1. Introduction
The Brewer Spent Grain (BSG) is the most abundant by-product of the brewing process. Based on the global beer production quantities, estimated at 1.82 billion hL, about 36.4 million tons of BSG would be available worldwide [
1]. Europe represents the third region in beer production (500.93 million hL), with about 10.019 million tons of BSG; in Italy, about 300,000 tonnes of BSG are produced per year and of these 70% is used in the livestock sector as animal feed, 10% is used in the biogas production, and the remaining 20% disposed in landfills [
2].
Considering that the production forecast for 2030 in EU is about 425 million hL of beer and 8.5 million tonnes of BSG per year, BSG is an interesting biomass resource for the future biorefineries [
1].
The chemical composition of BSG depends on several factors, primarily on the type of barley (or other raw materials) used in malting, the time and technique of harvesting, the quality of the malt, the additives applied in the mashing stage, etc. [
3].
BSG is a lignocellulosic material, its main components are hemicellulose, cellulose, lignin, proteins, and polysaccharides; among them, hemicellulose is the most abundant one. In [
4] BSG was hydrothermally treated to recover a stream concentrated in starch and protein. The obtained streams (83% and 48% solubilization of initial BSG starch and protein content, respectively) were applied as media for the cultivation of different edible fungi:
Aspergillus oryzae,
Neurospora intermedia, and
Rhizopus delemar. Among the tested strains, cultures performed with
N. intermedia produced the biomass with the highest protein content (59.62%). A recent review [
2] reported a detailed chemical composition of BSG in term of major component, minerals, non-essential and essential ammino acids and vitamins, highlighting that BSG is an attractive source of nutrients to obtain products with high added value by means of extraction processes and fermentation. The use of microbial fermentation to enhance brewers’ spent grain nutritional value producing single cell proteins, to enhance its techno-functional properties, and to increase its commercial value has been proposed [
5,
6,
7] in recent years. A fractionation process, combining wet milling, chemical and enzymatic treatment was proposed by [
5] to valorize BSG and simultaneously produce high protein product (HPP) and high fiber product (HFP). Alcalase activity, at the optimal enzyme concentration (20 µL/g BSG), allowed a high protein separation efficiency, 84%, and a protein concentration in HPP of 43% (w/w), almost double than the protein content (23%, w/w) in original BSG. Proteolytic enzymes have been also used in the valorization treatment of spent brewer’s yeast (SBY) [
7]. The protein hydrolysates represent a valuable source of protein and peptides and a main application is in the food industry. The protein hydrolysate obtained by sequential hydrolysis using commercial enzymes, Brauzyn
® and Alcalase™, showed the highest antioxidant properties and total solids content. BSG has been also used as a food additive due to its beneficial nutritional properties, it was demonstrated that the water-extractable components of BSG, which includes arabinoxylans [
8] and β-glucans [
9] contribute to regulate the gut microbiota. In [
10] nutrients, extracted from BSG were used to formulate a novel growth media for yeasts, both unfermented and
Aspergillus oryzae fermented extract were tested on
Rhodosporidium toruloides, a carotenogenic yeast. Growth media, prepared using fermented BSG, were able to fulfill the requirement as a nitrogen source for
R. toruloides growth. Fatty acid and carotenoid production of
R. toruloides in fermented BSG media were comparable to those obtained in yeast extract-peptone (2% w/w glucose) YPD.
Rhizopus oligosporus CCT 4134 was grown on BSG in solid state fermentation (SSF) cultures, under different initial moisture contents and nitrogen sources [
11]. The protein content of fermented BSG was approximately twofold compared to the crude protein content; moreover, the growth of
Rhizopus oligosporus increased the BSG levels of amino acids, vitamins and antioxidants, and reduced the levels of carbohydrates, fats, and dietary fiber.
BSG was used as raw material for
Lactobacillus delbrueckii,
L. pentosus and
L. rhamnosus fermentations to produce lactic acid [
2] or with
Clostridium beijerinckii DSM 6422 for the production of bioethanol and biobutanol [
12].
In recent years, to overcome the use of synthetic pigments that can be hazardous to human health and to the environment, the production of natural pigments from plants and microbial sources has been exploited.
An isolate of
R. glutinis was used in the work of [
13], optimization of the chemical-physical parameters (temperature, initial pH, salt concentration, C/N ratio) in synthetic medium was achieved in flask fermentation, the highest cellular (861 μg·g–1) and volumetric (1.9 mg·L–1) carotenoids content were obtained after 5 days of fermentation.
[
14] reported the production of red pigments by
Monascus purpureus, in a submerged fermentation system using hydrolyzed and detoxified BSG as the raw substrate, for food and pharmaceutical application. Maximum red pigment production (22.25 UA500) was obtained on the 7th day of fermentation at an initial pH value of 6.5 and monosodium glutamate (MSG) as the most effective nitrogen source.
The carotenoid production has been obtained with different strains of
Rhodotorula glutinis and
R. mucilaginosa, cultured on BSG as it is and on BSG syrups [
15]. Submerged batch fermentation of
R. mucilaginosa, in 500 mL flasks, was carried out by [
16], the highest concentrations of total carotenoids (1248.5 μg/L) and biomass (7.9 g/L) were obtained in the medium containing 70 g/L sugar cane molasses and 3.4 g/L corn steep liquor at 168 h. In the fed-batch production (96 hours feed) 30 g/L sugar cane molasses and 6.5 g/L corn steep liquor, a total carotenoid production of 3726 μg/L and biomass concentration of 16 g/L were obtained.
[
17] reported the optimization of carotenoids production by
R. mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor. It was found that Onion peels and mung bean husk are potential cheap substrates to obtain carotenoids in bioreactor fermentation. In the study, the influence of aeration in carotenogenesis from
R. mucilaginosa using agro-industrial waste as substrate was clearly demonstrated. The maximum amount of carotenoid obtained in aerobic fermentation conditions was 819.23 mg/g in comparison to 717.35 mg/g in shake flask under similar conditions. In previous studies, carried out with
R. mucilaginosa [
18] the effects of pH, temperature, aeration rate, initial sugar and ammonium sulphate concentrations, and activator (cotton seed oil and Tween 80) was evaluated. The highest carotenoid concentration (89.0 mg/L) was obtained in presence of molasses sucrose (20 g/L), while the highest product yield (35.0 mg carotenoid/g cell) was achieved when whey lactose (13.2 g/L) was used as the carbon source.
The influence of agitation on total carotenoids concentration and carotenoids production yield were reported for
R. glutinis on different substrate (glucose, molasses sucrose and whey lactose) [
19]. An increase in initial glucose and molasses sucrose concentrations prolonged the yeast growth and enhanced the total carotenoids production. Total carotenoids concentration and carotenoids production yield significantly enhanced with the increasing of the aeration rate up to 2.4 vvm.
The capacity of various oleaginous yeasts,
Rhodotorula spp. and
Sporobolomyces roseus, to co-synthesize high-value biomolecules such as lipids and carotenoids on different raw materials (BSG, pasta processing waste and bakery waste) was investigated in the work of [
20]. An enzymatic pre-treatment of agro-food wastes was applied to obtain a C and N feedstock and recovered sugars were used to formulate a waste-based medium for simultaneous production of carotenoids and lipids. The hydrolysates used as fermentation media provide the necessary nutrients to synthesize carotenoids by selected strains, on the contrary, obtained lipid titers were lower than in the control medium.
In the present work, R. mucilaginosa CBS316 was cultured on BSG extracts and syrups to produce carotenoids in submerged fermentations. The biomass and carotenoid production were optimized in Erlenmeyer flasks and then scaled-up in a lab-scale bioreactor.
3. Results and Discussion
The growth and carotenoid production by
R. mucilaginosa on different BSG extracts and syrups, carried out in multiwell plates, were reported in a previous work. Among the tested media, the cultures with Fs syrup, used as it is or supplemented (s-Fs), were scaled-up in submerged fermentation carried out in 500 mL Erlenmeyer flasks with baffles [
21].
Based on these previously obtained results, i.e., produced biomass and carotenoids, in the present work a new syrup, obtained by extraction in autoclave, supplemented (s-Fsa) or not (Fsa) has been applied in flask tests and then the process has been scaled-up in a stirred tank bioreactor.
3.1. Shake Flask Cultures
The fermentation in flask tests was carried out with Fs and Fsa (total protein 70. 19 ± 7.82 and 154.5±23.8 mg/g BSG, respectively), as it is or supplemented (s-Fs and s-Fsa), using YM medium as control. The culture volume in flask was 200 mL and the temperature was controlled at 25± 0,1°C.
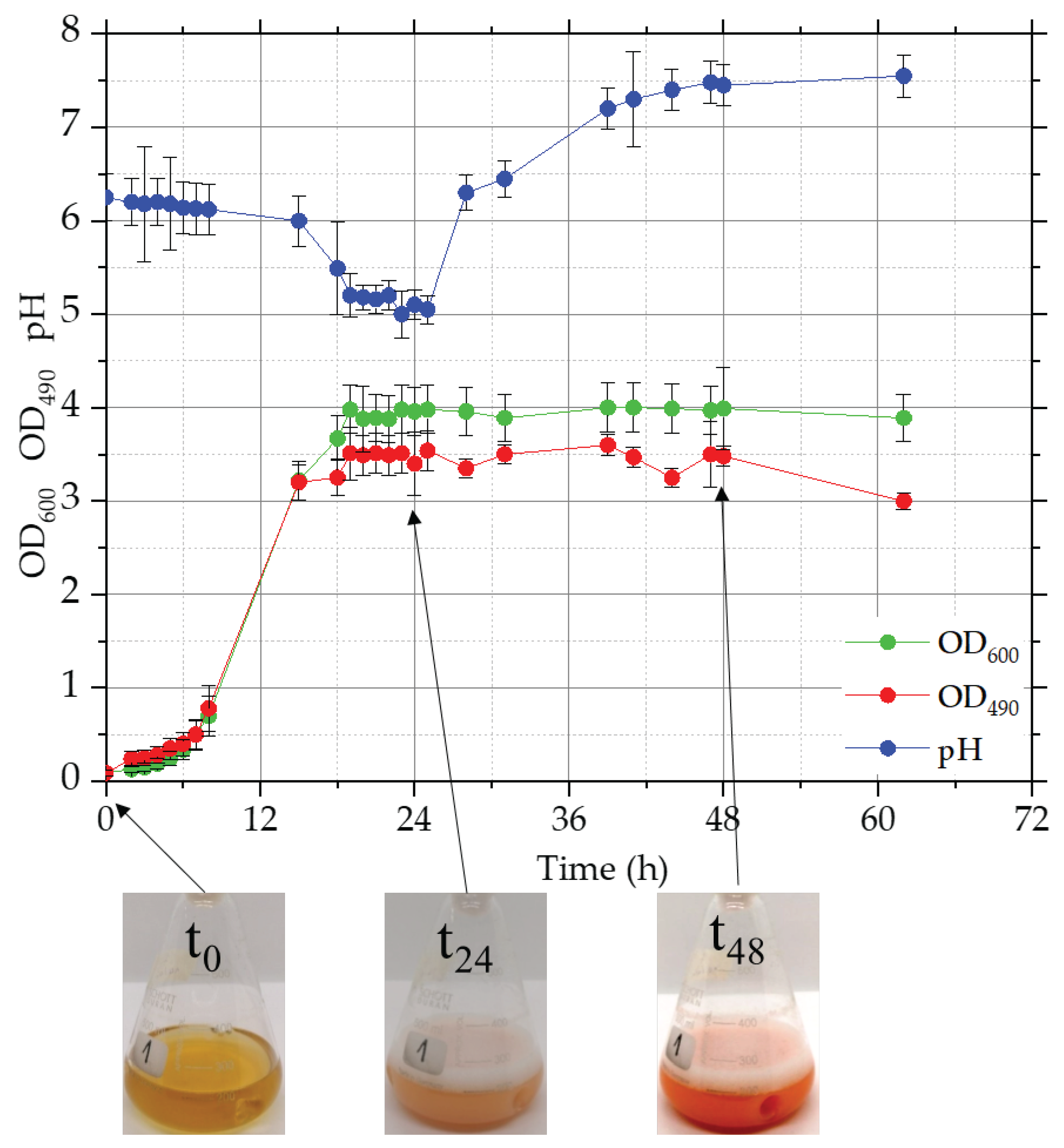
In
Figure 1, the growth behavior in YM culture was reported: the curve showed a lag phase of about 5-7 h and the stationary phase was reached at about 20 h with an OD
600 value of 3.9. The maximum value of biomass dry weight (14,14 ± 0,061) was obtained at 40 h of fermentation. The behavior of the carotenoid curve is very similar to that of biomass, a maximum OD
490 of 3.4 was obtained at 15 h of fermentation and remained constant until the end of the test (72 hours). In
Figure 1, the color intensity of YM cultures, related to carotenoid content, was also compared at three different fermentation times, 0, 24, and 48 h. Finally, as regard the pH is concerned, the initial value was about 6.25 and it decreases until 25 hours with a minimum value of 5.05, after that the pH grows up to 7.5.
In YM medium, the maximum value of total carotenoids, 2098 µg/L, and yield, YC/X, 141.81 µg/g, were obtained after 63 h of fermentation.
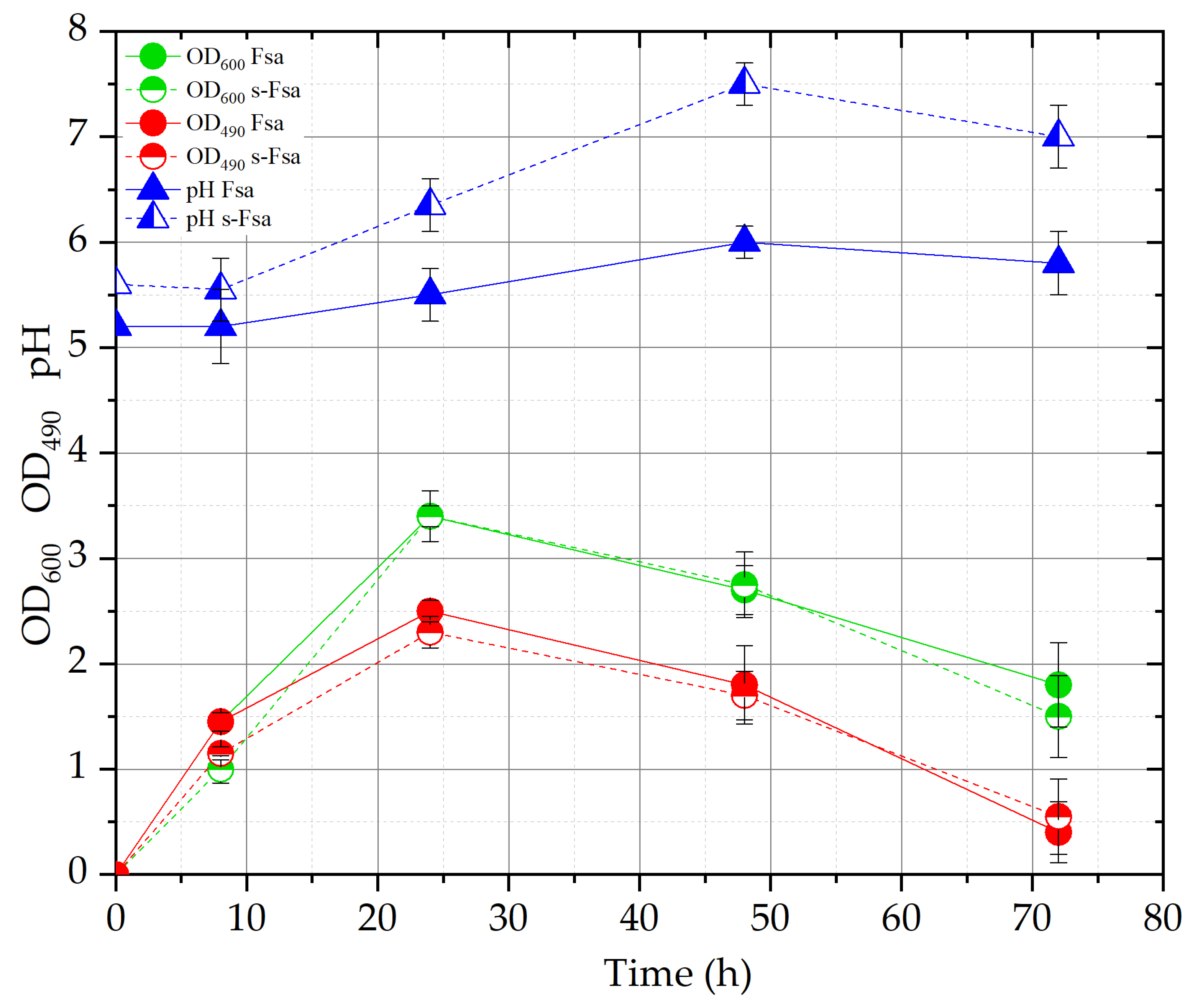
The obtained growth curves for Fsa and s-Fsa were very similar, as previously observed for Fs and s-Fs cultures [
21]. In both cases, the lag phase is almost absent and OD
600 values reach the maximum (about 3.5) at 24 h (
see Figure 2) confirming that, as previously reported for Fs syrup [
21], the addition of YE+PEP to Fsa don’t improve significantly the yeast growth. As it is possible to observe, the initial pH value of the two media was very similar (5.2 and 5.6 for Fsa and s-Fsa, respectively). On the contrary, during the fermentation, the pH behavior was different: in Fsa cultures pH slightly increases (maximum value of 6 at 48 h of fermentation), while in s-Fsa, pH diminishes in the first 8 h, then it increases to 7.5. As reported by [
22] and [
23], the pH value has an influence on the carotenoid production and an acidic pH (4.5-5.5) favors the pigment accumulation; in particular, in the work of [
22], among the tested pH, highest carotenoids contents, 282.5 µg/g and 317.6 µg/g, were obtained at pH equal to 4 and 5, respectively. In the present work, Fsa and s-Fsa syrups, shows very similar initial pH values; nevertheless, Fsa seems to be more advantageous than s-Fsa, as pH control is not necessary in this medium.
For both the tested media, the maximum biomass concentration has been observed at 48 h of incubation, in particular 5.3 and 6.2 g/L for Fsa and s-Fsa were obtained. After that, the biomass concentration diminished in both the media. The maximum values of dry weight biomass are comparable with those previously reported by [
22] on sugar cane molasses, 7.8 g/L, and by [
16], 7.9 g/L in shake-flask cultures in a medium containing sugar cane molasses as substrate and corn steep liquor.
As reported in Material and Methods, the BSG used in the present work was obtained from a recipe composed by 70% of malt and 30% of wheat malt. Considering this complex substrate, a rapid method to measure dissolved sugar content of the aqueous solution was applied, i.e., °Brix determination. In the tested media, the initial values of °Br were comparable, 6.4 for Fsa and 6.8 for s-Fsa as the carbohydrate consumption rate. In fact, at the end of the fermentation a decrease of 1°Br was measured in both the culture media. Finally, as regard the carotenoids absorbance curves (OD490), the highest values were registered at 24 h (2.5 and 2.3 for Fsa and s-Fsa); after that, a decrease was observed in both the cases.
In Fsa medium, the maximum concentration of total carotenoids was obtained at 24 h (832,82 ± 52,38 μg/L), while in s-Fsa it was reached at 72 h (1159,05 ± 70,27 μg/L).
In order to identify the most promising syrup for the process scale-up in bioreactor, the main parameters, obtained in shake flasks fermentation (YM, Fs, Fsa, s-Fs, and s-Fsa) and reported in
Table 2, have been compared.
The maximum carotenoid Yield (YC/X) has been obtained at different time: Fs, s-Fs, and s-Fsa reached the maximum at 72 h of fermentation, while in Fsa culture it was reached earlier, at 24 h. All the obtained YC/X values in BSG syrups were in the range 160-230 μg/g, higher than that obtained in the control medium YM (142 μg/g). The highest value of specific productivity P (μg/g*h) was obtained for Fsa syrup (7.16), two or three-fold higher than that of the other BSG media and YM.
The yield (Y
C/X), obtained in flask test (172 μg/g) at 24 h with
R. mucilaginosa grown on Fsa, was comparable with that of
R. glutinis in molasses medium (144 μg/g), while the carotenoid volumetric productivity was double (0.0346 mg/L*h and 0.015 mg/L*h, respectively) [
24].
The Y
C/X value was also comparable with that reported in the work of [
25], with
R. glutinis (167.23 μg/g), at 120 h of fermentation, in the medium cassava wastewater, supplemented with minerals. Moreover, the concentration of carotenoids was very similar (0.84 mg/L and 0.98 mg/L for
R. mucilaginosa and
R. glutinis, respectively). In the work of [
16], using
R. mucilaginosa, a maximum Y
C/X value of 158 μg/g at 168 h of fermentation was obtained, while [
22] reported a maximum of 321 μg/g after 216 hours.
3.2. Bioreactor Test
Among the specific productivity values, obtained in shake flask tests (see
Table 2), the Fsa syrup showed the higher one 7.16 μg/g*h, consequently it was selected for the scale-up of carotenoid production in a stirred thank bioreactor.
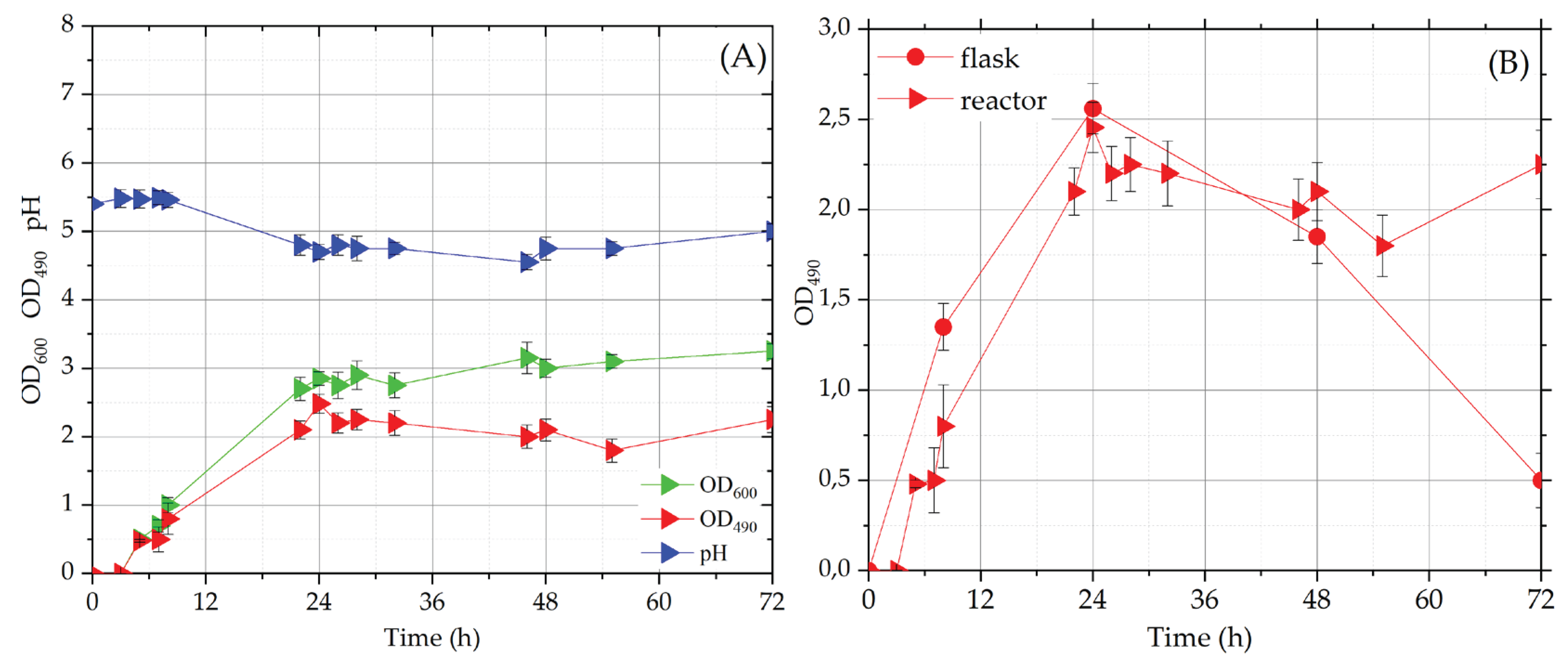
In bioreactor fermentation, temperature was controlled at 25 ± 0,2 °C and pH (initial value 5.5) was monitored, but no external control was applied. During the fermentation, to avoid the foam formation in the bioreactor, the initial aeration rate (200 L/h) was diminished to 100 L/h, after 8 hours. The behavior of biomass, carotenoid, °Brix and pH during fermentation is reported in
Figure 3A. As concerns the biomass (OD
600) and carotenoid (OD
490) curves, it is possible to observe a lag-phase of about 3 hours, after that an exponential phase is evident for both OD values. At 24 hours of fermentation, the stationary phase was reached, when OD
600 and OD
490 values were 2.9 and 2.2, respectively. As expected, pH value diminished in the first 24 hours (4.75) and the minimum (4.6) was reached at 46 hours; also in bioreactor, during the time course of fermentation, the pH value remained in the optimal range for carotenoid production [
22]. The initial value of °Br was 6.4, and, at the end of the fermentation, it diminished to 5.6, similarly to the decrease registered in flask tests.
In
Figure 3B, the behavior of OD
490 in flask and bioreactor tests has been compared: in the first 48 h of fermentation the carotenoid curves showed a very similar shape with comparable OD
490 values, after that the values diminished in flask culture (0.4) while remained the same in bioreactor one (2.2). These results suggest that the acidic pH value influences not only the carotenoid synthesis [
22] but also their maintenance in yeast cells.
In bioreactor test, the maximum biomass concentration (4.29 g/L) was obtained at 72 hours of fermentation, at the same time a carotenoid concentration of 396 µg/L was measured, corresponding to a maximum value of total carotenoid of 871 µg.
The best fermentation parameters in shake flask and bioreactor tests, with Fsa syrup, were obtained at 24 and 48 h, respectively. In flask, the biomass (3.12 g/L) and carotenoid concentration (833 µg/L) were higher than that obtained in bioreactor (2.11 g/L and 238 µg/L), while the total carotenoid value was higher in bioreactor than in flask test (524 and 150 μg, respectively). The lower concentration of the biomass and carotenoids, obtained in bioreactor, was probably due to the sub-optimal aeration of the culture medium (initial value 1.5 vvm), lower than the value reported by [
19] for
R. glutinis, on glucose and molasses sucrose (2.4 vvm). This consideration was confirmed by a previous bioreactor test, carried out at 0.3 vvm (data not reported), in which biomass and carotenoid concentrations, at 24 h, were lower than those obtained in the present test. In this concern, it could be essential to increase the air flow rate, eventually adding a suitable compound to avoid foam formation.
Since the carotenoids are intracellular products, their concentration in bioreactor is dependent from that of the biomass. In order to increase the obtained volumetric productivity P (4.96 μg/L*h), a fed-batch process could be investigated, as reported by [
16].
The carotenoid concentration value obtained in the bioreactor test at 48 hours (238.43 μg/L) was comparable with that of maximum β-carotene (201 μg/l), reported by [
26], with
R. glutinis in fermented radish brine, as a sole substrate, after 24-h fermentation. In the work of [
20], among the different strains tested for the carotenoid production on a BSG hydrolysate medium,
Sporobolomyces roseus CFGU-S005 showed the highest carotenoid yield 270 μg/g, about double of that reported in the present work, 113 μg/g at 48 h, but obtained after a longer fermentation time (96 h).
4. Conclusions
In the present work, a process for the valorization of brewery spent grain was presented. BSG, a lignocellulosic by-product, was applied to produce syrups without any chemical or enzymatic pre-treatment and solely using distilled water as solvent, the extraction was performed at 25°C (Fs) or at 120°C (Fsa). The different type of syrups, supplemented or not with yeast extract and peptone, were used as fermentation media. A process optimization was first carried out in shake flask tests; among the tested media, non-supplemented Fsa syrup allowed to obtain the highest specific productivity value. For this reason, Fsa medium was selected for the process scale-up in a stirred tank bioreactor.
The reported preliminary results, obtained in flask and bioreactor tests, confirmed the opportunity to use BSG syrup for the carotenoids production with the yeast R. mucilaginosa. The volumetric productivity value, obtained in bioreactor test in a batch process, could to be improved applying a fed-batch one.
The proposed carotenoid production process exhibits two main advantages from the environmental and economic perspective: the valorization of a lignocellulosic by-product without the need of pre-treatments and the application of BSG syrup in fermentation as it is, without supplements and pH control.