1. Introduction
Ultrasonography (US) plays a crucial role in the detection of solid pancreatic lesions (SPLs) [
1,
2,
3,
4]. Identification of small hypoechoic masses or dilation of the main pancreatic duct is the key to the early diagnosis of pancreatic cancer [
5,
6]; however, the artifacts generated by abdominal gas and ultrasound attenuation owing to the distance from the body surface have limited the diagnostic application of US. Endoscopic ultrasonography (EUS) is integral to the differential diagnosis and screening of SPLs [
7,
8,
9] owing to its superior spatial resolution and reduced abdominal gas interference. Nevertheless, differentiating various types of SPLs based on the findings of conventional EUS is challenging.
Most lesions are visualized as hypoechoic masses in B-mode EUS, and the dilation of the main pancreatic duct is not sufficient for diagnosing pancreatic cancer [
10]. Computed tomography (CT) is used for the diagnosis of SPL [
2,
11]; however, EUS has not been applied for the vascular evaluation of SPL. Although Doppler imaging provides information regarding the major vessels surrounding the pancreas [
12], the details of minute vessels within the SPLs cannot be obtained via this modality [
13].
Contrast-enhanced EUS (CE-EUS) is used for the differential diagnosis of SPLs [
11,
14,
15,
16,
17,
18,
19,
20]. However, some pancreatic cancer lesions are misdiagnosed as mass-forming pancreatitis (MFP) as they appear iso- or hypervascular on CE-EUS owing to its high sensitivity. Evaluation of the “wash-out sign” [
21] has been recommended to mitigate this issue; nevertheless, these limitations of CE-EUS hinder its usage.
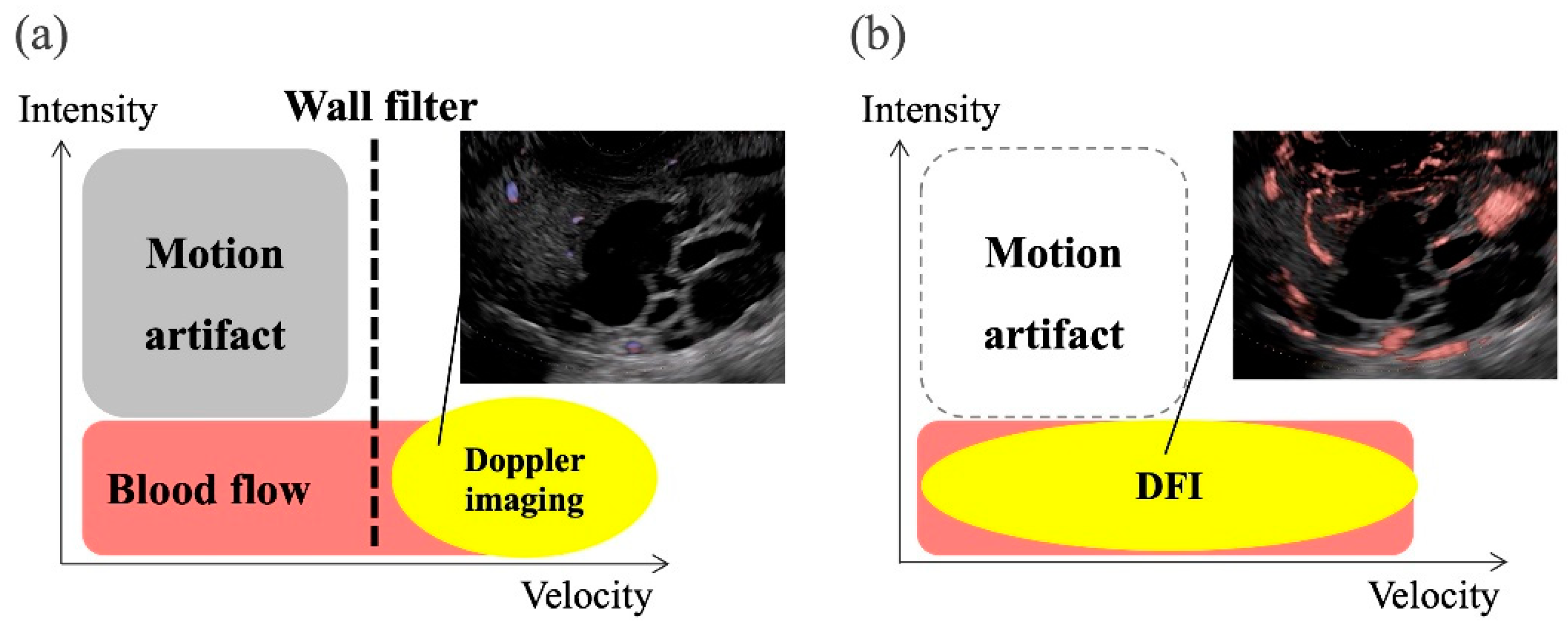
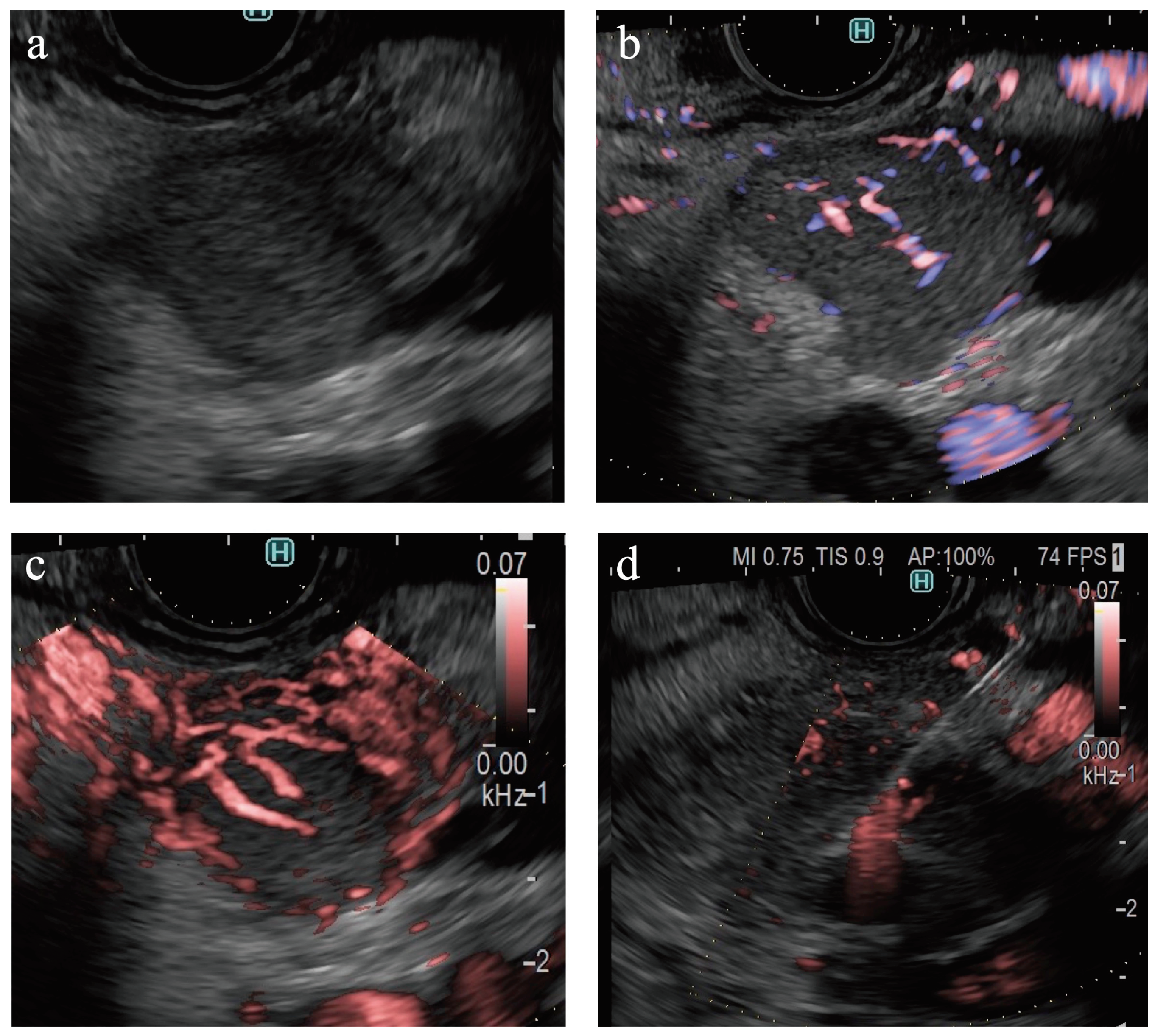
Recently, a novel technology using US, such as superb microvascular imaging (SMI) (Canon Medical System Corporation, Tokyo, Japan), B-flow imaging (GE Healthcare, Milwaukee, WI, US), and detective flow imaging (DFI) (Fujifilm Healthcare, Tokyo, Japan) were developed for the detection of slow-flow vessels (
Figure 1) [
22,
23,
24,
25,
26,
27,
28]. These modalities, which are characterized by a high frame rate, have enabled the visualization of minute tumor vessels while minimizing the incidence of motion artifacts. DFI-EUS is sensitive to the detection of vessels; however, DFI has not been applied for the differential diagnosis of SPLs [
29,
30,
31].
This study aimed to investigate DFI-EUS findings of SPLs and analyze their differential diagnostic accuracy for pancreatic cancer.
2. Materials and Methods
Patients
Medical records of 104 patients with pathologically confirmed SPLs who had undergone EUS examinations between April 2021 and June 2023 were retrospectively analyzed in this study. The inclusion criteria required participants to be over 18 years old, with archived B-mode, eFLOW, and DFI images. Pathological findings were acquired via surgery, EUS-guided tissue acquisition (EUS-TA), and fluoroscopic biopsy using endoscopic retrograde cholangiopancreatography. The diagnosis of benign lesions was confirmed using imaging modalities other than EUS-TA. This study was approved by the Ethics Committee of Yokohama City University (F220900020) and adhered to the ethical standards outlined by the Institutional Research Committee and the latest version of the Declaration of Helsinki. As this study was retrospective, obtaining patient consent was not required, and information was shared through an opt-out process.
EUS Examination
All endoscopic examinations were performed or supervised by experienced endosonographers. EUS was performed using ARIETTA 850 (FUJIFILM Healthcare, Tokyo, Japan) and GF-UCT260 (Olympus Medical Systems, Tokyo, Japan). Propofol, midazolam, and/or diazepam were administered to induce sedation. The doses were adjusted according to the body weights and ages of the patients. The SPLs, including the surrounding pancreatic parenchyma, were scanned using B-mode to acquire high-quality ultrasound images of the scan region. eFocusing was implemented during the acquisition. eFLOW was set to approximately 5.0 cm/s in the velocity range. Subsequently, DFI was used to evaluate the vascular structure within and surrounding the SPLs. The optimal color gain was adjusted based on the size and depth of the SPLs. A region of interest (ROI), including the SPLs and adjacent pancreatic parenchyma, was defined for each SPL. All images were stored in a digital format on an ARIETTA 850 hard disk by each endosonographer. In addition, static vessel images of the largest plane of each SPL were acquired for blinded reading.
Image Evaluation
The long diameter, depth of the SPLs, and frame rates for eFLOW and DFI were determined from the static images. All B-mode, eFLOW, and DFI images were numbered. Three expert endosonographers, each with over 5 years of experience in EUS and 1 year of experience in DFI-EUS, evaluated the images. Clinical information and final diagnoses were blinded.
Figure 1 presents the evaluation criteria. The images were classified as fair or poor regarding evaluability. Echogenicity (hyperechoic, isoechoic, or hypoechoic), border detection (well-defined or indistinct), contour shape (smooth or irregular), and internal echo of the SPLs (homogeneous or heterogeneous) were classified on B-mode images. The presence of vessels (present or absent), vessel distribution (peritumoral or intratumoral), and vessel form (spotty or linear) in the SPLs was classified on eFLOW images. The presence of vessels (present or absent), vascularity (hypervascular or hypovascular), vessel distribution (peritumoral or intratumoral), and vessel shape (spotted or linear) in the SPLs were classified on the images acquired via DFI (
Figure 2). The eFLOW and DFI findings were evaluated only in cases where vessels were present within the SPLs. The “vascularity” on DFI was classified as hypovascular in cases where vessels were not present within the SPLs. When a discrepancy was observed in the findings made by the three readers, the findings selected by the majority of the two were adopted. Diagnostic criteria for pancreatic cancer were formulated based on these findings. The findings of lesions other than pancreatic cancer were also evaluated. Neuroendocrine neoplasms (NEN) and metastasis from renal cell carcinoma (RCC) were collectively classified as “hypervascular tumors” based on historical results [
32,
33,
34].
Statistical Analysis
All statistical analyses were performed using the JMP Pro 17 (SAS Institute Inc., Cary, NC, USA). Categorical variables are presented as frequencies with percentages. Continuous variables are presented as medians and ranges. The frame rate and vessel detection rate of eFLOW and DFI were compared using Student’s t-test and McNemar’s test. Categorical variables for the characteristics and US findings of pancreatic cancer and those of other lesions were compared using the chi-square. Statistical significance was set at p-value < 0.05. Diagnostic criteria for pancreatic cancer were established for each modality based on the significant findings identified via Fisher’s exact test. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were determined based on the diagnostic criteria for pancreatic cancer.
3. Results
Patients’ Characteristics
Table 1 presents the patient characteristics. Among the 104 patients included in this study, 79 (76%), 9 (8.7%), 6 (5.8%), 3 (2.9%), 2 (1.9%), and 1 (1.0%) were diagnosed with pancreatic cancer, MFP, NEN, pancreatic metastasis from renal cell carcinoma, intrapancreatic accessory spleen (IPAS), and malignant lymphoma, respectively. The lesions were located in the pancreatic head and body or tail in 50 (48%) and 54 (52%) patients, respectively. Fifty-six (54%) patients underwent transgastric scanning. The median lesion diameter of lesions was 21 mm (range: 6–53 mm). The depth of the lesions (distance from the echoendoscope to the bottom of the tumor) was 27.5 mm (range: 13–53 mm).
Frame Rate of eFLOW and DFI
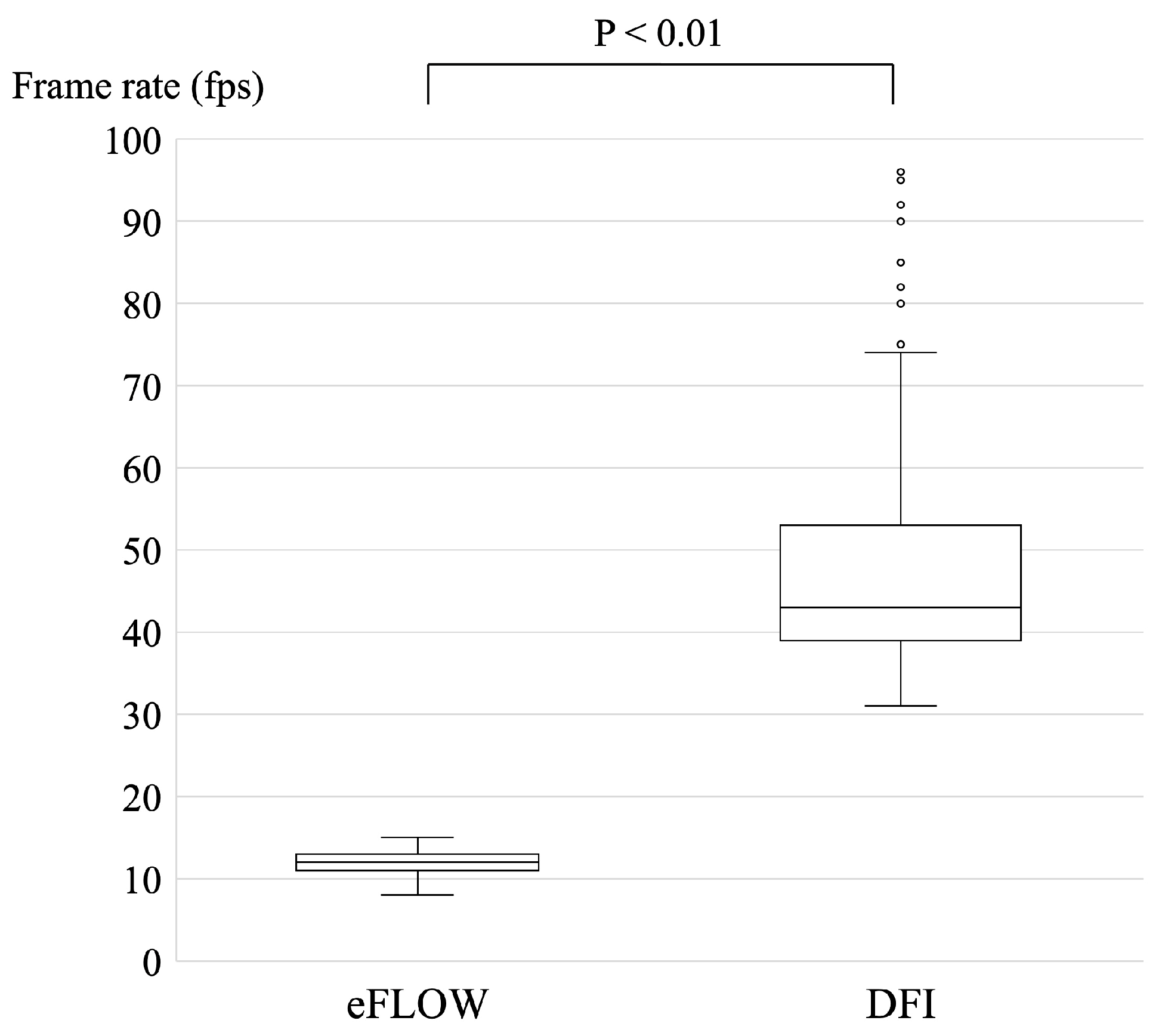
The frame rate was set as 20 frames per second (fps) for B-mode; in contrast, the frame rate was automatically modified according to the size of the ROI for eFLOW and DFI. Each frame rate was recorded and analyzed retrospectively (
Figure 3). The median and range of frame rate of DFI (43 fps [31–96 fps]) were significantly higher than those of eFLOW (12 fps [8–15 fps]) (p < 0.01).
Vessel Detection Rate of eFLOW and DFI
DFI-EUS visualized vessels within the SPLs in 96% (100) of cases, whereas eFLOW achieved this in only 27% (28 patients) of cases. Thus, the vessel detection sensitivity of DFI-EUS was significantly higher than that of eFLOW (p < 0.01).
Findings of Pancreatic Cancer
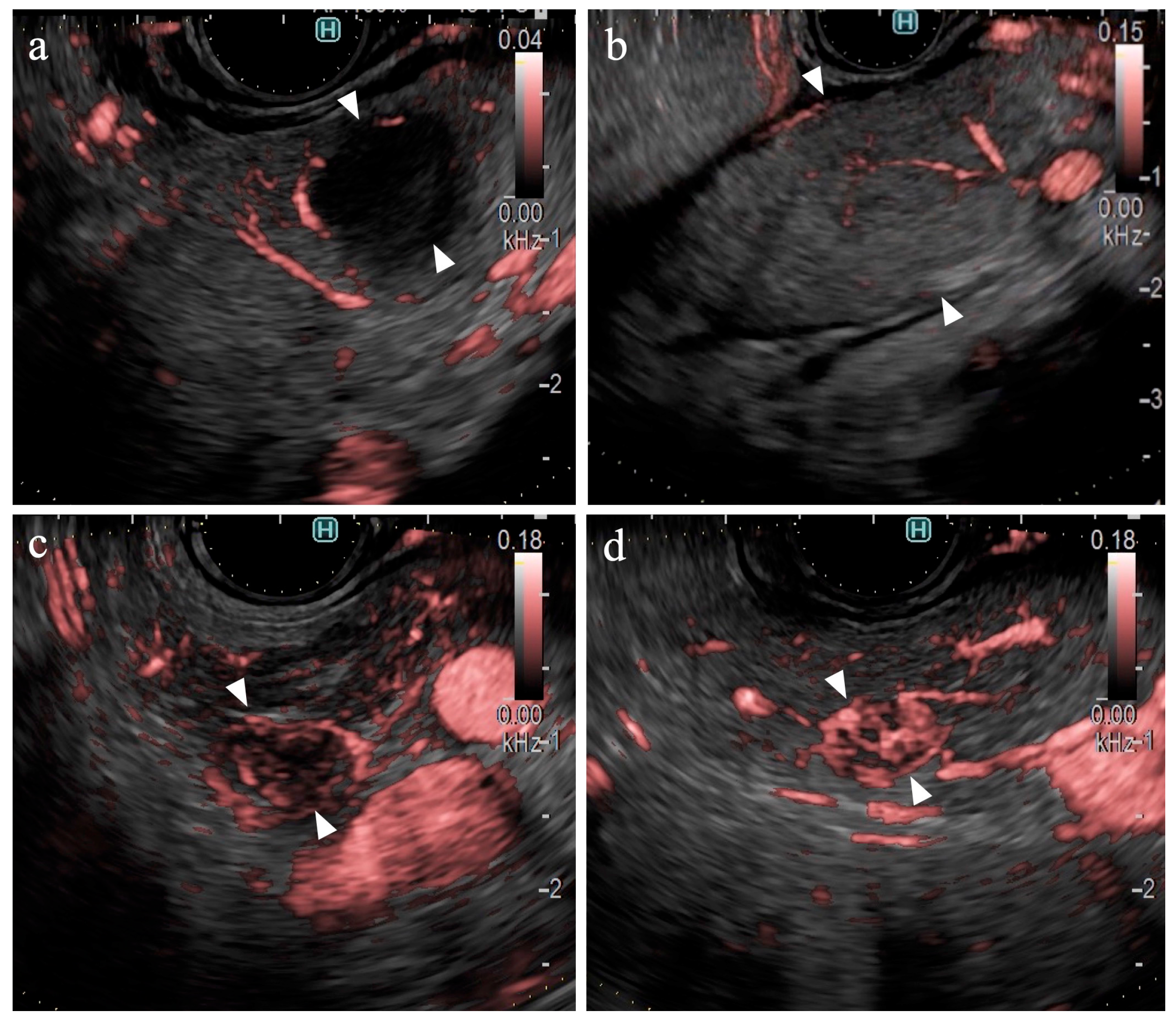
Table 2 presents the result of the univariate analysis of the characteristics and findings of pancreatic cancer and other lesions. Pancreatic cancer was observed significantly more frequently in the pancreatic head (54%, p=0.013) and in areas deeper than 25 mm (63%, p=0.042). The number of large SPLs (> 20 mm) was greater in patients with pancreatic cancer than in other types of pancreatic lesions; however, the difference was not statistically significant. The majority of lesions were classified as hypoechoic lesions on B-mode (98% (102/104), p=0.473). The borders were “well-defined” in 70% of the pancreatic cancer lesions and 81% of other types of lesions (p=0.362). “Irregular contour” and “heterogenic internal echo” were observed in 88% (73/83) and 83% (69/83) of pancreatic cancer lesions, respectively (p < 0.01). eFLOW detected the presence of vessels in 27% (28/104) of lesions only. The shape and distribution of the vessels were classified in cases where vessels were present within the lesion; however, no significant differences were observed. DFI detected the presence of vessels in 96% (100/104) of the lesions. Thus, the detection rate of DFI was significantly higher than that of eFLOW (p < 0.01). Four lesions without vessels were classified as “hypovascular”. Hypovascular lesions were significantly more frequent in patients with pancreatic cancer (p < 0.01). The distribution and shape of the vessels were evaluated in the 100 cases where vessels were present within the lesions. Pancreatic cancer lesions were accompanied by “peritumoral” and “spotty” vessels in 84% (66/79) and 86% (68/79) of cases (
Figure 4a), respectively, significantly more than in other lesions, presenting 38% (8/21) and 43% (9/21) of cases, respectively (p < 0.01).
Diagnostic Criteria for Pancreatic Cancer
The diagnostic criteria for pancreatic cancer were set as “irregular or heterogeneous” and “hypovascular or with peritumoral or spotty vessels” on B-mode and DFI, respectively.
Table 2 presents the number of cases satisfying each criterion.
Table 3 presents the diagnostic accuracy. Although no significant differences were observed, the “absence of vessels” on eFLOW was set as a finding of pancreatic cancer. The sensitivity, specificity, and accuracy of these diagnostic criteria for pancreatic cancer were 94%, 19%, and 44% on B-mode; 76%, 38%, and 68% on eFLOW; and 99%, 43%, and 88% on DFI, respectively.
Findings on Lesions Other Than Pancreatic Cancer
Table 4 presents the findings in the NEN (n=6) and MFP (n=9) groups. No significant differences were observed between the two groups in terms of lesion characteristics. All lesions were hypoechoic on B-mode. No significant differences were observed between the B-mode and eFLOW findings. DFI could visualize vessels within the SPLs in all lesions. Notably, 83% (5/6) of the NEN lesions were hypervascular, whereas all MFP lesions were hypovascular (
Figure 4b), with a statistically significant difference (p < 0.01).
4. Discussion
To the best of our knowledge, this study is the first to demonstrate the utility of DFI-EUS for the differential diagnosis of SPLs. The ability of DFI-EUS to detect vessels within the lesions was significantly higher than that of eFLOW. Pancreatic cancer lesions are characterized by the presence of hypovascular and spotty vessels in the peritumoral region. The sensitivity, specificity, and accuracy of DFI-EUS for the detection of pancreatic cancer were 99%, 43%, and 88%, respectively, which were higher than those of B-mode and eFLOW.
Ultrasound techniques for detecting slow-velocity vessels are unique. Lu R et al. and Bakdik S et al. have reported that SMI was used for the differential diagnosis of thyroid nodules and breast cancer [
22,
26]. The precision of DFI for the identification of vessels is similar to that of SMI; however, only a few studies have been conducted in this field. A novel algorithm that can eliminate motion artifacts from the feature amount of movement derived by evaluating the received signals in the ROI, which vary according to the signal intensity, was used in DFI [
29]. The frame rate used in the DFI setting at 43 fps (range: 31–96 fps) was higher than that of eFLOW in this study.
The ability of eFLOW and DFI to detect the presence of vessels within SPLs was compared in this study. eFLOW facilitates imaging with high spatial resolution without the occurrence of the blooming artifacts in Color Doppler imaging [
12,
13,
35]. However, the detection of vessels within SPLs with eFLOW remains challenging as most pancreatic cancer lesions are hypovascular. A dense fibrotic stroma is observed in pancreatic cancer lesions. Thus, the blood flow within the lesion is limited compared with that in the surrounding pancreatic parenchyma. [
36,
37,
38] The vessel detection rate of eFLOW was only 27% in this study, which is lower than that reported in previous studies. This finding may be attributed to the large proportion of pancreatic cancer lesions and the relatively small size of the hypervascular lesions in the study cohort. The vessel detection rate of DFI was significantly higher (96%) than that of eFLOW. The sensitivity of DFI for the detection of vessels has been reported in several studies; however, data related to SPLs are absent. This study's findings may facilitate the adoption of a new approach for assessing the structure of the vessels within SPLs using DFI-EUS.
Criteria for the classification of vessels within SPLs remain unestablished. In DFI images, heterogeneity, which is useful in CE-EUS [
14,
21], cannot be evaluated. Thus, the distribution and shape of the vessels within SPLs were evaluated in this study. The distribution of the vessels within the SPLs was classified as “peritumoral” or “intratumoral” based on whether the vessels were detected throughout the SPLs. The shape of the vessels was classified as “spotty” or “linear” based on the continuity of the vessel signals. The diagnostic criteria for pancreatic cancer were set to maximize the sensitivity of B-mode, eFLOW, and DFI; thus, the specificity was relatively low. The lower specificity of these criteria may be attributed to the MFP findings. MFP lesions presented with irregular margins and heterogeneity on B-mode and hypovascular with spotty vessels on DFI. These results indicate that DFI can be used to differentiate pancreatic cancer from NEN. However, further studies are warranted to differentiate pancreatic cancer from MFP.
The findings of this study demonstrate the utility of DFI for purposes other than the differential diagnosis of SPLs. DFI enabled the identification of small hypervascular lesions within 10 mm, which was unexpected (
Figure 4c, 4d). The frame rate of DFI is higher than those of B-mode and eFLOW; thus, DFI can be used as a screening evaluation for patients with Multiple Endocrine Neoplasia type 1 or von Hippel-Lindau disease [
39,
40]. Hypersensitivity to vascular structures aided the avoidance of thick vessels during the EUS fine-needle biopsy (EUS-FNB) procedure (
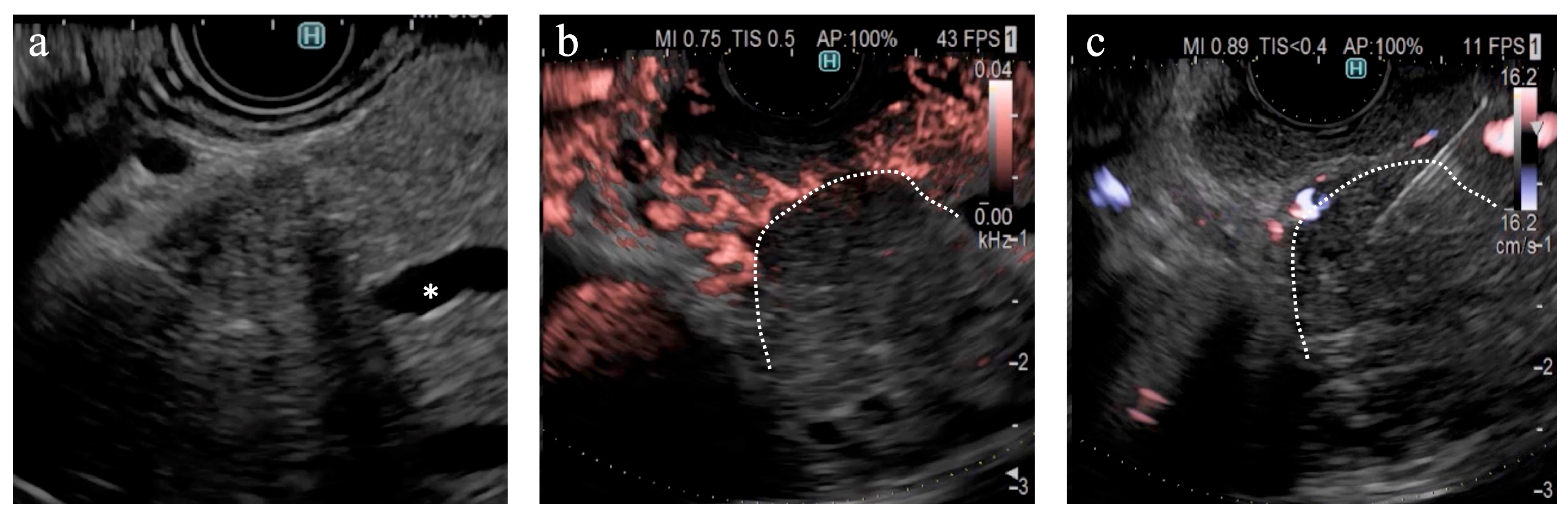
Figure 5). DFI, rather than eFLOW, can be used to define the vessels more precisely in patients with NEN. This technique enables the effective acquisition of tissue samples without major bleeding. The absence of linear vessels in pancreatic cancer lesions can aid the detection of tumor margins (
Figure 6). A pancreatic cancer lesion without a detectable margin was observed on B-mode and eFLOW in this case, and the initial EUS-FNB result was a false negative. Subsequently, DFI was performed, and the normal vessels in the surrounding pancreatic parenchyma near the tumor margin disappeared. The diagnosis was confirmed by puncturing the areas without vessels on DFI. Thus, DFI can be considered a novel tool for determining the differential diagnosis of pancreatic cancer in the future.
This study had some limitations. First, this was a single-center retrospective study with a small sample size; particularly, the number of patients with the benign disease was inadequate. Differences in the epidemiological frequencies of pancreatic cancer and other benign lesions may have affected the results of the statistical analysis. Therefore, further prospective studies must be conducted in the future to establish the diagnostic criteria of pancreatic cancer using DFI-EUS. Second, the images evaluated in this study were static vessel images with the largest planes of the SPLs. The vessel distribution was not homogeneous; thus, the results could differ in other planes. Third, the final diagnosis of the SPLs was not confirmed surgically or by FNB. Although benign lesions can be detected using other imaging modalities, misdiagnosis can alter the diagnostic criteria.
5. Conclusions
In conclusion, DFI-EUS is a superior modality for the detection of pancreatic cancer as it significantly enhances the visualization of vascular structures within the SPLs. This novel vascular imaging modality, which does not require a contrast agent, is more useful for differential diagnosis than B-mode or eFLOW.
Author Contributions
H.M. drafted and prepared the manuscript; H.M., K.S., S.Y., H.Y., K.E., R.O., A.F., H.T., and T.K. contributed to data acquisition; K.S., K.E., and T.K. contributed to the interpretation of the data; K.N. and M.S. contributed to revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Yokohama City University (F220900020).
Informed Consent Statement
Patient consent was waived because this was a retrospective study. Information was shared through an opt-out process.
Data Availability Statement
The datasets generated during and/or analyzed during this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank all the patients involved in this study. We would also like to thank Editage (www.editage. com) for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martínez-Noguera, A.; D’Onofrio, M. Ultrasonography of the Pancreas. 1. Conventional Imaging. Abdom. Imaging 2007, 32, 136–149. [CrossRef]
- D’Onofrio, M.; Gallotti, A.; Pozzi Mucelli, R. Imaging Techniques in Pancreatic Tumors. Expert Rev. Med. Devices 2010, 7, 257–273. [CrossRef]
- Zamboni, G.A.; Ambrosetti, M.C.; D’Onofrio, M.; Pozzi Mucelli, R. Ultrasonography of the Pancreas. Radiol. Clin. North Am. 2012, 50, 395–406. [CrossRef]
- Ciaravino, V.; D’Onofrio, M. Pancreatic Ultrasound: State of the Art. J. Ultrasound Med. 2019, 38, 1125–1137. [CrossRef]
- Tanaka, S.; Nakaizumi, A.; Ioka, T.; Oshikawa, O.; Uehara, H.; Nakao, M.; Yamamoto, K.; Ishikawa, O.; Ohigashi, H.; Kitamra, T. Main Pancreatic Duct Dilatation: A Sign of High Risk for Pancreatic Cancer. Jpn. J. Clin. Oncol. 2002, 32, 407–411. [CrossRef]
- Hanada, K.; Amano, H.; Abe, T. Early Diagnosis of Pancreatic Cancer: Current Trends and Concerns. Ann Gastroenterol Surg 2017, 1, 44–51. [CrossRef]
- Fusaroli, P.; Kypraios, D.; Caletti, G.; Eloubeidi, M.A. Pancreatico-Biliary Endoscopic Ultrasound: A Systematic Review of the Levels of Evidence, Performance and Outcomes. World J. Gastroenterol. 2012, 18, 4243–4256. [CrossRef]
- Teshima, C.W.; Sandha, G.S. Endoscopic Ultrasound in the Diagnosis and Treatment of Pancreatic Disease. World J. Gastroenterol. 2014, 20, 9976–9989. [CrossRef]
- Dietrich, C.F.; Shi, L.; Koch, J.; Löwe, A.; Dong, Y.; Cui, X.; Worni, M.; Jenssen, C. Early Detection of Pancreatic Tumors by Advanced EUS Imaging. Minerva Gastroenterol. 2022, 68, 133–143. [CrossRef]
- Osher, E.; Scapa, E.; Klausner, J.; Greenman, Y.; Tordjman, K.; Melhem, A.; Nachmany, I.; Sofer, Y.; Geva, R.; Blachar, A.; et al. Pancreatic Incidentaloma: Differentiating nonfunctioning pancreatic neuroendocirne tumors from intrapancreatic accessory spleen. Endocr. Pract. 2016, 22, 773–779. [CrossRef]
- Yan, X.; Lv, K.; Xiao, M.; Tan, L.; Gui, Y.; Zhang, J.; Chen, X.; Jia, W.; Li, J. The Diagnostic Performance of Contrast-Enhanced Ultrasound versus Contrast-Enhanced Computed Tomography for Pancreatic Carcinoma: A Systematic Review and Meta-Analysis. Transl. Cancer Res. 2022, 11, 3645–3656. [CrossRef]
- Das, K.; Kudo, M.; Kitano, M.; Sakamoto, H.; Komaki, T.; Takagi, T.; Yamao, K. Diagnostic Value of Endoscopic Ultrasound-Guided Directional EFLOW in Solid Pancreatic Lesions. J. Med. Ultrason. 2013, 40, 211–218. [CrossRef]
- Kobayashi, G.; Fujita, N.; Noda, Y.; Ito, K.; Horaguchi, J.; Koshida, S.; Kanno, Y.; Ogawa, T.; Masu, K.; Michikawa, Y. Vascular Image in Autoimmune Pancreatitis by Contrast-Enhanced Color-Doppler Endoscopic Ultrasonography: Comparison with Pancreatic Cancer. Endosc Ultrasound 2014, 3, S13. [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of Small Solid Tumors in the Pancreas: The Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [CrossRef]
- Kwek, B.E.A.; Ang, T.L.; Seo, D.W.; Imazu, H. Contrast-Enhanced Harmonic Endoscopic Ultrasonography of Solid Pancreatic Lesions. Endosc Ultrasound 2013, 2, 142–147. [CrossRef]
- Kitano, M.; Kamata, K.; Imai, H.; Miyata, T.; Yasukawa, S.; Yanagisawa, A.; Kudo, M. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Pancreatobiliary Diseases. Dig. Endosc. 2015, 27 Suppl 1, 60–67. [CrossRef]
- Dietrich, C.F.; Sahai, A.V.; D’Onofrio, M.; Will, U.; Arcidiacono, P.G.; Petrone, M.C.; Hocke, M.; Braden, B.; Burmester, E.; Möller, K.; et al. Differential Diagnosis of Small Solid Pancreatic Lesions. Gastrointest. Endosc. 2016, 84, 933–940. [CrossRef]
- Kitano, M.; Yamashita, Y. New Imaging Techniques for Endoscopic Ultrasonography: Contrast-Enhanced Endoscopic Ultrasonography. Gastrointest. Endosc. Clin. N. Am. 2017, 27, 569–583. [CrossRef]
- He, X.-K.; Ding, Y.; Sun, L.-M. Contrast-Enhanced Endoscopic Ultrasound for Differential Diagnosis of Pancreatic Cancer: An Updated Meta-Analysis. Oncotarget 2017, 8, 66392–66401. [CrossRef]
- Omoto, S.; Kitano, M.; Fukasawa, M.; Ashida, R.; Kato, H.; Shiomi, H.; Sugimori, K.; Kanno, A.; Chiba, Y.; Takano, S.; et al. Tissue Harmonic versus Contrast-Enhanced Harmonic Endoscopic Ultrasonography for the Diagnosis of Pancreatic Tumors: Prospective Multicenter Study. Dig. Endosc. 2022, 34, 198–206. [CrossRef]
- Miwa, H.; Numata, K.; Sugimori, K.; Kaneko, T.; Sakamaki, K.; Ueda, M.; Fukuda, H.; Tanaka, K.; Maeda, S. Differential Diagnosis of Solid Pancreatic Lesions Using Contrast-Enhanced Three-Dimensional Ultrasonography. Abdom. Imaging 2014, 39, 988–999. [CrossRef]
- Lu, R.; Meng, Y.; Zhang, Y.; Zhao, W.; Wang, X.; Jin, M.; Guo, R. Superb Microvascular Imaging (SMI) Compared with Conventional Ultrasound for Evaluating Thyroid Nodules. BMC Med. Imaging 2017, 17, 65. [CrossRef]
- Yamanaka, Y.; Ishida, H.; Naganuma, H.; Komatsuda, T.; Miyazawa, H.; Miyauchi, T.; Takahashi, S.; Tozawa, T.; Enomoto, K. Superb Microvascular Imaging (SMI) Findings of Splenic Artery Pseudoaneurysm: A Report of Two Cases. J. Med. Ultrason. 2018, 45, 515–523. [CrossRef]
- Liu, W.; Zhou, P.; Zhao, Y.; Tian, S.; Wu, X. Superb Microvascular Imaging Compared with Contrast-Enhanced Ultrasound for Assessing Laser Ablation Treatment of Benign Thyroid Nodules. Biomed Res. Int. 2018, 2018, 1025657. [CrossRef]
- Miwa, H.; Numata, K.; Sugimori, K.; Kaneko, T.; Maeda, S. Vascular Evaluation Using Transabdominal Ultrasound for Gallbladder Polyps. J. Med. Ultrason. 2021, 48, 159–173. [CrossRef]
- Bakdik, S.; Arslan, S.; Oncu, F.; Durmaz, M.S.; Altunkeser, A.; Eryilmaz, M.A.; Unlu, Y. Effectiveness of Superb Microvascular Imaging for the Differentiation of Intraductal Breast Lesions. Med. Ultrason. 2018, 20, 306–312. [CrossRef]
- Hofmann, A.G.; Mlekusch, I.; Wickenhauser, G.; Assadian, A.; Taher, F. Clinical Applications of B-Flow Ultrasound: A Scoping Review of the Literature. Diagnostics (Basel) 2023, 13. [CrossRef]
- Matsui, M.; Jikuzono, T.; Kure, S.; Ishibashi, O.; Akasu, H.; Sugitani, I. Usefulness of Ultrasonographic Detective Flow Imaging to Diagnose Parathyroid Tumors: Two Case Reports. J. Nippon Med. Sch. 2022. [CrossRef]
- Yamashita, Y.; Yoshikawa, T.; Yamazaki, H.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Ida, Y.; Maekita, T.; et al. A Novel Endoscopic Ultrasonography Imaging Technique for Depicting Microcirculation in Pancreatobiliary Lesions without the Need for Contrast-Enhancement: A Prospective Exploratory Study. Diagnostics (Basel) 2021, 11. [CrossRef]
- Yamashita, Y.; Yoshikawa, T.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ida, Y.; Maekita, T.; Iguchi, M.; Murata, S.I.; et al. Novel Endoscopic Ultrasonography Imaging Technique for Visualizing Microcirculation without Contrast Enhancement in Subepithelial Lesions: Prospective Study. Dig. Endosc. 2021, 33, 955–961. [CrossRef]
- Miwa, H.; Sugimori, K.; Maeda, S. Vessel Images of Gallbladder Polypoid Lesions on Detective Flow Imaging Endoscopic Ultrasonography. Dig. Endosc. 2023, 35, e61–e62. [CrossRef]
- Balaban, D.V.; Coman, L.; Marin, F.S.; Balaban, M.; Tabacelia, D.; Vasilescu, F.; Costache, R.S.; Jinga, M. Metastatic Renal Cell Carcinoma to Pancreas: Case Series and Review of the Literature. Diagnostics (Basel) 2023, 13. [CrossRef]
- Ciaravino, V.; De Robertis, R.; Tinazzi Martini, P.; Cardobi, N.; Cingarlini, S.; Amodio, A.; Landoni, L.; Capelli, P.; D’Onofrio, M. Imaging Presentation of Pancreatic Neuroendocrine Neoplasms. Insights Imaging 2018, 9, 943–953. [CrossRef]
- Shankar, P.R.; Wasnik, A.P.; Al-Hawary, M.M.; Francis, I.R.; Kaza, R.K. Hypervascular Pancreatic “Lesions”: A Pattern-Based Approach to Differentiation. Abdom Radiol (NY) 2018, 43, 1013–1028. [CrossRef]
- Hwang, J.S.; Seo, D.-W.; So, H.; Ko, S.W.; Joo, H.D.; Oh, D.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; et al. Clinical Utility of Directional EFLOW Compared with Contrast-Enhanced Harmonic Endoscopic Ultrasound for Assessing the Vascularity of Pancreatic and Peripancreatic Masses. Pancreatology 2022, 22, 130–135. [CrossRef]
- Erkan, M.; Hausmann, S.; Michalski, C.W.; Schlitter, A.M.; Fingerle, A.A.; Dobritz, M.; Friess, H.; Kleeff, J. How Fibrosis Influences Imaging and Surgical Decisions in Pancreatic Cancer. Front. Physiol. 2012, 3, 389. [CrossRef]
- Erkan, M.; Hausmann, S.; Michalski, C.W.; Fingerle, A.A.; Dobritz, M.; Kleeff, J.; Friess, H. The Role of Stroma in Pancreatic Cancer: Diagnostic and Therapeutic Implications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 454–467. [CrossRef]
- Fukui, H.; Onishi, H.; Nakamoto, A.; Tsuboyama, T.; Ota, T.; Yano, K.; Enchi, Y.; Yamada, D.; Takeda, Y.; Kobayashi, S.; et al. Pancreatic Fibrosis by Extracellular Volume Fraction Using Contrast-Enhanced Computed Tomography and Relationship with Pancreatic Cancer. Eur. J. Radiol. 2022, 156, 110522. [CrossRef]
- Al-Salameh, A.; Cadiot, G.; Calender, A.; Goudet, P.; Chanson, P. Clinical Aspects of Multiple Endocrine Neoplasia Type 1. Nat. Rev. Endocrinol. 2021, 17, 207–224. [CrossRef]
- Zwolak, A.; Świrska, J.; Tywanek, E.; Dudzińska, M.; Tarach, J.S.; Matyjaszek-Matuszek, B. Pancreatic Neuroendocrine Tumours in Patients with von Hippel-Lindau Disease. Endokrynol. Pol. 2020, 71, 256–259. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).