Submitted:
25 March 2024
Posted:
26 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Recent Diverse Theories of Control Mechanisms of Tension Development in Cardiac Sarcomeres
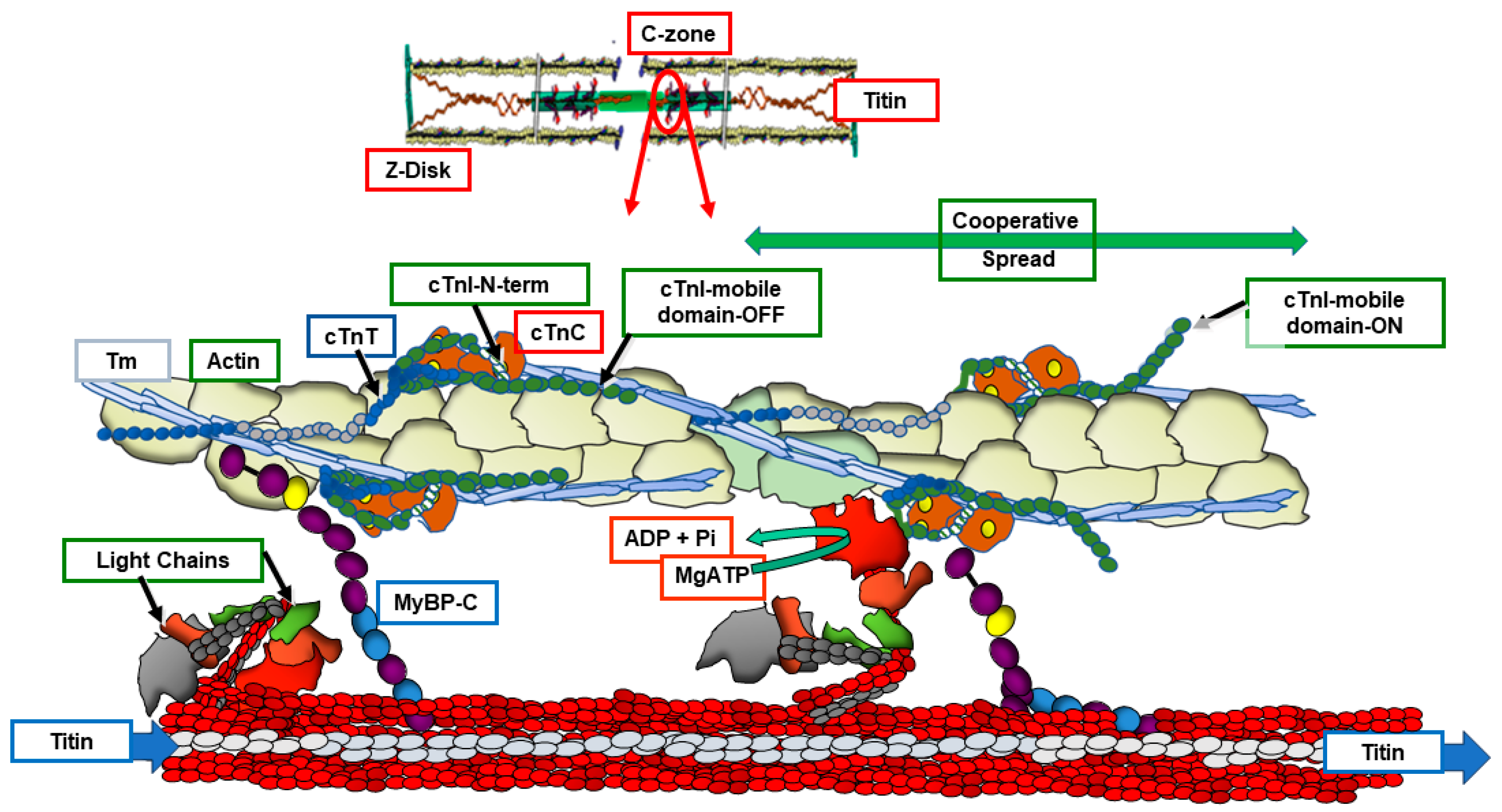
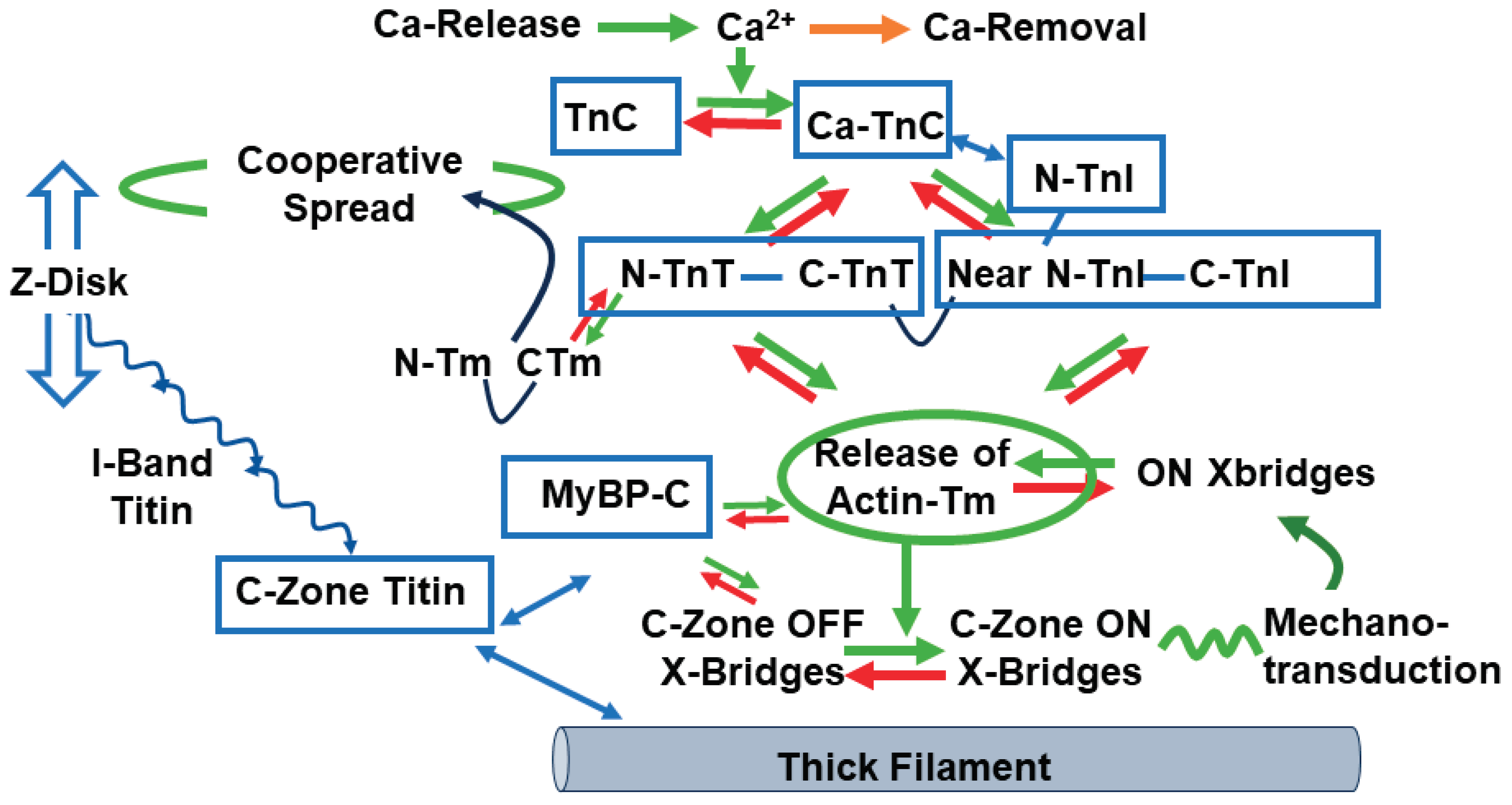
3. Cross-Bridge ON/OFF States and Control of Tension/Wall Stress
4. Implications of the On/Off Theory in Modulation of Tension and Dynamics by Dominant Myocardial Regulatory Mechanisms
4.1. Regulation by Sarcomere Length and Load
4.2. Regulation by Sarcomere Protein Phosphorylation
5. Research Challenges in the Quest to Develop Sarcomere Targeted Drugs Treating DCM
5.1. Targeting Thin Filaments in DCM Therapy
5.2. Targeting the Troponin Complex Avoiding PDE III Inhibition
5.3. Myosin Heavy Chain as a Drug Target
5.4. Cardiac Myosin Binding Protein C as a Drug Target
6. Future Directions and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNally EM, Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ Res. 2017;121(7):731-48.
- Fatkin D, Huttner IG, Kovacic JC, Seidman JG, Seidman CE. Precision Medicine in the Management of Dilated Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74(23):2921-38.
- Koslow M, Mondaca-Ruff D, Xu X. Transcriptome studies of inherited dilated cardiomyopathies. Mammalian Genome. 2023;34(2):312-22. [CrossRef]
- Bretherton RC, Reichardt IM, Zabrecky KA, Goldstein AJ, Bailey LRJ, Bugg D, et al. Correcting dilated cardiomyopathy with fibroblast-targeted p38 deficiency. bioRxiv. 2023.
- Merlo M, Cannatá A, Vitagliano A, Zambon E, Lardieri G, Sinagra G. Clinical management of dilated cardiomyopathy: current knowledge and future perspectives. Expert Rev Cardiovasc Ther. 2016;14(2):137-40. [CrossRef]
- von Lewinski D, Gasser R, Rainer PP, Huber MS, Wilhelm B, Roessl U, et al. Functional effects of glucose transporters in human ventricular myocardium. Eur J Heart Fail. 2010;12(2):106-13. [CrossRef]
- Chandra Mohan N, Foley P, Chandrasekaran B. A case report of successful physiological pacing in a patient with lamin A/C cardiomyopathy. Eur Heart J Case Rep. 2022;6(8):ytac324. [CrossRef]
- Fairuz S, Ang CW, Mraiche F, Goh JK. Current Targets and Future Directions of Positive Inotropes for Heart Failure. Curr Med Chem. 2023. [CrossRef]
- Powers JD, Kooiker KB, Mason AB, Teitgen AE, Flint GV, Tardiff JC, et al. Modulating the tension-time integral of the cardiac twitch prevents dilated cardiomyopathy in murine hearts. JCI Insight. 2020;5(20). [CrossRef]
- Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N Engl J Med. 2021;384(2):105-16.
- Tang W, Unrath WC, Desetty R, Yengo CM. Dilated cardiomyopathy mutation in the converter domain of human cardiac myosin alters motor activity and response to omecamtiv mecarbil. J Biol Chem. 2019;294(46):17314-25. [CrossRef]
- Kooiker KB, Mohran S, Turner KL, Ma W, Flint G, Qi L, et al. Danicamtiv increases myosin recruitment and alters the chemomechanical cross bridge cycle in cardiac muscle. bioRxiv. 2023.
- Nag S, Gollapudi SK, Del Rio CL, Spudich JA, McDowell R. Mavacamten, a precision medicine for hypertrophic cardiomyopathy: From a motor protein to patients. Sci Adv. 2023;9(30):eabo7622. [CrossRef]
- Coats CJ, Maron MS, Abraham TP, Olivotto I, Lee MMY, Arad M, et al. Exercise Capacity in Patients With Obstructive Hypertrophic Cardiomyopathy: SEQUOIA-HCM Baseline Characteristics and Study Design. JACC Heart Fail. 2023.
- Paratz ED, Mundisugih J, Rowe SJ, Kizana E, Semsarian C. Gene therapy in cardiology: is a cure for hypertrophic cardiomyopathy on the horizon? Can J Cardiol. 2023.
- Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, et al. A Tension-Based Model Distinguishes Hypertrophic versus Dilated Cardiomyopathy. Cell. 2016;165(5):1147-59. [CrossRef]
- Sandler H, Dodge HT. LEFT VENTRICULAR TENSION AND STRESS IN MAN. Circ Res. 1963;13:91-104. [CrossRef]
- Vikhorev PG, Vikhoreva NN, Yeung W, Li A, Lal S, Dos Remedios CG, et al. Titin-truncating mutations associated with dilated cardiomyopathy alter length-dependent activation and its modulation via phosphorylation. Cardiovasc Res. 2022;118(1):241-53. [CrossRef]
- LeWinter MM, Granzier HL. Titin is a major human disease gene. Circulation. 2013;127(8):938-44.
- Solaro RJ. Advances in understanding the state of titin truncation variants in dilated cardiomyopathy. Pflugers Arch. 2022;474(3):265-6. [CrossRef]
- Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J. 2011;100(8):1969-76.
- Nag S, Trivedi DV. To lie or not to lie: Super-relaxing with myosins. eLife. 2021;10. [CrossRef]
- Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115(35):E8143-e52. [CrossRef]
- McNamara JW, Singh RR, Sadayappan S. Cardiac myosin binding protein-C phosphorylation regulates the super-relaxed state of myosin. Proc Natl Acad Sci U S A. 2019;116(24):11731-6. [CrossRef]
- Schmid M, Toepfer CN. Cardiac myosin super relaxation (SRX): a perspective on fundamental biology, human disease and therapeutics. Biol Open. 2021;10(2). [CrossRef]
- Day SM, Tardiff JC, Ostap EM. Myosin modulators: emerging approaches for the treatment of cardiomyopathies and heart failure. J Clin Invest. 2022;132(5). [CrossRef]
- Kawana M, Spudich JA, Ruppel KM. Hypertrophic cardiomyopathy: Mutations to mechanisms to therapies. Front Physiol. 2022;13:975076. [CrossRef]
- Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, et al. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 2015;528(7581):276-9. [CrossRef]
- Reconditi M, Linari M, Lucii L, Stewart A, Sun YB, Narayanan T, et al. Structure-function relation of the myosin motor in striated muscle. Ann N Y Acad Sci. 2005;1047:232-47. [CrossRef]
- Park-Holohan SJ, Brunello E, Kampourakis T, Rees M, Irving M, Fusi L. Stress-dependent activation of myosin in the heart requires thin filament activation and thick filament mechanosensing. Proc Natl Acad Sci U S A. 2021;118(16). [CrossRef]
- Caremani M, Pinzauti F, Powers JD, Governali S, Narayanan T, Stienen GJM, et al. Inotropic interventions do not change the resting state of myosin motors during cardiac diastole. J Gen Physiol. 2019;151(1):53-65. [CrossRef]
- Ma W, Jani VP, Song T, Gao C, Gong H, Sadayappan S, et al. The structural OFF and ON states of myosin can be decoupled from the biochemical super-relaxed and disordered-relaxed states. bioRxiv. 2023. [CrossRef]
- Tamborrini D, Wang Z, Wagner T, Tacke S, Stabrin M, Grange M, et al. Structure of the native myosin filament in the relaxed cardiac sarcomere. Nature. 2023. [CrossRef]
- Tonino P, Kiss B, Gohlke J, Smith JE, 3rd, Granzier H. Fine mapping titin's C-zone: Matching cardiac myosin-binding protein C stripes with titin's super-repeats. J Mol Cell Cardiol. 2019;133:47-56.
- Chen L, Liu J, Rastegarpouyani H, Janssen PML, Pinto JR, Taylor KA. Structure of mavacamten-free human cardiac thick filaments within the sarcomere by cryoelectron tomography. Proc Natl Acad Sci U S A. 2024;121(9):e2311883121. [CrossRef]
- Sequeira V, Maack C, Reil GH, Reil JC. Exploring the Connection Between Relaxed Myosin States and the Anrep Effect. Circ Res. 2024;134(1):117-34. [CrossRef]
- dos Remedios CG, Law KYC, McNamara JW, Kraft T, Peckham M, van der Velden J, et al., editors. The Molecular Basis of the Frank-Starling Law of the Heart: A Possible Role for PIEZO1?2024; Cham: Springer International Publishing.
- Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424(6944):35-41. [CrossRef]
- Yamada Y, Namba K, Fujii T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat Commun. 2020;11(1):153. [CrossRef]
- Brunello E, Fusi L. Regulating Striated Muscle Contraction: Through Thick and Thin. Annu Rev Physiol. 2023. [CrossRef]
- Solís C, Solaro RJ. Novel insights into sarcomere regulatory systems control of cardiac thin filament activation. J Gen Physiol. 2021;153(7). [CrossRef]
- Rynkiewicz MJ, Pavadai E, Lehman W. Modeling Human Cardiac Thin Filament Structures. Front Physiol. 2022;13:932333. [CrossRef]
- Risi CM, Belknap B, Atherton J, Leite Coscarella I, White HD, Bryant Chase P, et al. Troponin structural dynamics in the native cardiac thin filament revealed by cryo electron microscopy. Journal of Molecular Biology. 2024:168498. [CrossRef]
- Risi C, Schäfer LU, Belknap B, Pepper I, White HD, Schröder GF, et al. High-Resolution Cryo-EM Structure of the Cardiac Actomyosin Complex. Structure. 2021;29(1):50-60.e4. [CrossRef]
- Tobacman LS. Troponin Revealed: Uncovering the Structure of the Thin Filament On-Off Switch in Striated Muscle. Biophys J. 2021;120(1):1-9.
- Mun JY, Previs MJ, Yu HY, Gulick J, Tobacman LS, Beck Previs S, et al. Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism. Proc Natl Acad Sci U S A. 2014;111(6):2170-5. [CrossRef]
- Risi C, Belknap B, Forgacs-Lonart E, Harris SP, Schröder GF, White HD, et al. N-Terminal Domains of Cardiac Myosin Binding Protein C Cooperatively Activate the Thin Filament. Structure. 2018;26(12):1604-11.e4. [CrossRef]
- Ponnam S, Sevrieva I, Sun YB, Irving M, Kampourakis T. Site-specific phosphorylation of myosin binding protein-C coordinates thin and thick filament activation in cardiac muscle. Proc Natl Acad Sci U S A. 2019;116(31):15485-94. [CrossRef]
- Sevrieva IR, Ponnam S, Yan Z, Irving M, Kampourakis T, Sun YB. Phosphorylation-dependent interactions of myosin-binding protein C and troponin coordinate the myofilament response to protein kinase A. J Biol Chem. 2023;299(1):102767. [CrossRef]
- Loescher CM, Breitkreuz M, Li Y, Nickel A, Unger A, Dietl A, et al. Regulation of titin-based cardiac stiffness by unfolded domain oxidation (UnDOx). Proc Natl Acad Sci U S A. 2020;117(39):24545-56. [CrossRef]
- Ratti J, Rostkova E, Gautel M, Pfuhl M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem. 2011;286(14):12650-8.
- Jung HS, Komatsu S, Ikebe M, Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol Biol Cell. 2008;19(8):3234-42. [CrossRef]
- Sevrieva I, Knowles AC, Kampourakis T, Sun YB. Regulatory domain of troponin moves dynamically during activation of cardiac muscle. J Mol Cell Cardiol. 2014;75:181-7. [CrossRef]
- Brunello E, Fusi L, Ghisleni A, Park-Holohan SJ, Ovejero JG, Narayanan T, et al. Myosin filament-based regulation of the dynamics of contraction in heart muscle. Proc Natl Acad Sci U S A. 2020;117(14):8177-86. [CrossRef]
- Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch. 2005;449(5):449-57. [CrossRef]
- Colson BA, Bekyarova T, Locher MR, Fitzsimons DP, Irving TC, Moss RL. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ Res. 2008;103(3):244-51.
- Mamidi R, Gresham KS, Verma S, Stelzer JE. Cardiac Myosin Binding Protein-C Phosphorylation Modulates Myofilament Length-Dependent Activation. Front Physiol. 2016;7:38. [CrossRef]
- Ait-Mou Y, Hsu K, Farman GP, Kumar M, Greaser ML, Irving TC, et al. Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci U S A. 2016;113(8):2306-11. [CrossRef]
- Li KL, Methawasin M, Tanner BCW, Granzier HL, Solaro RJ, Dong WJ. Sarcomere length-dependent effects on Ca(2+)-troponin regulation in myocardium expressing compliant titin. J Gen Physiol. 2019;151(1):30-41. [CrossRef]
- Groen M, López-Dávila AJ, Zittrich S, Pfitzer G, Stehle R. Hypertrophic and Dilated Cardiomyopathy-Associated Troponin T Mutations R130C and ΔK210 Oppositely Affect Length-Dependent Calcium Sensitivity of Force Generation. Front Physiol. 2020;11:516. [CrossRef]
- Reda SM, Chandra M. Dilated cardiomyopathy mutation (R174W) in troponin T attenuates the length-mediated increase in cross-bridge recruitment and myofilament Ca(2+) sensitivity. Am J Physiol Heart Circ Physiol. 2019;317(3):H648-h57. [CrossRef]
- Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100(10):1486-93.
- Colson BA, Patel JR, Chen PP, Bekyarova T, Abdalla MI, Tong CW, et al. Myosin binding protein-C phosphorylation is the principal mediator of protein kinase A effects on thick filament structure in myocardium. J Mol Cell Cardiol. 2012;53(5):609-16.
- Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262(5569):615-7. [CrossRef]
- Solaro RJ, Henze M, Kobayashi T. Integration of troponin I phosphorylation with cardiac regulatory networks. Circ Res. 2013;112(2):355-66. [CrossRef]
- Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+. Am J Physiol. 1996;271(1 Pt 1):C391-7. [CrossRef]
- Kranias EG, Garvey JL, Srivastava RD, Solaro RJ. Phosphorylation and functional modifications of sarcoplasmic reticulum and myofibrils in isolated rabbit hearts stimulated with isoprenaline. Biochem J. 1985;226(1):113-21. [CrossRef]
- Alves ML, Dias FAL, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, et al. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ Cardiovasc Genet. 2014;7(2):132-43. [CrossRef]
- Messer AE, Marston SB. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca(2+)-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol. 2014;5:315. [CrossRef]
- Pavadai E, Rynkiewicz MJ, Yang Z, Gould IR, Marston SB, Lehman W. Modulation of cardiac thin filament structure by phosphorylated troponin-I analyzed by protein-protein docking and molecular dynamics simulation. Archives of biochemistry and biophysics. 2022;725:109282. [CrossRef]
- Sevrieva IR, Brandmeier B, Ponnam S, Gautel M, Irving M, Campbell KS, et al. Cardiac myosin regulatory light chain kinase modulates cardiac contractility by phosphorylating both myosin regulatory light chain and troponin I. J Biol Chem. 2020;295(14):4398-410. [CrossRef]
- Landim-Vieira M, Knollmann BC. Danicamtiv Recruits Myosin Motors to Aid the Failing Heart. Circ Res. 2023;133(5):444-6. [CrossRef]
- Kooiker KB, Mohran S, Turner KL, Ma W, Martinson A, Flint G, et al. Danicamtiv Increases Myosin Recruitment and Alters Cross-Bridge Cycling in Cardiac Muscle. Circ Res. 2023;133(5):430-43. [CrossRef]
- Hitsumoto T, Tsukamoto O, Matsuoka K, Li J, Liu L, Kuramoto Y, et al. Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin. Circulation. 2023;147(25):1902-18. [CrossRef]
- He H, Baka T, Balschi J, Motani AS, Nguyen KK, Liu Q, et al. Novel Small-Molecule Troponin Activator Increases Cardiac Contractile Function Without Negative Impact on Energetics. Circ Heart Fail. 2022;15(3):e009195. [CrossRef]
- Bunch TA, Guhathakurta P, Thompson AR, Lepak VC, Carter AL, Thomas JJ, et al. Drug discovery for heart failure targeting myosin-binding protein C. bioRxiv. 2023. [CrossRef]
- Solaro RJ, Rüegg JC. Stimulation of Ca++ binding and ATPase activity of dog cardiac myofibrils by AR-L 115BS, a novel cardiotonic agent. Circ Res. 1982;51(3):290-4. [CrossRef]
- Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, et al. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73(6):981-90. [CrossRef]
- Wang X, Li MX, Spyracopoulos L, Beier N, Chandra M, Solaro RJ, et al. Structure of the C-domain of human cardiac troponin C in complex with the Ca2+ sensitizing drug EMD 57033. J Biol Chem. 2001;276(27):25456-66. [CrossRef]
- Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113(2):305-15. [CrossRef]
- Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331(6023):1439-43. [CrossRef]
- Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118(12):3893-903.
- Sorsa T, Pollesello P, Solaro RJ. The contractile apparatus as a target for drugs against heart failure: interaction of levosimendan, a calcium sensitiser, with cardiac troponin c. Mol Cell Biochem. 2004;266(1-2):87-107. [CrossRef]
- Haikala H, Kaivola J, Nissinen E, Wall P, Levijoki J, Lindén IB. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol. 1995;27(9):1859-66.
- Lee JA, Allen DG. Calcium sensitisers. Bmj. 1990;300(6724):551-2.
- Pollesello P, Papp Z, Papp JG. Calcium sensitizers: What have we learned over the last 25 years? Int J Cardiol. 2016;203:543-8.
- Brunkhorst D, v der Leyen H, Meyer W, Nigbur R, Schmidt-Schumacher C, Scholz H. Relation of positive inotropic and chronotropic effects of pimobendan, UD-CG 212 Cl, milrinone and other phosphodiesterase inhibitors to phosphodiesterase III inhibition in guinea-pig heart. Naunyn Schmiedebergs Arch Pharmacol. 1989;339(5):575-83. [CrossRef]
- Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, et al. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995;77(1):107-13.
- Cleland JG, Nikitin N, McGowan J. Levosimendan: first in a new class of inodilator for acute and chronic severe heart failure. Expert Rev Cardiovasc Ther. 2004;2(1):9-19. [CrossRef]
- Remme WJ. Inodilator therapy for heart failure. Early, late, or not at all? Circulation. 1993;87(5 Suppl):Iv97-107.
- Nonaka M, Morimoto S, Murayama T, Kurebayashi N, Li L, Wang YY, et al. Stage-dependent benefits and risks of pimobendan in mice with genetic dilated cardiomyopathy and progressive heart failure. British journal of pharmacology. 2015;172(9):2369-82. [CrossRef]
- Solaro RJ. Translational medicine with a capital T, troponin T, that is. Circ Res. 2007;101(2):114-5. [CrossRef]
- Boyle KL, Leech E. A review of the pharmacology and clinical uses of pimobendan. J Vet Emerg Crit Care (San Antonio). 2012;22(4):398-408. [CrossRef]
- Alves ML, Warren CM, Simon JN, Gaffin RD, Montminy EM, Wieczorek DF, et al. Early sensitization of myofilaments to Ca2+ prevents genetically linked dilated cardiomyopathy in mice. Cardiovasc Res. 2017;113(8):915-25. [CrossRef]
- Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol. 2000;3(Pt 3):541-9. [CrossRef]
- Li MX, Mercier P, Hartman JJ, Sykes BD. Structural Basis of Tirasemtiv Activation of Fast Skeletal Muscle. J Med Chem. 2021;64(6):3026-34. [CrossRef]
- Cai F, Kampourakis T, Parijat P, Cockburn KT, Sykes BD. Conversion of a Cardiac Muscle Modulator from an Inhibitor to an Activator. ACS Med Chem Lett. 2023;14(4):530-3. [CrossRef]
- Collibee SE, Romero A, Muci AR, Hwee DT, Chuang C, Hartman JJ, et al. Cardiac Troponin Activator CK-963 Increases Cardiac Contractility in Rats. Journal of Medicinal Chemistry. 2024. [CrossRef]
- Parijat P, Ponnam S, Attili S, Campbell KS, El-Mezgueldi M, Pfuhl M, et al. Discovery of novel cardiac troponin activators using fluorescence polarization-based high throughput screening assays. Sci Rep. 2023;13(1):5216. [CrossRef]
- Mahmud Z, Tikunova S, Belevych N, Wagg CS, Zhabyeyev P, Liu PB, et al. Small Molecule RPI-194 Stabilizes Activated Troponin to Increase the Calcium Sensitivity of Striated Muscle Contraction. Front Physiol. 2022;13:892979. [CrossRef]
- Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617-21. [CrossRef]
- El Oumeiri B, Dewachter L, Van de Borne P, Hubesch G, Melot C, Jespers P, et al. Altered Left Ventricular Rat Gene Expression Induced by the Myosin Activator Omecamtiv Mecarbil. Genes (Basel). 2023;14(1). [CrossRef]
- Amesz JH, Langmuur SJJ, Bierhuizen MFA, de Groot NMS, Manintveld OC, Taverne YJHJ. Omecamtiv mecarbil in precision-cut living heart failure slices: A story of a double-edged sword. Journal of Molecular and Cellular Cardiology Plus. 2023:100040. [CrossRef]
- Qu Y, Gao B, Arimura Z, Fang M, Vargas HM. Comprehensive in vitro pro-arrhythmic assays demonstrate that omecamtiv mecarbil has low pro-arrhythmic risk. Clin Transl Sci. 2021;14(4):1600-10. [CrossRef]
- Utter MS, Ryba DM, Li BH, Wolska BM, Solaro RJ. Omecamtiv Mecarbil, a Cardiac Myosin Activator, Increases Ca2+ Sensitivity in Myofilaments With a Dilated Cardiomyopathy Mutant Tropomyosin E54K. J Cardiovasc Pharmacol. 2015;66(4):347-53. [CrossRef]
- Nagy L, Kovács Á, Bódi B, Pásztor ET, Fülöp G, Tóth A, et al. The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br J Pharmacol. 2015;172(18):4506-18. [CrossRef]
- Halas M, Langa P, Warren CM, Goldspink PH, Wolska BM, Solaro RJ. Effects of Sarcomere Activators and Inhibitors Targeting Myosin Cross-Bridges on Ca(2+)-Activation of Mature and Immature Mouse Cardiac Myofilaments. Mol Pharmacol. 2022;101(5):286-99. [CrossRef]
- Kieu TT, Awinda PO, Tanner BCW. Omecamtiv Mecarbil Slows Myosin Kinetics in Skinned Rat Myocardium at Physiological Temperature. Biophys J. 2019;116(11):2149-60. [CrossRef]
- Broughton KM, Li J, Sarmah E, Warren CM, Lin YH, Henze MP, et al. A myosin activator improves actin assembly and sarcomere function of human-induced pluripotent stem cell-derived cardiomyocytes with a troponin T point mutation. Am J Physiol Heart Circ Physiol. 2016;311(1):H107-17. [CrossRef]
- Barrick SK, Garg A, Greenberg L, Zhang S, Lin CY, Stitziel NO, et al. Functional assays reveal the pathogenic mechanism of a de novo tropomyosin variant identified in patient with dilated cardiomyopathy. J Mol Cell Cardiol. 2023;176:58-67. [CrossRef]
- Lehman SJ, Crocini C, Leinwand LA. Targeting the sarcomere in inherited cardiomyopathies. Nat Rev Cardiol. 2022;19(6):353-63. [CrossRef]
- Lakdawala NK, Dellefave L, Redwood CS, Sparks E, Cirino AL, Depalma S, et al. Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: the distinctive natural history of sarcomeric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55(4):320-9. [CrossRef]
- Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48(10):1977-85. [CrossRef]
- Eijgenraam TR, Silljé HHW, de Boer RA. Current understanding of fibrosis in genetic cardiomyopathies. Trends in cardiovascular medicine. 2020;30(6):353-61. [CrossRef]
- Frangogiannis NG. Editorial commentary: Myocardial fibrosis in genetic cardiomyopathies: A cause of dysfunction, or simply an epiphenomenon? Trends in cardiovascular medicine. 2020;30(6):362-3. [CrossRef]
- Solís C, Warren CM, Dittloff K, DiNello E, Solaro RJ, Russell B. Cardiomyocyte external mechanical unloading activates modifications of α-actinin differently from sarcomere-originated unloading. The FEBS journal. 2023;290(22):5322-39. [CrossRef]
- Langa P, Wolska BM, Solaro RJ. The Hippo Signaling Pathway as a Drug Target in Familial Dilated Cardiomyopathy. International Journal of Drug Discovery and Pharmacology. 2022;1(1):4. [CrossRef]
- Chowdhary A, Garg P, Das A, Nazir MS, Plein S. Cardiovascular magnetic resonance imaging: emerging techniques and applications. Heart. 2021;107(9):697-704. [CrossRef]
- Nomura S, Ono M. Precision and genomic medicine for dilated and hypertrophic cardiomyopathy. Frontiers in cardiovascular medicine. 2023;10:1137498. [CrossRef]
- Jordan E, Kinnamon DD, Haas GJ, Hofmeyer M, Kransdorf E, Ewald GA, et al. Genetic Architecture of Dilated Cardiomyopathy in Individuals of African and European Ancestry. Jama. 2023;330(5):432-41. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




