Submitted:
01 April 2024
Posted:
01 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- studies in which a valid measurement of epigenetic changes that were associated with a significant stress factor (death of a loved one, emotional, physical, sexual abuse, etc.) was performed;
- studies that assessed whether the epigenetic change was correlated with a diagnosis of an affective disorder;
- subjects with somatic comorbidities,
- subjects under the age of 18 years old.
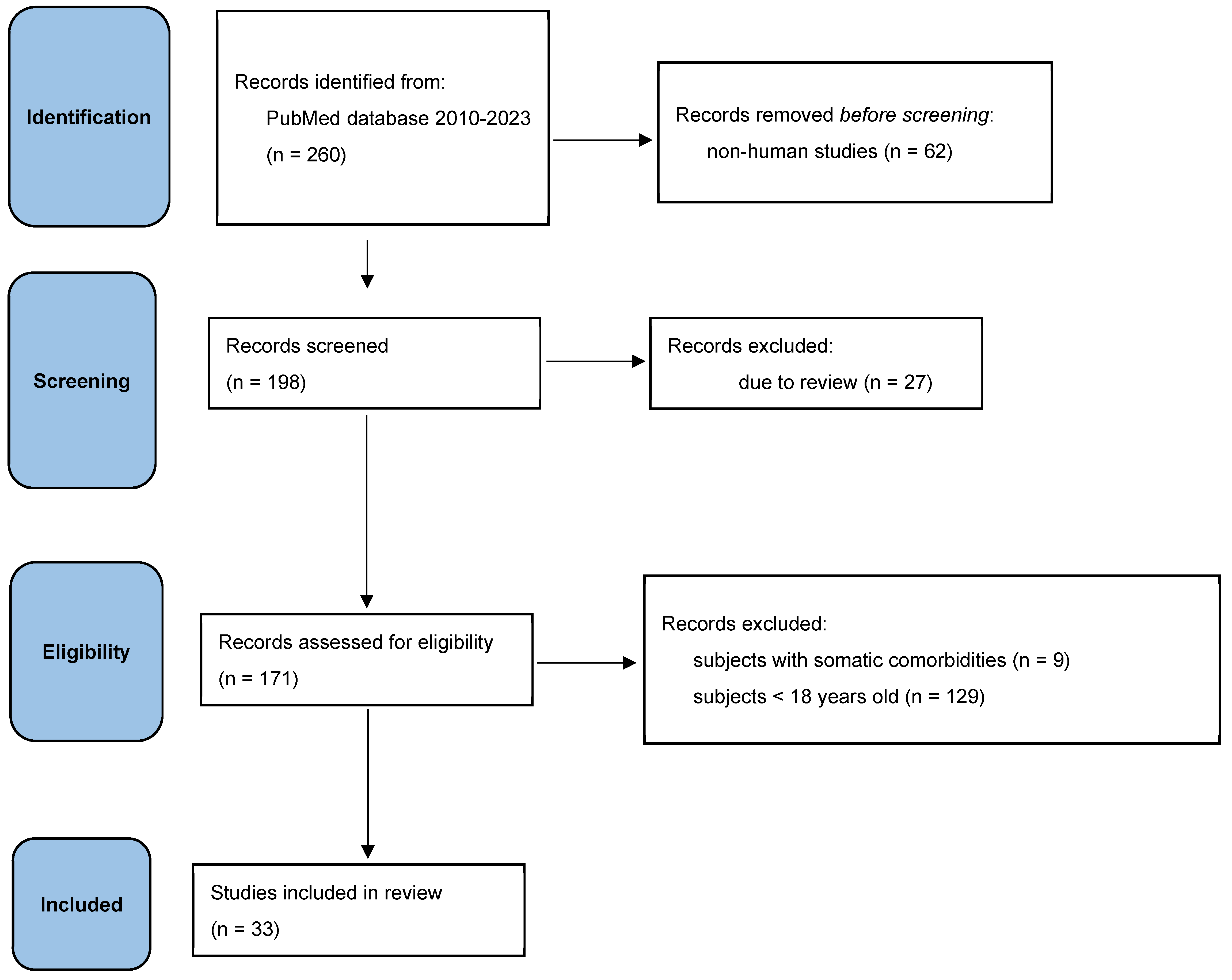
3. Results
- NR3C1 (human glucocorticoid receptor gene);
- SLC6A4 (serotonergic transporter gene);
- BDNF (brain-derived neurotrophic factor);
- FKBP5 (FK506 5 binding protein gene);
- SKA2 (kinetochore protein gene);
- OXTR (oxytocin receptor) and genes encoding oligodendrocytes.
| GENE | STUDY | STRESS | AFFECTIVE DISORDER | QUESTIONNAIRE |
|---|---|---|---|---|
| NR3C1 | Bustamante et al. [18] | Positive association | Negative association | CTS (child trauma screen), CTQ (childhood trauma questionnaire) |
| Radtke et al. [19] | Didn’t report | Didn’t report | KERF 1 | |
| Perroud et al. [20] | Positive association | Didn’t report | CTQ | |
| De Assis Pinheiro et al. [21] | Negative association | Positive association | BDI-II (Beck Depression Inventory II) | |
| Comtois-Cabana et al. [22] | Positive association | Negative associaton | CTQ-SF (child trauma screen – short form), BDI-II | |
| FKPB 5 | Weder et al. [23] | Positive association | Negative association | CTQ |
| Tyrka et al. [24] | Negative association | Positive association | Interview | |
| Flasbeck&Brüne [25] | Negative association | Positive association | CTQ | |
| SLC6A4 | Sanwald et al. [26] | Positive association | Positive association | MADRS (Montgomery–ÅsbergDepression Rating Scale) |
| Swartz et al. [27] | Positive association | Positive association | Interview | |
| Booji et al. [28] | Positive association | Didn’t report | CTQ | |
| Lei et al. [29] | Positive association | Positive association | Non-standard 11 item scale | |
| Kang et al. [30] | Positive association | Didn’t report | Non-standard childhood adversity | |
| Alaasari et al. [31] | Negative association | Positive association | Karasek-Job Content Questionnaire | |
| Comtois-Cabana et al. [22] | Positive association | Positive association | CTQ-SF, BDI-II | |
| BDNF | Song et al. [32] | Positive association | Negative association | Non-standardself-reportquestionnaire |
| SKA2 | Weder et al. [23] | Didn’t report | Didn’t report | Report of parental abuse and neglect |
| Sadeh et al. [33] | Didn’t report | Positive association | PTSD (post-traumatic stress disorder) scale administered by clinicians | |
| Sadeh et al. [34] | Positive association | Didn’t report | PTSD scale administered by clinicians | |
| OXTR, LINGO3, POU3F1, ITGB1. | Smearman et al. [35] | Positive association | Didn’t report | CTQ |
| Lutz et al. [36] | Positive association | Negative association | CECA (Childhood Experience of Care and Abuse), Interview | |
| Ludwig et al. [37] | Didn’t report | Positive association | CTQ, HAM-D (Hamilton Depression Rating Scale) | |
| Kogan et al. [38] | Positive association | Positive association | ACE (Adverse Childhood Experiences) |
| STUDY | NUMBER OF PATIENTS | GENDER | AGE (average) | QUESTIONNARE | RESULTS |
|---|---|---|---|---|---|
| Kasi et al. [40] | 162, MDD (major depressive disorder) and GAD (generalized anxiety disorder) | 74.4% M; 25.3% F | It does not specify | COPE (Coping Orientation to Problems Experienced Inventory) | In patients diagnosed with generalised anxiety disorder or major depressive disorder, “religion-oriented” was the most common coping mechanism identified. |
| Horwitz et al. [41] | 286, MDD | 41%M; 59% F | 18 | COPE, C-SSRS (Columbia Suicide Severity Rating Scale) | Active coping was correlated with lower C-SSRS sores at follow-up. |
| Fletcher et al. [42] | 379, BD (bipolar disorder) + MDD | 41% M 59%F | 39 | COPE, RPA (Responses to Positive Affect), CIPM (Coping Styles in Prodrome of Bipolar Mania), RSQ (Response Style Questionnaire),CERQ (Cognitive Emotion Regulation Questionnaire) | A number of differences were found between the group of patients with unipolar depression and the group with bipolar depression, the former being oriented towards active coping, focused on the problem. |
| Au CH et al. [43] | 115, BD | 37%M; 63%F | 47 | SCOS (Stigma Coping Orientation Scale) | It has been reported that low self-esteem is crucial to social functioning. Dysfunctional coping predominates among these patients. |
| Nitzburg et al. [44] | 92, BD | 48%M; 42%F | 45 | COPE | Dysfunctional coping is a predictive factor for many disabilities, while active coping is associated with resilience. Likewise, behavioral disengagement and guilt are predictors of disability. |
| Paans et al. [45] | 90, BD | 45%M; 55%F | 67 | UCL | The authors reported positive associations between better cognitive functioning and active coping. |
| Lin J et al. [7] | 310, MDD with suicidal risk | It does not specify | 30 | SCSQ (Simplified Coping Style Questionnaire) | Patients at risk of suicide had negative coping strategies and an inadequate social support network. |
| Kuiper et al. [45] | 89, MDD | It does not specify | 20 | COPE | Problem-centred coping has been shown to correlate with better functionality. Emotion-centred coping and dysfunctional coping have been associated with low resilience |
| Orzechowska et al. [46] | 80, MDD and BD | 48 women, 32 men | 49 | COPE | Unlike healthy people, depressed patients in stressful situations more often use strategies based on avoidance, denial and have more difficulty in finding positive aspects of stressful events. |
| Roohafza HR et al. [47] | 4685, MDD and GAD | It does not specify | 49 | COPE | The results show that positive interpretation and growth, active coping and a supportive social network are protective factors in major depressive disorder and generalised anxiety disorder. |
4. Discussion
Epigenetic Mechanisms and Affective Disorders
Coping Mechanisms and Affective Disorders
Individual Variability and Resilience
Clinical Implications and Future Directions
- Heterogeneity of study designs: The review encompasses studies with varying methodologies, including cross-sectional and longitudinal designs, which may limit the generalizability of findings.
- Sample characteristics: Studies included in the review involve diverse patient populations with variations in age, gender, and clinical characteristics, potentially confounding the interpretation of results.
- Measurement of epigenetic changes: The review primarily focuses on DNA methylation as a proxy for epigenetic alterations, overlooking other mechanisms such as histone modifications or microRNA regulation, which could also contribute to the pathophysiology of affective disorders.
- Causality and directionality: Most reviewed studies establish associations between epigenetic changes, stressors, coping mechanisms, and affective disorders, but causality and directionality remain unclear. Longitudinal studies are necessary to elucidate temporal relationships and causal pathways.
- Publication bias: The review may be subject to publication bias, as studies reporting statistically significant findings are more likely to be published, potentially skewing the evidence synthesis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiele, M.A.; Gottschalk, M.G.; Domschke, K. The Applied Implications of Epigenetics in Anxiety, Affective and Stress-Related Disorders—A Review and Synthesis on Psychosocial Stress, Psychotherapy and Prevention. Clin. Psychol. Rev. 2020, 77, 101830. [Google Scholar] [CrossRef] [PubMed]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Knapik, A.; Krzystanek, E.; Szefler–Derela, J.; Siuda, J.; Rottermund, J.; Plinta, R.; Brzęk, A. Affective Disorder and Functional Status as Well as Selected Sociodemographic Characteristics in Patients with Multiple Sclerosis, Parkinson’s Disease and History of Stroke. Medicina 2020, 56, 117. [Google Scholar] [CrossRef]
- Post, R.M. Mechanisms of Illness Progression in the Recurrent Affective Disorders. Neurotox. Res. 2010, 18, 256–271. [Google Scholar] [CrossRef]
- Saunders, K.E.A.; Goodwin, G.M. The Course of Bipolar Disorder. Adv. Psychiatr. Treat. 2010, 16, 318–328. [Google Scholar] [CrossRef]
- Huang, Y.; Wongpakaran, T.; Wongpakaran, N.; Bhatarasakoon, P.; Pichayapan, P.; Worland, S. Depression and Its Associated Factors among Undergraduate Engineering Students: A Cross-Sectional Survey in Thailand. Healthcare 2023, 11, 2334. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Su, Y.; Lv, X.; Liu, Q.; Wang, G.; Wei, J.; Zhu, G.; Chen, Q.; Tian, H.; Zhang, K.; et al. Perceived Stressfulness Mediates the Effects of Subjective Social Support and Negative Coping Style on Suicide Risk in Chinese Patients with Major Depressive Disorder. J. Affect. Disord. 2020, 265, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.T.; Holmes, S.E.; Pietrzak, R.H.; Esterlis, I. Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress 2017, 1, 247054701771091. [Google Scholar] [CrossRef]
- Enatescu, V.-R.; Bernad, E.; Gluhovschi, A.; Papava, I.; Romosan, R.; Palicsak, A.; Munteanu, R.; Craina, M.; Enatescu, I. Perinatal Characteristics and Mother’s Personality Profile Associated with Increased Likelihood of Postpartum Depression Occurrence in a Romanian Outpatient Sample. J Ment Health 2017, 26, 212–219. [Google Scholar] [CrossRef]
- Park, H.G.; Jeong, S.; Kwon, M. Factors Related to Depression According to the Degree of Loneliness in Adolescents with Severe Friend-Relationship Stress. Healthcare 2023, 11, 1354. [Google Scholar] [CrossRef]
- Răchită, A.; Strete, G.E.; Suciu, L.M.; Ghiga, D.V.; Sălcudean, A.; Mărginean, C. Psychological Stress Perceived by Pregnant Women in the Last Trimester of Pregnancy. Int. J. Environ. Res. Public. Health 2022, 19, 8315. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.V.; Kessing, L.V. Clinical Use of Coping in Affective Disorder, a Critical Review of the Literature. Clin. Pract. Epidemiol. Ment. Health 2005. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pan, F.; Tang, Y.; Huang, J.H. Editorial: Early Life Stress-Induced Epigenetic Changes Involved in Mental Disorders. Front. Genet. 2021, 12, 684844. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.S.; Marazita, M.L.; Zubenko, W.N.; Spiker, D.G.; Giles, D.E.; Kaplan, B.B.; Zubenko, G.S. Genetic Segregation Analysis of Recurrent, Early-onset Major Depression: Evidence for Single Major Locus Transmission. Am. J. Med. Genet. 2002, 114, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhao, Y.; Tan, Z.; Lai, J.; Chen, J.; Zhang, X.; Sun, J.; Chen, L.; Lu, K.; Cao, L.; et al. Differentiation between Bipolar Disorder and Major Depressive Disorder in Adolescents: From Clinical to Biological Biomarkers. Front. Hum. Neurosci. 2023, 17, 1192544. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.A.; Marwaha, S. Epidemiology and Risk Factors for Bipolar Disorder. Ther. Adv. Psychopharmacol. 2018, 8, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Thorup, A.A.E.; Jepsen, J.R.; Ellersgaard, D.V.; Burton, B.K.; Christiani, C.J.; Hemager, N.; Skjærbæk, M.; Ranning, A.; Spang, K.S.; Gantriis, D.L.; et al. The Danish High Risk and Resilience Study—VIA 7—A Cohort Study of 520 7-Year-Old Children Born of Parents Diagnosed with Either Schizophrenia, Bipolar Disorder or Neither of These Two Mental Disorders. BMC Psychiatry 2015, 15, 233. [Google Scholar] [CrossRef]
- Bustamante, A.C.; Aiello, A.E.; Galea, S.; Ratanatharathorn, A.; Noronha, C.; Wildman, D.E.; Uddin, M. Glucocorticoid Receptor DNA Methylation, Childhood Maltreatment and Major Depression. J. Affect. Disord. 2016, 206, 181–188. [Google Scholar] [CrossRef]
- Radtke, K.M.; Schauer, M.; Gunter, H.M.; Ruf-Leuschner, M.; Sill, J.; Meyer, A.; Elbert, T. Epigenetic Modifications of the Glucocorticoid Receptor Gene Are Associated with the Vulnerability to Psychopathology in Childhood Maltreatment. Transl. Psychiatry 2015, 5, e571. [Google Scholar] [CrossRef]
- Perroud, N.; Paoloni-Giacobino, A.; Prada, P.; Olié, E.; Salzmann, A.; Nicastro, R.; Guillaume, S.; Mouthon, D.; Stouder, C.; Dieben, K.; et al. Increased Methylation of Glucocorticoid Receptor Gene (NR3C1) in Adults with a History of Childhood Maltreatment: A Link with the Severity and Type of Trauma. Transl. Psychiatry 2011, 1, e59. [Google Scholar] [CrossRef]
- De Assis Pinheiro, J.; Freitas, F.V.; Borçoi, A.R.; Mendes, S.O.; Conti, C.L.; Arpini, J.K.; Dos Santos Vieira, T.; De Souza, R.A.; Dos Santos, D.P.; Barbosa, W.M.; et al. Alcohol Consumption, Depression, Overweight and Cortisol Levels as Determining Factors for NR3C1 Gene Methylation. Sci. Rep. 2021, 11, 6768. [Google Scholar] [CrossRef]
- Comtois-Cabana, M.; Barr, E.; Provençal, N.; Ouellet-Morin, I. Association between Child Maltreatment and Depressive Symptoms in Emerging Adulthood: The Mediating and Moderating Roles of DNA Methylation. PLoS ONE 2023, 18, e0280203. [Google Scholar] [CrossRef] [PubMed]
- Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; Perepletchikova, F.; et al. Child Abuse, Depression, and Methylation in Genes Involved With Stress, Neural Plasticity, and Brain Circuitry. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 417–424. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Parade, S.H.; Welch, E.S.; Ridout, K.K.; Price, L.H.; Marsit, C.; Philip, N.S.; Carpenter, L.L. Methylation of the Leukocyte Glucocorticoid Receptor Gene Promoter in Adults: Associations with Early Adversity and Depressive, Anxiety and Substance-Use Disorders. Transl. Psychiatry 2016, 6, e848. [Google Scholar] [CrossRef]
- Flasbeck, V.; Brüne, M. Association between Childhood Maltreatment, Psychopathology and DNA Methylation of Genes Involved in Stress Regulation: Evidence from a Study in Borderline Personality Disorder. PLoS ONE 2021, 16, e0248514. [Google Scholar] [CrossRef]
- Sanwald, S.; Widenhorn-Müller, K.; Schönfeldt-Lecuona, C.; GenEmo Research Group; Connemann, B.J.; Gahr, M.; Kammer, T.; Montag, C.; Kiefer, M. Factors Related to Age at Depression Onset: The Role of SLC6A4 Methylation, Sex, Exposure to Stressful Life Events and Personality in a Sample of Inpatients Suffering from Major Depression. BMC Psychiatry 2021, 21, 167. [Google Scholar] [CrossRef]
- Swartz, J.R.; Hariri, A.R.; Williamson, D.E. An Epigenetic Mechanism Links Socioeconomic Status to Changes in Depression-Related Brain Function in High-Risk Adolescents. Mol. Psychiatry 2017, 22, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Booij, L.; Szyf, M.; Carballedo, A.; Frey, E.-M.; Morris, D.; Dymov, S.; Vaisheva, F.; Ly, V.; Fahey, C.; Meaney, J.; et al. DNA Methylation of the Serotonin Transporter Gene in Peripheral Cells and Stress-Related Changes in Hippocampal Volume: A Study in Depressed Patients and Healthy Controls. PLoS ONE 2015, 10, e0119061. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.-K.; Beach, S.R.H.; Simons, R.L.; Philibert, R.A. Neighborhood Crime and Depressive Symptoms among African American Women: Genetic Moderation and Epigenetic Mediation of Effects. Soc. Sci. Med. 2015, 146, 120–128. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kim, J.-M.; Stewart, R.; Kim, S.-Y.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Shin, M.-G.; Yoon, J.-S. Association of SLC6A4 Methylation with Early Adversity, Characteristics and Outcomes in Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 23–28. [Google Scholar] [CrossRef]
- Alasaari, J.S.; Lagus, M.; Ollila, H.M.; Toivola, A.; Kivimäki, M.; Vahtera, J.; Kronholm, E.; Härmä, M.; Puttonen, S.; Paunio, T. Environmental Stress Affects DNA Methylation of a CpG Rich Promoter Region of Serotonin Transporter Gene in a Nurse Cohort. PLoS ONE 2012, 7, e45813. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Miyaki, K.; Suzuki, T.; Sasaki, Y.; Tsutsumi, A.; Kawakami, N.; Shimazu, A.; Takahashi, M.; Inoue, A.; Kan, C.; et al. Altered DNA Methylation Status of Human Brain Derived Neurotrophis Factor Gene Could Be Useful as Biomarker of Depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014, 165, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, N.; Spielberg, J.M.; Logue, M.W.; Wolf, E.J.; Smith, A.K.; Lusk, J.; Hayes, J.P.; Sperbeck, E.; Milberg, W.P.; McGlinchey, R.E.; et al. SKA2 Methylation Is Associated with Decreased Prefrontal Cortical Thickness and Greater PTSD Severity among Trauma-Exposed Veterans. Mol. Psychiatry 2016, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, N.; Wolf, E.J.; Logue, M.W.; Hayes, J.P.; Stone, A.; Griffin, L.M.; Schichman, S.A.; Miller, M.W. EPIGENETIC VARIATION AT SKA2 PREDICTS SUICIDE PHENOTYPES AND INTERNALIZING PSYCHOPATHOLOGY: 2015 Donald F Klein Investigator Award Finalist: Epigenetic Variation at SKA2 Predicts Suicide Phenotypes. Depress. Anxiety 2016, 33, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Smearman, E.L.; Almli, L.M.; Conneely, K.N.; Brody, G.H.; Sales, J.M.; Bradley, B.; Ressler, K.J.; Smith, A.K. Oxytocin Receptor Genetic and Epigenetic Variations: Association With Child Abuse and Adult Psychiatric Symptoms. Child Dev. 2016, 87, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.-E.; Tanti, A.; Gasecka, A.; Barnett-Burns, S.; Kim, J.J.; Zhou, Y.; Chen, G.G.; Wakid, M.; Shaw, M.; Almeida, D.; et al. Association of a History of Child Abuse With Impaired Myelination in the Anterior Cingulate Cortex: Convergent Epigenetic, Transcriptional, and Morphological Evidence. Am. J. Psychiatry 2017, 174, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Carlberg, L.; Kienesberger, K.; Swoboda, P.; Swoboda, M.M.M.; Bernegger, A.; Koller, R.; Inaner, M.; Fuxjäger, M.; Zotter, M.; et al. Oxytocin Receptor Gene Methylation as a Molecular Marker for Severity of Depressive Symptoms in Affective Disorder Patients. BMC Psychiatry 2022, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.M.; Bae, D.; Cho, J.; Smith, A.K.; Nishitani, S. Pathways Linking Adverse Environments to Emerging Adults’ Substance Abuse and Depressive Symptoms: A Prospective Analysis of Rural African American Men. Dev. Psychopathol. 2021, 33, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Reiner, I.C.; Gimpl, G.; Beutel, M.E.; Bakermans-Kranenburg, M.J.; Frieling, H. OXTR-Related Markers in Clinical Depression: A Longitudinal Case–Control Psychotherapy Study. J. Mol. Neurosci. 2022, 72, 695–707. [Google Scholar] [CrossRef]
- Kasi, P.M.; Naqvi, H.A.; Afghan, A.K.; Khawar, T.; Khan, F.H.; Khan, U.Z.; Khuwaja, U.B.; Kiani, J.; Khan, H.M. Coping Styles in Patients with Anxiety and Depression. ISRN Psychiatry 2012, 2012, 128672. [Google Scholar] [CrossRef]
- Horwitz, A.G.; Czyz, E.K.; Berona, J.; King, C.A. Prospective Associations of Coping Styles With Depression and Suicide Risk Among Psychiatric Emergency Patients. Behav. Ther. 2018, 49, 225–236. [Google Scholar] [CrossRef]
- Fletcher, K.; Parker, G.B.; Manicavasagar, V. Coping Profiles in Bipolar Disorder. Compr. Psychiatry 2013, 54, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Au, C.-H.; Wong, C.S.-M.; Law, C.-W.; Wong, M.-C.; Chung, K.-F. Self-Stigma, Stigma Coping and Functioning in Remitted Bipolar Disorder. Gen. Hosp. Psychiatry 2019, 57, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Nitzburg, G.C.; Russo, M.; Cuesta-Diaz, A.; Ospina, L.; Shanahan, M.; Perez-Rodriguez, M.; McGrath, M.; Burdick, K.E. Coping Strategies and Real-World Functioning in Bipolar Disorder. J. Affect. Disord. 2016, 198, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Paans, N.P.G.; Dols, A.; Comijs, H.C.; Stek, M.L.; Schouws, S.N.T.M. Associations between Cognitive Functioning, Mood Symptoms and Coping Styles in Older Age Bipolar Disorder. J. Affect. Disord. 2018, 235, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, A.; Zajączkowska, M.; Talarowska, M.; Gałecki, P. Depression and Ways of Coping with Stress: A Preliminary Study. Med. Sci. Monit. 2013, 19, 1050–1056. [Google Scholar] [CrossRef]
- Roohafza, H.R.; Afshar, H.; Keshteli, A.H.; Mohammadi, N.; Feizi, A.; Taslimi, M.; Adibi, P. What’s the Role of Perceived Social Support and Coping Styles in Depression and Anxiety? J. Res. Med. Sci. 2014. [Google Scholar]
- Bagot, R.C.; Labonté, B.; Peña, C.J.; Nestler, E.J. Epigenetic Signaling in Psychiatric Disorders: Stress and Depression. Dialogues Clin. Neurosci. 2014, 16, 281–295. [Google Scholar] [CrossRef]
- Lee, J.S.; Jaini, P.A.; Papa, F. An Epigenetic Perspective on Lifestyle Medicine for Depression: Implications for Primary Care Practice. Am. J. Lifestyle Med. 2022, 16, 76–88. [Google Scholar] [CrossRef]
- Martins De Carvalho, L.; Chen, W.-Y.; Lasek, A.W. Epigenetic Mechanisms Underlying Stress-Induced Depression. In International Review of Neurobiology; Elsevier, 2021; Vol. 156, pp 87–126. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





