1. Introduction
Traditionally, a root canal treatment is indicated when the pulp tissue becomes severely inflamed or necrotic due to caries, dental trauma or developmental aberrations. During the endodontic treatment procedure, root canals are mechanically shaped, disinfected with appropriate irrigating solutions and filled with synthetic obturation materials, resulting in a total loss of all pulp functions. This situation can be highly detrimental to immature permanent teeth, as pulp tissue removal at an early stage of root development prevents subsequent root maturation and apex closure [
1]. Conventional endodontic treatment leaves immature permanent teeth with short roots and thin dentinal walls, and there is a significantly increased risk of root fracture from a long-term perspective [
2]. Although implant therapy is now considered a safe and reliable method of replacing missing teeth, it can only be considered after the completion of growth in early adulthood and has a lower survival rate than natural teeth [
3]. Therefore, attempts to preserve natural teeth and improve their long-term prognosis have led to the development of regenerative endodontic treatment approaches. The new treatment strategy focuses on three main goals: (I) resolution of clinical symptoms and periapical bone destruction and restoration of normal tooth function; (II) continuation and completion of root development; and (III) regeneration of pulp or pulp-like tissue [
1].
Biological elements required for the success of regenerative endodontic procedures are based on a triad of endogenously or exogenously derived stem cells, scaffolds and growth factors [
2]. The most clinically used approach is tooth revitalization, where the scaffold is a blood clot generated by inducing apical bleeding into the root canal space. This host-derived cross-linked fibrin network serves as a scaffold for cell homing. During bleeding, various types of cells, including stem cells, mainly originating from the apical papilla and periodontal ligament, migrate into the root canal space [
1,
4]. Pulp tissue regeneration highly depends on the survival, attachment, proliferation and differentiation of the recruited stem cells, which is controlled by signaling molecules and growth factors [
5,
6]. Thus, proper root canal disinfection and the release of endogenous growth factors from root canal dentin play a decisive role in the success of regenerative endodontic procedures [
4].
The release of growth factors, which are embedded in the extracellular matrix of dentin [
4], is mainly achieved by applying conventional chelating agents, such as ethylenediaminetetraacetic acid (EDTA) or citric acid [
7]. However, various intracanal medicaments used between visits to improve the disinfection of the entire root canal system may also release bioactive molecules from dentin [
4]. Calcium hydroxide paste (Ca(OH)
2), which has been the main intracanal dressing material applied in endodontics for decades [
8], was shown to allow a higher amount of TGF-β1 release compared to EDTA alone (4). Therefore, using Ca(OH)
2 in regenerative endodontic procedures appears to be preferable regarding root canal decontamination and subsequent growth factor release.
Nevertheless, Ca(OH)
2 has limitations [
9], the main of which is cytotoxicity. When Ca(OH)
2 is accidentally extruded beyond the apex, the release of reactive hydroxide causes tissue necrosis [
10,
11]. This scenario can be extremely detrimental in regenerative endodontic procedures, owing to the compromised viability of stem cells, which typically reside near the root apex and guide the root formation process jointly with Hertwig’s epithelial root sheath [
11,
12]. Unfortunately, the younger patients and immature teeth, the primary regenerative endodontic procedures receivers [
13], are directly associated with a higher risk of inadvertent extrusions due to the limited patient cooperation and open root apices [
14]. Moreover, the complete Ca(OH)
2 removal from various root canal irregularities has also been reported to be difficult [
15], resulting in blockage of dentinal tubules due to the remnants on the root canal surface. Another possible intracanal medicament for regenerative endodontic procedures is triple antibiotic paste (TAP). However, TAP has several disadvantages over Ca(OH)
2, including discolouration, higher cytotoxicity to stem cells, lower promotion of growth factor release and even more difficult removal from root canals [
4,
16].
Recently, the first temporary, ready-to-use calcium silicate-based intracanal medicament Bio-C Temp (Angelus, Londrina, Brasil) has been introduced to the market, opening up new perspectives for potentially improving root canal disinfection and conditioning between appointments. Bio-C Temp is composed of glycol salicylate, titanium oxide, calcium tungsten, calcium aluminate, calcium oxide, and calcium silicate [
17]. The paste does not harden in root canals when delivered and releases Ca2+ and OH− ions via hydration of the material [
17]. The concept of using hydraulic calcium silicate-based materials is not new, as these permanent filling materials have already been successfully applied in various endodontic procedures. Although evidence-based data on Bio-C Temp properties is still limited, initial studies have shown that Bio-C Temp is more biocompatible and easier to remove from root canals than conventional Ca(OH)
2 paste [
18]. Based on these observations, it has been speculated that a calcium silicate-based intracanal medicament may be a particularly attractive alternative to Ca(OH)
2 in regenerative endodontic procedures.
However, the applicability of calcium silicate-based intracanal medicament in regenerative endodontic procedures has not been investigated. Thus, it is important to determine whether Bio-C Temp induces the release of growth factors from root canal dentin. Since the transforming growth factor β1 (TGF-β1) has been reported to be abundant and particularly important for odontoblast mineralization and differentiation [
2], the present study aimed to investigate and compare the effect of calcium silicate-based temporary intracanal medicament Bio-C Temp and calcium hydroxide-based material UltraCal XS (Ultradent Products Inc., USA) on TGF-β1 release from root canal dentin. The null hypothesis tested was that there is no difference in the effect on growth factors release promoted by calcium silicate-based temporary intracanal medicament and calcium hydroxide.
2. Materials and Methods
2.1. Teeth Selection
Twenty-two intact and fully developed human premolars from patients aged 15 – 18 years were collected under the approval of Lithuania’s national biomedical research ethics committee (protocol no. 158200-16-860-369). Vital teeth were freshly extracted for reasons unrelated to the study, cleaned with periodontal curettes to remove soft tissue, rinsed with sterile phosphate-buffered saline (PBS), and stored in 0.5% Chloramine-T trihydrate solution at 4 °C for up to 4 months. Prior to tooth preparation, storage solution was changed to distilled water and left for 24 hours at room temperature. Only single-rooted premolars with a single root canal on preoperative radiographs and no anatomical malformations were selected.
2.2. Preparation and Conditioning of Root Canal Dentine
Standard endodontic access cavities were prepared using high-speed Endo Access burs (Dentsply Sirona, Ballaiques, Switzerland) under water cooling. A size 10 K-file (Dentsply Sirona, Ballaiques, Switzerland) was inserted into the root canal to determine the working length (WL) at 0.5 mm from apical foramen (mean WL = 19.86 ± 1.25). The teeth were then fixed in prefabricated A-silicone (3M ESPE, Seefeld, Germany) blocks up to the cement-enamel junction to mimic the surrounding tissues and prevent the irrigating solution from overflowing beyond the apex.
Root canal shaping was performed with ReciprocBlue instruments (VDW, Munich, Germany) at the determined working length in the following sequence: R25 (25/0.08), R40 (40/0.06), R50 (50/0.05). After the use of each instrument, root canals were repeatedly irrigated with 2 mL 2% sodium hypochlorite (NaOCl; Cerkamed, Stalowa Wola, Poland) using 29-G NaviTip needles (Ultradent Products Inc., South Jordan, USA) attached to disposable syringes. The final rinse of the root canals at the end of mechanical preparation was performed as follows: 20 mL 2% NaOCl for 5 min, 5 mL PBS, 20 mL 17% EDTA (Cerkamed, Stalowa Wola, Poland) for 5 min. The shaped and cleaned root canals were dried with sterile paper points.
Subsequently, all specimens were carefully grooved longitudinally on the buccal and lingual surfaces without penetrating the root canal. The teeth were gently split into two halves using a small chisel. The paired halves of the same root were randomly divided into two experimental groups, according to the intracanal medicament used (n = 13 per group):
Bio-C Temp - the root canal dentin was covered with 0.05 ± 0.01 g calcium silicate-based paste Bio-C Temp.
Ca(OH)2 - the root canal dentin of the paired half was covered with 0.05 ± 0.01 g Ca(OH)2 paste UltraCal XS.
The control group consisted of 9 randomly selected halves that were not paired with the experimental groups. After splitting the teeth, the control root canal dentine was left untreated.
Specimens from all three groups were incubated separately in tissue culture dishes at 37 °C and 5% CO2 for 3 weeks. The external surface of each root half was immersed in gelatinized Hank’s balanced salt solution (HBSS) to avoid the dehydration of the specimen, while the internal surface was left uncovered attempting to avoid the material washout.
After the specified time, the bulk of intracanal medicament was carefully removed with the spatula and the root canal dentine was rinsed with 500 μL 17% EDTA. The internal remnants-free root surface was then fully immersed in 300 μL of fresh 17% EDTA and incubated at 37 °C for 24 hours. The EDTA contact with external root surface was avoided. The collected solution was immediately frozen at -80 °C until further use.
2.3. Quantification of TGF-β1 Release
The thawed samples were subjected to quantification of released TGF-β1 by performing an ELISA assay. Each sample was measured in triplicate. Quantification was performed according to the protocol provided by the manufacturer of the selected ELISA test system (TGF-β1 Human ELISA Kit; Thermo Fisher Scientific, USA). The resulting absorbance values were within the range of the standard supplied with the ELISA kit.
2.4. Statistical Analysis
Statistical analysis was performed using RStudio software v.4.3.1 (RStudio Inc., Boston, USA). The assumption of normality was assessed by using the Shapiro-Wilk test and Q-Q plots. Since the Bio-C Temp group showed a non-normal distribution of TGF-β1 measurements, the logarithmic transformation was applied. The assumption of normality was then accepted, and Levene’s test confirmed the homogeneity of variances. One-way analysis of variance (ANOVA), followed by Tukey’s test, was selected to determine the statistically significant differences between the groups at the 0.95 confidence level.
4. Discussion
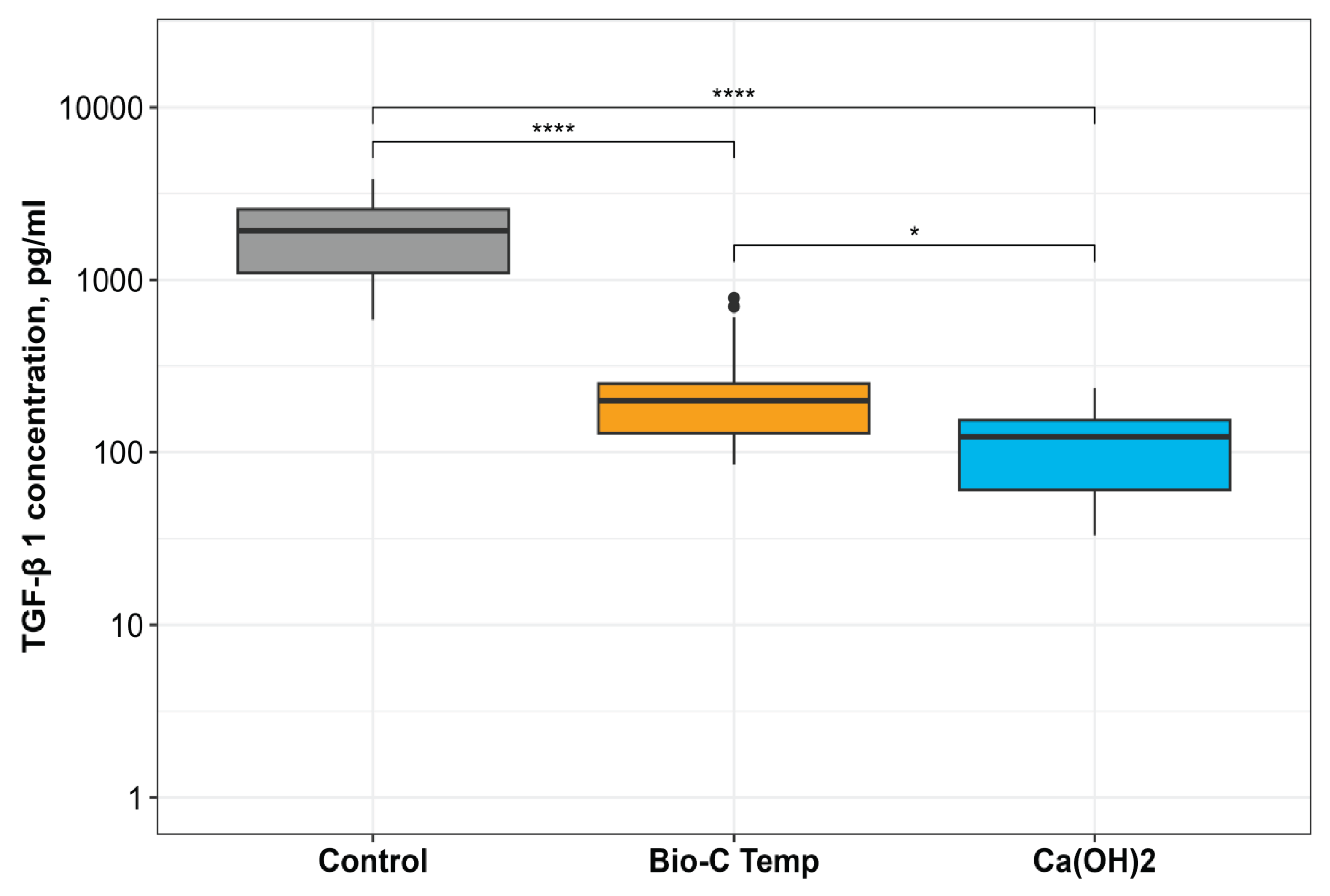
This study investigated the effect of temporary intracanal disinfecting medicaments - calcium silicate-based material Bio-C Temp and Ca(OH)2 paste - on transforming growth factor TGF-β 1 release from root dentin compared to the nonmedicated samples. The results demonstrate that both intracanal materials tested interfere with growth factor release. The calcium silicate-based temporary material Bio-C Temp promotes the release of higher TGF-β1 amounts than Ca(OH)2. Therefore, the null hypothesis tested was rejected.
Dentine is a complex structure of 70–72% weight composed of inorganic materials, 20 % of organic components (90% of which is type 1 collagen and the remaining 10% - dentin-specific proteins), and 8–10% water [
19]. Among these specific proteins, dentin contains a wide variety of entrapped growth factors, predominantly TGF-β family and angiogenesis-promoting bioactive substances [
20,
21]. Growth factors are bioactive signaling molecules that control a variety of cellular responses through the specific binding of transmembrane or intracellular receptors in a target cell [
22]. These molecules can modulate the recruitment, proliferation and differentiation of cells at very low concentration and therefore play an indispensable role in regenerative endodontic procedures [
22]. Although published studies continue to present various strategies for growth factor delivery systems relevant to dentin-pulp engineering, none of these efforts have yet reached the clinical application [
22,
23]. Therefore, the dentin matrix, containing a plethora of growth factors, remains the main clinically available source of the required signaling molecules for regenerative endodontic approaches such as revitalization.
It has been proposed that 1.5% NaOCl irrigation followed by the rinse of chelating solution should be used as a standard irrigation protocol for regenerative endodontic procedures [
24]. As numerous studies have demonstrated the ability of various organic acids to demineralize the dentin and promote the release of embedded bioactive components [
4,
12,
25], the current clinical recommendations for revitalization procedures suggest the use of a 17% EDTA as the final rinse to expose the entrapped growth factors and subsequentially induce the differentiation of blood clot-derived stem cells [
20]. However, the application of intracanal medicament between appointments may also affect the release of growth factors, and thus, the amount of bioactive molecules liberated from root canal dentin is highly dependent on the synergistic effect of the irrigating solutions and intracanal dressing materials used in regenerative endodontic procedures [
4,
25]. These observations were corroborated by the results of our study, as both intracanal medicaments tested had an effect on the amount of TGF-β1 released. Irrespective of the same irrigation protocol used in the groups, Bio-C Temp promoted a significantly higher release of growth factors than the Ca(OH)
2 - main intracanal dressing material currently used in the endodontic clinical practice.
However, the highest amount of released TGF-β1 was detected in the control group, where the root canal dentin was conditioned with 17% EDTA alone and no medicament was placed. This finding is consistent with a well-documented ability of EDTA to extract signaling molecules by demineralization of the dentin extracellular matrix [
2,
20]. Interestingly, the combined effect of EDTA and intracanal medicaments reduced the liberated amounts of TGF-β1, contradicting some previous studies in which Ca(OH)
2 did not affect the TGF-β1 release as compared to EDTA alone [
4]. One of the possible explanations could be related to the different incubation times of the specimens. As the short-term application of intracanal medicament is not recommended or supported by any current clinical regenerative endodontic procedures protocol [
16], our study followed the statements and incubated samples for 3 weeks, whereas 48 hours was chosen in the experiment of Galler et al [
4]. However, it should be mentioned that the longer incubation time may result in deeper penetration of intracanal medicaments into the dentinal tubules and more difficulty in their further removal, thereby limiting the release of growth factors due to the physical barrier created by medicament residues [
16]. Additionally, some of the released growth factors may be attached to the intracanal medicament and be subsequently removed with it. Nevertheless, the use of intracanal medicaments is unavoidable, as root canal disinfection is crucial for the survival of the delivered stem cells and, thus, for the overall success of regenerative endodontic procedures [
16,
26].
Based on the solid scientific evidence for the use of disinfecting intracanal agents between appointments and the previously confirmed acceptable biological properties of Bio-C Temp [
18,
27], our results suggest that the newly introduced calcium silicate-based temporary intracanal material has more potential for use in regenerative endodontic procedures than Ca(OH)
2. However, the results regarding the ability of Bio-C Temp to influence or modulate the release of growth factor TGF-β1 cannot be compared with other studies due to the lack of data. Despite the advantageous aspects of our study, such as paired samples and an organotypic experimental model of intact human premolars with a narrow age range, further experimental
in vitro investigations and well-designed clinical trials are warranted to explore broader implications of Bio-C Temp use in regenerative endodontic procedures. Understanding the effects of this new disinfecting material on stem cells and growth factors is critical to advancing in the field of revitalization procedures and translating these findings into practical clinical protocols.
Author Contributions
Conceptualization, G.B., S.D. and M.W.; methodology, G.B., S.D., E.S. and M.W.; software, E.S.; validation, S.D.; formal analysis, G.B. and E.S.; investigation, G.B and S.D.; resources, G.B., E.S. and S.S.; data curation, G.B..; writing—original draft preparation, S.D. and G.B.; writing—review and editing, E.S., M.W. and S.S.; visualization, E.S.; supervision, S.D. and E.S.; project administration, S.D. All authors have read and agreed to the published version of the manuscript.