Submitted:
28 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Translation
2.3. Statistical Analysis
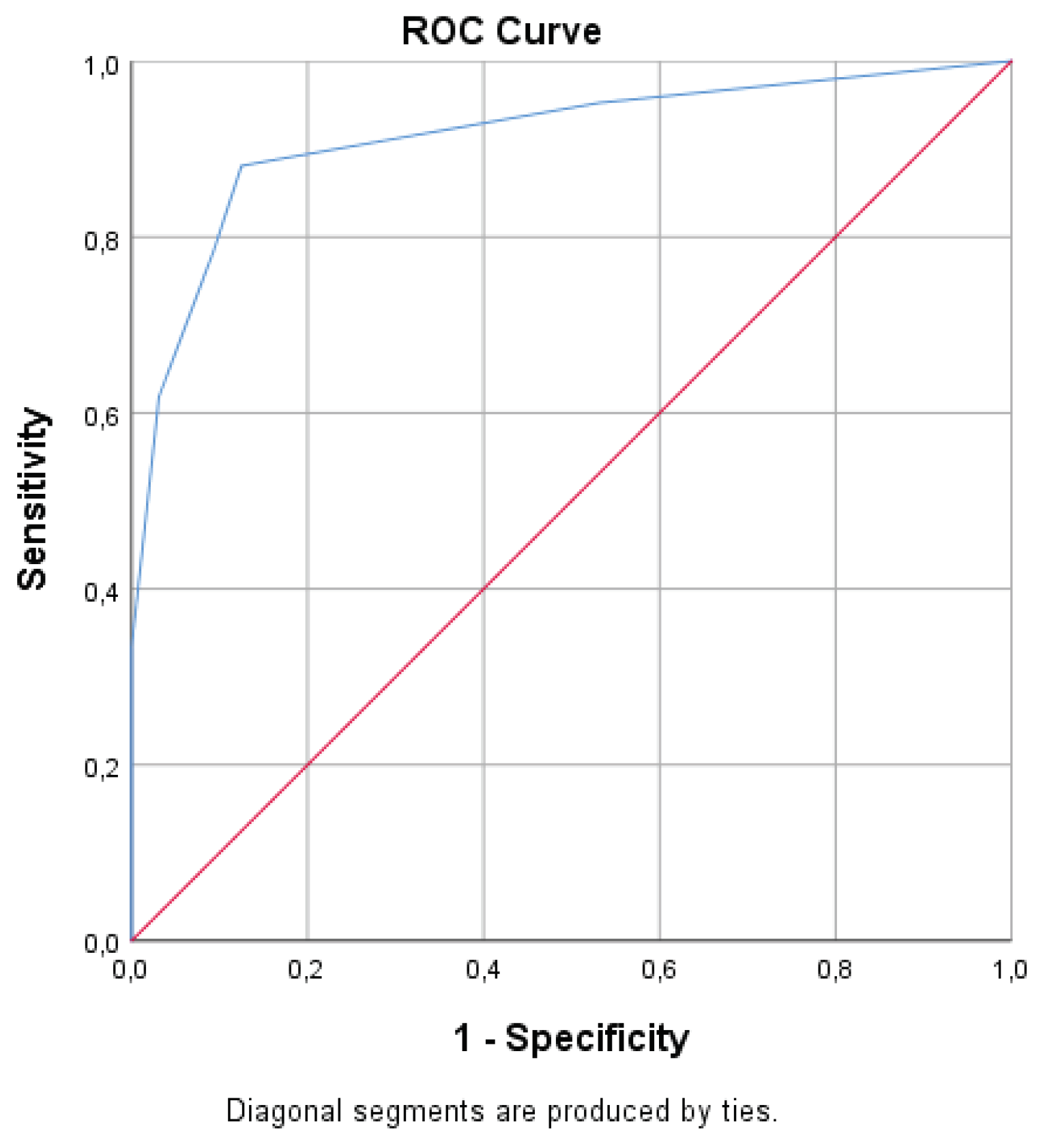
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly People. The Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Mañas, L.; García-Sánchez, I.; Hendry, A.; Bernabei, R.; Roller-Wirnsberger, R.; Gabrovec, B.; Liew, A.; Carriazo, A.M.; Redon, J.; Galluzzo, L.; Viña, J.; Antoniadou, E.; Targowski, T.; di Furia, L.; Lattanzio, F.; Bozdog, E.; Telo, M. Key Messages for a Frailty Prevention and Management Policy in Europe from the Advantage Joint Action Consortium. The journal of nutrition, health & aging 2018, 22, 892–897. [Google Scholar] [CrossRef]
- Santos-Eggimann, B.; Cuenoud, P.; Spagnoli, J.; Junod, J. Prevalence of Frailty in Middle-Aged and Older Community-Dwelling Europeans Living in 10 Countries. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2009, 64A, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Gabrovec, B.; Antoniadou, E. European Guide for Management of Frailty at Individual Level Including Recommendations and Roadmap. ADVANTAGE project, 2019; ISBN 978-961-7002-88-1. [Google Scholar]
- Leng, S.; Chen, X.; Mao, G. Frailty Syndrome: An Overview. Clinical Interventions in Aging 2014, 9, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; McBurnie, M.A. Frailty in Older Adults: Evidence for a Phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Dupoux, F. World Report on Ageing and HeAltH; 2015. Available online: http://apps.who.int/iris/bitstream/10665/186463/1/9789240694811_eng.pdf?ua=1.
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; Fried, L.P.; Guralnik, J.M.; Katz, P.R.; Malmstrom, T.K.; McCarter, R.J.; Gutierrez Robledo, L.M.; Rockwood, K.; von Haehling, S.; Vandewoude, M.F.; Walston, J. Frailty Consensus: A Call to Action. Journal of the American Medical Directors Association 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty Index as a Predictor of Mortality: A Systematic Review and Meta-Analysis. Age and Ageing 2017, 47, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Graham, J.E.; Mogilner, A.J.; Rockwood, K. ; Frailty, Fitness and Late-Life Mortality in Relation to Chronological and Biological Age. BMC Geriatrics 2002, 2. [Google Scholar] [CrossRef]
- Rockwood, K. A Global Clinical Measure of Fitness and Frailty in Elderly People. Canadian Medical Association Journal 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Fugate Woods, N.; LaCroix, A.Z.; Gray, S.L.; Aragaki, A.; Cochrane, B.B.; Brunner, R.L.; Masaki, K.; Murray, A.; Newman, A.B. Frailty: Emergence and Consequences in Women Aged 65 and Older in the Women’s Health Initiative Observational Study. Journal of the American Geriatrics Society 2005, 53, 1321–1330. [Google Scholar] [CrossRef]
- Gutiérrez-Valencia, M.; Izquierdo, M.; Cesari, M.; Casas-Herrero, Á.; Inzitari, M.; Martínez-Velilla, N. The Relationship between Frailty and Polypharmacy in Older People: A Systematic Review. British Journal of Clinical Pharmacology 2018, 84, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. Journal of the American Geriatrics Society 2010, 58, 681–687. [Google Scholar] [CrossRef]
- Watts, P.N.; Blane, D.; Netuveli, G. Minimum Income for Healthy Living and Frailty in Adults over 65 Years Old in the English Longitudinal Study of Ageing: A Population-Based Cohort Study. BMJ Open 2019, 9, e025334. [Google Scholar] [CrossRef]
- Shrier, W.; Dewar, C.; Parrella, P.; Hunt, D.; Hodgson, L.E. Agreement and Predictive Value of the Rockwood Clinical Frailty Scale at Emergency Department Triage. Emergency Medicine Journal 2020, 38. [Google Scholar] [CrossRef]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A Scoping Review of the Clinical Frailty Scale. BMC Geriatrics 2020, 20. [Google Scholar] [CrossRef]
- Basic, D.; Shanley, C. Frailty in an Older Inpatient Population. Journal of Aging and Health 2014, 27, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Szewieczek, J.; Bieniek, J.; Wilczyński, K. Fried Frailty Phenotype Assessment Components as Applied to Geriatric Inpatients. Clinical Interventions in Aging 2016, 11, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, O.; O’Neill, A.; Barry, L.; Leahy, A.; Robinson, K.; O’Connor, M.; Galvin, R. The Diagnostic and Predictive Accuracy of the PRISMA-7 Screening Tool for Frailty in Older Adults: A Systematic Review Protocol. HRB Open Research 2020, 3, 26. [Google Scholar] [CrossRef]
- Lawley, D.N.; Maxwell, A.E. Factor Analysis as a Statistical Method, 2nd ed.; Butterworths: London, 1971. [Google Scholar]
- Bryant, F.B.; Yarnold, P.R. Principal-components analysis and exploratory and confirmatory factor analysis. In Reading and understanding multivariate statistics; Grimm, L.G., Yarnold, P.R., Eds.; American Psychological Association, 1995; pp. 99–136. [Google Scholar]
- Suhr, D. Exploratory or Confirmatory Factor Analysis. In SAS Users Group International Conference, San Fransisco, March 2006, Cary: SAS Institute Inc, pp. 1–17.
- Tsang, S.; Royse, C.; Terkawi, A. Guidelines for Developing, Translating, and Validating a Questionnaire in Perioperative and Pain Medicine. Saudi Journal of Anaesthesia 2017, 11, 80. [Google Scholar] [CrossRef]
- Vrettos, I.; Voukelatou, P.; Panayiotou, S.; Kyvetos, A.; Kalliakmanis, A.; Makrilakis, K.; Sfikakis, P.P.; Niakas, D. Validation of the Revised 9-Scale Clinical Frailty Scale (CFS) in Greek Language. BMC Geriatrics 2021, 21. [Google Scholar] [CrossRef]
- Li, F.; He, H. Assessing the Accuracy of Diagnostic Tests. Shanghai Archives of Psychiatry 2018, 30, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F. Multivariate Data Analysis; Prentice-Hall: Upper Saddle River, Nj, Englewood Cliffs, 1998. [Google Scholar]
- Costello, A.B.; Osborne, J.W. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Practical Assessment. Research & Evaluation 2005, 10, 1–9. [Google Scholar]
- Raîche, M.; Hébert, R.; Dubois, M.-F. PRISMA-7: A case-finding tool to identify older adults with moderate to severe disabilities. Archives of Gerontology and Geriatrics 2008, 47, 9–18. [Google Scholar]
- EMA. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-physical-frailty-instruments-baseline-characterisation-older-populations-clinical-trials-first-version_en.pdf (accessed on 12 Marrch 2024).
- Melchiorre, M.G.; Chiatti, C.; Lamura, G.; Torres-Gonzales, F.; Stankunas, M.; Lindert, J.; Ioannidi-Kapolou, E.; Barros, H.; Macassa, G.; Soares, J.F.J. Social Support, Socio-Economic Status, Health and Abuse among Older People in Seven European Countries. PLoS ONE 2013, 8, e54856. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Ilhan, B.; Erdogan, T.; Catikkas, N.M.; Karan, M.A.; Drey, M.; Gasowski, J.; Kotsani, M.; Piotrowicz, K.; Morley, J. Simpler Modified Fried Frailty Scale as a Practical Tool to Evaluate Physical Frailty: Methodological Report for Its Cross-Cultural Adaptation and Validation. Experimental Gerontology 2022, 166, 111887. [Google Scholar] [CrossRef] [PubMed]
- Fairhall, N.; Aggar, C.; Kurrle, S.E.; Sherrington, C.; Lord, S.; Lockwood, K.; Monaghan, N.; Cameron, I.D. Frailty Intervention Trial (FIT). BMC Geriatrics 2008, 8. [Google Scholar] [CrossRef]
- Van der Elst, M.C.J.; Schoenmakers, B.; Op Het Veld, L.P.M.; De Roeck, E.E.; Van der Vorst, A.; Schols, J.M.G.A.; De Lepeleire, J.; Kempen, G.I.J.M. D-SCOPE Consortium. Validation of replacement questions for slowness and weakness to assess the Fried Phenotype: a cross-sectional study. Eur Geriatr Med. 2020, 11, 793–801. [Google Scholar] [CrossRef] [PubMed]
- IPAQ - . Available online: https://sites.google.com/view/ipaq/download?authuser=0. (accessed on 12 March 2024).
- Sylvia, L.G.; Bernstein, E.E.; Hubbard, J.L.; Keating, L.; Anderson, E.J. Practical guide to measuring physical activity. J Acad Nutr Diet. 2014, 114, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Barthel Index (BI) – Strokengine. Available online: https://strokengine.ca/en/assessments/barthel-index-bi. (accessed on 12 March 2024).
- Sainsbury, A.; Seebass, G.; Bansal, A.; Young, J.B. Reliability of the Barthel Index when used with older people. Age Ageing. 2005, 34, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Kotsani, M.; Avgerinou, C.; Haidich, A.B.; Smyrnakis, E.; Soulis, G.; Papageorgiou, D.I.; Andreou, M.; Zeimbekis, D.; Kokkali, S.; Gavana, M. Aristotle University of Thessaloniki Primary Health Care Research Network. Feasibility and impact of a short training course on frailty destined for primary health care professionals. Eur Geriatr Med. 2021, 12, 333–346. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) | |
| Sex | Male | 30 (40.5) |
| Female | 44 (59.5) | |
| Age (mean±SD; 80.47±7.45 years) | 65-70 | 8 (10.8) |
| 71-75 | 11 (14.9) | |
| 76-80 | 21 (28.4) | |
| 81-85 | 13 (17.6) | |
| 86-90 | 13 (17.6) | |
| >90 | 8 (10.8) | |
| Marital status | Single | 3 (4.1) |
| Married | 49 (66.2) | |
| Widowed | 20 (27.0) | |
| Divorced | 2 (2.7) | |
| Educational level | Primary school | 16 (21.6) |
| Junior/High school | 31 (41.9) | |
| Higher (Technical school) | 7 (9.5) | |
| Highest (University) | 16 (21.6) | |
| Post graduate/PhD | 4 (5.4) | |
| Smoking | Never | 36 (48.6) |
| Ex | 26 (35.1) | |
| Current | 12 (16.2) | |
| Orthostatic hypotension | Yes | 11 (14.9) |
| No | 63 (85.1) | |
| Rotated Component Matrix | ||
| Component | ||
| 1 | 2 | |
| Prisma1 | 0.675 | |
| Prisma3 | 0.837 | |
| Prisma4 | 0.855 | |
| Prisma5 | 0.727 | |
| Prisma7 | 0.818 | |
| Prisma2 | 0.995 | |
| Measures | Mean | SD | Min-Max |
|---|---|---|---|
| PRISMA 7 | 2.43 | 2.00 | 0 – 6 |
| CFS | 3.68 | 1.07 | 1 – 7 |
| FRIED | 1.11 | 1.28 | 0 – 5 |
| SPPB | 7.89 | 3.70 | 1 – 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





