivation of the Proteasome Augments Alveolar Macrophage Secretion of Vesicular SOCS3. Cells 2020;9(7). [CrossRef]
- Hanahan D and Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell 2011;144(5):646–674. [CrossRef]
- Haoran D, Xuefang L, Wanchun Z, et al. Three Tiaobu Feishen Formulae Reduces Cigarette Smoke-Induced in-Flammation in Human Airway Epithelial Cells. J Tradit Chin Med 2020;40(3):386–392.
- Hartwig A. Role of DNA Repair in Particle- and Fiber-Induced Lung Injury. Inhal Toxicol 2002;14(1):91–100. [CrossRef]
- Hayes P and Knaus UG. Balancing Reactive Oxygen Species in the Epigenome: NADPH Oxidases as Target and Perpetrator. Antioxid Redox Signal 2013;18(15):1937–1945. [CrossRef]
- He J and Jiang BH. Interplay between Reactive Oxygen Species and MicroRNAs in Cancer. Curr Pharmacol Rep 2016;2(2):82–90. [CrossRef]
- Herath H, Piao MJ, Kang KA, et al. Hesperidin Exhibits Protective Effects against PM(2.5)-Mediated Mitochondrial Damage, Cell Cycle Arrest, and Cellular Senescence in Human HaCaT Keratinocytes. Molecules 2022;27(15). [CrossRef]
- Van Den Heuvel R, Den Hond E, Govarts E, et al. Identification of PM10 Characteristics Involved in Cellular Responses in Human Bronchial Epithelial Cells (Beas-2B). Environ Res 2016;149:48–56. [CrossRef]
- Housseiny H Al, Singh M, Emile S, et al. Identification of Toxicity Parameters Associated with Combustion Produced Soot Surface Chemistry and Particle Structure by in Vitro Assays. Biomedicines 2020;8(9). [CrossRef]
- Hrelia S and Angeloni C. New Mechanisms of Action of Natural Antioxidants in Health and Disease II. Antioxidants 2021;10(8):1200. [CrossRef]
- Huang H, Ji Y, Zhang J, et al. Aberrant DNA Methylation in Radon and/or Cigarette Smoke-Induced Malignant Transformation in BEAS-2B Human Lung Cell Line. J Toxicol Environ Health A 2017;80(23–24):1321–1330. [CrossRef]
- Huang W, Zhang Y, Zhang Y, et al. Development of an Automated Sampling-Analysis System for Simultaneous Measurement of Reactive Oxygen Species (ROS) in Gas and Particle Phases: GAC-ROS. Atmos Environ 2016;134:18–26. [CrossRef]
- Hunyadi A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med Res Rev 2019;39(6):2505–2533. [CrossRef]
- Ito Y, Oshinden K, Kutsuzawa N, et al. Heat-Not-Burn Cigarette Induces Oxidative Stress Response in Primary Rat Alveolar Epithelial Cells. PLoS One 2020;15(11 November). [CrossRef]
- Jang JH, Lee JH, Chand HS, et al. APO-9’-Fucoxanthinone Extracted from Undariopsis Peteseniana Protects Oxidative Stress-Mediated Apoptosis in Cigarette Smoke-Exposed Human Airway Epithelial Cells. Mar Drugs 2016;14(7). [CrossRef]
- Janssen BG, Madlhoum N, Gyselaers W, et al. Cohort Profile: The ENVIRonmental Influence ON Early AGEing (ENVIR ON AGE): A Birth Cohort Study. Int J Epidemiol 2017;dyw269. [CrossRef]
- Jantzen K, Roursgaard M, Desler C, et al. Oxidative Damage to DNA by Diesel Exhaust Particle Exposure in Co-Cultures of Human Lung Epithelial Cells and Macrophages. Mutagenesis 2012;27(6):693–701. [CrossRef]
- Ji D, Hu C, Ning J, et al. N(6)-Methyladenosine Mediates Nrf2 Protein Expression Involved in PM2.5-Induced Pulmonary Fibrosis. Ecotoxicol Environ Saf 2023;254:114755. [CrossRef]
- Jiang, Ahmed, Canchola, et al. Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols. Atmosphere (Basel) 2019;10(10):571. [CrossRef]
- Jiang P, Hao S, Xie L, et al. LncRNA NEAT1 Contributes to the Acquisition of a Tumor Like-Phenotype Induced by PM 2.5 in Lung Bronchial Epithelial Cells via HIF-1α Activation. Environ Sci Pollut Res Int 2021;28(32):43382–43393. [CrossRef]
- Jin S, Yoon SJ, Jung NY, et al. Antioxidants Prevent Particulate Matter-Induced Senescence of Lung Fibroblasts. Heliyon 2023;9(3):e14179. [CrossRef]
- Kietzmann T, Petry A, Shvetsova A, et al. The Epigenetic Landscape Related to Reactive Oxygen Species Formation in the Cardiovascular System. Br J Pharmacol 2017;174(12):1533–1554. [CrossRef]
- Kodali V, Afshari A, Meighan T, et al. In Vivo and in Vitro Toxicity of a Stainless-Steel Aerosol Generated during Thermal Spray Coating. Arch Toxicol 2022;96(12):3201–3217. [CrossRef]
- Kryston TB, Georgiev AB, Pissis P, et al. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutat Res 2011;711(1–2):193–201. [CrossRef]
- Lee DH, Woo JK, Heo W, et al. Citrus Junos Tanaka Peel Extract and Its Bioactive Naringin Reduce Fine Dust-Induced Respiratory Injury Markers in BALB/c Male Mice. Nutrients 2022a;14(5). [CrossRef]
- Lee JY, Lee WK and Kim DS. Particulate Matter-Induced Hypomethylation of Alu and LINE1 in Normal Human Bronchial Epithelial Cells and Epidermal Keratinocytes. Genes Environ 2022b;44(1):8. [CrossRef]
- Lee KY, Ho SC, Sun WL, et al. Lnc-IL7R Alleviates PM(2.5)-Mediated Cellular Senescence and Apoptosis through EZH2 Recruitment in Chronic Obstructive Pulmonary Disease. Cell Biol Toxicol 2022c;38(6):1097–1120. [CrossRef]
- Li H, Zhao Z, Luo XS, et al. Insight into Urban PM2.5 Chemical Composition and Environmentally Persistent Free Radicals Attributed Human Lung Epithelial Cytotoxicity. Ecotoxicol Environ Saf 2022;234. [CrossRef]
- Li J, Wang J, Li Y, et al. Effective-Component Compatibility of Bufei Yishen Formula Protects COPD Rats against PM2.5-Induced Oxidative Stress via MiR-155/FOXO3a Pathway. Ecotoxicol Environ Saf 2021;228. [CrossRef]
- Li J, Zhou Q, Liang Y, et al. MiR-486 Inhibits PM2.5-Induced Apoptosis and Oxidative Stress in Human Lung Alveolar Epithelial A549 Cells. Ann Transl Med 2018;6(11):209. [CrossRef]
- Li YJ, Yu CH, Li JB, et al. Andrographolide Antagonizes Cigarette Smoke Extract-Induced Inflammatory Response and Oxidative Stress in Human Alveolar Epithelial A549 Cells through Induction of MicroRNA-218. Exp Lung Res 2013;39(10):463–471. [CrossRef]
- Lin Y-H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int J Mol Sci 2019;20(18):4497.
- Liu H, Nie H, Lai W, et al. Different Exposure Modes of PM2.5 Induces Bronchial Asthma and Fibrosis in Male Rats through Macrophage Activation and Immune Imbalance Induced by TIPE2 Methylation. Ecotoxicol Environ Saf 2022a;247. [CrossRef]
- Liu J, Guo Z-N, Yan X-L, et al. Crosstalk Between Autophagy and Ferroptosis and Its Putative Role in Ischemic Stroke. Front Cell Neurosci 2020a;14. [CrossRef]
- Liu J, Kuang F, Kroemer G, et al. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem Biol 2020b;27(4):420–435. [CrossRef]
- Liu L, Shi Q, Wang K, et al. Fibroblast Growth Factor 10 Protects against Particulate Matter-Induced Lung Injury by Inhibiting Oxidative Stress-Mediated Pyroptosis via the PI3K/Akt/Nrf2 Signaling Pathway. Int Immunopharmacol 2022b;113. [CrossRef]
- Liu T, Zhang L, Joo D, et al. NF-ΚB Signaling in Inflammation. Signal Transduct Target Ther 2017;2. [CrossRef]
- Liu Y, Luo F, Xu Y, et al. Epithelial-Mesenchymal Transition and Cancer Stem Cells, Mediated by a Long Non-Coding RNA, HOTAIR, Are Involved in Cell Malignant Transformation Induced by Cigarette Smoke Extract. Toxicol Appl Pharmacol 2015;282(1):9–19. [CrossRef]
- LOGAN W. MORTALITY IN THE LONDON FOG INCIDENT, 1952. The Lancet 1953;261(6755):336–338. [CrossRef]
- Lu L, Luo F, Liu Y, et al. Posttranscriptional Silencing of the LncRNA MALAT1 by MiR-217 Inhibits the Epithelial-Mesenchymal Transition via Enhancer of Zeste Homolog 2 in the Malignant Transformation of HBE Cells Induced by Cigarette Smoke Extract. Toxicol Appl Pharmacol 2015;289(2):276–285. [CrossRef]
- Lu L, Xu H, Luo F, et al. Epigenetic Silencing of MiR-218 by the LncRNA CCAT1, Acting via BMI1, Promotes an Altered Cell Cycle Transition in the Malignant Transformation of HBE Cells Induced by Cigarette Smoke Extract. Toxicol Appl Pharmacol 2016;304:30–41. [CrossRef]
- Luo J, Li L, Hu D, et al. LINC00612/MiR-31-5p/Notch1 Axis Regulates Apoptosis, Inflammation, and Oxidative Stress in Human Pulmonary Microvascular Endothelial Cells Induced by Cigarette Smoke Extract. Int J Chron Obstruct Pulmon Dis 2020;15:2049–2060. [CrossRef]
- Lushchak VI and Storey KB. Oxidative Stress Concept Updated: Definitions, Classifications, and Regulatory Pathways Implicated. EXCLI J 2021;20:956–967. [CrossRef]
- Lv XJ, Du YW, Hao YQ, et al. RNA-Binding Motif Protein 5 Inhibits the Proliferation of Cigarette Smoke-Transformed BEAS-2B Cells through Cell Cycle Arrest and Apoptosis. Oncol Rep 2016;35(4):2315–2327. [CrossRef]
- Mahalanobish S, Dutta S, Saha S, et al. Melatonin Induced Suppression of ER Stress and Mitochondrial Dysfunction Inhibited NLRP3 Inflammasome Activation in COPD Mice. Food and Chemical Toxicology 2020;144. [CrossRef]
- Marchetti S, Gualtieri M, Pozzer A, et al. On Fine Particulate Matter and COVID-19 Spread and Severity: An in Vitro Toxicological Plausible Mechanism. Environ Int 2023;179:108131. [CrossRef]
- Marques dos Santos M, Tan Pei Fei M, Li C, et al. Cell-Line and Culture Model Specific Responses to Organic Contaminants in House Dust: Cell Bioenergetics, Oxidative Stress, and Inflammation Endpoints. Environ Int 2022;167. [CrossRef]
- Martikainen MV, Tossavainen T, Täubel M, et al. Toxicological and Microbiological Characterization of Cow Stable Dust. Toxicology in Vitro 2021;75. [CrossRef]
- Meganathan V, Hamilton CE, Natarajan K, et al. NADPH and Xanthine Oxidases Control Induction of Inflammatory Mediator Expression by Organic Dust in the Lung. The FASEB Journal 2022;36(7). [CrossRef]
- Millar MW, Fazal F and Rahman A. Therapeutic Targeting of NF-ΚB in Acute Lung Injury: A Double-Edged Sword. Cells 2022;11(20):3317. [CrossRef]
- Ngo V and Duennwald ML. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022;11(12):2345. [CrossRef]
- Niu BY, Li WK, Li JS, et al. Effects of Dna Damage and Oxidative Stress in Human Bronchial Epithelial Cells Exposed to Pm2.5 from Beijing, China, in Winter. Int J Environ Res Public Health 2020a;17(13):1–14. [CrossRef]
- Niu BY, Li WK, Li JS, et al. Effects of Dna Damage and Oxidative Stress in Human Bronchial Epithelial Cells Exposed to Pm2.5 from Beijing, China, in Winter. Int J Environ Res Public Health 2020b;17(13):1–14. [CrossRef]
- Oberdörster G, Oberdörster E and Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ Health Perspect 2005a;113(7):823–839. [CrossRef]
- Oberdörster G, Oberdörster E and Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ Health Perspect 2005b;113(7):823–839. [CrossRef]
- Pan K, Lu J and Song Y. Artesunate Ameliorates Cigarette Smoke-Induced Airway Remodelling via PPAR-γ/TGF-Β1/Smad2/3 Signalling Pathway. Respir Res 2021;22(1). [CrossRef]
- Pang Y, Huang W, Luo XS, et al. In-Vitro Human Lung Cell Injuries Induced by Urban PM2.5 during a Severe Air Pollution Episode: Variations Associated with Particle Components. Ecotoxicol Environ Saf 2020;206. [CrossRef]
- Pradhan SH, Gibb M, Kramer AT, et al. Peripheral (Lung-to-Brain) Exposure to Diesel Particulate Matter Induces Oxidative Stress and Increased Markers for Systemic Inflammation. Environ Res 2023;231. [CrossRef]
- Prunicki M, Cauwenberghs N, Ataam JA, et al. Immune Biomarkers Link Air Pollution Exposure to Blood Pressure in Adolescents. Environ Health 2020;19(1). [CrossRef]
- Puthussery J V., Zhang C and Verma V. Development and Field Testing of an Online Instrument for Measuring the Real-Time Oxidative Potential of Ambient Particulate Matter Based on Dithiothreitol Assay. Atmos Meas Tech 2018;11(10):5767–5780. [CrossRef]
- Qiao D, Hu C, Li Q, et al. Circ-RBMS1 Knockdown Alleviates CSE-Induced Apoptosis, Inflammation and Oxidative Stress via Up-Regulating FBXO11 Through MiR-197-3p in 16HBE Cells. Int J Chron Obstruct Pulmon Dis 2021;16:2105–2118. [CrossRef]
- Quezada-Maldonado EM, Chirino YI, Gonsebatt ME, et al. Nucleotide Excision Repair Pathway Activity Is Inhibited by Airborne Particulate Matter (PM(10)) through XPA Deregulation in Lung Epithelial Cells. Int J Mol Sci 2022;23(4). [CrossRef]
- Quezada-Maldonado EM, Sánchez-Pérez Y, Chirino YI, et al. Airborne Particulate Matter Induces Oxidative Damage, DNA Adduct Formation and Alterations in DNA Repair Pathways. Environ Pollut 2021;287:117313. [CrossRef]
- Quintana R, Serrano J, Gómez V, et al. The Oxidative Potential and Biological Effects Induced by PM10 Obtained in Mexico City and at a Receptor Site during the MILAGRO Campaign. Environmental Pollution 2011;159(12):3446–3454. [CrossRef]
- Quintana-Belmares R A-MEG-CCG-VVV-LIS-SMR-PIO-VA. Evaluation of the Oxidative Potential of Urban PM and Its Relation to in Vitro Induced DNA Damage: A Spatial and Temporal Comparison. Revista internacional de contaminación ambiental 2015;31(2):145–154.
- Ren F, Xu J, Zhang J, et al. PM2.5 Induced Lung Injury through Upregulating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis. Immunobiology 2022;227(3). [CrossRef]
- Rout-Pitt N, Farrow N, Parsons D, et al. Epithelial Mesenchymal Transition (EMT): A Universal Process in Lung Diseases with Implications for Cystic Fibrosis Pathophysiology. Respir Res 2018;19(1):136. [CrossRef]
- Ryu YS, Kang KA, Piao MJ, et al. Particulate Matter-Induced Senescence of Skin Keratinocytes Involves Oxidative Stress-Dependent Epigenetic Modifications. Exp Mol Med 2019;51(9):1–14. [CrossRef]
- Saha P, Durugkar S, Jain S, et al. Piperine Attenuates Cigarette Smoke-Induced Oxidative Stress, Lung Inflammation, and Epithelial–Mesenchymal Transition by Modulating the SIRT1/Nrf2 Axis. Int J Mol Sci 2022;23(23). [CrossRef]
- Saha S, Buttari B, Panieri E, et al. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020;25(22):5474. [CrossRef]
- Santibáñez-Andrade M, Quezada-Maldonado EM, Rivera-Pineda A, et al. The Road to Malignant Cell Transformation after Particulate Matter Exposure: From Oxidative Stress to Genotoxicity. Int J Mol Sci 2023;24(2). [CrossRef]
- Sarker AH, Chatterjee A, Williams M, et al. NEIL2 Protects against Oxidative DNA Damage Induced by Sidestream Smoke in Human Cells. PLoS One 2014;9(3):e90261. [CrossRef]
- Shi Q, Qian Y, Wang B, et al. Glycyrrhizin Protects against Particulate Matter-Induced Lung Injury via Regulation of Endoplasmic Reticulum Stress and NLRP3 Inflammasome-Mediated Pyroptosis through Nrf2/HO-1/NQO1 Signaling Pathway. Int Immunopharmacol 2023;120. [CrossRef]
- Shi T, Schins RPF, Knaapen AM, et al. Hydroxyl Radical Generation by Electron Paramagnetic Resonance as a New Method to Monitor Ambient Particulate Matter Composition. Journal of Environmental Monitoring 2003;5(4):550. [CrossRef]
- Shrestha D, Bhat SM, Massey N, et al. Pre-Exposure to Hydrogen Sulfide Modulates the Innate Inflammatory Response to Organic Dust. Cell Tissue Res 2021;384(1):129–148. [CrossRef]
- Sies H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol 2017;11:613–619. [CrossRef]
- da Silva Araújo NP, de Matos NA, Leticia Antunes Mota S, et al. Quercetin Attenuates Acute Lung Injury Caused by Cigarette Smoke Both In Vitro and In Vivo. COPD: Journal of Chronic Obstructive Pulmonary Disease 2020;17(2):205–214. [CrossRef]
- Smith MT, Guyton KZ, Gibbons CF, et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ Health Perspect 2016;124(6):713–721. [CrossRef]
- So B, Park J, Jang J, et al. Effect of Aerobic Exercise on Oxidative Stress and Inflammatory Response During Particulate Matter Exposure in Mouse Lungs. Front Physiol 2022;12. [CrossRef]
- Son ES, Park JW, Kim YJ, et al. Effects of Antioxidants on Oxidative Stress and Inflammatory Responses of Human Bronchial Epithelial Cells Exposed to Particulate Matter and Cigarette Smoke Extract. Toxicology in Vitro 2020;67. [CrossRef]
- Song L, Li D, Gu Y, et al. Let-7a Modulates Particulate Matter (≤ 2.5 Μm)-Induced Oxidative Stress and Injury in Human Airway Epithelial Cells by Targeting Arginase 2. J Appl Toxicol 2016;36(10):1302–1310. [CrossRef]
- Song L, Li D, Li X, et al. Exposure to PM2.5 Induces Aberrant Activation of NF-ΚB in Human Airway Epithelial Cells by Downregulating MiR-331 Expression. Environ Toxicol Pharmacol 2017;50:192–199. [CrossRef]
- Takizawa M, Nakano M, Fukami T, et al. Decrease in ADAR1 Expression by Exposure to Cigarette Smoke Enhances Susceptibility to Oxidative Stress. Toxicol Lett 2020;331:22–32. [CrossRef]
- Tanaka KI, Nakaguchi S, Shiota S, et al. Preventive Effect of Epigallocatechin Gallate, the Main Component of Green Tea, on Acute Lung Injury Caused by Air Pollutants. Biomolecules 2022;12(9). [CrossRef]
- Thangavel P, Park D and Lee Y-C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int J Environ Res Public Health 2022;19(12):7511. [CrossRef]
- Tian X, Xue Y, Xie G, et al. (−)-Epicatechin Ameliorates Cigarette Smoke-Induced Lung Inflammation via Inhibiting ROS/NLRP3 Inflammasome Pathway in Rats with COPD. Toxicol Appl Pharmacol 2021;429. [CrossRef]
- Upadhyay S, Chakraborty A, Thimraj TA, et al. Establishment of Repeated In Vitro Exposure System for Evaluating Pulmonary Toxicity of Representative Criteria Air Pollutants Using Advanced Bronchial Mucosa Models. Toxics 2022a;10(6). [CrossRef]
- Upadhyay S, Chakraborty A, Thimraj TA, et al. Establishment of Repeated In Vitro Exposure System for Evaluating Pulmonary Toxicity of Representative Criteria Air Pollutants Using Advanced Bronchial Mucosa Models. Toxics 2022b;10(6). [CrossRef]
- Vattanasit U, Navasumrit P, Khadka MB, et al. Oxidative DNA Damage and Inflammatory Responses in Cultured Human Cells and in Humans Exposed to Traffic-Related Particles. Int J Hyg Environ Health 2014;217(1):23–33. [CrossRef]
- Veerappan I, Sankareswaran SK and Palanisamy R. Morin Protects Human Respiratory Cells from PM(2.5) Induced Genotoxicity by Mitigating ROS and Reverting Altered MiRNA Expression. Int J Environ Res Public Health 2019;16(13). [CrossRef]
- Wang C, Meng X, Meng M, et al. Oxidative Stress Activates the TRPM2-Ca2+-NLRP3 Axis to Promote PM2.5-Induced Lung Injury of Mice. Biomedicine and Pharmacotherapy 2020;130. [CrossRef]
- Wang HL, Chen FQ and Wu LJ. Ephedrine Ameliorates Chronic Obstructive Pulmonary Disease (COPD) through Restraining Endoplasmic Reticulum (ER) Stress in Vitro and in Vivo. Int Immunopharmacol 2022a;103. [CrossRef]
- Wang J, Jia J, Wang D, et al. Zn(2+) Loading as a Critical Contributor to the Circ_0008553-Mediated Oxidative Stress and Inflammation in Response to PM(2.5) Exposures. J Environ Sci (China) 2023a;124:451–461. [CrossRef]
- Wang Q, Shi Q, Liu L, et al. FGF10 Mediates Protective Anti-Oxidative Effects in Particulate Matter-Induced Lung Injury through Nrf2 and NF-ΚB Signaling. Ann Transl Med 2022b;10(22):1203–1203. [CrossRef]
- Wang X, Zhu H, Sun G, et al. Linc01515 Regulates PM(2.5)-Induced Oxidative Stress via Targeting NRF2 in Airway Epithelial Cells. Environ Pollut 2023b;331(Pt 2):121798. [CrossRef]
- Wang Y, Liao S, Pan Z, et al. Hydrogen Sulfide Alleviates Particulate Matter-Induced Emphysema and Airway Inflammation by Suppressing Ferroptosis. Free Radic Biol Med 2022c;186:1–16. [CrossRef]
- Wang Y, Zuo X, Jiang F, et al. A Comparative Study on the Model of PM2.5 Direct or Indirect Interaction with Bronchial Epithelial Cells. Environmental Science and Pollution Research 2022d;29(27):41567–41576. [CrossRef]
- Wang Z, Zuo Y and Gao Z. CircANKRD11 Knockdown Protects HPMECs from Cigarette Smoke Extract-Induced Injury by Regulating MiR-145-5p/BRD4 Axis. Int J Chron Obstruct Pulmon Dis 2021;16:887–899. [CrossRef]
- Wei H, Feng Y, Liang F, et al. Role of Oxidative Stress and DNA Hydroxymethylation in the Neurotoxicity of Fine Particulate Matter. Toxicology 2017;380:94–103. [CrossRef]
- Wei H, Liang F, Meng G, et al. Redox/Methylation Mediated Abnormal DNA Methylation as Regulators of Ambient Fine Particulate Matter-Induced Neurodevelopment Related Impairment in Human Neuronal Cells. Sci Rep 2016;6:33402. [CrossRef]
- Weichenthal S, Lavigne E, Evans G, et al. Ambient PM2.5 and Risk of Emergency Room Visits for Myocardial Infarction: Impact of Regional PM2.5 Oxidative Potential: A Case-Crossover Study. Environmental Health 2016;15(1):46. [CrossRef]
- World Health Organization, (ed). WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. 2021.
- Xiong R, Wu Y, Wu Q, et al. Integration of Transcriptome Analysis with Pathophysiological Endpoints to Evaluate Cigarette Smoke Toxicity in an in Vitro Human Airway Tissue Model. Arch Toxicol 2021a;95(5):1739–1761. [CrossRef]
- Xiong R, Wu Y, Wu Q, et al. Integration of Transcriptome Analysis with Pathophysiological Endpoints to Evaluate Cigarette Smoke Toxicity in an in Vitro Human Airway Tissue Model. Arch Toxicol 2021b;95(5):1739–1761. [CrossRef]
- Xu Z, Ding W and Deng X. PM2.5, Fine Particulate Matter: A Novel Player in the Epithelial-Mesenchymal Transition? Front Physiol 2019;10. [CrossRef]
- Xue M, Peng N, Zhu X, et al. Hsa_circ_0006872 Promotes Cigarette Smoke-Induced Apoptosis, Inflammation and Oxidative Stress in HPMECs and BEAS-2B Cells through the MiR-145-5p/NF-ΚB Axis. Biochem Biophys Res Commun 2021a;534:553–560. [CrossRef]
- Xue Z, Gao X, Yu W, et al. Biochanin A Alleviates Oxidative Damage Caused by the Urban Particulate Matter. Food Funct 2021b;12(5):1958–1972. [CrossRef]
- Yang DQ, Zuo QN, Wang T, et al. Mitochondrial-Targeting Antioxidant SS-31 Suppresses Airway Inflammation and Oxidative Stress Induced by Cigarette Smoke. Oxid Med Cell Longev 2021;2021. [CrossRef]
- Yang L, Wang Y, Lin Z, et al. Mitochondrial OGG1 Protects against PM2.5-Induced Oxidative DNA Damage in BEAS-2B Cells. Exp Mol Pathol 2015;99(2):365–373. [CrossRef]
- Yu C and Zhang L. Methylprednisolone Up-Regulates Annexin A1 (ANXA1) to Inhibit the Inflammation, Apoptosis and Oxidative Stress of Cigarette Smoke Extract (CSE)-Induced Bronchial Epithelial Cells, a Chronic Obstructive Pulmonary Disease in Vitro Model, through the Formyl Peptide Receptor 2 (FPR2) Receptors and the Adenosine 5’-Monophosphate (AMP)-Activated Protein Kinase (AMPK) Pathway. Bioengineered 2022;13(2):4028–4038. [CrossRef]
- Zeng Y, Zhu G, Zhu M, et al. Edaravone Attenuated Particulate Matter-Induced Lung Inflammation by Inhibiting ROS-NF- κ B Signaling Pathway. Oxid Med Cell Longev 2022;2022. [CrossRef]
- Zhang C, Gu S and Kang X. CircRNA Circ_0006892 Regulates MiR-24/PHLPP2 Axis to Mitigate Cigarette Smoke Extract-Induced Bronchial Epithelial Cell Injury. Biotechnol Appl Biochem 2022;69(2):735–748. [CrossRef]
- Zhang MY, Jiang YX, Yang YC, et al. Cigarette Smoke Extract Induces Pyroptosis in Human Bronchial Epithelial Cells through the ROS/NLRP3/Caspase-1 Pathway. Life Sci 2021;269. [CrossRef]
- Zhao C, Wang Y, Su Z, et al. Respiratory Exposure to PM2.5 Soluble Extract Disrupts Mucosal Barrier Function and Promotes the Development of Experimental Asthma. Science of the Total Environment 2020;730. [CrossRef]
- Zhongyin Z, Wei W, Juan X, et al. Epigallocatechin Gallate Relieved PM2.5-Induced Lung Fibrosis by Inhibiting Oxidative Damage and Epithelial-Mesenchymal Transition through AKT/MTOR Pathway. Oxid Med Cell Longev 2022;2022. [CrossRef]
- Zhou F, Cao C, Chai H, et al. Circ-HACE1 Aggravates Cigarette Smoke Extract-Induced Injury in Human Bronchial Epithelial Cells via Regulating Toll-Like Receptor 4 by Sponging MiR-485-3p. Int J Chron Obstruct Pulmon Dis 2021;16:1535–1547. [CrossRef]
- Zhou W, Yuan X, Zhang L, et al. Overexpression of HO-1 Assisted PM2.5-Induced Apoptosis Failure and Autophagy-Related Cell Necrosis. Ecotoxicol Environ Saf 2017;145:605–614. [CrossRef]
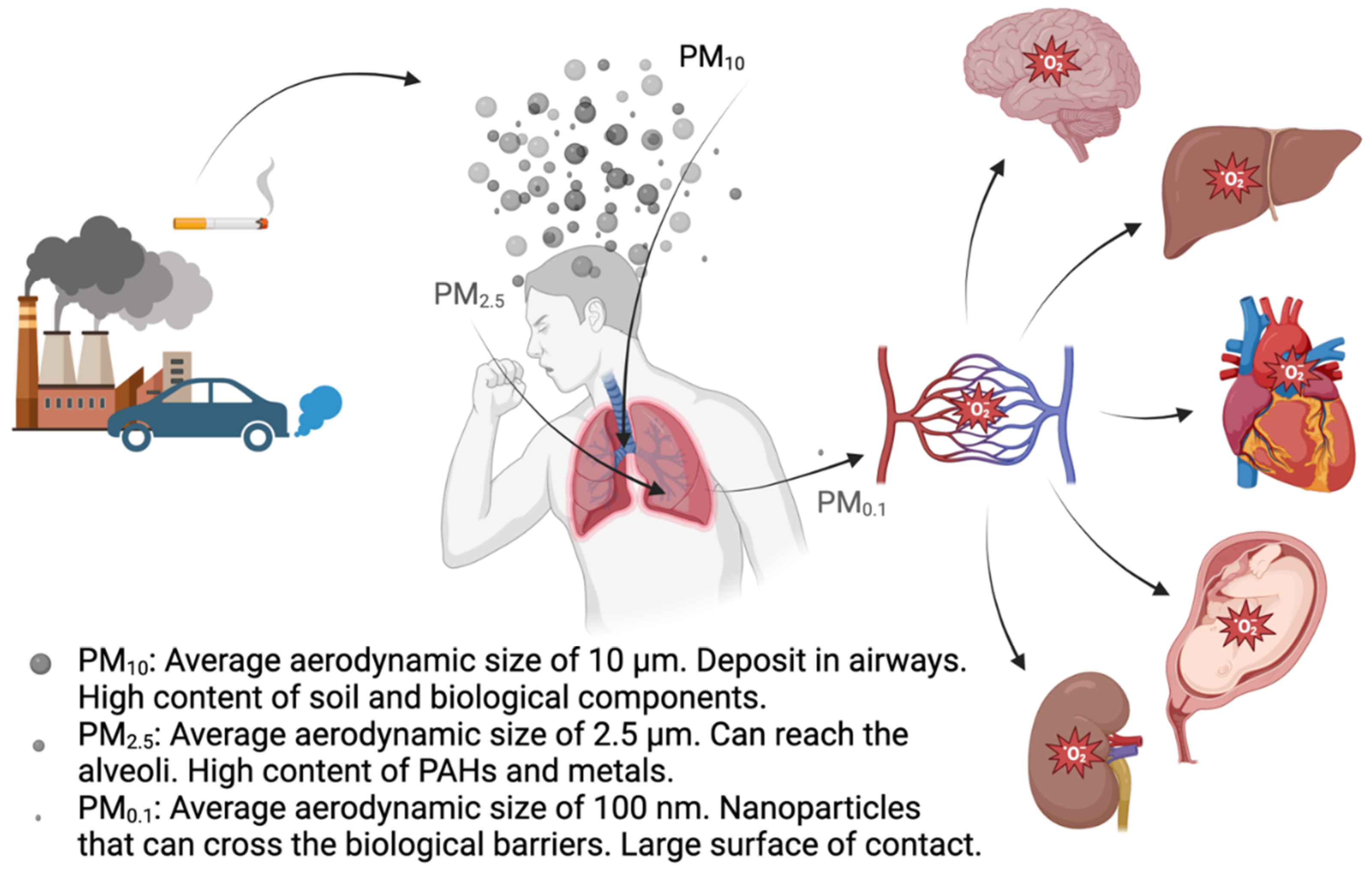
| PM size | Average aero-dynamic size | Surface area per particle (µm2) | Particles fitting in a 10 µm diameter sphere | Equivalent area vs a 10 µm diameter sphere (µm2) | Main sources | Main components |
|---|---|---|---|---|---|---|
| PM10 | 10 µm | 314 | 1 | 314 | Soil, industry, traffic, construction | Soil products, salts and oxides, biological components (i.e. bacteria and fungi) |
| PM2.5 | 2.5 µm | 19.63 | 64 | 1256 | Fuel combustion, traffic (gasoline and diesel engines) | Black carbon particles, PAHs and other organic compounds |
| PM0.1 | 0.1 µm | 0.0314 | 1 *106 | 31400 | Cigarette smoke, fuel combustion, waste incineration, wear off of materials containing NPs | Carbon derived nanomaterials, metal derived nanomaterials |
| Cells/ cell line | Type of PM, conc., time of exposure, condition | Main oxidative stress-related effects | Reference |
|---|---|---|---|
| A549 | CSE, 3 and 6%, 12h, SUB | - LC3B-II, protein carbonylation, translocation of ADAR1 from nucleus to cytosol - ADAR1 (but not mRNA, so it’s post-transcriptional), CYP1A1, RNA editing levels of AhR, SOD act. |
(Takizawa et al., 2020) |
| A549 | CSE (commercial), 0.25 & 1mg/mL, 24h, SUB | - IL-6, IL-8, MCP-1, CCL5, CYBA, SOD, GPx, CAT, NOX, Nrf2, ATG5, ATG12, ATG16, beclin-1, LC3B-II/LC3B-I, autophagosome formation, FOXO1, nuclear FOXO3a - FOXO3a, mTOR No change in cell viability, ANXV+ or Pi+ cells (= no necrosis, no apoptosis) |
(Bagam et al., 2021) |
| A549 | PM10 SRM 1648a water-soluble fraction, 400mg/mL, 24h, SUB | - MDA, NO, MEK5, ERK5, p-ERK5, Nrf2, HO-1 - cell viability, SOD act., CAT, GSH |
(Xue et al., 2021b) |
| A549 | PM SRM 1648a, 25-200mg/cm2 (119-950mg/mL), 24h, SUB | - ROS, p-AMPKa, Sestrin2 (oxidative stress suppressor), IL-8, TNF-a, COX-2 - cell viability, mitochondrial function |
(So et al., 2022) |
| A549 | PM SRM 1649b Organic extractable fraction, 100mg/mL, 24h, SUB | - wound healing, cell migration, vimentin, fibronectin, ETS-1, p-p65 NF-κb - E-cadherin |
(Chen et al., 2020) |
| A549 | PM2.5 (Water-soluble fraction in simulated lung fluid), 50-200mg/mL, 24h, SUB | - LDH, DNA damage, proline expression - cell viability, TAC |
(Barzgar et al., 2023) |
| A549 | PM2.5 (brake-derived) w/ ¹ Cu conc., 50-500mg/mL, 48h, SUB |
- ROS, % apoptotic cells, MitoMP, IL-8, IL-1a, IL-6, TNF-a, HO-1 - Cell viability, Bcl-2 |
(Figliuzzi et al., 2020) |
| A549 | PM2.5 Urban vs industrial, 80mg/mL, 24h, SUB | - ROS, TNF-a (non-pollution), IL-6 (industrial) - Cell viability, NOQ1 |
(Pang et al., 2020) |
| A549 | PM2.5, 80mg/mL, 24h, SUB | - ROS, IL-6, TNF-a, LDH - Cell viability (significant but not relevant) |
(Li et al., 2022) |
| A549+HUVEC on chip | PM2.5, 100mg/mL, 24h, SUB | - ROS, IL-1a, IL-1β, IL-6, INF-a, % apoptotic cells, BIP, PERK, p-eIF2a, CHOP, caspase-3 | (Guan et al., 2021) |
| A549, SD-1 |
PM2.5, 100mg/mL, 12h, SUB | - ROS, Ca2+, IL-1b, IL-6, TNF-a, NLRP3, caspase-1, TRPM2 | (Wang et al., 2020) |
| A549, RAW 264.7 |
PM, 50mg/mL, 24h, SUB | - ROS, NO, O2-, IL-6, TNF-a, cells in G2/M, % apoptotic cells - viability |
(Guerra e Oliveira et al., 2022) |
| A549+ diff THP-1 | Cow stable dust, 25-100mg/mL, 18h, SUB | - ROS, IL-6, TNF-a, cells in G1/G0 - metabolic act, cell in S-G2/M |
(Martikainen et al., 2021) |
| A549, BEAS-2B |
CSE, 3 (A549) 1.38% (BEAS-2B), 48h, SUB | - p-NF-kb/NF-kb, vimentin, N-cadherin, a-SMA - Cell viability, Nrf2, SIRT1, p-b-catenin/b-catenin, E-cadherin |
(Saha et al., 2022) |
| BEAS-2B | 3¹ functionalized carbon black vs carbon black (PM2.5), 1.56-25mg/mL, 24h, SUB | - IL-1b, IL-6, protein carbonylation - Cell viability, SOD2, Nrf2 |
(Housseiny et al., 2020) |
| BEAS-2B | CSE, 8%, 24h, SUB |
- ROS, MDA, ERK p-p38 MAPK, IL-6, TNF-a, MMP-9, mitochondrial fission factor - SOD and GPx act, OPA1 |
(Yang et al., 2021) |
| BEAS-2B | CSE, 5%, 24h, SUB |
- ROS, apoptotic cells, Bax, cleaved caspase-3/caspase-3, cleaved PARP/ PARP, MDA, TNFa, IL-6, IL-1b - cell viability, Bcl-2, SOD, GSH-Px, ANXA1, FRP2, pAMPK/AMPK |
(Yu and Zhang, 2022) |
| BEAS-2B | CSE, 5%, 24h, SUB | - ROS, MDA, Nrf2, HO-1, NQO1, TRIM25, caspase-1, LDH, NLRP3, GSDMD-N, IL-1b, IL-18 - cell viability, SOD-1, SOD-2, SOD-3, Keap-1 |
(Tian et al., 2021) |
| BEAS-2B | CSE (1%, 7days) & PM10 (SRM 1648 100mg/mL, 24h) alone vs combined, SUB |
- ROS (combined exposure), LDH (not CSE), MDA, IL-6, IL-8, p-ERK, p-JNK, Nrf2, IL-1β, IL-6, IL-8, TNF-α, MCP-1, CXCL-1, HO-1, NQO1 - Cell viability (not CSE), GSH, TXN |
(Son et al., 2020) |
| BEAS-2B | PM SRM 1649b, 200μg/mL, 24h, SUB | - ROS, IL-6, IL-8, p-IκBα/IκBα, p-p65/p65 NF-κb, Nrf2, HO-1, NQO1 - Keap1 |
(Wang et al., 2022b) |
| BEAS-2B | PM SRM 1649b, 200mg/mL, 24h, SUB | - ROS, Pi+ cells, NLRP3, ASC, GSDM-N/GSDMD, cleaved caspase-1/caspase-1, caspase-1 act., LDH release, mature IL-1b/IL-1b, mature IL-18/IL-18, Nrf2 (total + nuclear), NQO1, HO-1, p-Akt/Akt - cell viability |
(Liu et al., 2022b) |
| BEAS-2B | PM2.5 (SRM 2786), 20μg/cm2, 36h, SUB | - Lipid ROS, ROS, MitoMP, Mitochondrial ROS, NADP+/NADPH, COX2, MDA, IL-6, IL-8, TNF-a, Fe2+ accumulation, LC3B-II, NCOA4, FTH1 - cell viability, GPX4, GSH, GPx, Nrf2, PPAR-γ |
(Wang et al., 2022c) |
| BEAS-2B | PM2.5 (China) soluble extract, 300μg/mL (~94 μg/cm2), up to 24h, SUB | - ROS, IL-1b, IL-6, IL-8, GM-CSF, cleaved PARP, cleaved caspase-3, Bax, %apoptotic cells, COX2, p-p65 NF-κb, p-ERK, p-p38 MAPK/ERK, p-JNK - cell viability, ZO-1, E-cadherin, Bcl-2, GSH act, p-mTOR |
(Zhao et al., 2020) |
| BEAS-2B | PM2.5, 25-200mg/mL, 24h, SUB | - Nrf2, NF-kb, IL-1, IL-6, IL-8, a-SMA - Cell viability (lower in direct exp), E-cadherin |
(Wang et al., 2022d) |
| BEAS-2B | PM2.5-0.3 vs organic extractable & non-extractable fractions, 12mgEq. PM/cm2, 6-48h, SUB | - ROS, Nrf2, Nrf2 binding act, Keap-1, NQO1, HO, SOD, GSSG/GSH, DNA damage protein carbonylation, 8-isoprostane, TNF-a, IL-6, IL-8, MCP-1, caspase 3/7, caspase 8, caspase 9 - cell viability, ATG5, Beclin, LC3B-II |
(Badran et al., 2020) |
| BEAS-2B, WL-38, primary rat alveolar macrophages |
PM2.5, 70mg/mL, 24h SUB | BEAS-2B: - ROS, apoptosis rate, collagen I/III, a-SMA, TGF-b1, p-Smad2 - cell viability WL-38: - ROS, apoptosis rate, collagen I/III, a-SMA, TGF-b1, p-Smad2 - cell viability Alveolar macrophages: - ROS, apoptosis rate, M2 phenotype, mTORC1, TIPE2 - cell viability, M1 phenotype |
(Liu et al., 2022a) |
| BEAS-2B, Primary mouse tracheal epithelial cells |
PM2.5, 100mg/mL, 24h, SUB | - ROS, MDA, miR-155 - SOD, GPx, FOXO3a, SOD2, CAT |
(Li et al., 2021) |
| BEAS-2B, primary human small airway epithelial cells |
Polycarbonate (PC) vs polyurethane (PU) incinerated thermoplastics & derivatives w/ 3% carbon nanotubes (CNT), 0.6 or 1.2mg/cm2, 48h, ND | - ROS (only for PC-CNT and results in DNA damage), LDH, CYP1 act, cells in G2 - viability, cells in G1, MitoMP |
(Coyle et al., 2020) |
| BEAS-2B, NHBE cells | Poultry organic dust extract, 0.25%, up to 24h, SUB | - ROS, mitoROS, pro-IL-1b, IL-8, IL-6, PTGS2, ICAM-1, p-p65 NF-κb, p-STAT-3 - p47phox (indicates NOX2 activation) |
(Meganathan et al., 2022) |
| BEAS-2B, THP-1 |
Organic dust extract, 5%, 24h, SUB | BEAS-2B: - ROS, RNS, Nrf2, IL-1β, IL-6, IL-8, IL-10 THP-1: - ROS, RNS, iNOS, Nrf2, Trl2, Trl4, IL-6, IL-8, NF-kb |
(Shrestha et al., 2021) |
| 16-HBE | CSE, 5%, 24h, SUB | - ROS, LDH, IL-1b, IL-18, Pi+ cells, caspase-1 act, NLRP3 - GSDMD |
(Zhang et al., 2021) |
| 16-HBE | PM2.5 (China); 67.5, 116.9, 202.5mg/mL; 4 & 24h, SUB | - ROS, LDH, MDA, HO-1, DNA damage - Cell viability, GSH |
(Niu et al., 2020a) |
| 16-HBE14o-, NuLi-1 | SRM 2585 (Organic extract of house dust), 0.2mg/mL, SUB | - ROS, mitochondrial dysfunction - TEER |
(Marques dos Santos et al., 2022) |
| HBECs | PM SRM 1649b, 300μg/mL, 24h, SUB | - ROS, IL-6, IL-1a, IL-1b, COX2, p-p65/p65 NF-κb - MitoMP |
(Zeng et al., 2022) |
| HBECs | PM SRM 1649b, 200mg/mL, 24h, SUB |
- ROS, ATF4, BIP/GRP78, CHOP, ATF6, cleaved caspase-3, NLRP3, a, GSDMD-N, IL-1β, caspase-1, IL-18, IL-6, IL-8, apoptotic and necrotic cells, Nrf2 (total and nuclear), HO-1, NQO1. | (Shi et al., 2023) |
| HBECs | CSE, 2%, 48h, SUB | - ROS, apoptosis rate, IL-8, IL-6, TNF-a, cleaved caspase-3, p-NF-kb, Keap-1, BIP/GRP78, p-PERK, p-IRE1a, ATF6, ATF4, CHOP, NOX1, NOX2, NOX4, XO, Keap-1 - Cell viability, HO-1, NQO-1, SOD, GCLM, Nrf-2 |
(Wang et al., 2022a) |
| HBSM | CSE, 2.5% 24h, SUB |
- proliferation rate, BrdU incorporation (into newly synthesized DNA of actively proliferating cells), cyclin D1, a-SMA, p-SMAD2, p-SMAD3, TGF-b1 - PPAR- γ |
(Pan et al., 2021) |
| J774A.1 | CSE, 0.5%, 24h, SUB | - ROS, NO - Cell viability |
(da Silva Araújo et al., 2020) |
| L-132 | CSE, 10%, 24h, SUB | - TXNIP, NLRP3, mitoROS, LDH release - cell viability, mitophagy (mitochondria clearance) |
(Mahalanobish et al., 2020) |
| MH-S | CSE, 3%, 1h, SUB | - ROS, EVs conc, vesicular (not intracellular) SOCS3 - 20S proteasome act. |
(Haggadone et al., 2020) |
| MLE-12 | PM2.5, 100mg/mL, 24h, SUB | - a-SMA, Txnip, p-mTOR - cell viability, E-cadherin, Txnrd1 |
(Zhongyin et al., 2022) |
| NCI-H292 | CSE, 10%, 48h, SUB | - IL-8, TNF-a, MMP-9, STAT3, JAK1, JAK2 - SOD, TIMP-1, PPAR |
(Haoran et al., 2020) |
| NCI-H292, HPAEC |
PM2.5, 10mg/cm2, up to 24h, SUB |
- ROS (sub-urban), HO-1, SOD-2, IL-8 in both cell types but higher in endothelial | (Crobeddu et al., 2020) |
| NCI-H460 | PM10, 400mg/mL, 12h, SUB | - ROS - cell viability |
(Lee et al., 2022a) |
| NHBE | CS diluted in clean air, 0.5-4L/min, 40’/day * 3x/week * 4weeks followed by a 20day-recovery phase (RP), ALI |
- HO-1 (back to basal after RP), IL-1β, IL-1 receptor antagonist, IL-6, IL-8, G-CSF, RANTES, CK6, involucrin, TEER, PPARg - GSH/GSSG (acute exposure), IL-7, MCP-1, MMP-1, MMP-2, MMP-3, MMP-7, MMP-10, MMP-13, MUC5AC, MUC5B, CCSP, cilliated cells, goblet cells, number of cilia, cilia lenght, cilia beating frequency, |
(Xiong et al., 2021a) |
| Primary human bronchial epithelial cells | DEP alone (12.5 μg/cm2, 3’/day x3days) vs single combined exposure w/ NO2 (0.1ppm) and w/ SO2 (0.2ppm), aerosol | -Alone: -IL-6, IL-8, TNF-a, GSTA1, HO, SOD3 - IL-8, MMP-9 Combined: -TNF-a, GSTA1, SOD3, MMP-9 |
(Upadhyay et al., 2022a) |
| Primary human CCR6+Th17 cells | CSE, 5%, 48h, SUB | - ROS, SA-b gal+ cells, p16INK4a + cells, VEGFa, p-ERK+ cells, HO-1, NQO1 | (Baskara et al., 2020) |
| Primary rat alveolar epithelial cells | CSE (Heat-not-burn), 20% vs CSE (conventional), 10%, up to 24h, SUB |
-Nrf2, HO-1, GSTA1, GSTA3, NQO1 | (Ito et al., 2020) |
| Rat ATII cells, NR8383 | PM2.5, 50mg/mL, 24h, SUB | - ROS, IL-6, TNF-a, apoptosis or necrosis Data related to immunomodulation is normalized to PM2.5 making it not possible to understand the effects of PM2.5 relative to control. |
(Gao et al., 2021) |
| RAW 264.7 | PMET720 (common stainless-steel wire) aerosols collected @50 or 60 psi, up to 200mg/mL, 24h, SUB vs. GMA-SS, MMA-SS welding particles |
PMET720(60) @200mg/mL: -LDH, NF-kb (>3.12ug/mL) - Cell viability PMET720(50&60) @100mg/mL: - ROS, NF-kb (>3.12ug/mL) |
(Kodali et al., 2022) |
| RAW 264.7 | PM2.5, 400mg/mL, 24h, SUB | - ROS, MDA, NLRP3, NF-kb, Bax, apoptotic rate, caspase-1, caspase-3, GSDMD, IL-1β, %cells in G2 - Bcl-2, SOD act., %cells in G1 |
(Ren et al., 2022) |
| RAW 264.7 | PM (China) urban aerossol, 30mg/cm2, 24h, SUB | - ROS, TNF-a, IL-1b, IL-6, MIP-2 | (Tanaka et al., 2022) |
| diff U937 (as alveolar macrophages), HMC3 (microglia) | DPM SRM 2975, 25mg/mL, 24h, SUB Conditioned serum, 48h, SUB |
-U937: - ROS, H2O2, MCP-1, IL-1b, IL-6, IL-8, TNF-a HMC3: - ROS, H2O2, IL-6, IL-8, IL-1b, TNF-a, CD-14 activation |
(Pradhan et al., 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





