Submitted:
28 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Angiogenesis
1.2. Chemokines
2. Chemokine CX3CL1 (Fractalkine, FKN)
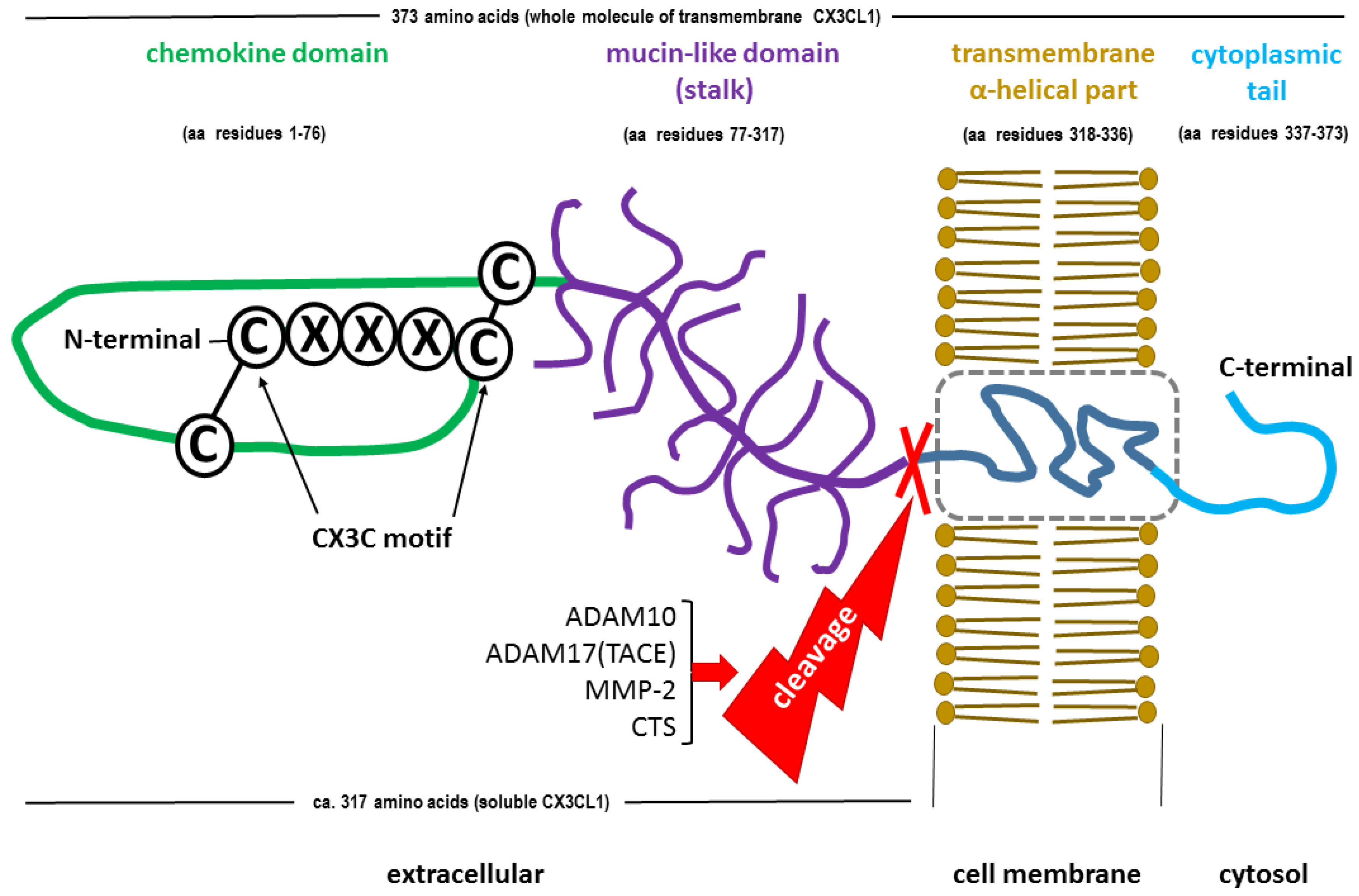
2.1. CX3CL1 Structure
2.2. CX3CL1 Function
2.2.1. Membrane-Bound CX3CL1 (mFKN) as an Adhesion Molecule
2.2.2. Soluble CX3CL1 (sFKN) as a Chemoattractant
3. CX3CR1 – the Sole Fractalkine Receptor
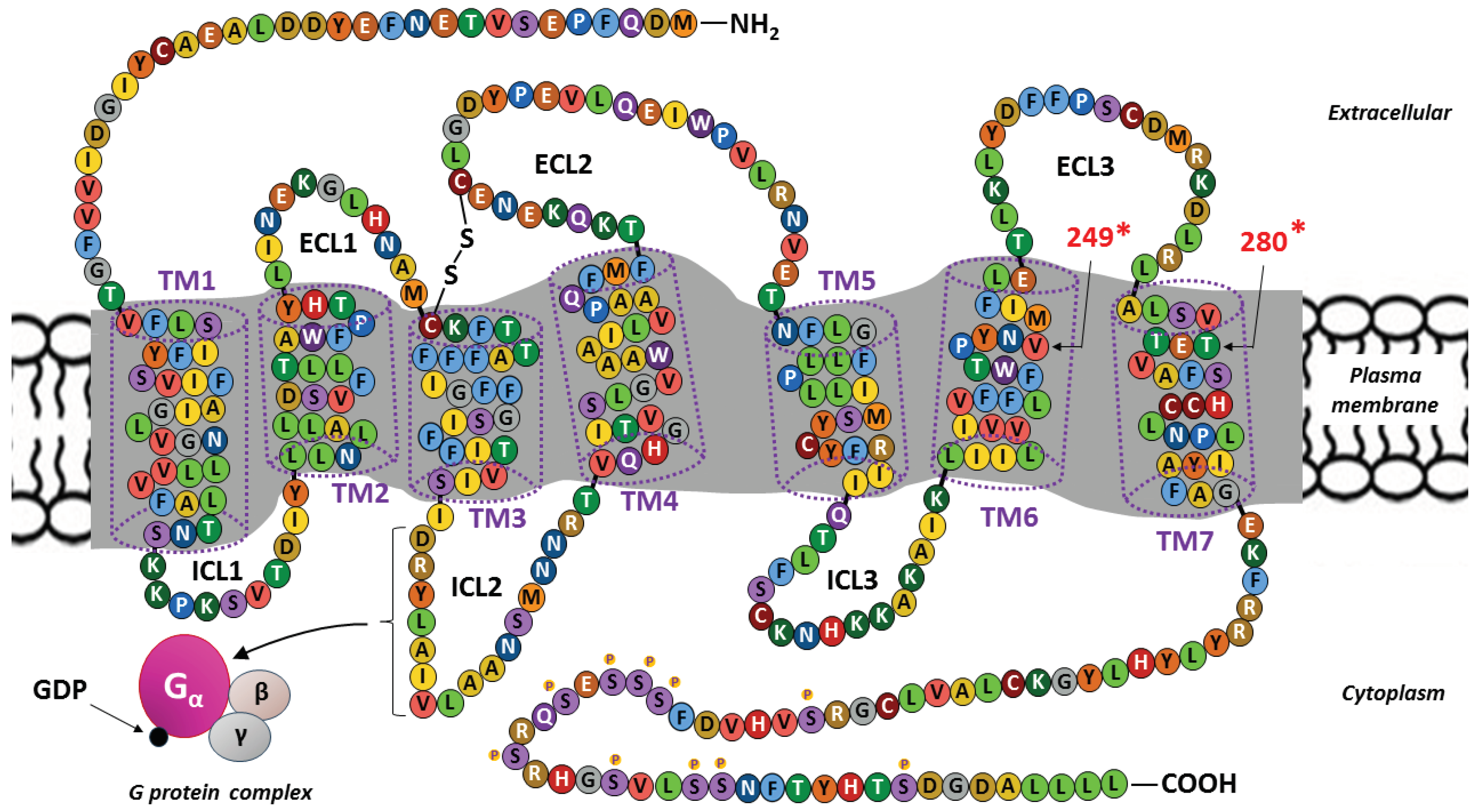
3.1. CX3CR1 Structure
3.2. FKN signaling via CX3CR1
3.2.1. Conformational Rearrangements Following FKN Binding
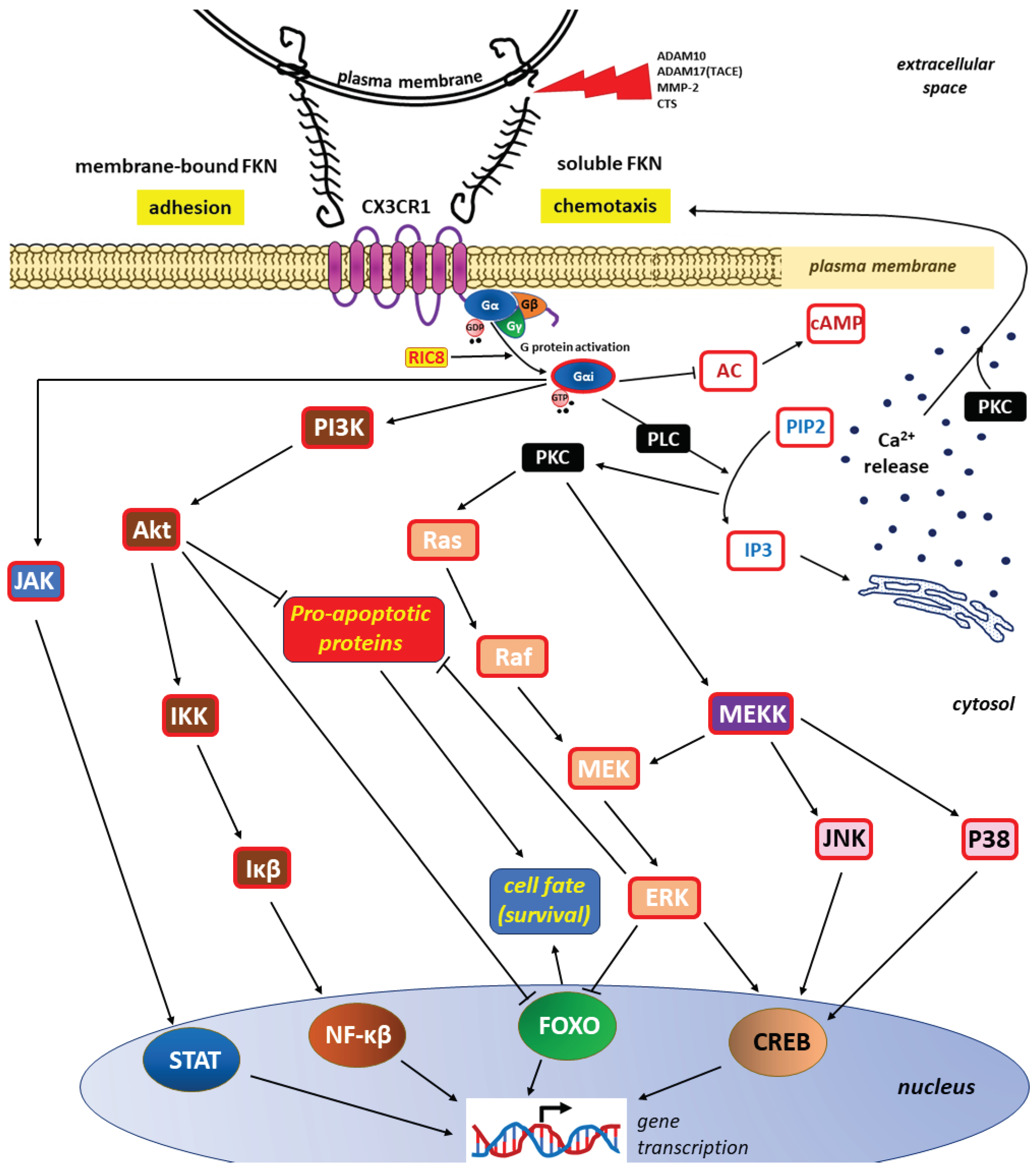
3.2.2. Main FKN/CX3CR1 Signaling Pathways
4. FKN/CX3CR1 Axis and Inflammation
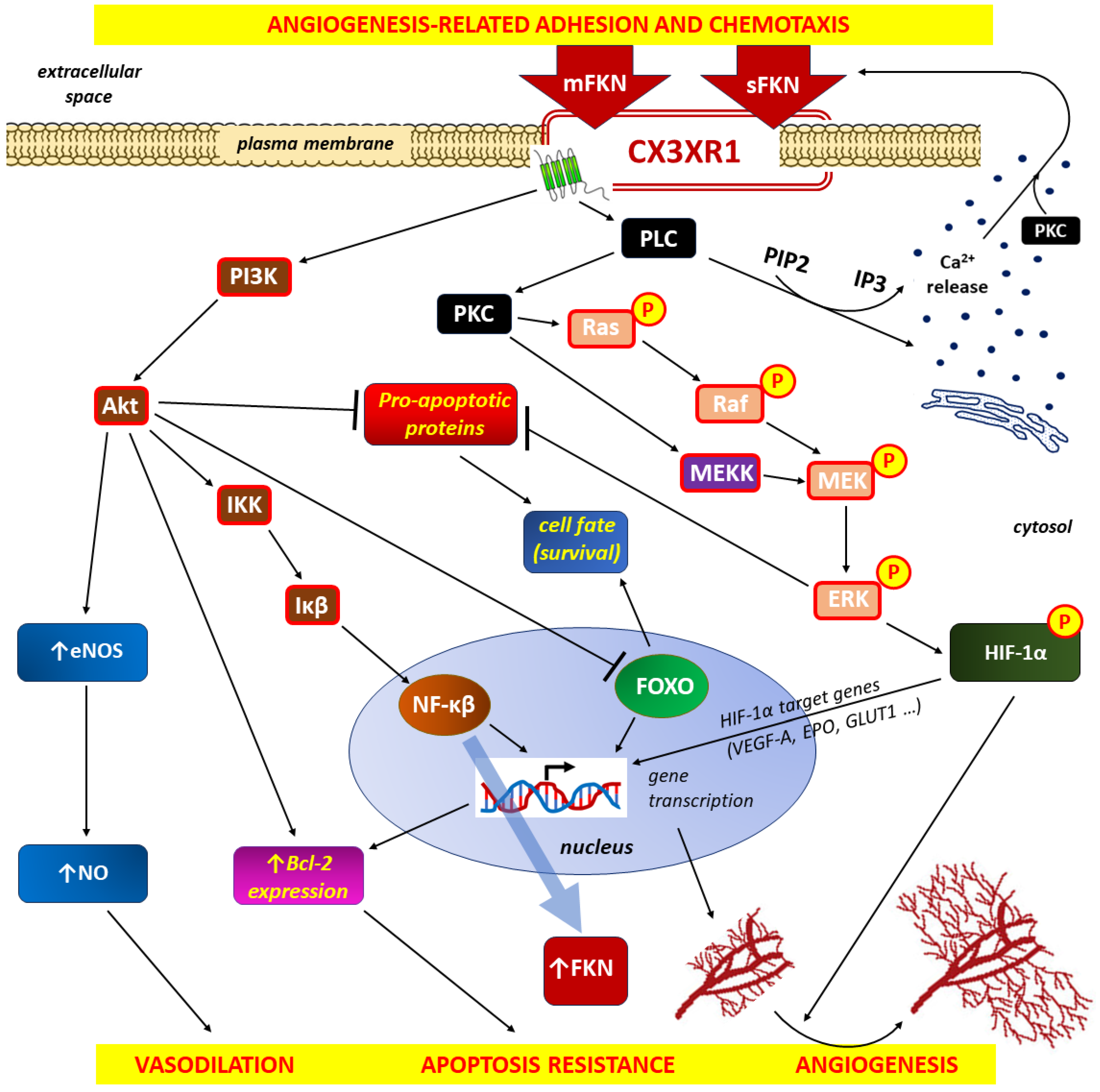
4.1. FKN/CX3CR1 Axis and Inflammation-Induced Angiogenesis
4.1.1. Interdependence of Inflammation and Angiogenesis
4.1.2. Proangiogenic Effects of FKN/CX3CR1 Signaling on the Inflammatory Response
5. FKN/CX3CR1 Axis and Tumorigenesis
5.1. Hypoxia and Angiogenesis in the Tumor Microenvironment (TME)
5.2. FKN in the TME
5.2.1. FKN/CX3CR1 Signaling May Promote Tumorigenesis
5.2.2. FKN/CX3CR1 Signaling May Be a Good Prognostic Factor in Cancer
5.2.3. Possible Reasons for the Contradictory Results of FKN/CX3CR1 Signaling in Cancer
6. Concluding Remarks
Abbreviations
Funding Statement
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Overview of Angiogenesis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53238.
- Szewczyk G, Maciejewski TM, Szukiewicz D. Current progress in the inflammatory background of angiogenesis in gynecological cancers. Inflamm Res. 2019;68(4):247-260. [CrossRef]
- Yoder, MC. Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012 ;2(7): a006692. [CrossRef]
- Marçola M, Rodrigues CE. Endothelial progenitor cells in tumor angiogenesis: another brick in the wall. Stem Cells Int. 2015;2015:832649. [CrossRef]
- Ng CY, Cheung C. Origins and functional differences of blood endothelial cells. Semin Cell Dev Biol. 2024;155(Pt C):23-29. [CrossRef]
- Fujisawa T, Tura-Ceide O, Hunter A, Mitchell A, Vesey A, Medine C, Gallogly S, Hadoke PWF, Keith C, Sproul A, Roddie H, McQuaker G, Wilmut I, Mills NL, Brittan M. Endothelial Progenitor Cells Do Not Originate From the Bone Marrow. Circulation. 2019;140(18):1524-1526. [CrossRef]
- Li Z, Solomonidis EG, Meloni M, Taylor RS, Duffin R, Dobie R, Magalhaes MS, Henderson BEP, Louwe PA, D’Amico G, Hodivala-Dilke KM, Shah AM, Mills NL, Simons BD, Gray GA, Henderson NC, Baker AH, Brittan M. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur Heart J. 2019;40(30):2507-2520. [CrossRef]
- Ackermann M, Houdek JP, Gibney BC, Ysasi A, Wagner W, Belle J, Schittny JC, Enzmann F, Tsuda A, Mentzer SJ, Konerding MA. Sprouting and intussusceptive angiogenesis in postpneumonectomy lung growth: mechanisms of alveolar neovascularization. Angiogenesis. 2014;17(3):541-51. [CrossRef]
- Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217-57. [CrossRef]
- Lin S, Chai Y, Zheng X, Xu X. The role of HIF in angiogenesis, lymphangiogenesis, and tumor microenvironment in urological cancers. Mol Biol Rep. 2023;51(1):14. [CrossRef]
- Yuan X, Ruan W, Bobrow B, Carmeliet P, Eltzschig HK. Targeting hypoxia-inducible factors: therapeutic opportunities and challenges. Nat Rev Drug Discov. 2023. [CrossRef]
- Wang Y, Yang Y, Yang Y, Dang Y, Guo Z, Zhuang Q, Zheng X, Wang F, Cheng N, Liu X, Guo W, Zhao B. Hypoxia induces hepatocellular carcinoma metastasis via the HIF-1α/METTL16/lnc-CSMD1-7/RBFOX2 axis. iScience. 2023;26(12):108495. [CrossRef]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5’-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26(14):5336-47. [CrossRef]
- Feng Y, Luo S, Fan D, Guo X, Ma S. The role of vascular endothelial cells in tumor metastasis. Acta Histochem. 2023;125(6):152070. [CrossRef]
- Ebeling S, Kowalczyk A, Perez-Vazquez D, Mattiola I. Regulation of tumor angiogenesis by the crosstalk between innate immunity and endothelial cells. Front Oncol. 2023;13:1171794. [CrossRef]
- Bisht M, Dhasmana DC, Bist SS. Angiogenesis: Future of pharmacological modulation. Indian J Pharmacol. 2010;42(1):2-8. [CrossRef]
- Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36(15):2187-2203. [CrossRef]
- Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3(8):a005090. [CrossRef]
- Nan W, He Y, Wang S, Zhang Y. Molecular mechanism of VE-cadherin in regulating endothelial cell behaviour during angiogenesis. Front Physiol. 2023;14:1234104. [CrossRef]
- Wen JH, Choi O, Taylor-Weiner H, Fuhrmann A, Karpiak JV, Almutairi A, Engler AJ. Haptotaxis is cell type specific and limited by substrate adhesiveness. Cell Mol Bioeng. 2015; 8(4):530-542. [CrossRef]
- Kazerounian S, Lawler J. Integration of pro- and anti-angiogenic signals by endothelial cells. J Cell Commun Signal. 2018;12(1):171-179. [CrossRef]
- Geindreau M, Bruchard M, Vegran F. Role of Cytokines and Chemokines in Angiogenesis in a Tumor Context. Cancers (Basel). 2022;14(10):2446. [CrossRef]
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745-1770. [CrossRef]
- Balogh E, Biniecka M, Fearon U, Veale DJ, Szekanecz Z. Angiogenesis in Inflammatory Arthritis. Isr Med Assoc J. 2019;21(5):345-352.
- Lu E, Li C, Wang J, Zhang C. Inflammation and angiogenesis in the corpus luteum. J Obstet Gynaecol Res. 2019;45(10):1967-1974. [CrossRef]
- Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL. Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation. 2016;23(2):95-121. [CrossRef]
- Walsh DA, Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001;3(3):147-53. [CrossRef]
- Tsoupras A, Lordan R, Zabetakis I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients. 2018 May 12;10(5):604. [CrossRef]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204-7218. [CrossRef]
- Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552-8. [CrossRef]
- Jeong JH, Ojha U, Lee YM. Pathological angiogenesis and inflammation in tissues. Arch Pharm Res. 2021;44(1):1-15. [CrossRef]
- Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109-19. [CrossRef]
- Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost. 2007;97(5):755-62.
- Miller MC, Mayo KH. Chemokines from a Structural Perspective. Int J Mol Sci. 2017; 18(10):2088. [CrossRef]
- Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16): 2944-2971. [CrossRef]
- Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol. 2015;93(4):372-83. [CrossRef]
- Legler DF, Thelen M. New insights in chemokine signaling. F1000Res. 2018;7:95. [CrossRef]
- Stone MJ, Hayward JA, Huang C, E Huma Z, Sanchez J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int J Mol Sci. 2017;18(2):342. [CrossRef]
- Portella L, Bello AM, Scala S. CXCL12 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1302:51-70. [CrossRef]
- Nazari A, Khorramdelazad H, Hassanshahi G. Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes in the pathogenesis of bladder cancer. Int J Clin Oncol. 2017; 22(6):991-1000. [CrossRef]
- Shakir M, Tang D, Zeh HJ, Tang SW, Anderson CJ, Bahary N, Lotze MT. The chemokine receptors CXCR4/CXCR7 and their primary heterodimeric ligands CXCL12 and CXCL12/high mobility group box 1 in pancreatic cancer growth and development: finding flow. Pancreas. 2015;44(4):528-34. [CrossRef]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91(4):521-30. [CrossRef]
- Wojdasiewicz P, Poniatowski LA, Kotela A, Deszczyński J, Kotela I, Szukiewicz D. The chemokine CX3CL1 (fractalkine) and its receptor CX3CR1: occurrence and potential role in osteoarthritis. Arch Immunol Ther Exp (Warsz). 2014;62(5):395-403. [CrossRef]
- Loh SX, Ekinci Y, Spray L, Jeyalan V, Olin T, Richardson G, Austin D, Alkhalil M, Spyridopoulos I. Fractalkine Signalling (CX3CL1/CX3CR1 Axis) as an Emerging Target in Coronary Artery Disease. J Clin Med. 2023;12(14):4821. [CrossRef]
- Imaizumi T, Yoshida H, Satoh K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb. 2004;11(1):15-21. [CrossRef]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640-4. [CrossRef]
- Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611-7. Erratum in: Nature 1997 Sep 4;389(6646):100. [CrossRef]
- Hortsch M, Patel NH, Bieber AJ, Traquina ZR, Goodman CS. Drosophila neurotactin, a surface glycoprotein with homology to serine esterases, is dynamically expressed during embryogenesis. Development. 1990;110(4):1327-40. [CrossRef]
- Zhuang Q, Cheng K, Ming Y. CX3CL1/CX3CR1 Axis, as the Therapeutic Potential in Renal Diseases: Friend or Foe? Curr Gene Ther. 2017;17(6):442-452. [CrossRef]
- D’Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets. 2010;14(2):207-19. [CrossRef]
- Wu CY, Peng PW, Renn TY, Lee CJ, Chang TM, Wei AI, Liu JF. CX3CL1 induces cell migration and invasion through ICAM-1 expression in oral squamous cell carcinoma cells. J Cell Mol Med. 2023;27(11):1509-1522. [CrossRef]
- White GE, Greaves DR. Fractalkine: a survivor’s guide: chemokines as antiapoptotic mediators. Arterioscler Thromb Vasc Biol. 2012;32(3):589-94. [CrossRef]
- Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2001;158(3):855-66. [CrossRef]
- Jones BA, Beamer M, Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol Interv. 2010;10(5):263-70. [CrossRef]
- O’Sullivan SA, Gasparini F, Mir AK, Dev KK. Fractalkine shedding is mediated by p38 and the ADAM10 protease under pro-inflammatory conditions in human astrocytes. J Neuroinflammation. 2016;13(1):189. [CrossRef]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102(4):1186-95. [CrossRef]
- Poniatowski ŁA, Wojdasiewicz P, Krawczyk M, Szukiewicz D, Gasik R, Kubaszewski Ł, Kurkowska-Jastrzębska I. Analysis of the Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Traumatic Brain and Spinal Cord Injury: Insight into Recent Advances in Actions of Neurochemokine Agents. Mol Neurobiol. 2017;54(3):2167-2188. [CrossRef]
- Jones BA, Riegsecker S, Rahman A, Beamer M, Aboualaiwi W, Khuder SA, Ahmed S. Role of ADAM-17, p38 MAPK, cathepsins, and the proteasome pathway in the synthesis and shedding of fractalkine/CX₃ CL1 in rheumatoid arthritis. Arthritis Rheum. 2013;65(11):2814-25. [CrossRef]
- Bourd-Boittin K, Basset L, Bonnier D, L’helgoualc’h A, Samson M, Théret N. CX3CL1/fractalkine shedding by human hepatic stellate cells: contribution to chronic inflammation in the liver. J Cell Mol Med. 2009;13(8A):1526-35. [CrossRef]
- Uchida M, Ito T, Nakamura T, Igarashi H, Oono T, Fujimori N, Kawabe K, Suzuki K, Jensen RT, Takayanagi R. ERK pathway and sheddases play an essential role in ethanol-induced CX3CL1 release in pancreatic stellate cells. Lab Invest. 2013;93(1):41-53. [CrossRef]
- Lu, X. Lu X. Structure and Function of Ligand CX3CL1 and its Receptor CX3CR1 in Cancer. Curr Med Chem. 2022;29(41):6228-6246. [CrossRef]
- Iemmolo M, Ghersi G, Bivona G. The Cytokine CX3CL1 and ADAMs/MMPs in Concerted Cross-Talk Influencing Neurodegenerative Diseases. Int J Mol Sci. 2023;24(9):8026. [CrossRef]
- Turner SL, Mangnall D, Bird NC, Blair-Zajdel ME, Bunning RA. Effects of pro-inflammatory cytokines on the production of soluble fractalkine and ADAM17 by HepG2 cells. J Gastrointestin Liver Dis. 2010;19(3):265-71.
- Fonović UP, Jevnikar Z, Kos J. Cathepsin S generates soluble CX3CL1 (fractalkine) in vascular smooth muscle cells. Biol Chem. 2013;394(10):1349-52. [CrossRef]
- Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, Winter U, Paliga K, Reiss K, Saftig P, Weber C, Ludwig A. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol. 2007;178(12):8064-72. [CrossRef]
- Scarselli M, Donaldson JG. Constitutive internalization of G protein-coupled receptors and G proteins via clathrin-independent endocytosis. J Biol Chem. 2009;284(6):3577-85. [CrossRef]
- Hattermann K, Gebhardt H, Krossa S, Ludwig A, Lucius R, Held-Feindt J, Mentlein R. Transmembrane chemokines act as receptors in a novel mechanism termed inverse signaling. Elife. 2016;5:e10820. [CrossRef]
- Shimaoka T, Nakayama T, Fukumoto N, Kume N, Takahashi S, Yamaguchi J, Minami M, Hayashida K, Kita T, Ohsumi J, Yoshie O, Yonehara S. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. 2004;75(2):267-74. [CrossRef]
- Ostuni MA, Hermand P, Saindoy E, Guillou N, Guellec J, Coens A, Hattab C, Desuzinges-Mandon E, Jawhari A, Iatmanen-Harbi S, Lequin O, Fuchs P, Lacapere JJ, Combadière C, Pincet F, Deterre P. CX3CL1 homo-oligomerization drives cell-to-cell adherence. Sci Rep. 2020;10(1): 9069. [CrossRef]
- Lee M, Lee Y, Song J, Lee J, Chang SY. Tissue-specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018;18(1):e5. [CrossRef]
- Ni Y, Zhuge F, Ni L, Nagata N, Yamashita T, Mukaida N, Kaneko S, Ota T, Nagashimada M. CX3CL1/CX3CR1 interaction protects against lipotoxicity-induced nonalcoholic steatohepatitis by regulating macrophage migration and M1/M2 status. Metabolism. 2022;136:155272. [CrossRef]
- Guo S, Dong L, Li J, Chen Y, Yao Y, Zeng R, Shushakova N, Haller H, Xu G, Rong S. C-X3-C motif chemokine ligand 1/receptor 1 regulates the M1 polarization and chemotaxis of macrophages after hypoxia/reoxygenation injury. Chronic Dis Transl Med. 2021;7(4):254-265. [CrossRef]
- Cormican S, Griffin MD. Fractalkine (CX3CL1) and Its Receptor CX3CR1: A Promising Therapeutic Target in Chronic Kidney Disease? Front Immunol. 2021;12:664202. [CrossRef]
- Kerfoot SM, Lord SE, Bell RB, Gill V, Robbins SM, Kubes P. Human fractalkine mediates leukocyte adhesion but not capture under physiological shear conditions; a mechanism for selective monocyte recruitment. Eur J Immunol. 2003;33(3):729-39. [CrossRef]
- Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom ET, Domae N, Umehara H. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164(8):4313-20. [CrossRef]
- Flierl U, Bauersachs J, Schäfer A. Modulation of platelet and monocyte function by the chemokine fractalkine (CX3 CL1) in cardiovascular disease. Eur J Clin Invest. 2015;45(6):624-33. [CrossRef]
- Umehara H, Imai T. Role of fractalkine in leukocyte adhesion and migration and in vascular injury. Drug News Perspect. 2001;14(8):460-4. [CrossRef]
- Hermand P, Pincet F, Carvalho S, Ansanay H, Trinquet E, Daoudi M, Combadière C, Deterre P. Functional adhesiveness of the CX3CL1 chemokine requires its aggregation. Role of the transmembrane domain. J Biol Chem. 2008;283(44):30225-34. [CrossRef]
- Ostuni MA, Guellec J, Hermand P, Durand P, Combadière C, Pincet F, Deterre P. CX3CL1, a chemokine finely tuned to adhesion: critical roles of the stalk glycosylation and the membrane domain. Biol Open. 2014;3(12):1173-82. [CrossRef]
- Huang YW, Su P, Liu GY, Crow MR, Chaukos D, Yan H, Robinson LA. Constitutive endocytosis of the chemokine CX3CL1 prevents its degradation by cell surface metalloproteases. J Biol Chem. 2009;284(43):29644-53. [CrossRef]
- Wong HS, Jaumouillé V, Heit B, Doodnauth SA, Patel S, Huang YW, Grinstein S, Robinson LA. Cytoskeletal confinement of CX3CL1 limits its susceptibility to proteolytic cleavage by ADAM10. Mol Biol Cell. 2014;25(24):3884-99. [CrossRef]
- Liu GY, Kulasingam V, Alexander RT, Touret N, Fong AM, Patel DD, Robinson LA. Recycling of the membrane-anchored chemokine, CX3CL1. J Biol Chem. 2005;280(20):19858-66. [CrossRef]
- Miller AF, Falke JJ. Chemotaxis receptors and signaling. Adv Protein Chem. 2004;68:393-444. [CrossRef]
- Conroy MJ, Lysaght J. CX3CL1 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1231:1-12. [CrossRef]
- Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705-16. [CrossRef]
- Richmond, A. Richmond A. Chemokine research moves on. Exp Cell Res. 2011;317(5):553-5. [CrossRef]
- Mortier A, Van Damme J, Proost P. Overview of the mechanisms regulating chemokine activity and availability. Immunol Lett. 2012;145(1-2):2-9. [CrossRef]
- Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Semin Immunol. 2002;14(2):83-92. [CrossRef]
- Nanki T, Imai T, Nagasaka K, Urasaki Y, Nonomura Y, Taniguchi K, Hayashida K, Hasegawa J, Yoshie O, Miyasaka N. Migration of CX3CR1-positive T cells producing type 1 cytokines and cytotoxic molecules into the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 2002;46(11):2878-83. [CrossRef]
- Hamann I, Unterwalder N, Cardona AE, Meisel C, Zipp F, Ransohoff RM, Infante-Duarte C. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology. 2011 ;133(1):62-73. [CrossRef]
- Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, Magnan A, Dombrowicz D, Glaichenhaus N, Julia V. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med. 2010;16(11):1305-12. [CrossRef]
- Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197(12):1701-7. [CrossRef]
- Papadopoulos EJ, Fitzhugh DJ, Tkaczyk C, Gilfillan AM, Sassetti C, Metcalfe DD, Hwang ST. Mast cells migrate, but do not degranulate, in response to fractalkine, a membrane-bound chemokine expressed constitutively in diverse cells of the skin. Eur J Immunol. 2000;30(8):2355-61. [CrossRef]
- Beck GCh, Ludwig F, Schulte J, van Ackern K, van der Woude FJ, Yard BA. Fractalkine is not a major chemoattractant for the migration of neutrophils across microvascular endothelium. Scand J Immunol. 2003;58(2):180-7. [CrossRef]
- Hall JD, Kurtz SL, Rigel NW, Gunn BM, Taft-Benz S, Morrison JP, Fong AM, Patel DD, Braunstein M, Kawula TH. The impact of chemokine receptor CX3CR1 deficiency during respiratory infections with Mycobacterium tuberculosis or Francisella tularensis. Clin Exp Immunol. 2009;156(2):278-84. [CrossRef]
- Volin MV, Huynh N, Klosowska K, Reyes RD, Woods JM. Fractalkine-induced endothelial cell migration requires MAP kinase signaling. Pathobiology. 2010;77(1):7-16. [CrossRef]
- Liu JF, Tsao YT, Hou CH. Fractalkine/CX3CL1 induced intercellular adhesion molecule-1-dependent tumor metastasis through the CX3CR1/PI3K/Akt/NF-κB pathway in human osteosarcoma. Oncotarget. 2016;8(33):54136-54148. [CrossRef]
- Tang J, Xiao L, Cui R, Li D, Zheng X, Zhu L, Sun H, Pan Y, Du Y, Yu X. CX3CL1 increases invasiveness and metastasis by promoting epithelial-to-mesenchymal transition through the TACE/TGF-α/EGFR pathway in hypoxic androgen-independent prostate cancer cells. Oncol Rep. 2016;35(2):1153-62. [CrossRef]
- Garin A, Pellet P, Deterre P, Debré P, Combadière C. Cloning and functional characterization of the human fractalkine receptor promoter regions. Biochem J. 2002;368(Pt 3):753-60. [CrossRef]
- Raport CJ, Schweickart VL, Eddy RL Jr, Shows TB, Gray PW. The orphan G-protein-coupled receptor-encoding gene V28 is closely related to genes for chemokine receptors and is expressed in lymphoid and neural tissues. Gene. 1995;163(2):295-9. [CrossRef]
- Combadiere C, Gao J, Tiffany HL, Murphy PM. Gene cloning, RNA distribution, and functional expression of mCX3CR1, a mouse chemotactic receptor for the CX3C chemokine fractalkine. Biochem Biophys Res Commun. 1998;253(3):728-32. [CrossRef]
- Zhuang Q, Ou J, Zhang S, Ming Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr Protein Pept Sci. 2019;20(8):844-854. [CrossRef]
- Schwarz N, Pruessmeyer J, Hess FM, Dreymueller D, Pantaler E, Koelsch A, Windoffer R, Voss M, Sarabi A, Weber C, Sechi AS, Uhlig S, Ludwig A. Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1. Cell Mol Life Sci. 2010;67(24):4233-48. [CrossRef]
- Raucci R, Costantini S, Castello G, Colonna G. An overview of the sequence features of N- and C-terminal segments of the human chemokine receptors. Cytokine. 2014;70(2):141-50. [CrossRef]
- Szpakowska M, Perez Bercoff D, Chevigné A. Closing the ring: a fourth extracellular loop in chemokine receptors. Sci Signal. 2014;7(341):pe21. [CrossRef]
- Nomiyama H, Yoshie O. Functional roles of evolutionary conserved motifs and residues in vertebrate chemokine receptors. J Leukoc Biol. 2015;97(1):39-47. [CrossRef]
- Mafi A, Kim SK, Goddard WA 3rd. The mechanism for ligand activation of the GPCR-G protein complex. Proc Natl Acad Sci U S A. 2022;119(18):e2110085119. [CrossRef]
- Tardáguila M, Mañes S. Tardáguila M, Mañes S. The complex role of chemokines in cancer: The case of the CX3CL1/CX3CR1 axis. In Oncology Theory & Practice, 1st ed.; iConcept Press Ltd.: Madrid, Spain, 2014; Chapter 8.
- Chaudhri A, Bu X, Wang Y, Gomez M, Torchia JA, Hua P, Hung SH, Davies MA, Lizee GA, von Andrian U, Hwu P, Freeman GJ. The CX3CL1-CX3CR1 chemokine axis can contribute to tumor immune evasion and blockade with a novel CX3CR1 monoclonal antibody enhances response to anti-PD-1 immunotherapy. Front Immunol. 2023;14:1237715. [CrossRef]
- Goode-Romero G, Dominguez L. Computational study of the conformational ensemble of CX3C chemokine receptor 1 (CX3CR1) and its interactions with antagonist and agonist ligands. J Mol Graph Model. 2022;117:108278. [CrossRef]
- Mizoue LS, Bazan JF, Johnson EC, Handel TM. Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1. Biochemistry. 1999;38(5):1402-14. [CrossRef]
- Nakayama T, Watanabe Y, Oiso N, Higuchi T, Shigeta A, Mizuguchi N, Katou F, Hashimoto K, Kawada A, Yoshie O. Eotaxin-3/CC chemokine ligand 26 is a functional ligand for CX3CR1. J Immunol. 2010;185(11):6472-9. [CrossRef]
- Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debré P, Théodorou I, Combadière C. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287(5461):2274-7. [CrossRef]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254-8. [CrossRef]
- Ludeman JP, Stone MJ. The structural role of receptor tyrosine sulfation in chemokine recognition. Br J Pharmacol. 2014;171(5):1167-79. [CrossRef]
- Villaseca S, Romero G, Ruiz MJ, Pérez C, Leal JI, Tovar LM, Torrejón M. Gαi protein subunit: A step toward understanding its non-canonical mechanisms. Front Cell Dev Biol. 2022; 10:941870. [CrossRef]
- Lu M, Zhao W, Han S, Lin X, Xu T, Tan Q, Wang M, Yi C, Chu X, Yang W, Zhu Y, Wu B, Zhao Q. Activation of the human chemokine receptor CX3CR1 regulated by cholesterol. Sci Adv. 2022; 8(26):eabn8048. [CrossRef]
- Laganà M, Schlecht-Louf G, Bachelerie F. The G Protein-Coupled Receptor Kinases (GRKs) in Chemokine Receptor-Mediated Immune Cell Migration: From Molecular Cues to Physiopathology. Cells. 2021;10(1):75. [CrossRef]
- Niessner A, Marculescu R, Haschemi A, Endler G, Zorn G, Weyand CM, Maurer G, Mannhalter C, Wojta J, Wagner O, Huber K. Opposite effects of CX3CR1 receptor polymorphisms V249I and T280M on the development of acute coronary syndrome. A possible implication of fractalkine in inflammatory activation. Thromb Haemost. 2005;93(5):949-54. [CrossRef]
- Wu J, Yin RX, Lin QZ, Guo T, Shi GY, Sun JQ, Shen SW, Li Q. Two polymorphisms in the Fractalkine receptor CX3CR1 gene influence the development of atherosclerosis: a meta-analysis. Dis Markers. 2014;2014:913678. [CrossRef]
- Chamera K, Szuster-Głuszczak M, Basta-Kaim A. Shedding light on the role of CX3CR1 in the pathogenesis of schizophrenia. Pharmacol Rep. 2021;73(4):1063-1078. [CrossRef]
- Sakai M, Takeuchi H, Yu Z, Kikuchi Y, Ono C, Takahashi Y, Ito F, Matsuoka H, Tanabe O, Yasuda J, Taki Y, Kawashima R, Tomita H. Polymorphisms in the microglial marker molecule CX3CR1 affect the blood volume of the human brain. Psychiatry Clin Neurosci. 2018;72(6):409-422. [CrossRef]
- Imai T, Yasuda N. Therapeutic intervention of inflammatory/immune diseases by inhibition of the fractalkine (CX3CL1)-CX3CR1 pathway. Inflamm Regen. 2016;36:9. [CrossRef]
- Mirzadegan T, Benkö G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42(10):2759-67. [CrossRef]
- Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3(11):RESEARCH0063. [CrossRef]
- Zhou XE, Melcher K, Xu HE. Structure and activation of rhodopsin. Acta Pharmacol Sin. 2012; 33(3):291-9. [CrossRef]
- Lefkowitz, RJ. Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25(8):413-22. [CrossRef]
- Vischer HF, Hulshof JW, de Esch IJ, Smit MJ, Leurs R. Virus-encoded G-protein-coupled receptors: constitutively active (dys)regulators of cell function and their potential as drug target. Ernst Schering Found Symp Proc. 2006;(2):187-209. [CrossRef]
- Burg JS, Ingram JR, Venkatakrishnan AJ, Jude KM, Dukkipati A, Feinberg EN, Angelini A, Waghray D, Dror RO, Ploegh HL, Garcia KC. Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science. 2015;347(6226): 1113-7. [CrossRef]
- Hjortø GM, Kiilerich-Pedersen K, Selmeczi D, Kledal TN, Larsen NB. Human cytomegalovirus chemokine receptor US28 induces migration of cells on a CX3CL1-presenting surface. J Gen Virol. 2013;94(Pt 5):1111-1120. [CrossRef]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639-50. [CrossRef]
- Palczewski, K. Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743-67. [CrossRef]
- Gustavsson M, Zheng Y, Handel TM. Production of Chemokine/Chemokine Receptor Complexes for Structural Biophysical Studies. Methods Enzymol. 2016;570:233-60. [CrossRef]
- Miles TF, Spiess K, Jude KM, Tsutsumi N, Burg JS, Ingram JR, Waghray D, Hjorto GM, Larsen O, Ploegh HL, Rosenkilde MM, Garcia KC. Viral GPCR US28 can signal in response to chemokine agonists of nearly unlimited structural degeneracy. Elife. 2018;7:e35850. [CrossRef]
- Tsutsumi N, Maeda S, Qu Q, Vögele M, Jude KM, Suomivuori CM, Panova O, Waghray D, Kato HE, Velasco A, Dror RO, Skiniotis G, Kobilka BK, Garcia KC. Atypical structural snapshots of human cytomegalovirus GPCR interactions with host G proteins. Sci Adv. 2022;8(3):eabl5442. [CrossRef]
- Zhou Q, Yang D, Wu M, Guo Y, Guo W, Zhong L, Cai X, Dai A, Jang W, Shakhnovich EI, Liu ZJ, Stevens RC, Lambert NA, Babu MM, Wang MW, Zhao S. Common activation mechanism of class A GPCRs. Elife. 2019;8:e50279. [CrossRef]
- Syrovatkina V, Alegre KO, Dey R, Huang XY. Regulation, Signaling, and Physiological Functions of G-Proteins. J Mol Biol. 2016;428(19):3850-68. [CrossRef]
- Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7(4):339-57. Erratum in: Nat Rev Drug Discov. 2008;7(6):542. [CrossRef]
- Cancellieri C, Vacchini A, Locati M, Bonecchi R, Borroni EM. Atypical chemokine receptors: from silence to sound. Biochem Soc Trans. 2013;41(1):231-6. [CrossRef]
- Wingler LM, Lefkowitz RJ. Conformational Basis of G Protein-Coupled Receptor Signaling Versatility. Trends Cell Biol. 2020;30(9):736-747. [CrossRef]
- Hoffmann C, Zürn A, Bünemann M, Lohse MJ. Conformational changes in G-protein-coupled receptors-the quest for functionally selective conformations is open. Br J Pharmacol. 2008; 153 Suppl 1(Suppl 1):S358-66. [CrossRef]
- Hamza A, Samad A, Parray ZA, Ara S, Ahmed A, Almajhdi FN, Hussain T, Islam A, Parveen S. Mutation in the CX3C Motif of G Protein Disrupts Its Interaction with Heparan Sulfate: A Calorimetric, Spectroscopic, and Molecular Docking Study. Int J Mol Sci. 2022;23(4):1950. [CrossRef]
- Darbandi-Tehrani K, Hermand P, Carvalho S, Dorgham K, Couvineau A, Lacapère JJ, Combadière C, Deterre P. Subtle conformational changes between CX3CR1 genetic variants as revealed by resonance energy transfer assays. FASEB J. 2010;24(11):4585-98. [CrossRef]
- Ishizuka K, Fujita Y, Kawabata T, Kimura H, Iwayama Y, Inada T, Okahisa Y, Egawa J, Usami M, Kushima I, Uno Y, Okada T, Ikeda M, Aleksic B, Mori D, Someya T, Yoshikawa T, Iwata N, Nakamura H, Yamashita T, Ozaki N. Rare genetic variants in CX3CR1 and their contribution to the increased risk of schizophrenia and autism spectrum disorders. Transl Psychiatry. 2017;7 (8): e1184. [CrossRef]
- Lee S, Latha K, Manyam G, Yang Y, Rao A, Rao G. Role of CX3CR1 signaling in malignant transformation of gliomas. Neuro Oncol. 2020;22(10):1463-1473. [CrossRef]
- Rivas-Fuentes S, Salgado-Aguayo A, Arratia-Quijada J, Gorocica-Rosete P. Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: A mini-review. J Cancer. 2021;12(2):571-583. [CrossRef]
- Kehlen A, Haegele M, Böhme L, Cynis H, Hoffmann T, Demuth HU. N-terminal pyroglutamate formation in CX3CL1 is essential for its full biologic activity. Biosci Rep. 2017; 37(4):BSR20170712. [CrossRef]
- Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163(3):1628-35.
- Pallandre JR, Krzewski K, Bedel R, Ryffel B, Caignard A, Rohrlich PS, Pivot X, Tiberghien P, Zitvogel L, Strominger JL, Borg C. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood. 2008;112(12):4420-4. [CrossRef]
- Foussat A, Coulomb-L’Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30(1): 87-97. [CrossRef]
- Chidambaram H, Das R, Chinnathambi S. Interaction of Tau with the chemokine receptor, CX3CR1 and its effect on microglial activation, migration and proliferation. Cell Biosci. 2020; 10:109. [CrossRef]
- Chen Y, Green SR, Almazan F, Quehenberger O. The amino terminus and the third extracellular loop of CX3CR1 contain determinants critical for distinct receptor functions. Mol Pharmacol. 2006;69(3):857-65. [CrossRef]
- Kharche S, Joshi M, Chattopadhyay A, Sengupta D. Conformational plasticity and dynamic interactions of the N-terminal domain of the chemokine receptor CXCR1. PLoS Comput Biol. 2021;17(5):e1008593.
- Srivastava D, Gakhar L, Artemyev NO. Structural underpinnings of Ric8A function as a G-protein α-subunit chaperone and guanine-nucleotide exchange factor. Nat Commun. 2019;10(1):3084. [CrossRef]
- Wright SJ, Inchausti R, Eaton CJ, Krystofova S, Borkovich KA. RIC8 is a guanine-nucleotide exchange factor for Galpha subunits that regulates growth and development in Neurospora crassa. Genetics. 2011;189(1):165-76. [CrossRef]
- Weis WI, Kobilka BK. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu Rev Biochem. 2018;87:897-919. [CrossRef]
- Arnoux I, Audinat E. Fractalkine Signaling and Microglia Functions in the Developing Brain. Neural Plast. 2015;2015:689404. [CrossRef]
- Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Keshwani M, Perkins G, Dong H, Kayali AG, Sweet IR, Olefsky J. The fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell. 2013;153(2):413-25. [CrossRef]
- Wang A, Yang T, Zhang L, Jia L, Wu Q, Yao S, Xu J, Yang H. IP3-Mediated Calcium Signaling Is Involved in the Mechanism of Fractalkine-Induced Hyperalgesia Response. Med Sci Monit. 2018;24:8804-8811. [CrossRef]
- Wojdasiewicz P, Turczyn P, Dobies-Krzesniak B, Frasunska J, Tarnacka B. Role of CX3CL1/CX3CR1 Signaling Axis Activity in Osteoporosis. Mediators Inflamm. 2019;2019:7570452. [CrossRef]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110(5):1547-56. [CrossRef]
- Park J, Song KH, Ha H. Fractalkine increases mesangial cell proliferation through reactive oxygen species and mitogen-activated protein kinases. Transplant Proc. 2012;44(4):1026-8. [CrossRef]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem. 2001;276(41):37993-8001. [CrossRef]
- Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, Chung HT, Kwon YG, Kim YM. Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. Am J Physiol Heart Circ Physiol. 2006;291(6):H2836-46. [CrossRef]
- Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. [CrossRef]
- Wang H, Cai J, Du S, Guo Z, Xin B, Wang J, Wei W, Shen X. Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochem Funct. 2017;35(6):315-326. [CrossRef]
- Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113(4):963-72. [CrossRef]
- Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225). Curr Opin Oncol. 2001;13(6):506-13. [CrossRef]
- Cai Z, Zhang H, Liu J, Berezov A, Murali R, Wang Q, Greene MI. Targeting erbB receptors. Semin Cell Dev Biol. 2010;21(9):961-6. [CrossRef]
- Ledonne A, Mercuri NB. Insights on the Functional Interaction between Group 1 Metabotropic Glutamate Receptors (mGluRI) and ErbB Receptors. Int J Mol Sci. 2020;21(21):7913. [CrossRef]
- Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. [CrossRef]
- Thomas SM, Bhola NE, Zhang Q, Contrucci SC, Wentzel AL, Freilino ML, Gooding WE, Siegfried JM, Chan DC, Grandis JR. Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer Res. 2006;66(24):11831-9. [CrossRef]
- Cantor AJ, Shah NH, Kuriyan J. Deep mutational analysis reveals functional trade-offs in the sequences of EGFR autophosphorylation sites. Proc Natl Acad Sci U S A. 2018;115(31):E7303-E7312. [CrossRef]
- Jurišić V, Obradovic J, Pavlović S, Djordjevic N. Epidermal Growth Factor Receptor Gene in Non-Small-Cell Lung Cancer: The Importance of Promoter Polymorphism Investigation. Anal Cell Pathol (Amst). 2018;2018:6192187. [CrossRef]
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117(8):2051-8. [CrossRef]
- Tardáguila M, Mira E, García-Cabezas MA, Feijoo AM, Quintela-Fandino M, Azcoitia I, Lira SA, Mañes S. CX3CL1 promotes breast cancer via transactivation of the EGF pathway. Cancer Res. 2013;73(14):4461-73. [CrossRef]
- Bai Q, Wang J, Zhou X. EGFR exon20 insertion mutations in non-small cell lung cancer: Clinical implications and recent advances in targeted therapies. Cancer Treat Rev. 2023; 120:102605. [CrossRef]
- Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20(13):1594-600. [CrossRef]
- Burch ML, Osman N, Getachew R, Al-Aryahi S, Poronnik P, Zheng W, Hill MA, Little PJ. G protein coupled receptor transactivation: extending the paradigm to include serine/threonine kinase receptors. Int J Biochem Cell Biol. 2012;44(5):722-7. [CrossRef]
- Chandrasekar B, Mummidi S, Perla RP, Bysani S, Dulin NO, Liu F, Melby PC. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J. 2003;373(Pt 2):547-58. [CrossRef]
- Szukiewicz D, Wojciechowska M, Bilska A, Stangret A, Szewczyk G, Mittal TK, Watroba M, Kochanowski J. Aspirin Action in Endothelial Cells: Different Patterns of Response Between Chemokine CX3CL1/CX3CR1 and TNF-α/TNFR1 Signaling Pathways. Cardiovasc Drugs Ther. 2015;29(3):219-29. [CrossRef]
- Zwijnenburg AJ, Pokharel J, Varnaitė R, Zheng W, Hoffer E, Shryki I, Comet NR, Ehrström M, Gredmark-Russ S, Eidsmo L, Gerlach C. Graded expression of the chemokine receptor CX3CR1 marks differentiation states of human and murine T cells and enables cross-species interpretation. Immunity. 2023;56(8):1955-1974.e10. [CrossRef]
- Ren M, Zhang J, Dai S, Wang C, Chen Z, Zhang S, Xu J, Qin X, Liu F. CX3CR1 deficiency exacerbates immune-mediated hepatitis by increasing NF-κB-mediated cytokine production in macrophage and T cell. Exp Biol Med (Maywood). 2023;248(2):117-129. [CrossRef]
- Wojdasiewicz P, Poniatowski ŁA, Kotela A, Skoda M, Pyzlak M, Stangret A, Kotela I, Szukiewicz D. Comparative Analysis of the Occurrence and Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Hemophilic Arthropathy and Osteoarthritis. J Immunol Res. 2020;2020:2932696. Erratum in: J Immunol Res. 2020;2020:7179283. [CrossRef]
- White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovasc Res. 2010;85(4):825-35. [CrossRef]
- White GE, McNeill E, Channon KM, Greaves DR. Fractalkine promotes human monocyte survival via a reduction in oxidative stress. Arterioscler Thromb Vasc Biol. 2014;34(12):2554-62. [CrossRef]
- Raei Sadigh A, Darabi M, Salmassi A, Hamdi K, Farzadi L, Ghasemzadeh A, Fattahi A, Nouri M. Fractalkine and apoptotic/anti-apoptotic markers in granulosa cells of women with polycystic ovarian syndrome. Mol Biol Rep. 2020;47(5):3593-3603. [CrossRef]
- Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3(12):130181. [CrossRef]
- Pawelec P, Ziemka-Nalecz M, Sypecka J, Zalewska T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells. 2020;9(10):2277. [CrossRef]
- Paolicelli RC, Bisht K, Tremblay MÈ. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. [CrossRef]
- Camacho-Hernández NP, Peña-Ortega F. Fractalkine/CX3CR1-Dependent Modulation of Synaptic and Network Plasticity in Health and Disease. Neural Plast. 2023;2023:4637073. [CrossRef]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979(1-2):65-70. [CrossRef]
- Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int J Mol Sci. 2018;19(1):318. [CrossRef]
- Subbarayan MS, Joly-Amado A, Bickford PC, Nash KR. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol Ther. 2022;231:107989. [CrossRef]
- Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, Perego C, De Simoni MG, Fredholm BB, Eusebi F, Limatola C. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31(45):16327-35. [CrossRef]
- Luo P, Chu SF, Zhang Z, Xia CY, Chen NH. Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases. Brain Res Bull. 2019;146:12-21. [CrossRef]
- Bivona G, Iemmolo M, Ghersi G. CX3CL1 Pathway as a Molecular Target for Treatment Strategies in Alzheimer’s Disease. Int J Mol Sci. 2023;24(9):8230. [CrossRef]
- Nash KR, Moran P, Finneran DJ, Hudson C, Robinson J, Morgan D, Bickford PC. Fractalkine over expression suppresses α-synuclein-mediated neurodegeneration. Mol Ther. 2015;23(1):17-23. [CrossRef]
- Juliani J, Vassileff N, Spiers JG. Inflammatory-Mediated Neuron-Glia Communication Modulates ALS Pathophysiology. J Neurosci. 2021;41(6):1142-1144. [CrossRef]
- Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol. 2010;177(5):2549-62. [CrossRef]
- Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286(37):32713-22. [CrossRef]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010;13(4):411-3. [CrossRef]
- Eugenín J, Eugenín-von Bernhardi L, von Bernhardi R. Age-dependent changes on fractalkine forms and their contribution to neurodegenerative diseases. Front Mol Neurosci. 2023;16:1249320. [CrossRef]
- Stratoulias V, Venero JL, Tremblay MÈ, Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019;38(17):e101997. [CrossRef]
- Jacquelin S, Licata F, Dorgham K, Hermand P, Poupel L, Guyon E, Deterre P, Hume DA, Combadière C, Boissonnas A. CX3CR1 reduces Ly6Chigh-monocyte motility within and release from the bone marrow after chemotherapy in mice. Blood. 2013;122(5):674-83. [CrossRef]
- Hoshino A, Ueha S, Hanada S, Imai T, Ito M, Yamamoto K, Matsushima K, Yamaguchi A, Iimura T. Roles of chemokine receptor CX3CR1 in maintaining murine bone homeostasis through the regulation of both osteoblasts and osteoclasts. J Cell Sci. 2013;126(Pt 4):1032-45. [CrossRef]
- Kuboi Y, Kuroda Y, Ohkuro M, Motoi S, Tomimori Y, Yasuda H, Yasuda N, Imai T, Matsuo K. The Fractalkine-CX3CR1 Axis Regulates Non-inflammatory Osteoclastogenesis by Enhancing Precursor Cell Survival. JBMR Plus. 2022;6(10):e10680. [CrossRef]
- Yano R, Yamamura M, Sunahori K, Takasugi K, Yamana J, Kawashima M, Makino H. Recruitment of CD16+ monocytes into synovial tissues is mediated by fractalkine and CX3CR1 in rheumatoid arthritis patients. Acta Med Okayama. 2007;61(2):89-98. [CrossRef]
- Marchica V, Toscani D, Corcione A, Bolzoni M, Storti P, Vescovini R, Ferretti E, Dalla Palma B, Vicario E, Accardi F, Mancini C, Martella E, Ribatti D, Vacca A, Pistoia V, Giuliani N. Bone Marrow CX3CL1/Fractalkine is a New Player of the Pro-Angiogenic Microenvironment in Multiple Myeloma Patients. Cancers (Basel). 2019;11(3):321. [CrossRef]
- Sciumè G, De Angelis G, Benigni G, Ponzetta A, Morrone S, Santoni A, Bernardini G. CX3CR1 expression defines 2 KLRG1+ mouse NK-cell subsets with distinct functional properties and positioning in the bone marrow. Blood. 2011;117(17):4467-75. [CrossRef]
- Zhang Y, Zheng J, Zhou Z, Zhou H, Wang Y, Gong Z, Zhu J. Fractalkine promotes chemotaxis of bone marrow-derived mesenchymal stem cells towards ischemic brain lesions through Jak2 signaling and cytoskeletal reorganization. FEBS J. 2015;282(5):891-903. [CrossRef]
- Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101(51):17795-800. [CrossRef]
- Ma J, Luo J, Sun Y, Zhao Z. Cytokines associated with immune response in atherosclerosis. Am J Transl Res. 2022;14(9):6424-6444.
- Riopel M, Vassallo M, Ehinger E, Pattison J, Bowden K, Winkels H, Wilson M, de Jong R, Patel S, Balakrishna D, Bilakovics J, Fanjul A, Plonowski A, Larson CJ, Ley K, Cabrales P, Witztum JL, Olefsky JM, Lee YS. CX3CL1-Fc treatment prevents atherosclerosis in Ldlr KO mice. Mol Metab. 2019;20:89-101. [CrossRef]
- Liu H, Jiang D. Fractalkine/CX3CR1 and atherosclerosis. Clin Chim Acta. 2011;412(13-14):1180-6. [CrossRef]
- Elliott MR, Koster KM, Murphy PS. Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J Immunol. 2017;198(4):1387-1394. [CrossRef]
- Lucas AD, Bursill C, Guzik TJ, Sadowski J, Channon KM, Greaves DR. Smooth muscle cells in human atherosclerotic plaques express the fractalkine receptor CX3CR1 and undergo chemotaxis to the CX3C chemokine fractalkine (CX3CL1). Circulation. 2003;108(20):2498-504. [CrossRef]
- Harman JL, Jørgensen HF. The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br J Pharmacol. 2019;176(19):3741-3753. [CrossRef]
- Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol Sin. 2013;34(10):1251-6. [CrossRef]
- Skoda M, Stangret A, Szukiewicz D. Fractalkine and placental growth factor: A duet of inflammation and angiogenesis in cardiovascular disorders. Cytokine Growth Factor Rev. 2018;39:116-123. [CrossRef]
- Noels H, Weber C, Koenen RR. Chemokines as Therapeutic Targets in Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2019;39(4):583-592. [CrossRef]
- Flamant M, Mougenot N, Balse E, Le Fèvre L, Atassi F, Gautier EL, Le Goff W, Keck M, Nadaud S, Combadière C, Boissonnas A, Pavoine C. Early activation of the cardiac CX3CL1/CX3CR1 axis delays β-adrenergic-induced heart failure. Sci Rep. 2021;11(1):17982. [CrossRef]
- Njerve IU, Solheim S, Lunde K, Hoffmann P, Arnesen H, Seljeflot I. Fractalkine levels are elevated early after PCI-treated ST-elevation myocardial infarction; no influence of autologous bone marrow derived stem cell injection. Cytokine. 2014;69(1):131-5. [CrossRef]
- Yao K, Zhang S, Lu H, Hong X, Qian J, Sun A, Zou Y, Ge J. Changes in fractalkine in patients with ST-elevation myocardial infarction. Coron Artery Dis. 2015;26(6):516-20. [CrossRef]
- Xu B, Qian Y, Zhao Y, Fang Z, Tang K, Zhou N, Li D, Wang J. Prognostic value of fractalkine/CX3CL1 concentration in patients with acute myocardial infarction treated with primary percutaneous coronary intervention. Cytokine. 2019;113:365-370. [CrossRef]
- Boag SE, Das R, Shmeleva EV, Bagnall A, Egred M, Howard N, Bennaceur K, Zaman A, Keavney B, Spyridopoulos I. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest. 2015;125(8):3063-76. [CrossRef]
- Zhang J, Patel JM. Role of the CX3CL1-CX3CR1 axis in chronic inflammatory lung diseases. Int J Clin Exp Med. 2010;3(3):233-44.
- Balabanian K, Foussat A, Dorfmüller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165(10):1419-25. [CrossRef]
- Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, Latiri M, Dubois-Rande JL, Lipskaia L, Combadiere C, Adnot S. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 Chemokine Systems in Hypoxic Pulmonary Hypertension. Am J Respir Cell Mol Biol. 2017;56(5):597-608. [CrossRef]
- Tsai WH, Chang SC, Lin YC, Hsu HC. CX3CL1(+) Microparticles-Induced MFG-E8 Enhances Apoptotic Cell Clearance by Alveolar Macrophages. Cells. 2021;10(10):2583. [CrossRef]
- El-Shazly A, Berger P, Girodet PO, Ousova O, Fayon M, Vernejoux JM, Marthan R, Tunon-de-Lara JM. Fraktalkine produced by airway smooth muscle cells contributes to mast cell recruitment in asthma. J Immunol. 2006;176(3):1860-8. [CrossRef]
- Godwin MS, Jones M, Blackburn JP, Yu Z, Matalon S, Hastie AT, Meyers DA, Steele C. The chemokine CX3CL1/fractalkine regulates immunopathogenesis during fungal-associated allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2021;320(3):L393-L404. [CrossRef]
- Upton N, Jackson DJ, Nikonova AA, Hingley-Wilson S, Khaitov M, Del Rosario A, Traub S, Trujillo-Torralbo MB, Habibi M, Elkin SL, Kon OM, Edwards MR, Mallia P, Footitt J, Macintyre J, Stanciu LA, Johnston SL, Sykes A. Rhinovirus induction of fractalkine (CX3CL1) in airway and peripheral blood mononuclear cells in asthma. PLoS One. 2017;12(8):e0183864. [CrossRef]
- Efsen E, Grappone C, DeFranco RM, Milani S, Romanelli RG, Bonacchi A, Caligiuri A, Failli P, Annunziato F, Pagliai G, Pinzani M, Laffi G, Gentilini P, Marra F. Up-regulated expression of fractalkine and its receptor CX3CR1 during liver injury in humans. J Hepatol. 2002;37(1):39-47. [CrossRef]
- Sutti S, Locatelli I, Bruzzì S, Jindal A, Vacchiano M, Bozzola C, Albano E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin Sci (Lond). 2015; 129(9):797-808. [CrossRef]
- Nagata N, Chen G, Xu L, Ando H. An Update on the Chemokine System in the Development of NAFLD. Medicina (Kaunas). 2022;58(6):761. [CrossRef]
- Aoyama T, Inokuchi S, Brenner DA, Seki E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology. 2010;52(4):1390-400. [CrossRef]
- Zhang P, Wang BJ, Wang JZ, Xie XM, Tong QX. Association of CX3CL1 and CX3CR1 Expression with Liver Fibrosis in a Mouse Model of Schistosomiasis. Curr Med Sci. 2020;40(6):1121-1127. [CrossRef]
- Karlmark KR, Zimmermann HW, Roderburg C, Gassler N, Wasmuth HE, Luedde T, Trautwein C, Tacke F. The fractalkine receptor CX₃CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52(5):1769-82. [CrossRef]
- Wasmuth HE, Zaldivar MM, Berres ML, Werth A, Scholten D, Hillebrandt S, Tacke F, Schmitz P, Dahl E, Wiederholt T, Hellerbrand C, Berg T, Weiskirchen R, Trautwein C, Lammert F. The fractalkine receptor CX3CR1 is involved in liver fibrosis due to chronic hepatitis C infection. J Hepatol. 2008;48(2):208-15. [CrossRef]
- Hassan GS, Flores Molina M, Shoukry NH. The multifaceted role of macrophages during acute liver injury. Front Immunol. 2023;14:1237042. [CrossRef]
- Isse K, Harada K, Zen Y, Kamihira T, Shimoda S, Harada M, Nakanuma Y. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41(3):506-16. [CrossRef]
- Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, Kikuchi K, Nakanuma Y, Mackay IR, Gershwin ME, Akashi K. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51(2):567-75. [CrossRef]
- Joeris T, Müller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10(4):845-864. [CrossRef]
- Bain CC, Mowat AM. Intestinal macrophages - specialised adaptation to a unique environment. Eur J Immunol. 2011;41(9):2494-8. [CrossRef]
- Ferretti E, Pistoia V, Corcione A. Role of fractalkine/CX3CL1 and its receptor in the pathogenesis of inflammatory and malignant diseases with emphasis on B cell malignancies. Mediators Inflamm. 2014;2014:480941. [CrossRef]
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6(3):498-510. [CrossRef]
- Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15(10):929-937. [CrossRef]
- Sans M, Danese S, de la Motte C, de Souza HS, Rivera-Reyes BM, West GA, Phillips M, Katz JA, Fiocchi C. Enhanced recruitment of CX3CR1+ T cells by mucosal endothelial cell-derived fractalkine in inflammatory bowel disease. Gastroenterology. 2007;132(1):139-53. [CrossRef]
- Siwetz M, Blaschitz A, Kremshofer J, Bilic J, Desoye G, Huppertz B, Gauster M. Metalloprotease dependent release of placenta derived fractalkine. Mediators Inflamm. 2014;2014:839290. [CrossRef]
- Siwetz M, Sundl M, Kolb D, Hiden U, Herse F, Huppertz B, Gauster M. Placental fractalkine mediates adhesion of THP-1 monocytes to villous trophoblast. Histochem Cell Biol. 2015;143(6):565-74. [CrossRef]
- Siwetz M, Dieber-Rotheneder M, Cervar-Zivkovic M, Kummer D, Kremshofer J, Weiss G, Herse F, Huppertz B, Gauster M. Placental fractalkine is up-regulated in severe early-onset preeclampsia. Am J Pathol. 2015;185(5):1334-43. [CrossRef]
- Vishnyakova P, Poltavets A, Nikitina M, Muminova K, Potapova A, Vtorushina V, Loginova N, Midiber K, Mikhaleva L, Lokhonina A, Khodzhaeva Z, Pyregov A, Elchaninov A, Fatkhudinov T, Sukhikh G. Preeclampsia: inflammatory signature of decidual cells in early manifestation of disease. Placenta. 2021;104:277-283. [CrossRef]
- Szewczyk G, Pyzlak M, Pankiewicz K, Szczerba E, Stangret A, Szukiewicz D, Skoda M, Bierła J, Cukrowska B, Fijałkowska A. The potential association between a new angiogenic marker fractalkine and a placental vascularization in preeclampsia. Arch Gynecol Obstet. 2021;304(2): 365-376. [CrossRef]
- Ullah A, Zhao J, Singla RK, Shen B. Pathophysiological impact of CXC and CX3CL1 chemokines in preeclampsia and gestational diabetes mellitus. Front Cell Dev Biol. 2023;11:1272536. [CrossRef]
- Szukiewicz D, Kochanowski J, Pyzlak M, Szewczyk G, Stangret A, Mittal TK. Fractalkine (CX3CL1) and its receptor CX3CR1 may contribute to increased angiogenesis in diabetic placenta. Mediators Inflamm. 2013;2013:437576. [CrossRef]
- Szukiewicz D, Kochanowski J, Mittal TK, Pyzlak M, Szewczyk G, Cendrowski K. CX3CL1 (fractalkine) and TNFα production by perfused human placental lobules under normoxic and hypoxic conditions in vitro: the importance of CX3CR1 signaling. Inflamm Res. 2014;63(3):179-89. [CrossRef]
- Gokce S, Herkiloglu D, Cevik O, Turan V. Role of chemokines in early pregnancy loss. Exp Ther Med. 2022;23(6):397. [CrossRef]
- Tanaka Y, Hoshino-Negishi K, Kuboi Y, Tago F, Yasuda N, Imai T. Emerging Role of Fractalkine in the Treatment of Rheumatic Diseases. Immunotargets Ther. 2020 ;9:241-253. [CrossRef]
- Koizumi K, Saitoh Y, Minami T, Takeno N, Tsuneyama K, Miyahara T, Nakayama T, Sakurai H, Takano Y, Nishimura M, Imai T, Yoshie O, Saiki I. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol. 2009;183(12):7825-31. [CrossRef]
- Guo YN, Cui SJ, Tian YJ, Zhao NR, Zhang YD, Gan YH, Zhou YH, Wang XD. Chondrocyte apoptosis in temporomandibular joint osteoarthritis promotes bone resorption by enhancing chemotaxis of osteoclast precursors. Osteoarthritis Cartilage. 2022;30(8):1140-1153. [CrossRef]
- Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [CrossRef]
- Lu Z, Zhang A, Dai Y. CX3CL1 deficiency ameliorates inflammation, apoptosis and accelerates osteogenic differentiation, mineralization in LPS-treated MC3T3-E1 cells via its receptor CX3CR1. Ann Anat. 2023;246:152036. [CrossRef]
- Gao XW, Hu HL, Xie MH, Tang CX, Ou J, Lu ZH. CX3CL1/CX3CR1 axis alleviates inflammation and apoptosis in human nucleus pulpous cells via M2 macrophage polarization. Exp Ther Med. 2023; 26(1):359. [CrossRef]
- Folkman, J. Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1-18. [CrossRef]
- Akbarian M, Bertassoni LE, Tayebi L. Biological aspects in controlling angiogenesis: current progress. Cell Mol Life Sci. 2022;79(7):349. [CrossRef]
- Szade A, Grochot-Przeczek A, Florczyk U, Jozkowicz A, Dulak J. Cellular and molecular mechanisms of inflammation-induced angiogenesis. IUBMB Life. 2015;67(3):145-59. [CrossRef]
- Narkar, VA. Narkar VA. Exercise and Ischemia-Activated Pathways in Limb Muscle Angiogenesis and Vascular Regeneration. Methodist Debakey Cardiovasc J. 2023;19(5):58-68. [CrossRef]
- Yin CL, Ma YJ. The Regulatory Mechanism of Hypoxia-inducible Factor 1 and its Clinical Significance. Curr Mol Pharmacol. 2024;17:e18761429266116. [CrossRef]
- Zhou W, Yang L, Nie L, Lin H. Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am J Cancer Res. 2021;11(2):301-317.
- Perry BN, Arbiser JL. The duality of angiogenesis: implications for therapy of human disease. J Invest Dermatol. 2006;126(10):2160-6. [CrossRef]
- Edgar LT, Park H, Crawshaw JR, Osborne JM, Eichmann A, Bernabeu MO. Traffic Patterns of the Migrating Endothelium: How Force Transmission Regulates Vascular Malformation and Functional Shunting During Angiogenic Remodelling. Front Cell Dev Biol. 2022;10:840066. [CrossRef]
- Silvestre JS, Lévy BI, Tedgui A. Mechanisms of angiogenesis and remodelling of the microvasculature. Cardiovasc Res. 2008;78(2):201-2. [CrossRef]
- Niklason L, Dai G. Arterial Venous Differentiation for Vascular Bioengineering. Annu Rev Biomed Eng. 2018;20:431-447. [CrossRef]
- Sarabipour S, Kinghorn K, Quigley KM, Kovacs-Kasa A, Annex BH, Bautch VL, Mac Gabhann F. Trafficking dynamics of VEGFR1, VEGFR2, and NRP1 in human endothelial cells. PLoS Comput Biol. 2024;20(2):e1011798.
- Colotti G, Failla CM, Lacal PM, Ungarelli M, Ruffini F, Di Micco P, Orecchia A, Morea V. Neuropilin-1 is required for endothelial cell adhesion to soluble vascular endothelial growth factor receptor 1. FEBS J. 2022;289(1):183-198. [CrossRef]
- Alghamdi AAA, Benwell CJ, Atkinson SJ, Lambert J, Johnson RT, Robinson SD. NRP2 as an Emerging Angiogenic Player; Promoting Endothelial Cell Adhesion and Migration by Regulating Recycling of α5 Integrin. Front Cell Dev Biol. 2020;8:395. [CrossRef]
- Li T, Ran J, Miao Z, Yang M, Mou D, Jiang Y, Xu X, Xie Q, Jin K. Deficiency of inflammation-sensing protein neuropilin-2 in myeloid-derived macrophages exacerbates colitis via NF-κB activation. J Pathol. 2024;262(2):175-188. [CrossRef]
- Rahane D, Dhingra T, Chalavady G, Datta A, Ghosh B, Rana N, Borah A, Saraf S, Bhattacharya P. Hypoxia and its effect on the cellular system. Cell Biochem Funct. 2024;42(2):e3940. [CrossRef]
- Cui Y, Lin H, Zhao YH, Ma JX, Li JX. Tube Formation Capability and Chemotaxis of Skin Pericytes. Discov Med. 2024;36(181):308-322. [CrossRef]
- Yang S, Graham J, Kahn JW, Schwartz EA, Gerritsen ME. Functional roles for PECAM-1 (CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am J Pathol. 1999;155(3):887-95. [CrossRef]
- Granger DN, Senchenkova E. Inflammation and the Microcirculation. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 6, Angiogenesis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53377/.
- Ouarné M, Pena A, Franco CA. From remodeling to quiescence: The transformation of the vascular network. Cells Dev. 2021;168:203735. [CrossRef]
- Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7(13):2056-70. [CrossRef]
- Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of Angiogenesis to Inflammation and Cancer. Front Oncol. 2019;9:1399. [CrossRef]
- Britzen-Laurent N, Weidinger C, Stürzl M. Contribution of Blood Vessel Activation, Remodeling and Barrier Function to Inflammatory Bowel Diseases. Int J Mol Sci. 2023;24(6):5517. [CrossRef]
- Reyes ME, Pulgar V, Vivallo C, Ili CG, Mora-Lagos B, Brebi P. Epigenetic modulation of cytokine expression in gastric cancer: influence on angiogenesis, metastasis and chemoresistance. Front Immunol. 2024;15:1347530. [CrossRef]
- Lee YE, Lee SH, Kim WU. Cytokines, Vascular Endothelial Growth Factors, and PlGF in Autoimmunity: Insights From Rheumatoid Arthritis to Multiple Sclerosis. Immune Netw. 2024 ;24(1):e10. [CrossRef]
- Vofo BN, Chowers I. Suppressing Inflammation for the Treatment of Diabetic Retinopathy and Age-Related Macular Degeneration: Dazdotuftide as a Potential New Multitarget Therapeutic Candidate. Biomedicines. 2023;11(6):1562. [CrossRef]
- Fu J, Liang H, Yuan P, Wei Z, Zhong P. Brain pericyte biology: from physiopathological mechanisms to potential therapeutic applications in ischemic stroke. Front Cell Neurosci. 2023;17:1267785. [CrossRef]
- Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-kappaB. FASEB J. 2008;22(6): 1807-16. [CrossRef]
- Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014; 26(3):253-66. [CrossRef]
- Diep S, Maddukuri M, Yamauchi S, Geshow G, Delk NA. Interleukin-1 and Nuclear Factor Kappa B Signaling Promote Breast Cancer Progression and Treatment Resistance. Cells. 2022; 11(10):1673. [CrossRef]
- Chen D, Ireland SJ, Remington G, Alvarez E, Racke MK, Greenberg B, Frohman EM, Monson NL. CD40-Mediated NF-κB Activation in B Cells Is Increased in Multiple Sclerosis and Modulated by Therapeutics. J Immunol. 2016;197(11):4257-4265. [CrossRef]
- Seigner J, Basilio J, Resch U, de Martin R. CD40L and TNF both activate the classical NF-κB pathway, which is not required for the CD40L induced alternative pathway in endothelial cells. Biochem Biophys Res Commun. 2018;495(1):1389-1394.
- Yang C, Hwang HH, Jeong S, Seo D, Jeong Y, Lee DY, Lee K. Inducing angiogenesis with the controlled release of nitric oxide from biodegradable and biocompatible copolymeric nanoparticles. Int J Nanomedicine. 2018;13:6517-6530. [CrossRef]
- Heydari P, Kharaziha M, Varshosaz J, Kharazi AZ, Javanmard SH. Co-release of nitric oxide and L-arginine from poly (β-amino ester)-based adhesive reprogram macrophages for accelerated wound healing and angiogenesis in vitro and in vivo. Biomater Adv. 2024;158:213762. [CrossRef]
- Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468(1):53-8. [CrossRef]
- Ryu J, Lee CW, Hong KH, Shin JA, Lim SH, Park CS, Shim J, Nam KB, Choi KJ, Kim YH, Han KH. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovasc Res. 2008;78(2):333-40. [CrossRef]
- Park Y, Lee J, Kwak JY, Noh K, Yim E, Kim HK, Kim YJ, Broxmeyer HE, Kim JA. Fractalkine induces angiogenic potential in CX3CR1-expressing monocytes. J Leukoc Biol. 2018;103(1): 53-66. [CrossRef]
- Smith TL, Oubaha M, Cagnone G, Boscher C, Kim JS, El Bakkouri Y, Zhang Y, Chidiac R, Corriveau J, Delisle C, Andelfinger GU, Sapieha P, Joyal JS, Gratton JP. eNOS controls angiogenic sprouting and retinal neovascularization through the regulation of endothelial cell polarity. Cell Mol Life Sci. 2021;79(1):37. [CrossRef]
- Li B, Zhang Y, Yin R, Zhong W, Chen R, Yan J. Activating CD137 Signaling Promotes Sprouting Angiogenesis via Increased VEGFA Secretion and the VEGFR2/Akt/eNOS Pathway. Mediators Inflamm. 2020;2020:1649453. [CrossRef]
- Zarychta E, Ruszkowska-Ciastek B. Cooperation between Angiogenesis, Vasculogenesis, Chemotaxis, and Coagulation in Breast Cancer Metastases Development: Pathophysiological Point of View. Biomedicines. 2022;10(2):300. [CrossRef]
- Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem. 2020;295(8):2495-2505. [CrossRef]
- Folkman, J. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15-8. [CrossRef]
- Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2(3):213-9. [CrossRef]
- Tannock, IF. Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968;22(2):258-73. [CrossRef]
- Place TL, Domann FE, Case AJ. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic Biol Med. 2017; 113:311-322. Erratum in: Free Radic Biol Med. 2021 Jan;162:180. [CrossRef]
- Folkman, J. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182-6. [CrossRef]
- Welter M, Bartha K, Rieger H. Vascular remodelling of an arterio-venous blood vessel network during solid tumour growth. J Theor Biol. 2009;259(3):405-22. [CrossRef]
- Gerlee P, Anderson AR. Diffusion-limited tumour growth: simulations and analysis. Math Biosci Eng. 2010;7(2):385-400. [CrossRef]
- Martin JD, Seano G, Jain RK. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu Rev Physiol. 2019;81:505-534. [CrossRef]
- Lefler DS, Manobianco SA, Bashir B. Immunotherapy resistance in solid tumors: mechanisms and potential solutions. Cancer Biol Ther. 2024;25(1):2315655. [CrossRef]
- Bielenberg DR, Zetter BR. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015;21(4):267-73. [CrossRef]
- Schlößer HA, Theurich S, Shimabukuro-Vornhagen A, Holtick U, Stippel DL, von Bergwelt-Baildon M. Overcoming tumor-mediated immunosuppression. Immunotherapy. 2014;6(9):973-88. [CrossRef]
- Wan Y, Fu LH, Li C, Lin J, Huang P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv Mater. 2021;33(48):e2103978. [CrossRef]
- Mohamed OAA, Tesen HS, Hany M, Sherif A, Abdelwahab MM, Elnaggar MH. The role of hypoxia on prostate cancer progression and metastasis. Mol Biol Rep. 2023;50(4):3873-3884. [CrossRef]
- Liu Z, Liu X, Zhang W, Gao R, Wei H, Yu CY. Current advances in modulating tumor hypoxia for enhanced therapeutic efficacy. Acta Biomater. 2024;176:1-27. [CrossRef]
- Ma S, Pan X, Gan J, Guo X, He J, Hu H, Wang Y, Ning S, Zhi H. DNA methylation heterogeneity attributable to a complex tumor immune microenvironment prompts prognostic risk in glioma. Epigenetics. 2024;19(1):2318506. [CrossRef]
- Ge R, Wang Z, Cheng L. Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance. NPJ Precis Oncol. 2022;6(1):31. [CrossRef]
- Jia Q, Wang A, Yuan Y, Zhu B, Long H. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp Hematol Oncol. 2022;11(1):24. [CrossRef]
- Sidibe A, Ropraz P, Jemelin S, Emre Y, Poittevin M, Pocard M, Bradfield PF, Imhof BA. Angiogenic factor-driven inflammation promotes extravasation of human proangiogenic monocytes to tumours. Nat Commun. 2018;9(1):355. [CrossRef]
- Richards DM, Hettinger J, Feuerer M. Monocytes and macrophages in cancer: development and functions. Cancer Microenviron. 2013;6(2):179-91. [CrossRef]
- Li F, Wang Z, Liu Y, Li J. Down-regulation of fractalkine inhibits the in vitro and in vivo angiogenesis of the hepatocellular carcinoma HepG2 cells. Oncol Rep. 2010;24(3):669-75.
- Zheng J, Yang M, Shao J, Miao Y, Han J, Du J. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Mol Cancer. 2013;12(1):141. [CrossRef]
- Xu X, Wang Y, Chen J, Ma H, Shao Z, Chen H, Jin G. High expression of CX3CL1/CX3CR1 axis predicts a poor prognosis of pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2012; 16(8):1493-8. [CrossRef]
- Ren T, Chen Q, Tian Z, Wei H. Down-regulation of surface fractalkine by RNA interference in B16 melanoma reduced tumor growth in mice. Biochem Biophys Res Commun. 2007;364(4): 978-84. [CrossRef]
- Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64(14):4693-8. [CrossRef]
- Goto Y, Aoyama M, Sekiya T, Kakita H, Waguri-Nagaya Y, Miyazawa K, Asai K, Goto S. CXCR4+ CD45- Cells are Niche Forming for Osteoclastogenesis via the SDF-1, CXCL7, and CX3CL1 Signaling Pathways in Bone Marrow. Stem Cells. 2016;34(11):2733-2743. [CrossRef]
- Jamieson WL, Shimizu S, D’Ambrosio JA, Meucci O, Fatatis A. CX3CR1 is expressed by prostate epithelial cells and androgens regulate the levels of CX3CL1/fractalkine in the bone marrow: potential role in prostate cancer bone tropism. Cancer Res. 2008;68(6):1715-22. [CrossRef]
- Liu P, Liang Y, Jiang L, Wang H, Wang S, Dong J. CX3CL1/fractalkine enhances prostate cancer spinal metastasis by activating the Src/FAK pathway. Int J Oncol. 2018;53(4):1544-1556. [CrossRef]
- Liang Y, Yi L, Liu P, Jiang L, Wang H, Hu A, Sun C, Dong J. CX3CL1 involves in breast cancer metastasizing to the spine via the Src/FAK signaling pathway. J Cancer. 2018 ;9(19):3603-3612. [CrossRef]
- Liu W, Liang Y, Chan Q, Jiang L, Dong J. CX3CL1 promotes lung cancer cell migration and invasion via the Src/focal adhesion kinase signaling pathway. Oncol Rep. 2019;41(3):1911-1917. [CrossRef]
- Guo J, Chen T, Wang B, Zhang M, An H, Guo Z, Yu Y, Qin Z, Cao X. Chemoattraction, adhesion and activation of natural killer cells are involved in the antitumor immune response induced by fractalkine/CX3CL1. Immunol Lett. 2003;89(1):1-7. [CrossRef]
- Chen, CK. Chen CK. Inference of gene networks using gene expression data with applications. Heliyon. 2024;10(5):e26065. [CrossRef]
- Yu D, Lim J, Wang X, Liang F, Xiao G. Enhanced construction of gene regulatory networks using hub gene information. BMC Bioinformatics. 2017;18(1):186. [CrossRef]
- Liu Y, Liu C, Huang D, Ge C, Chen L, Fu J, Du J. Identification and prognostic analysis of candidate biomarkers for lung metastasis in colorectal cancer. Medicine (Baltimore). 2024; 103(11):e37484. [CrossRef]
- Yue Y, Zhang Q, Sun Z. CX3CR1 Acts as a Protective Biomarker in the Tumor Microenvironment of Colorectal Cancer. Front Immunol. 2022;12:758040. [CrossRef]
- Erreni M, Siddiqui I, Marelli G, Grizzi F, Bianchi P, Morone D, Marchesi F, Celesti G, Pesce S, Doni A, Rumio C, Roncalli MG, Laghi L, Mantovani A, Allavena P. The Fractalkine-Receptor Axis Improves Human Colorectal Cancer Prognosis by Limiting Tumor Metastatic Dissemination. J Immunol. 2016;196(2):902-14. [CrossRef]
- Dreyer TF, Kuhn S, Stange C, Heithorst N, Schilling D, Jelsma J, Sievert W, Seitz S, Stangl S, Hapfelmeier A, Noske A, Wege AK, Weichert W, Ruland J, Schmitt M, Dorn J, Kiechle M, Reuning U, Magdolen V, Multhoff G, Bronger H. The Chemokine CX3CL1 Improves Trastuzumab Efficacy in HER2 Low-Expressing Cancer In Vitro and In Vivo. Cancer Immunol Res. 2021;9(7):779-789. [CrossRef]
- Wege AK, Dreyer TF, Teoman A, Ortmann O, Brockhoff G, Bronger H. CX3CL1 Overexpression Prevents the Formation of Lung Metastases in Trastuzumab-Treated MDA-MB-453-Based Humanized Tumor Mice (HTM). Cancers (Basel). 2021;13(10):2459. [CrossRef]
- Kee JY, Arita Y, Shinohara K, Ohashi Y, Sakurai H, Saiki I, Koizumi K. Antitumor immune activity by chemokine CX3CL1 in an orthotopic implantation of lung cancer model in vivo. Mol Clin Oncol. 2013;1(1):35-40. [CrossRef]
- Liu J, Li Y, Zhu X, Li Q, Liang X, Xie J, Hu S, Peng W, Li C. Increased CX3CL1 mRNA expression level is a positive prognostic factor in patients with lung adenocarcinoma. Oncol Lett. 2019; 17(6):4877-4890. [CrossRef]
- Matsubara T, Ono T, Yamanoi A, Tachibana M, Nagasue N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J Surg Oncol. 2007;95(3):241-9. [CrossRef]
- Hyakudomi M, Matsubara T, Hyakudomi R, Yamamoto T, Kinugasa S, Yamanoi A, Maruyama R, Tanaka T. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+ T cells and natural killer cells in gastric adenocarcinoma. Ann Surg Oncol. 2008;15(6):1775-82. [CrossRef]
- Korbecki J, Simińska D, Kojder K, Grochans S, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Fractalkine/CX3CL1 in Neoplastic Processes. Int J Mol Sci. 2020;21(10):3723. [CrossRef]
- Shao D, Zhou H, Yu H, Zhu X. CX3CR1 is a potential biomarker of immune microenvironment and prognosis in epithelial ovarian cancer. Medicine (Baltimore). 2024;103(3):e36891. [CrossRef]
- Yao X, Qi L, Chen X, Du J, Zhang Z, Liu S. Expression of CX3CR1 associates with cellular migration, metastasis, and prognosis in human clear cell renal cell carcinoma. Urol Oncol. 2014;32(2):162-70. [CrossRef]
- Sun C, Hu A, Wang S, Tian B, Jiang L, Liang Y, Wang H, Dong J. ADAM17-regulated CX3CL1 expression produced by bone marrow endothelial cells promotes spinal metastasis from hepatocellular carcinoma. Int J Oncol. 2020;57(1):249-263. [CrossRef]
- Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, Kunzelmann C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69(3): 785-93. [CrossRef]
- Smith TM Jr, Tharakan A, Martin RK. Targeting ADAM10 in Cancer and Autoimmunity. Front Immunol. 2020;11:499. [CrossRef]
- Wang K, Xuan Z, Liu X, Zheng M, Yang C, Wang H. Immunomodulatory role of metalloproteinase ADAM17 in tumor development. Front Immunol. 2022;13:1059376. [CrossRef]
| Location in the body | Regulation of biological processes via FKN/CX3CR1 axis in normal and pathological conditions | References |
|---|---|---|
| Central nervous system (CNS) | - In brain tissues, FKN is mostly expressed in neurons while microglia express CX3CR1. - FKN/CX3CR1 signaling enables precise interactions between neurons, microglia, and immune cells. - Through its key role in microglia-neuron communication, the FKN/CX3CR1 axis regulates a broad spectrum of microglial properties including microglial cell migration and dynamic surveillance of the brain parenchyma, neuronal survival, synaptic plasticity, and a variety of synaptic functions, as well as neuronal excitability via cytokine release modulation, chemotaxis, and phagocytosis. - FKN suppresses lipopolysaccharide (LPS)-induced microglia activation by reducing the production of nitric oxide (NO), interleukin-6 (IL-6) and transforming growth factor alpha (TNF-α), and therefore inhibits neuronal cell death in response to LPS cytotoxicity in the brain tissue. - FKN/CX3CR1 signal disruption is one of the most important elements in the pathogenesis of CNS-related disorders, especially neurodegenerative diseases and brain traumatic injuries. However, the results of studies on the modulation of inflammation in the CNS by FKN/CX3CR1 are often ambiguous or contradictory. For example, disruption of FKN signaling is beneficial in limiting the effects of CNS ischemia, but detrimental in other neurodegenerative diseases, including Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS). Furthermore, deletion of CX3CR1 in Alzheimer’s disease may, possibly depending on the stage of disease progression, lead to both neuroprotective and detrimental effects. There is also no complete agreement on the importance of the involvement of FKN isoforms in the development of neuropathological processes. - The specific response, neurotoxic or neuroprotective, most likely depends on the type of destructive factor, the CNS area influencing the regional heterogeneity of microglial cells, and the local concentrations of FKN and CX3CR1. |
Sheridan and Murphy 2013 [188] Pawelec et al. 2020 [189] Paolicelli et al. 2014 [190]; Camacho-Hernández and Peña-Ortega 2023 [191] Mizuno et al. 2003 [192]; Lyons et al. 2009 [161]; Mecca et al. 2018 [193] Subbarayan et al. 2022 [194]; Cipriani et al. 2011 [195]; Poniatowski et al. 2017 [57]; Luo et al. 2019 [196]; Bivona et al. 2023 [197]; Nash et al. 2015 [198]; Juliani et al. 2021 [199]; Lee et al. 2010 [200], Cho et al. 2011 [201]; Fuhrmann et al. 2010 [202]; Pawelec et al. 2020 [189]; Eugenín et al. 2023 [203] Sheridan and Murphy 2013 [188]; Stratoulias et al. [204] |
| Bone marrow and immune tissue | - The expression of CX3XR1 increases with the maturation of myeloid cells and shows an inverse correlation with the Ly6C marker and the C-C chemokine receptor 2 (CCR2) in the blood. This may indicate that CX3CR1 reduces the motility of Ly6C(high) monocytes in the bone marrow and thereby controls their release into the bloodstream. - FKN-CX3CL1 axis plays a significant role in an early stage of osteoblast differentiation, possibly through their trans and cis interactions. - FKN regulates mouse osteoclast precursors (OCPs) survival and primes OCPs for subsequent osteoclast differentiation. - Autoimmune and inflammatory response in rheumatoid arthritis (RA) is positively correlated with concentration of FKN in the serum and synovial fluid. The associated chemotaxis primarily involves the recruitment of CD16+ monocytes into synovial tissues, as CX3CR1 expression in CD16+ monocytes is markedly higher compared to other populations (e.g., CD14+ and CD16-). - Bone marrow (BM) FKN levels are significantly increased in the multiple myeloma (MM) patients and positively correlated with BM microvessel density. - CX3CR1 expression is an additional marker of natural killer (NK) cells differentiation and is closely related to their ability to migrate to the central nervous system (CNS) from the periphery. - CX3CR1 is prevalently expressed on killer cell lectin-like receptor subfamily G member 1 (KLRG1)+ NK cells, a subset considered terminally differentiated. Therefore, CX3CR1 may represent a marker of a KLRG1(+) NK-cell population with unique properties that can irreversibly differentiate from the KLRG1(+)/CX3CR1(-) NK cells during steady state conditions in the bone marrow. - FKN activates Jak2-Stat5α-ERK1/2 signaling through CX3CR1, thereby triggering integrin-dependent machinery of the cytoskeleton reorganization to allow chemotactic migration of bone marrow-derived mesenchymal stem cells (BMSCs) towards an ischemic cerebral lesion. |
Jacquelin et al. 2013 [205] Hoshino et al. 2013 [206] Kuboi et al. 2022 [207] Yano et al. 2007 [208] Marchica et al. 2019 [209] Hamman et al. 2011 [90] Sciumè et al. 2011 [210] Zhang et al. 2015 [211] |
| Cardiovascular system | - FKN and CX3CR1 are expressed in atherosclerotic lesions and FKN is involved in the initiation step of atherosclerotic plaque formation. Monocyte-endothelial cell interactions are partly mediated by expression of monocyte CX3CR1 and endothelial cell FKN. Activation of these lymphocytes upon ligand/receptor binding leads to release of lysis granules that destroy vascular endothelium. - After endothelial damage, the release of FKN from apoptotic cells results in subsequent recruitment of macrophages, which promotes the removal of apoptotic debris; However, in more advanced stages of atherosclerosis, signaling through the FKN/CX3CR1 axis enhances foam cell formation, promoting the development of atherosclerotic plaques. - CX3CR1 expression on vascular smooth muscle cells (VSMCs) within atherosclerotic plaque causes the functional state of the FKN/CX3CR1 axis to play an important role in plaque stability. Events responsible for cardiovascular mortality and morbidity are predominantly caused by rupture of “vulnerable” atherosclerotic lesions. - FKN promotes the development of atherosclerotic lesions by activating platelets and causing their adhesion to the endothelium. - Early activation of the cardiac FKN/CX3CR1 axis delays β-adrenergic-induced heart failure. - FKN levels are markedly elevated during acute myocardial infarction (AMI), compared to patients with stable angina pectoris (AP), although they do not correlate with infarct size. The inverse pattern in gene expression of CX3CR1 might be here a compensatory mechanism. - In addition to demonstrating a positive correlation of FKN concentration with an increased risk of developing poorer cardiac function after AMI, levels of FKN had also been proven to be prognostic for the likelihood of developing major adverse cardiovascular events (MACEs) in an acute ST-elevation myocardial infarction (STEMI) patients. - Inhibition of the FKN/CX3CR1 interaction has a beneficial effect on the final infarct size after reperfusion, as it reduces the severity of an important complication - ischemia/reperfusion injury. This complication is directly related to the action of CX3CR1+ lymphocytes towards microvascular obstruction (MVO). |
Teupser et al. 2004 [212]; Ma et al. 2022 [213]; Riopel et al. 2019 [214]; Liu and Jiang 2011 [215] Elliott et al. 2017 [216]; White et al. 2014[186]; Landsman et al. 2009 [167] Lucas et al. 2003 [217]; Harman and Jørgensen 2019 [218]; Apostolakis and Spandidos 2013 [219]; Skoda et al. 2018 [220] Noels et al. 2019 [221]; Flierl et al. 2015 [76] Flamant et al. 2021 [222] Njerve et al. 2014 [223]; Yao et al. 2015 [224] Yao et al. 2015 [224];Xu et al. 2019 [225] Loh et al. 2023 [44]; Boag et al. 2015 [226] |
| Respiratory system | - CX3CR1+ leukocyte attachment to and migration through the lung vascular endothelium lead to mononuclear cell accumulation in the lung vessel walls and parenchyma. Infiltrated CX3CR1+ immune cells can release mediators to induce injury, stimulate proliferation, and/or chemoattract inflammatory cells. This contributes to structural destruction and remodeling in the development of inflammatory lung diseases. - FKN/CX3CR1 signaling may be involved in the pathophysiology of hypoxia-induced pulmonary arterial hypertension (PAH) developing due to chronic inflammation. Both increased FKN concentrations and upregulated CX3CR1 expression cause PAH progression with vascular remodeling and proliferation of pulmonary artery smooth muscle cells. - Soluble FKN chemoattracts and activates CX3CR1+ leukocytes such as CD8+, CD4+, and γδ T lymphocytes, natural killer cells, dendritic cells, and monocytes/macrophages leading to mononuclear cell accumulation in the lung vessel walls and parenchyma. During the resolution phase of acute lung injury, apoptotic cell-derived CX3CL1 attracts alveolar macrophages transmigration toward apoptotic cells for phagocytosis. - In allergic asthma CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung together with chemotaxis recruited mast cells into bronchial mucosa. - CX3CL1 (fractalkine) is elevated in both bronchoalveolar lavage fluid and sputum from human asthmatics sensitized to fungi, implicating an association with CX3CL1 in fungal asthma severity. However, CX3CL1/CX3CR1 axis preserves lung function during fungal-associated allergic airway inflammation through a nonclassical immunoregulatory mechanism. Hence, absence of CX3CR1 signaling resulted in a profound impairment in lung function during fungal-associated allergic airway inflammation. - In pulmonary infections, the role of FKN/CX3CR1 axis remains unclear. For example, FKN may be involved in both immunopathological and anti-viral immune responses to rhinovirus infection. |
Zhang and Patel 2010 [227] Balabanian et al. 2002 [228]; Amsellem et al. 2017 [229] Tsai et al. 2021 [230] Mionnet et al. 2010 [91]; El-Shazly et al. 2006 [231] Godwin et al. 2021 [232] Upton et al. 2017 [233] |
| Liver | - FKN/CX3CR1 is upregulated during liver damage including chronic inflammatory liver diseases such as chronic hepatitis C, nonalcoholic steatohepatitis (NASH)/nonalcoholic fatty liver disease (NAFLD) and cirrhosis. - Assessment of the impact of increased FKN/CX3CR1 activity on the severity of steatosis, inflammation and liver fibrosis is still ambiguous. In addition to reports indicating that FKN-CX3CR1 interaction inhibits inflammatory properties in Kupffer cells/macrophages, resulting in decreased liver inflammation and fibrosis, there are also contradictory research data. - FKN/CX3CR1 upregulation was reported in injured bile ducts of primary cirrhosis with its involvement in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Moreover, the correlation between primary biliary cirrhosis and FKN expression is significantly proportional. |
Efsen et al. 2002 [234]; Bourd-Boittin et al. 2009 [59]; Sutti et al. 2015 [235]; Nagata et al. 2022 [236] Aoyama et al. 2010 [237]; Zhang et al. 2020 [238]; Ni et al. 2022 [71]; Sutti et al. 2015 [235]; Karlmark et al. 2010 [239]; Wasmuth et al. 2008 [240]; Hassan et al. 2023 [241] Isse et al. 2005 [242]; Shimoda et al. 2010 [243] |
| Gut | - Most macrophages and some dendritic cell (DC) subsets express CX3CR1 in the gut. In resting intestinal mucosa, the role of lamina propria CX3CR1+ macrophage is to pass captured antigen via trans-epithelial dendrites or phagocytosis onto DC for transport to mesenteric lymph node (MLN) to prime immune responses like lamina propria DC. - Deletion of FKN or CX3CR1 leads to a specific and significant reduction in lamina propria macrophages with decreased translocation of bacteria to MLNs and their ability to take up pathogens. Therefore, CX3CR1 may be treated as a specific marker for lamina propria macrophages and a critical component in maintaining lamina propria macrophage homeostasis. Contradictory, it has been reported that CX3CR1 deficient mice shows normal number of macrophages. - Intestinal microbiota can influence local accumulation of CX3CR1+ phagocytes, and the number of CX3CR1+ cells is reduced in germ-free mouse. - Enhanced recruitment of CX3CR1+ T cells by mucosal human intestinal microvascular endothelial cell (HIMECs)-derived FKN has been demonstrated in inflammatory bowel disease (IBD). |
Joeris et al. 2017 [244]; Niess et al. 2005 [114]; Bain and Mowat 2011 [245]; Lee et al. 2018 [70] Ferretti et al. 2014 [246]; Bain et al. 2013 [247] Bain et al. 2014 [248] Sans et al. 2007 [249] |
| Placenta | - Human placenta is a source of FKN, which is expressed in the syncytiotrophoblast and can be released into the maternal circulation by constitutive MMP-dependent shedding. - FKN content within the apical microvillous plasma membrane increases significantly in the placenta of full-term pregnancy compared to the first trimester. - FKN/CX3CR1 axis mediates adhesion of monocytes to the villous trophoblast. - Increased expression and release of placental FKN may contribute to low grade systemic inflammatory responses in third trimester of normal pregnancy. - Placental FKN is upregulated in severe early-onset preeclampsia (PE). Significant underdevelopment of placental vascular network with significantly lowered the vascular/extravascular tissue index (V/EVTI) in PE is associated with dysregulation of the FKN/CX3CR1 system, especially in fetal growth restriction (FGR)-complicated pregnancies. - Increased average FKN content in the diabetic placenta is accompanied by an increase in the density of placental microvessels and a higher expression of CX3CR1, compared to the placenta from a normal pregnancy. Therefore, FKN/CX3CR1 signaling pathway is involved in the pathomechanism of placental microvasculature remodeling during diabetes class C (after White). - Placental hypoxia increases FKN production and upregulates CX3CR1 expression in the placental endothelial cells. Under these conditions, tumor necrosis factor alpha (TNFα) induces FKN, influencing a mechanism of FKN autoregulation by CX3CR1 expression. - Increased FKN concentration, accompanied by a higher mean FKN gene expression level in the tissues of pregnant women with missed abortion may be responsible for abnormal placental invasion. |
Siwetz et al. 2014 [250] Siwetz et al. 2015 [251] Siwetz et al. 2015 [252] Vishnyakova et al. 2021 [253] Szewczyk et al. 2021 [254]; Ullah et al. 2023 [255] Szukiewicz et al. 2013 [256]; Ullah et al. 2023 [255]; Szukiewicz et al. 2014 [257] Gokce et al. 2022 [258] |
| Joint and bone tissue | - The number of circulating CX3CR1high T cell is elevated in the circulation of rheumatoid arthritis (RA) patients. Joint-infiltrated CX3CR1high T cells strongly adhere to fibroblast-like synoviocytes (FLSs) in the synovium in an FKN-dependent manner. - FKN/CX3CR1 axis promotes inflammation-free osteoclastogenesis by enhancing precursor cell survival and differentiation. - Apoptosis of chondrocytes during joint osteoarthritis upregulates of the FKN-CX3CR1-p38 axis, which results in enhanced chemotaxis of osteoclast precursors (OCPs) and promotes bone resorption. - Development of osteoarthritis (OA) is largely driven by low-grade local background inflammation based on FKN-mediated chemotaxis. - FKN/CX3CR1 signaling in hemophilia is involved in the pathomechanism of irreversible joint degeneration (hemophilic arthropathy). - High concentrations of FKN in human blood serum are accompanied by increased concentrations of bone turnover and inflammatory factors in the serum, such as tartrate-resistant acid phosphatase 5b(TRACP-5b), cross-linked N-telopeptides of type I collagen (NTx) and interleukins (IL-1β, IL-6). - FKN knockdown ameliorates inflammation and apoptosis after exposure to LPS, and accelerates osteogenic differentiation. These effects related to FKN deficiency can be reversed by increased expression of CX3CR1. - FKN axis signaling alleviates intervertebral disc degeneration (IDD) by reducing inflammation and apoptosis of human nucleus pulposus cells (HNPCs) via macrophages. |
Tanaka et al. 2020 [259] Kuboi et al. 2022 [207]; Koizumi et al. 2009 [260] Koizumi et al. 2009 [260]; Guo et al. 2022 [261] Wojdasiewicz et al. 2014 [43,262] Wojdasiewicz et al. 2020 [184] Wojdasiewicz et al. 2019 [160] Lu et al. 2023 [263] Gao et al. 2023 [264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




