Submitted:
21 March 2024
Posted:
21 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Chemistry of Curcumin – Structure and Properties
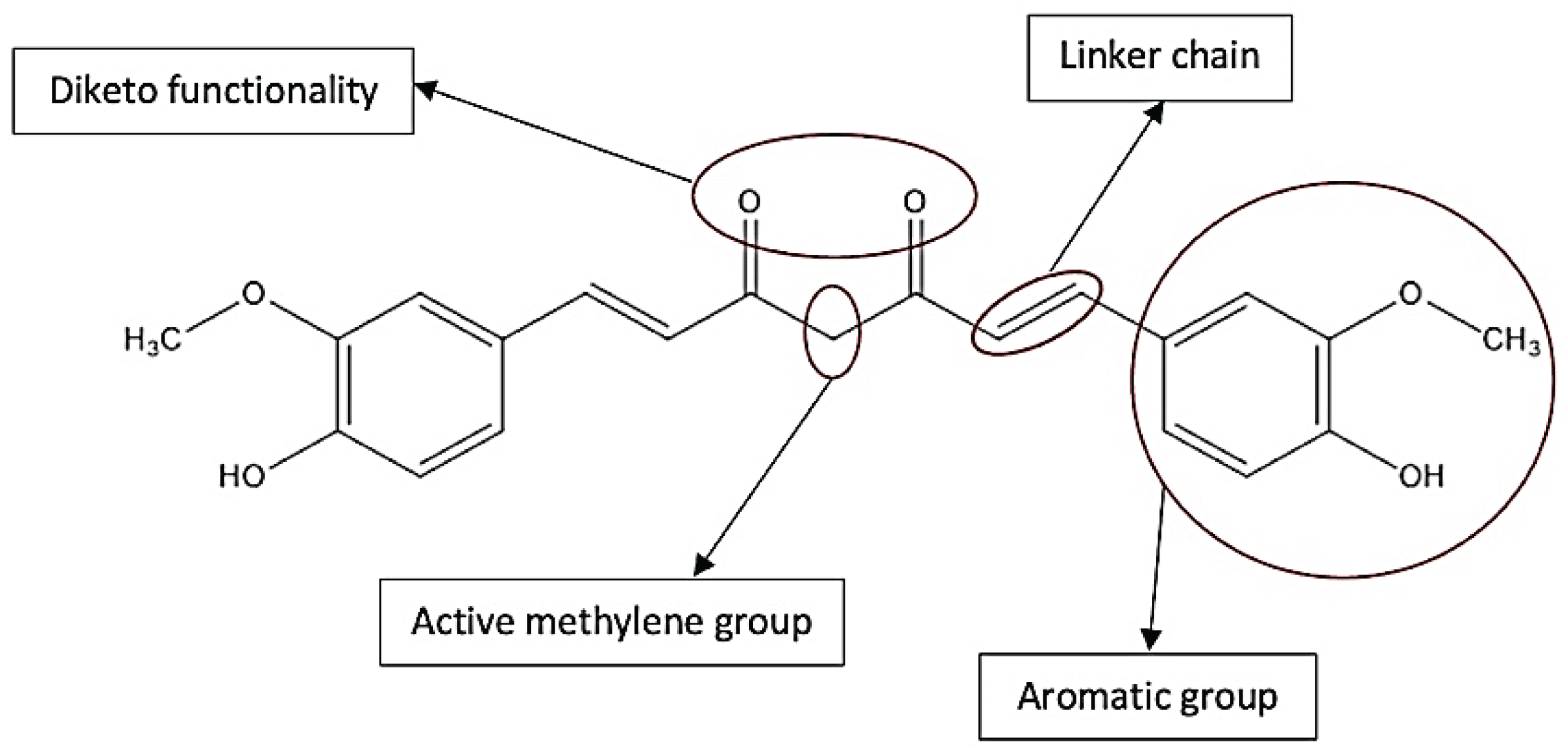
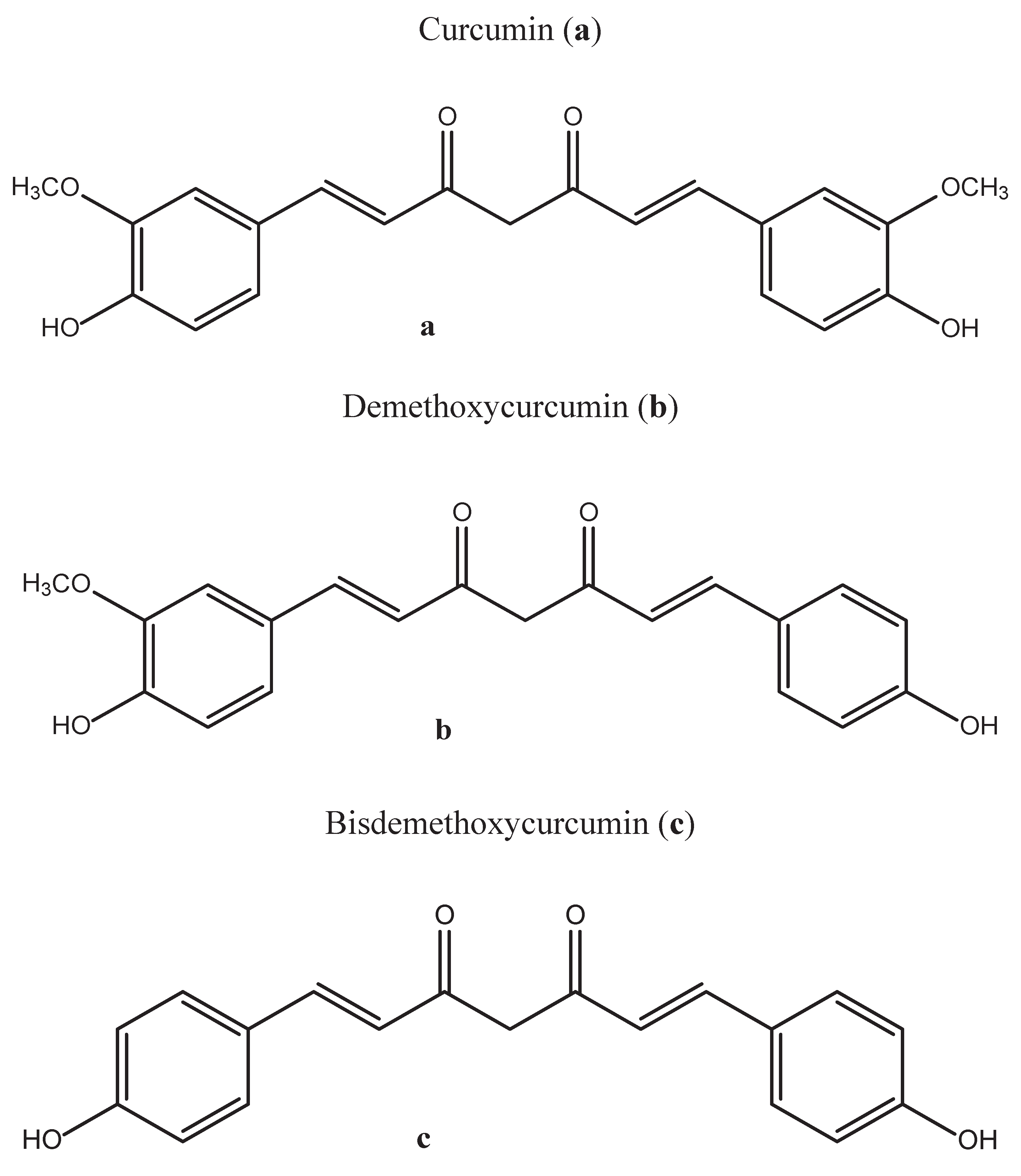
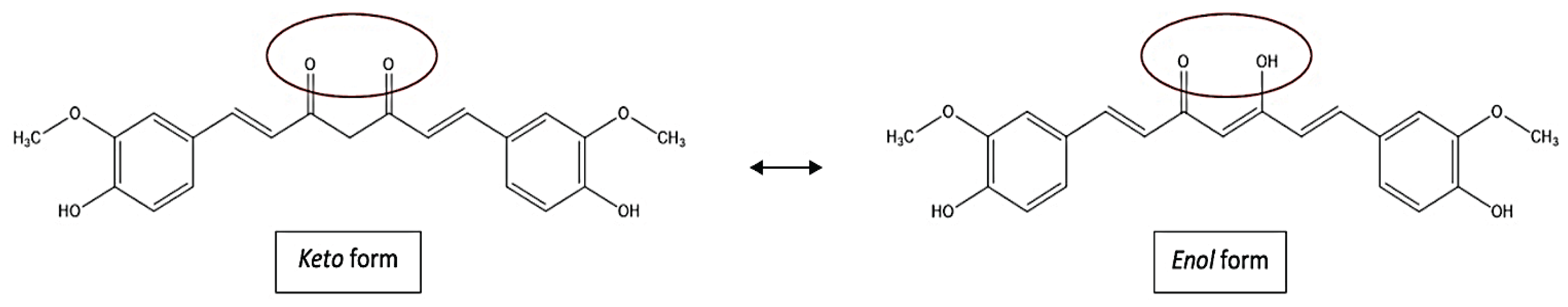
3. Methods
4. Strategies for Improving the Therapeutic Window of Curcumin
5. Novel Drug Delivery Systems
6. Structural Analogues of Curcumin and Their Anticancer, Antioxidant and Anti-Inflammatory Activity
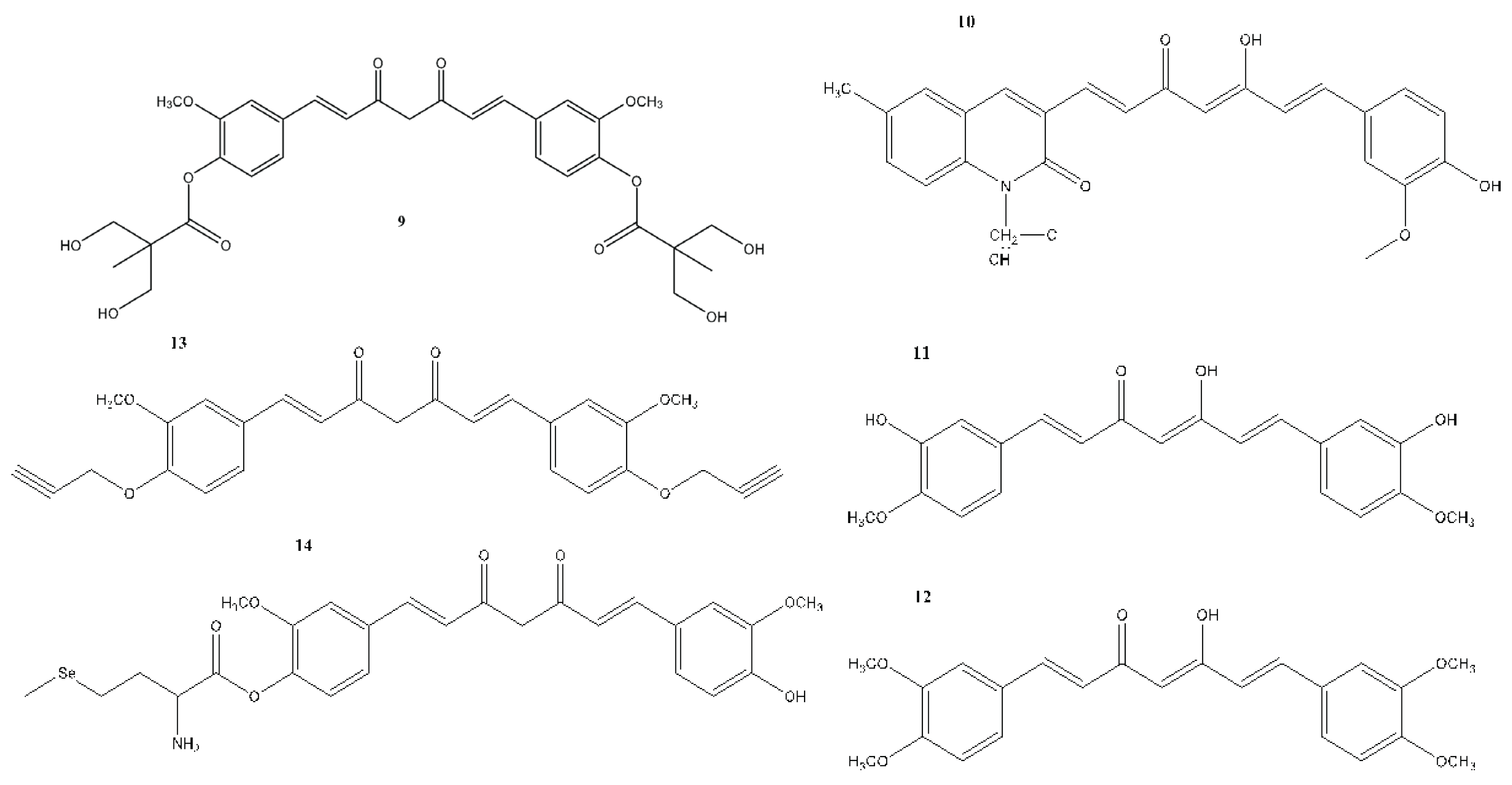
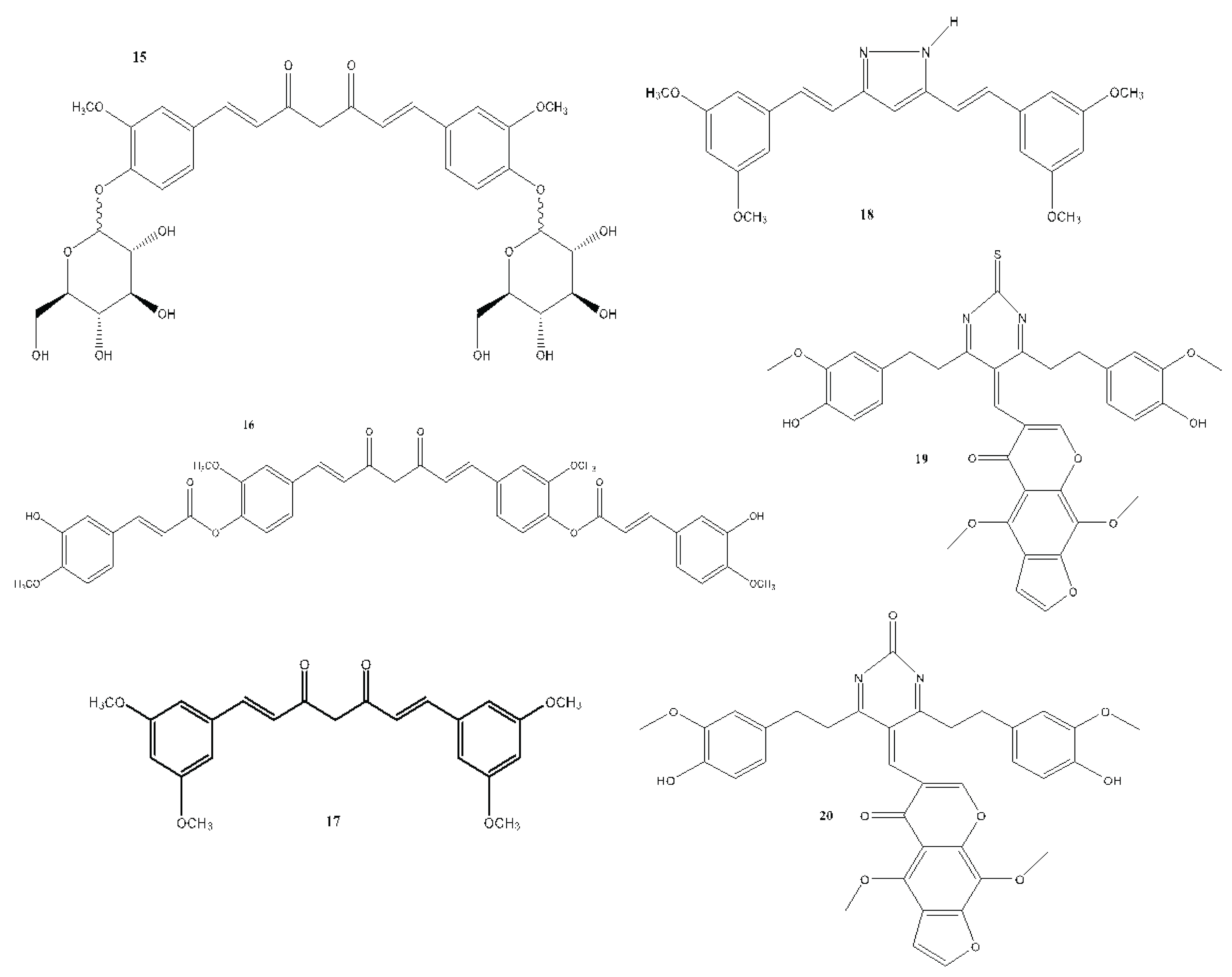
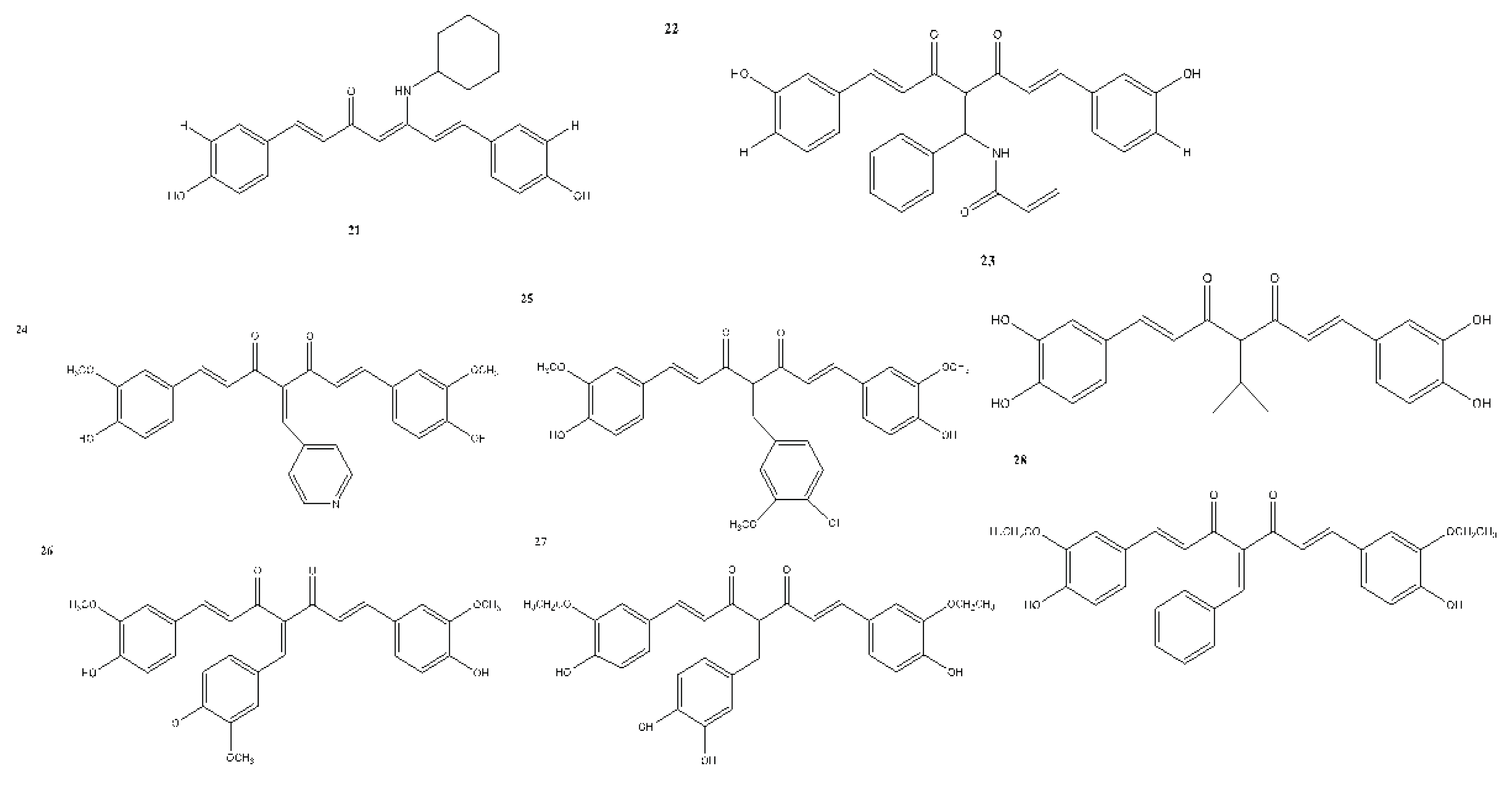
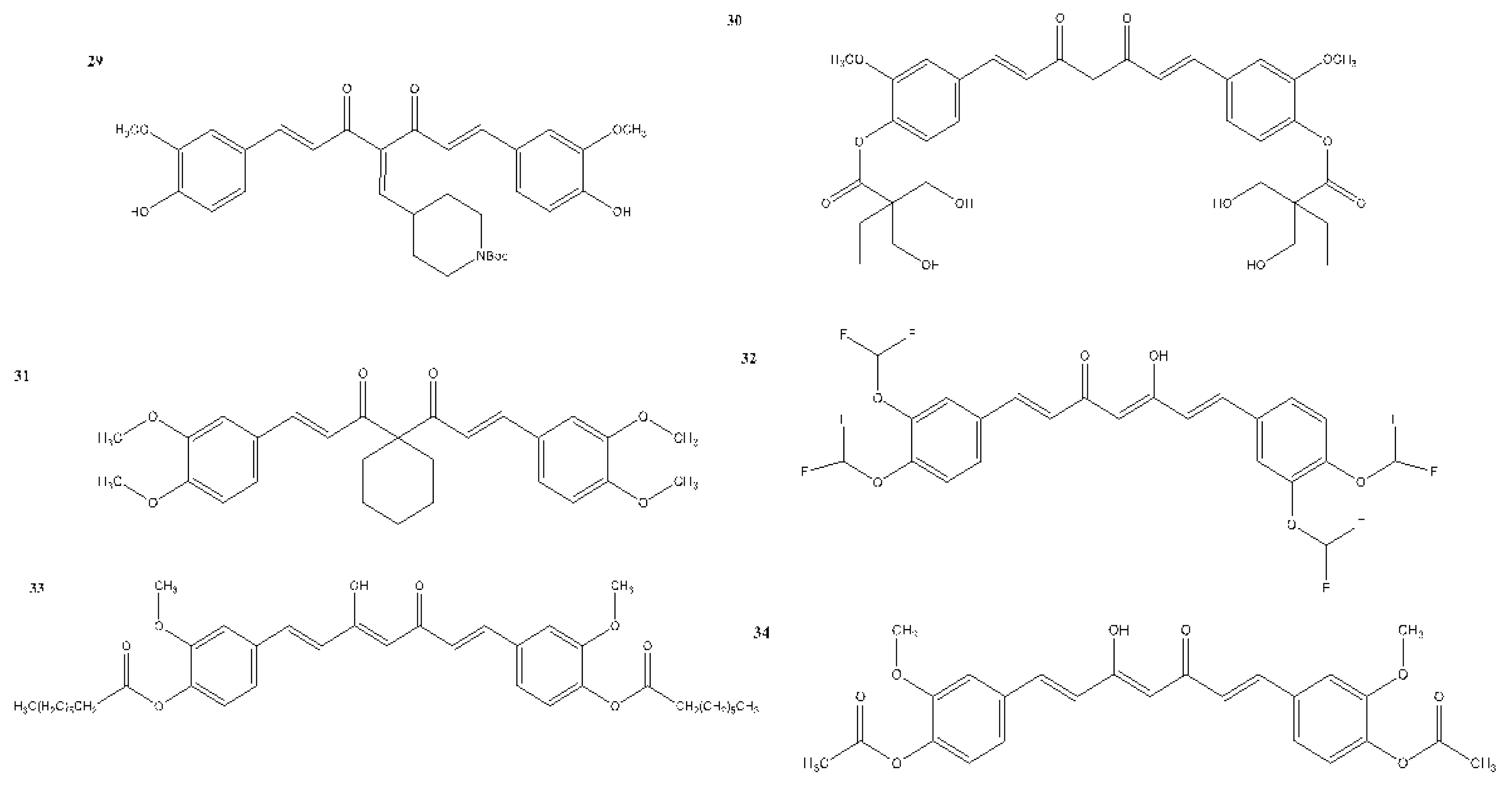
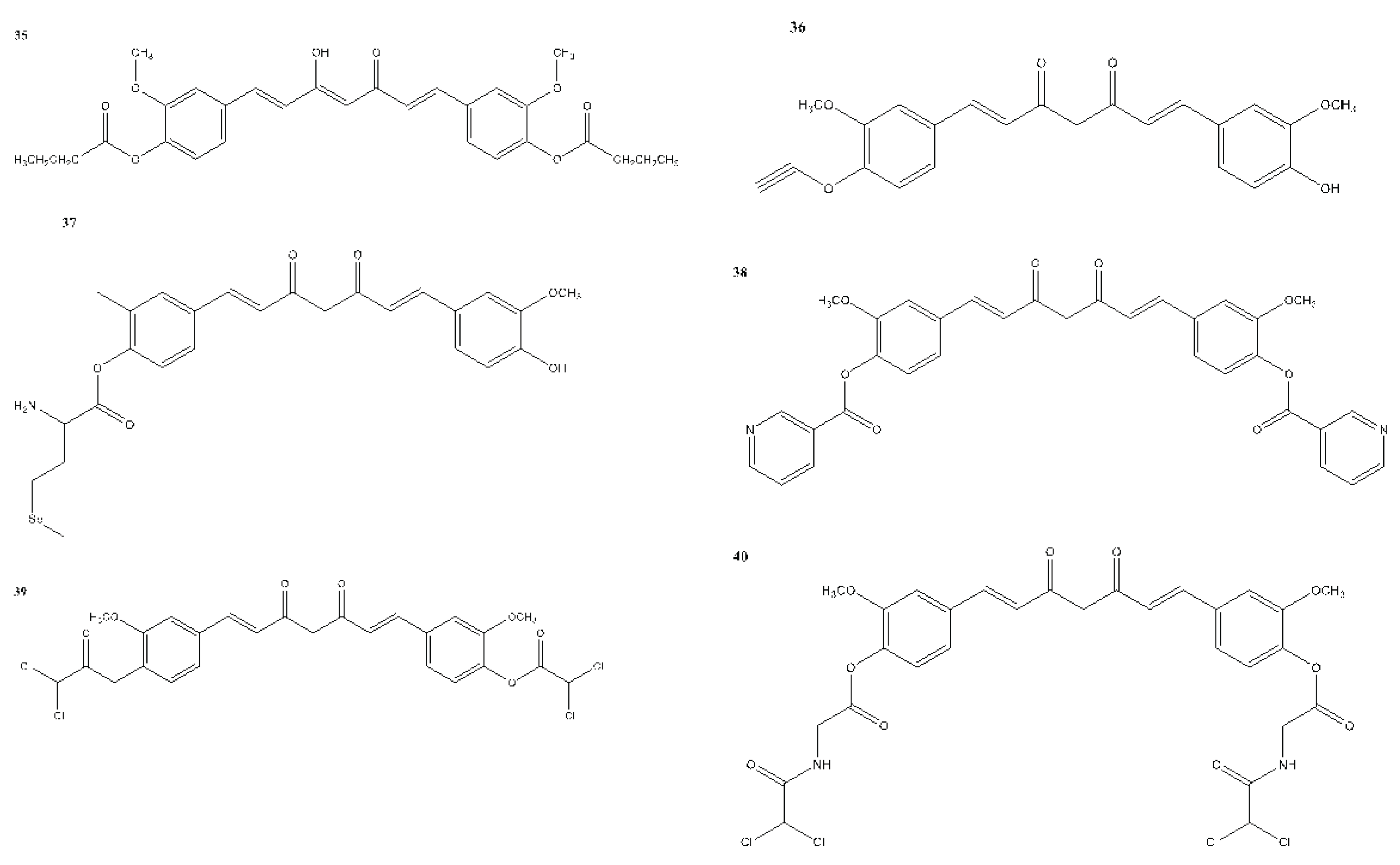
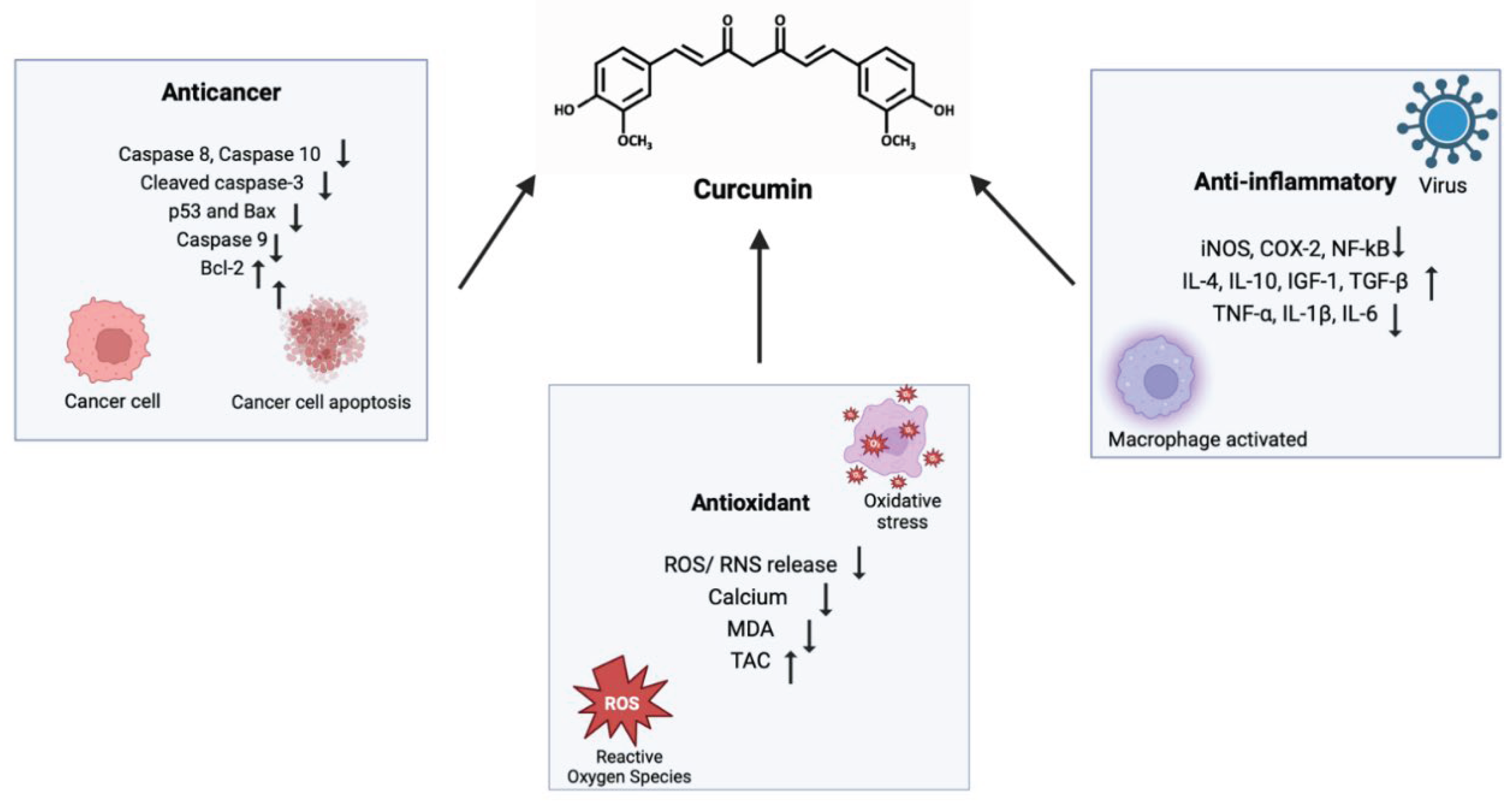
7. In Vitro and In Vivo Studies on the Antioxidant, Anti-inflammatory, and Anticancer Activities of Curcumin and Its Analogues
7.1. Antioxidant Activity
7.2. Anti-Inflammatory Activity
| Condition or Disease | Intervention/Treatment | Research Output |
|---|---|---|
| Rectal Cancer | Curcumin po bid +(Radiation therapy and capecitabine) for 11.5 weeks | To assess if curcumin can make tumor cells more sensitive to radiation therapy. |
| Colorectal Cancer Patients with Unresectable Metastasis | Curcumin 100mg po bid (+Avastin/FOLFIRI) | To assess Progression-free survival, overall survival rate, overall response rate, and safety and fatigue score. |
| Colon Cancer | Curcumin 500 mg po bid for 2 weeks. (Patients will continue curcumin at the same dose for an additional 6 weeks while being treated with 3 cycles of 5-Fu) | To test the safety and effects and find the Response Rate. |
| Advanced Breast Cancer | Paclitaxel +(curcumin or placebo) (300 mg i.v. once weekly for 12 weeks.) | To assess the adverse effects, Quality of life, Progression-free survival, Time to Disease Progression, and Time to treatment failure. |
| Metabolic Syndrome | Nanomicielle curcumin or placebo | To determine the effects of nano micelle curcumin on glycemic control, serum lipid profile, blood pressure and anthropometric measurements |
| Prostate Cancer | Curcumin or placebo (500 mg po bid) | To assess the efficacy. |
| Invasive Breast Cancer | Curcumin 500 mg po bid. (Curcumin will be given from when surgical resection is scheduled until the night before surgical resection.) | To determine whether curcumin causes biological changes in primary tumors of breast cancer patients. |
| Cervical Cancer | Cisplatin plus concomitant radiation therapy (teletherapy + high or low-rate brachytherapy) + Curcugreen (BCM95) or placebo 2000 mg daily (each 6 h) | To assess the efficacy and safety. |
| Metabolic syndrome | Curcumin 1 g daily 8 weeks |
↓ TNF-α, IL-6, TGF-β and MCP-1 |
| Male factor infertility | Curcumin nano micelle 80 mg daily 10 weeks |
↓ CRP, TNF-α |
| Osteoarthritis | Sinacurcumin® 80 mg daily 3 mouths |
↓ Visual Analog Score (VAS), CRP, CD4+ and CD8+ T cells, Th17 cells and B cells frequency |
| Crohn’s disease | Theracurmin® 360 mg daily 12 weeks |
Significant clinical and endoscopic efficacy together with a favourable safety profile. |
| Irritable Bowel Syndrome | IQP-CL-101(Each IQP-CL-101 soft gel contains a 330 mg proprietary mixture of curcuminoids and essential oils.) Two soft gels daily 8 weeks |
It is beneficial in improving the severity of IBS symptoms and the quality of life in patients suffering from abdominal pain and discomfort. |
| Knee osteoarthritis | Curcuma longa extract (CL) extract 500 mg along with Diclofenac twice a day 4 months |
It suppresses inflammation and brings clinical improvement in patients with KOA, which may be observed by decreased levels of IL-1β and VAS/WOMAC scores, respectively. |
| Knee osteoarthritis and knee effusion-synovitis | Curcuma longa extract 2 capsules of CL daily 12 weeks |
CL was more effective than placebo for knee pain but did not affect knee effusion–synovitis or cartilage composition. |
| Knee osteoarthritis | Herbal formulation “turmeric extract, black pepper, and ginger” Curcumin (300 mg), twice a day 4 weeks |
↓ PGE2 |
| Rheumatoid arthritis | Curcumin 500mg, twice daily, oral 8 weeks |
Improvement in overall DAS and ACR scores. |
| Psoriasis | Curcuminoid C3 Complex 4.5g 12 weeks |
The response rate was low, possibly due to a placebo effect or the natural history of psoriasis. |
| Major Depression | Curcumin 500–1500 mg/day 12 weeks |
Significant antidepressant effects. |
| COVID-19 | SinaCurcumin 40 mg, twice daily 2 weeks |
Significantly improve recovery time. |
| COVID-19 | Curcumin with Piperine Curcumin (525 mg) with piperine (2.5mg) in tablet form twice daily. 14 days |
Substantially reduce morbidity and mortality and ease the logistical and supply-related burdens on the healthcare system. |
| Knee osteoarthritis | Theracurmin® Six capsules of Theracurmin per day 6 months |
shows great potential for the treatment of human knee osteoarthritis. |
| Knee osteoarthritis | Curcumagalactomannoside complex (CurQfen) 400 mg, daily 6 weeks |
exerted beneficial effects in alleviating the pain and symptoms. |
| Osteoarthritis | CuraMed® Curamin® 500-mg capsules (333 mg curcuminoids) 500-mg capsules (350 mg curcuminoids and 150 mg boswellic acid) taken orally three times a day 12 weeks |
Reduces pain-related symptoms in patients with OA. |
| Knee osteoarthritis | LI73014F2 200, 400 mg/day 90 days |
Significant pain relief, improved physical function, and quality of life in OA patients. |
| Non-alcoholic fatty liver disease (NAFLD) | active ingredients formulated as soft gel capsules. 2 capsules/day 3 months |
Increased cholesterol, increased glucose, decreased Aspartate transaminase (AST) |
| COVID-19 | ArtemiC oral spray day 1 and day 2 twice daily |
Increased clinical improvement, SpO2 normalisation, decreased O2 supplementation, decreased fever, decreased hospital stays. |
| Dry eye syndrome | LCD capsule 1 tablet/day 8 weeks |
Increased Schirmer’s strip wetness length, tear volume, TBUT score, and SPEED score. Decreased OSDI score, corneal and conjunctival staining score, tear osmolarity, MMP- 9 positive score |
| Healthy subjects | iron + HydroCurc 18 mg +500 mg/day 65 mg +500 mg/ Day 6 weeks |
Decreased TBARS, TNF-α, GI side effects, fatigue, IL-6 |
| Structure or functional groups | Molecular pathway affected-Action Mechanism |
In vitro assay (Cell lines) and In vivo assay (animal models) (**Ex vivo assay) |
|---|---|---|
| -Removal of phenyl ring at the 7th position of the heptadiene backbone and addition of hydroxyl group CBA-iR: bis-demethylcurcumin (BDC) |
NF-KB |
Human myeloid leukemic cell line: KBM-5 Human prostate cancer cells: PC-3 Human multiple myeloma: U266 Human colorectal cancer cell line: HCT-116 and Human breast cancer cell line: MCF-7 |
| Heterocyclic curcumin analog CBA-iR: BAT3 |
NF-KB | Murine fibrosarcoma cells: L929A |
| EF-31, EF-24 | NF-KB |
Mouse RAW 264.7 macrophage cells A human ovarian carcinoma cell line: A2780 A mouse mammary carcinoma cell line: EMT6 |
| Symmetrical curcumin analogs CBA-iR: 2 |
NF-KB | Wistar rats |
| Phenolic 1,3-diketones: CBA-iR: bis-dimethoxycurcumin (GG6) and its cyclised pyrazole analog (GG9) |
NF-KB, TLR4 | Ba/F3 cells |
| Dimethoxycurcumin (DiMc) -increased number of methoxy group and conjugated double bond |
NF-KB, NO, iNOS | Murine and human macrophage cell lines: RAW264.7 |
| Curcuminoids dimethoxycurcumin (DMC), THC, DiMc and bis-dimethoxycurcumin (BDMC): -two methoxy groups and two hydroxy groups but lacks conjugated double bonds in the central seven-carbon chain -α, β-unsaturated carbonyl group |
Heme Oxygenase-1 | Murine and human macrophage cell lines: RAW264.7 |
| Mono-carbonyl analogs of curcumin CBA-iR: A01, A03, A13, B18, and C22 |
TNF-α, IL-1β, IL-6, MCP-1, COX-2, PGES, iNOS, p65, and NF-ΚB | Mouse J774A.1 macrophages |
| A13 | NO, TNF-α, and IL-6, | Mice |
| GL63 | COX-2 | H460 cells |
| Dibenzoylmethane (DBM): (2,2ʹ-diOAc-DBM) |
COX-2 | TPA-induced CD-1 mice ear edema |
| Unsymmetrical monocarbonyl curcumin analogs: -dimethoxy group, furanyl ring and vanillin moiety CBA-iR: 8b,8c,15a |
Prostaglandin E2 production signaling pathway | Murine and human macrophage cell lines RAW264.7 and U937 |
| -pharmachophore modification of the dienone functional group into monoketone and -side chain of aromatic rings with symmetrical or asymmetrical substituents |
COX-2 | - |
| Unsymmetrical dicarbonyl curcumin derivatives CBA-iR: 17f |
PGE2, COX-2 | Murine macrophages cell line: RAW264.7 |
| Pyrazole and isoxazole analogs CBA-iR: 4,7 |
COX-1, COX-2 | - |
| CBA-iR: HP109/HP102 - methoxy or methyl ester group on the phenyl ring |
COX-1 | Jurkat T-cells |
| A series of 1,5-diphenyl-1,4 pentadiene-3-ones and cyclic analogs with OH-groups in the para position of the phenyl rings and various meta substituents |
COX-1, COX-2 | - |
| -electron withdrawing substituent on amide ring and fluorine, trifluoromethyl substituent CBA-iR: 5f, 5j,5m,5h,5b,5d |
COX-2, TNF-α, and IL-6 |
Human pancreas carcinoma: Panc1 Human non cell lung carcinoma: H460 Human non cell lung carcinoma: Calu-1 Human colon carcinoma: HCT 116 Human renal cell carcinoma: ACHN |
| α,β-unsaturated carbonyl-based compounds -N-methyl-4-piperidone and 4-piperidone moieties CBA-iR: 3, 4, 12, 13,14 |
sPLA2, COX-1, LOX, IL-6, and TNF-α | Murine macrophages cell line: RAW264.7 |
| A series of novel curcumin diarylpentanoid analogs -N-methyl-N-(2-hydroxyethyl)-4-amino -diethyamine group at position 4 of the phenyl ring -2-methyl-N-ethyl-N-(2-cyanoethyl)-4-amino |
PLA2, COX, LOX, and mPGES-1 | - |
| Mono-carbonyl curcumin analogs -acryloyl group -1-naphthalene CBA-iR: 1b (BAT1) |
LOX, ALR2 |
Non small cell lung cancer: NCI-H460 Breast Cancer: MCF7 and Central Nervous System, glioma: SF268 Fisher-344 rats |
| C66 | IL-1β, TNF-α, IL-6, IL-12, COX-2, and iNOS |
Mouse primary peritoneal macrophage (MPM), C57BL/6 mice and Sprague– Dawley (SD) rats |
| C66 | - | Mice |
| Curcumin-related diarylpentanoid analogs -2,5-dimethoxylated and 2 hydroxylated phenyl groups CBA-iR: 2, 13, 33 |
NO | Murine macrophages cell line: RAW264.7 |
| Diarylpentanoid analogs: CBA-iR: 88, 97 |
NO | Murine macrophages cell line: RAW264.7 |
| Diarylpentanoid analogs CBA-iR: 5-methylthiophenyl-bearing analog |
NO | Murine macrophages cell line: RAW264.7 |
| Pentadienone oxime ester derivatives CBA-iR: 5j |
iNOS, COX-2, and NO, IL-6 | Murine macrophages cell line: RAW264.7 |
| N-substituted 3,5-bis(2-(trifluoromethyl)benzylidene)piperidin-4-ones CBA-iR: c6 and c10 |
TNF-α, IL-6, IL-1β, PGE2, NO | -Murine macrophages cell line: RAW264.7 -Rat |
| Mono-carbonyl analogs of Curcumin: -electron withdrawing groups in the benzene ring CBA-iR: AN1 and B82 |
TNF-α and IL-6 | Murine macrophages cell line: RAW264.7 |
| A series of 5-carbon linker-containing mono-carbonyl analogs of curcumin: N, N-dimethyl propoxy substituent CBA-iR: B75 and C12 |
TNF-α and IL-6 | Murine macrophages cell line: RAW264.7 |
| EF24 | TNF-α and IL-6 | JAWS II dendritic cells (DCs) |
| EF24 | - | Sprague Dawley rats |
| EF24 | TNF-α and IL-6 |
LPS-stimulated dendritic cells - rat |
| EF24 | NF-ΚB, IL-1R | JAWS II dendritic cells (DCs) |
| EF24 | NF-ΚB, COX2 | - Rat |
| EF24 | NF-ΚB, COX-2 | B cells - Rat |
| A series of asymmetric indole curcumin analogs CBA-iR: 5c, 5b, 5j, 5g, 5h |
TNF-α, IL-6, trypsin, b-glucuronidase | CCK-8 cells |
| C-5 Curcumin analogs | TNF-α/NF-ΚB pathway |
Chronic myeloid leukemia cell line: KBM5 Colon cancer cell line: HCT116 |
| The presence of NO2 on R1 and methoxy/hydroxy group on R2 and cyclohexanone CBA-iR: C26 |
TNF-α and IL-6 |
Mouse primary peritoneal macrophages (MPM cells) - ICR mice and Sprague–Dawley (SD) rats |
| Resveratrol-curcumin hybrids CBA-iR: a18 |
TNF-α and IL-6 |
Murine macrophages cell line: RAW264.7 - C57BL/6 mice |
| Diarylpentadienone derivatives CBA-iR: 3i |
TNF-α and IL-6 | Murine macrophages cell line: RAW264.7 |
| β-ionone-derived curcumin analogs CBA-iR: 1e |
TNF-α and IL-6 |
Murine macrophages cell line: RAW264.7 - C57BL/6 mice |
| 3ʹ-methoxy and cyclohexanone CBA-iR: 3c |
TNF-α and IL-6 | Mouse J774.1 macrophages |
| Mono-carbonyl analogs of curcumin: -dimethyl propoxyl and alkoxyl substituent |
TNF-α and IL-6 | Macrophages |
| Asymmetrical monocarbonyl analogs of curcumin CBA-iR: 3a, 3c |
TNF-α and IL-6 |
Murine macrophages cell line: RAW264.7 - C57BL/6 mice |
| Asymmetric mono-carbonyl analogs of curcumin (AMACs) CBA-iR: 3f |
TNF-α and IL-6 |
Mouse primary peritoneal macrophages (MPM cells) - C57BL/6 mice |
| PAC | IL-4 and IL-10 |
Breast cancer cells: MDA-MB231, MCF-10A, MCF-7 and T-47D Primary breast cancer cell culture: BEC114 - Balb-c mice |
| Benzylidenecyclopentanoneanaloguesofcurcumin CBA-iR: hydroxylmethoxybenzylidenecyclopentanone analog of curcumin |
Histamine | Rat basophilic leukemia cells: RBL-2H3 |
| 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien-3-one (hylin) | MRP5 | HEK293 cells |
| Diarylheptanoids | PGE2 | 3T3 cells |
| Enone analogs of Curcumin: -7-carbon dienone spacer, -5-carbon enone spacer with and without a ring -3-carbon enone spacer |
Nrf2 | Nrf2-ARE reporter-HepG2 stable cell line |
| FN1 (3E,5E)-3,5-bis(pyridin-2-methylene)-tetrahydrothiopyran-4-one |
Nrf2 |
Human hepatocellular cell line: HepG2-C8 - TRAMP mice |
| 1,3-dicarbonyl and acyclic series | TRP channels | HEK293 cells |
| Compounds with tetrahydroxyl groups: 2,6-bis (3,4-dihydroxybenzylidene)cyclohexanone (A2), 2,5-bis(3,4-dihydroxybenzylidene) cyclopentanone (B2), 1,5-bis(3,4-dihydroxyphenyl)-1,4-pentadiene-3-one (C2), and 3,5-bis(3,4-dihydroxybenzylidene)-4-piperidone (D2) | ALR2 | - |
| Curcumin analogs (MACs) CBA-iR: 17 and 28 |
MD2 |
Human Umbilical Vein Endothelial Cells (HUVECs) Murine macrophages cell line: RAW264.7 |
| Asymmetrical pyrazole curcumin analogs | - | - |
| Rosmarinic acid, tetrahydrocurcumin, dihydrocurucmin, and hexahydrocurcumin | Phospholipase A2 | - |
| 2,6-bis (3,4-dihydroxybenzylidene) cyclohexanone CBA-iR: A2 |
- |
Murine macrophages cell line: RAW264.7 - Mice |
| Curcumin | ↓ NO, IL-1β, IL-6, iNOS ↑ IL-4, IL-10, Arg-1 promoted microglial polarisation to the M2 phenotype |
LPS-induced BV2 cells |
| Curcumin | ↓ IL-1β, IL-6, iNOS, and TNF-α, CD86 protein, ↑ IL-10, TGF-β ↓ TLR4 signaling |
Subarachnoid hemorrhage mice models |
| Curcumin | ↑ PPAR-γ, ↓ NF-κB |
Cigarette smoke extract-treated Beas-2B cells |
| Curcumin | ↑ PPAR-γ, ↓ NF-κB, inflammation score ↓TNF-α, IL-6 |
Cigarette smoke-induced COPD rat models |
| Curcumin | ↓ MCP-1, IL-17 | Gp120-induced BV2 cells |
| Curcumin | ↓ MCP-1, TNF-α, iNOS, NO ↓ ROS |
LPS-induced inflammation in vascular smooth muscle cells |
| Curcumin | ↓ TNF-α, IL-6 ↓ ROS |
Palmitate-induced inflammation in skeletal muscle C2C12 cells |
| Curcumin | ↓ TLR4, NF-κB, IL-27 | TNBS-Induced Colitis Rats |
| Curcumin | ↓ IL-6, IL-17, IL-23 ↑ IL-10 regulating the Re-equilibration of Treg/Th17 |
dextran sulphate sodium-induced colitis mice |
| Curcumin | ↓ IL-1β, IL-6, MCP-1 | DSS-induced colitis mouse model |
| Curcumin | ↓ TNF-α, IL-6 | DSS-induced ulcerative colitis mice model |
| Curcumin | ↓ TNF-α, IL-6, IL-17 ↑ IL-10 |
DSS-induced acute colitis in mice |
| Curcumin Curcumin nanoparticles |
↓ MMP-1, MMP-3, MMP-13, ADAMTS5, IL-1β, TNF-α | Post-traumatic osteoarthritis mouse model |
| Acid-activatable curcumin polymer | ↓ IL-1β, TNF-α | Monoiodoacetic acid-induced osteoarthritis mouse model |
| Curcumin | ↓ TNF-α, IL-17, IL-1β and TGF-β | Collagen-induced rat arthritis model |
| Curcumin | ↓ IL-1β, TNF-ɑ | Anterior cruciate ligament transection rat model |
| Curcumin loaded hyalurosomes | ↑ IL-10 ↓ IL-6, IL-15, TNF-ɑ |
Fibroblast-like synovial cells |
| Curcumin | ↓ IL-1β, TNF-α, NLRP3, caspase-1 | Primary rat abdominal macrophages MSU-induced gouty arthritis rat model |
| Curcumin | ↓ IL-17, TNF-ɑ, IL-6, IFN-γ | Imiquimod-induced differentiated HaCaT cells |
| Curcumin | ↓ IFN-γ, TNF-α, IL-2, IL-12, IL-22, IL-23 | Transgenic mouse model of psoriasis |
| Curcumin | ↓ IFN-γ | TPA-induced K14-VEGF transgenic psoriasis |
| Curcumin nanohydrogel | ↓ TNF-α, iNOS | imiquimod-induced psoriasis model |
| Curcumin | ↓ IL-1β, IL-6, TNF-α ↓ NF-κB activation ↓stressed-induced P2X7R/NLRP3 inflammasome axis activation |
Chronic unpredictable mild stress-induced rats model |
| Curcumin | ↓ IL-6, TNF-α | Chronic unpredictable mild stress-induced rats model |
| Curcumin | ↓ IL-1β | CUMS depression model |
| Curcumin | ↓ TLR4, IL-1β, TNF-α, VCAM-1, ICAM-1, NF-κB, | ApoE-/- mice |
| Mannich Curcuminoids |
↓ NF-κB, IL-6, IL-4, TNF-α | TNBS-induced colitis rats’ model |
| TRB-N0224 | ↓IL-1β, IL-6, TNF-α, MMP-9, MMP-13 | A rabbit anterior cruciate ligament transection injury-induced model of OA. |
| Curcumin analogue AI-44 | ↓TNF-α, IL-1β | MSU-induced THP-1 cell |
| Curcumin diglutaric acid | ↓ NO, IL-6, TNF-α, iNOS, COX-2 | LPS-stimulated RAW 264.7 macrophage cells |
| Curcumin-galactomannoside (CGM) | ↓ COX-2, PGE2, iNOS, TLR4, IL-6, TNF-α | Acetic acid-induced colitis |
| Next Generation Ultrasol Curcumin (NGUC) | ↓ TNF-α, IL-1β, IL-6, COMP, CRP, MMP-3, 5-LOX, COX-2, NF-κB | MIA-induced OA |
| Curcumin | ↓ IL-1β, IL-6, TNF-α, p53, CRP | Mice |
| Demethoxycurcumin | ↓ IL-1β, IL-6, TNF-α, NO, IL-4, IL-13 | N9 microglial cells |
| curcumin-loaded tetrahedral framework nucleic acids (Cur-TFNAs) | ↓ NF-κB, ROS, NO and inflammatory factors (IL-6, IL-1β and TNF-α). | RAW264.7 cells |
7.3. Anticancer Activity
8. Future Directions
Author Contributions
Acknowledgements
Disclosure statement
Abbreviations
| ABTS | 2,2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid |
| AgCl | Silver chloride |
| AgNO3 | Silver nitrate |
| BDMC | Bisdemethoxycurcumin |
| CDF | Difluoro curcumin |
| CDFCD | Difluoro curcumin ß-cyclodextrin |
| Cisplatin | Cis-diamminedichloroplatinum (II) |
| Cu | Copper |
| CUPRAC | Cupric reducing antioxidant capacity |
| CUR | Curcumin |
| CUR-NS | Curcumin nanosuspension |
| DMC | Demethoxycurcumin |
| DMF | Dimethylformamide |
| DMSO | Dimethyl Sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| Fe | Iron |
| LPS | Lipopolysaccharides |
| Mg | Magnesium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium |
| NaOH | Sodium Hydroxide |
| NO | Nitric oxide |
| Pd | Palladium |
| Pt | Platinum |
| TNF | Tumor necrosis factor |
| THC | Tetrahydrocurcumin |
References
- Abd Razak, N., Akhtar, M. N., Abu, N., Ho, W. Y., Tan, S. W., Zareen, S., Nizam Bin Taj-Ud-Din, S., Long, K., Alitheen, N. B. & Yeap, S. K. 2017. The In Vivo Anti-Tumor Effect Of Curcumin Derivative (2 E, 6 E)-2, 6-Bis (4-Hydroxy-3-Methoxybenzylidene) Cyclohexanone (Bhmc) On 4t1 Breast Cancer Cells. Rsc Advances, 7, 36185-36192.
- Aggarwal, B. B. & Shishodia, S. 2006. Molecular Targets Of Dietary Agents For Prevention And Therapy Of Cancer. Biochemical Pharmacology, 71, 1397-1421.
- Agrawal, D. K. & Mishra, P. K. 2010. Curcumin And Its Analogues: Potential Anticancer Agents. Medicinal Research Reviews, 30, 818-860.
- Ananthakrishnan, P., Balci, F. L. & Crowe, J. P. 2012. Optimizing Surgical Margins In Breast Conservation. International Journal Of Surgical Oncology, 2012.
- Anitha, A., Deepagan, V., Rani, V. D., Menon, D., Nair, S. & Jayakumar, R. 2011. Preparation, Characterization, In Vitro Drug Release And Biological Studies Of Curcumin Loaded Dextran Sulphate–Chitosan Nanoparticles. Carbohydrate Polymers, 84, 1158-1164.
- Aggarwal, Bharat B, Anushree Kumar, and Alok C Bharti. “Anticancer Potential of Curcumin: Preclinical and Clinical Studies.” Anticancer research 23, no. 1/A (2003): 363-98.
- Allegra, Alessandro, Vanessa Innao, Sabina Russo, Demetrio Gerace, Andrea Alonci, and Caterina Musolino. “Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies.” Cancer investigation 35, no. 1 (2017): 1-22.
- Azad, Abul Kalam, Joanne Lai, Wan Mohd Azizi Wan Sulaiman, Hassan Almoustafa, Salah Abdalrazak Alshehade, Vinoth Kumarasamy, and Vetriselvan Subramaniyan. “The Fabrication of Polymer-Based Curcumin-Loaded Formulation as a Drug Delivery System: An Updated Review from 2017 to the Present.” Pharmaceutics 16, no. 2 (2024): 160.
- Banuppriya, Govindharasu, Ganeshan Shakambari, Rajendran Sribalan, Perumal Varalakshmi, and Vediappen Padmini. “Evaluation of Anticancer Activity of Water-Soluble Curcumin through the Induction of Apoptosis by P53 and P21 Modulation.” ChemistrySelect 3, no. 11 (2018): 2976-81.
- Bapat, Ranjeet A, Sumit V Bedia, Aarti S Bedia, Ho Jan Yang, Suyog Dharmadhikari, Anshad Mohamed Abdulla, Tanay V Chaubal, et al. “Current Appraises of Therapeutic Applications of Nanocurcumin: A Novel Drug Delivery Approach for Biomaterials in Dentistry.” Environmental Research (2023): 116971.
- Bhaskar Rao, Adari, Ernala Prasad, Seelam S Deepthi, and Imtiaz A Ansari. “Synthesis and Biological Evaluation of Glucosyl Curcuminoids.” Archiv der Pharmazie 347, no. 11 (2014): 834-39.
- Borik, Rita M, Nagwa M Fawzy, Sherifa M Abu-Bakr, and Magdy S Aly. “Design, Synthesis, Anticancer Evaluation and Docking Studies of Novel Heterocyclic Derivatives Obtained Via Reactions Involving Curcumin.” Molecules 23, no. 6 (2018): 1398.
- Banik, U., Parasuraman, S., Adhikary, A. K. & Othman, N. H. 2017. Curcumin: The Spicy Modulator Of Breast Carcinogenesis. Journal Of Experimental & Clinical Cancer Research, 36, 98.
- Borik, R. M., Fawzy, N. M., Abu-Bakr, S. M. & Aly, M. S. 2018. Design, Synthesis, Anticancer Evaluation And Docking Studies Of Novel Heterocyclic Derivatives Obtained Via Reactions Involving Curcumin. Molecules, 23, 1398.
- Chen, C.-L. & Zhang, D.-D. 2014. Anti-Inflammatory Effects Of 81 Chinese Herb Extracts And Their Correlation With The Characteristics Of Traditional Chinese Medicine. Evidence-Based Complementary And Alternative Medicine, 2014.
- Chen, X., Zou, L.-Q., Niu, J., Liu, W., Peng, S.-F. & Liu, C.-M. 2015. The Stability, Sustained Release And Cellular Antioxidant Activity Of Curcumin Nanoliposomes. Molecules, 20, 14293-14311.
- Chen, D., Dai, F., Chen, Z., Wang, S., Cheng, X., Sheng, Q., Lin, J. & Chen, W. 2016. Dimethoxy Curcumin Induces Apoptosis By Suppressing Survivin And Inhibits Invasion By Enhancing E-Cadherin In Colon Cancer Cells. Medical Science Monitor: International Medical Journal Of Experimental And Clinical Research, 22, 3215.
- Choi, H., Lim, J. & Hong, J. 2010. Curcumin Interrupts The Interaction Between The Androgen Receptor And Wnt/Β-Catenin Signaling Pathway In Lncap Prostate Cancer Cells. Prostate Cancer And Prostatic Diseases, 13, 343-349.
- Cridge, B., Larsen, L. & Rosengren, R. Curcumin And Its Derivatives In Breast Cancer: Current Developments And Potential Forthe Treatment Of Drug-Resistant Cancers. Oncology Discovery, 1.
- Cao, Ya-Kun, Hui-Jing Li, Zhi-Fang Song, Yang Li, and Qi-Yong Huai. “Synthesis and Biological Evaluation of Novel Curcuminoid Derivatives.” Molecules 19, no. 10 (2014): 16349-72.
- Cerletti, Chiara, Mario Colucci, Marianna Storto, Fabrizio Semeraro, Concetta T Ammollo, Francesca Incampo, Simona Costanzo, et al. “Randomised Trial of Chronic Supplementation with a Nutraceutical Mixture in Subjects with Non-Alcoholic Fatty Liver Disease.” British journal of nutrition 123, no. 2 (2020): 190-97.
- Chainoglou, Eirini, and Dimitra Hadjipavlou-Litina. “Curcumin Analogues and Derivatives with Anti-Proliferative and Anti-Inflammatory Activity: Structural Characteristics and Molecular Targets.” Expert Opinion on Drug Discovery 14, no. 8 (2019/08/03 2019): 821-42.
- Ciochina, Roxana, Chasity Savella, Brianna Cote, Davis Chang, and Deepa Rao. “Synthesis and Characterization of New Curcumin Derivatives as Potential Chemotherapeutic and Antioxidant Agents.” Drug Development Research 75, no. 2 (2014): 88-96.
- De Vreese, Rob, Charlotte Grootaert, Sander D’hoore, Atiruj Theppawong, Sam Van Damme, Maarten Van Bogaert, John Van Camp, and Matthias D’hooghe. “Synthesis of Novel Curcuminoids Accommodating a Central Β-Enaminone Motif and Their Impact on Cell Growth and Oxidative Stress.” European journal of medicinal chemistry 123 (2016): 727-36.
- Deshmukh, Tejshri R., Vagolu S. Krishna, Dharmarajan Sriram, Jaiprakash N. Sangshetti, and Bapurao B. Shingate. “Synthesis and Bioevaluation of A,A’-Bis(1h-1,2,3-Triazol-5-Ylmethylene) Ketones.” Chemical Papers 74, no. 3 (2020/03/01 2020): 809-20.
- Dall’acqua, S., Stocchero, M., Boschiero, I., Schiavon, M., Golob, S., Uddin, J., Voinovich, D., Mammi, S. & Schievano, E. 2016. New Findings On The In Vivo Antioxidant Activity Of Curcuma Longa Extract By An Integrated 1h Nmr And Hplc–Ms Metabolomic Approach. Fitoterapia, 109, 125-131.
- Dandawate, P. R., Vyas, A., Ahmad, A., Banerjee, S., Deshpande, J., Swamy, K. V., Jamadar, A., Dumhe-Klaire, A. C., Padhye, S. & Sarkar, F. H. 2012. Inclusion Complex Of Novel Curcumin Analogue Cdf And Β-Cyclodextrin (1: 2) And Its Enhanced In Vivo Anticancer Activity Against Pancreatic Cancer. Pharmaceutical Research, 29, 1775-1786.
- Dcodhar, S., Sethi, R. & Srimal, R. 2013. Preliminary Study On Antirheumatic Activity Of Curcumin (Diferuloyl Methane). Indian Journal Of Medical Research, 138.
- Ding, L., Ma, S., Lou, H., Sun, L. & Ji, M. 2015. Synthesis And Biological Evaluation Of Curcumin Derivatives With Water-Soluble Groups As Potential Antitumor Agents: An In Vitro Investigation Using Tumor Cell Lines. Molecules, 20, 21501-21514.
- Esatbeyoglu, T., Huebbe, P., Ernst, I. M., Chin, D., Wagner, A. E. & Rimbach, G. 2012. Curcumin—From Molecule To Biological Function. Angewandte Chemie International Edition, 51, 5308-5332.
- El-Gazzar, Marwa G, Nashwa H Zaher, Ebaa M El-Hossary, and Amel FM Ismail. “Radio-Protective Effect of Some New Curcumin Analogues.” Journal of Photochemistry and Photobiology B: Biology 162 (2016): 694-702.
- Engwa, Godwill Azeh. “Free Radicals and the Role of Plant Phytochemicals as Antioxidants against Oxidative Stress-Related Diseases.” Phytochemicals: source of antioxidants and role in disease prevention. BoD–Books on Demand 7 (2018): 49-74.
- Fan, Ying-juan, Yi-xiang Zhou, Lian-ru Zhang, Qiao-fa Lin, Ping-zhang Gao, Fang Cai, Li-ping Zhu, Bi Liu, and Jian-hua Xu. “C1206, a Novel Curcumin Derivative, Potently Inhibits Hsp90 and Human Chronic Myeloid Leukemia Cells in Vitro.” Acta Pharmacologica Sinica 39, no. 4 (2018): 649-58.
- Farzaei, Mohammad Hosein, Mahdi Zobeiri, Fatemeh Parvizi, Fardous F El-Senduny, Ilias Marmouzi, Ericsson Coy-Barrera, Rozita Naseri, et al. “Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective.” Nutrients 10, no. 7 (2018): 855.
- Feng, Ling, Yang Li, Zhi-fang Song, Hui-jing Li, and Qi-yong Huai. “Synthesis and Biological Evaluation of Curcuminoid Derivatives.” Chemical and Pharmaceutical Bulletin 63, no. 11 (2015): 873-81.
- Fadus, M. C., Lau, C., Bikhchandani, J. & Lynch, H. T. 2017. Curcumin: An Age-Old Anti-Inflammatory And Anti-Neoplastic Agent. Journal Of Traditional And Complementary Medicine, 7, 339-346.
- Feng, T., Wei, Y., Lee, R. J. & Zhao, L. 2017. Liposomal Curcumin And Its Application In Cancer. International Journal Of Nanomedicine, 6027-6044.
- Ganta, S., Devalapally, H. & Amiji, M. 2010. Curcumin Enhances Oral Bioavailability And Anti-Tumor Therapeutic Efficacy Of Paclitaxel Upon Administration In Nanoemulsion Formulation. Journal Of Pharmaceutical Sciences, 99, 4630-4641.
- Gong, Shunze, Chixiang Yuan, Hang Hu, and Defeng Xu. “Synthesis of Curcumin Derivatives for Suppression of Castration-Resistant Prostate Cancer.” Journal of Chemical Research 48, no. 1 (2024): 17475198241230042.
- Gao, Y., Li, Z., Sun, M., Li, H., Guo, C., Cui, J., Li, A., Cao, F., Xi, Y. & Lou, H. 2010. Preparation, Characterization, Pharmacokinetics, And Tissue Distribution Of Curcumin Nanosuspension With Tpgs As Stabilizer. Drug Development And Industrial Pharmacy, 36, 1225-1234.
- Gaurisankar, S., Tanya, D., Shuvomoy, B. & Juni, C. 2010. Curcumin: From Exotic Spice To Modern Anticancer Drug. Al Ameen Journal Of Medical Sciences, 3, 21-37.
- Gören, A. C., Çikrikçi, S., Çergel, M. & Bilsel, G. 2009. Rapid Quantitation Of Curcumin In Turmeric Via Nmr And Lc–Tandem Mass Spectrometry. Food Chemistry, 113, 1239-1242.
- Gorgannezhad, L., Dehghan, G., Ebrahimipour, S. Y., Naseri, A. & Dolatabadi, J. E. N. 2016. Complex Of Manganese (Ii) With Curcumin: Spectroscopic Characterization, Dft Study, Model-Based Analysis And Antiradical Activity. Journal Of Molecular Structure, 1109, 139-145.
- Grynkiewicz, G. & Ślifirski, P. 2012. Curcumin And Curcuminoids In Quest For Medicinal Status. Acta Biochimica Polonica, 59.
- Gupta, S. C., Patchva, S. & Aggarwal, B. B. 2013. Therapeutic Roles Of Curcumin: Lessons Learned From Clinical Trials. The Aaps Journal, 15, 195-218.
- Gupta, S. C., Prasad, S., Kim, J. H., Patchva, S., Webb, L. J., Priyadarsini, I. K. & Aggarwal, B. B. 2011. Multitargeting By Curcumin As Revealed By Molecular Interaction Studies. Natural Product Reports, 28, 1937-1955.
- Han, Y.-M., Shin, D.-S., Lee, Y.-J., Ismail, I. A., Hong, S.-H., Han, D. C. & Kwon, B.-M. 2011. 2-Hydroxycurcuminoid Induces Apoptosis Of Human Tumor Cells Through The Reactive Oxygen Species–Mitochondria Pathway. Bioorganic & Medicinal Chemistry Letters, 21, 747-751.
- Handler, N., Jaeger, W., Puschacher, H., Leisser, K. & Erker, T. 2007. Synthesis Of Novel Curcumin Analogues And Their Evaluation As Selective Cyclooxygenase-1 (Cox-1) Inhibitors. Chemical And Pharmaceutical Bulletin, 55, 64-71.
- Hatcher, H., Planalp, R., Cho, J., Torti, F. & Torti, S. 2008. Curcumin: From Ancient Medicine To Current Clinical Trials. Cellular And Molecular Life Sciences, 65, 1631-1652.=.
- Houssami, N., Macaskill, P., Marinovich, M. L., Dixon, J. M., Irwig, L., Brennan, M. E. & Solin, L. J. 2010. Meta-Analysis Of The Impact Of Surgical Margins On Local Recurrence In Women With Early-Stage Invasive Breast Cancer Treated With Breast-Conserving Therapy. European Journal Of Cancer, 46, 3219-3232.
- Howells, L. M., Mitra, A. & Manson, M. M. 2007. Comparison Of Oxaliplatin-And Curcumin-Mediated Antiproliferative Effects In Colorectal Cell Lines. International Journal Of Cancer, 121, 175-183.
- Hu, C., Li, M., Guo, T., Wang, S., Huang, W., Yang, K., Liao, Z., Wang, J., Zhang, F. & Wang, H. 2019. Anti-Metastasis Activity Of Curcumin Against Breast Cancer Via The Inhibition Of Stem Cell-Like Properties And Emt. Phytomedicine, 58, 152740.
- Hackler Jr, László, Béla Ózsvári, Márió Gyuris, Péter Sipos, Gabriella Fábián, Eszter Molnár, Annamária Marton, et al. “The Curcumin Analog C-150, Influencing Nf-Κb, Upr and Akt/Notch Pathways Has Potent Anticancer Activity in Vitro and in Vivo.” PloS one 11, no. 3 (2016): e0149832.
- Hegde, Mangala, Sosmitha Girisa, Bandari BharathwajChetty, Ravichandran Vishwa, and Ajaikumar B Kunnumakkara. “Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far?”. ACS omega 8, no. 12 (2023): 10713-46.
- Hellou, Elias, Jameel Mohsin, Ameer Elemy, Fahed Hakim, Mona Mustafa-Hellou, and Shadi Hamoud. “Effect of Artemic in Patients with Covid-19: A Phase Ii Prospective Study.” Journal of Cellular and Molecular Medicine 26, no. 11 (2022): 3281-89.
- Hsieh, Min-Tsang, Ling-Chu Chang, Hsin-Yi Hung, Hui-Yi Lin, Mei-Hui Shih, Chang-Hai Tsai, Sheng-Chu Kuo, and Kuo-Hsiung Lee. “New Bis (Hydroxymethyl) Alkanoate Curcuminoid Derivatives Exhibit Activity against Triple-Negative Breast Cancer in Vitro and in Vivo.” European Journal of Medicinal Chemistry 131 (2017): 141-51.
- Indira Priyadarsini, K. 2013. Chemical And Structural Features Influencing The Biological Activity Of Curcumin. Current Pharmaceutical Design, 19, 2093-2100.
- Jakubczyk, Karolina, Aleksandra Drużga, Janda Katarzyna, and Karolina Skonieczna-Żydecka. “Antioxidant Potential of Curcumin—a Meta-Analysis of Randomized Clinical Trials.” Antioxidants 9, no. 11 (2020): 1092.
- Jankun, J., Aleem, A. M., Malgorzewicz, S., Szkudlarek, M., Zavodszky, M. I., Dewitt, D. L., Feig, M., Selman, S. H. & Skrzypczak-Jankun, E. 2006. Synthetic Curcuminoids Modulate The Arachidonic Acid Metabolism Of Human Platelet 12-Lipoxygenase And Reduce Sprout Formation Of Human Endothelial Cells. Molecular Cancer Therapeutics, 5, 1371-1382.
- Ji, H.-F. & Shen, L. 2014. Can Improving Bioavailability Improve The Bioactivity Of Curcumin? Trends In Pharmacological Sciences, 6, 265-266.
- Kapoor, N., Sharma, A. K., Dwivedi, V., Kumar, A., Pati, U. & Misra, K. 2007. Telomerase Targeted Anticancer Bioactive Prodrug By Antisense-Based Approach. Cancer Letters, 248, 245-250.
- Kato, C., Itaya-Takahashi, M., Miyazawa, T., Ito, J., Parida, I. S., Yamada, H., Abe, A., Shibata, M., Someya, K. & Nakagawa, K. 2023. Effects Of Particle Size Of Curcumin Solid Dispersions On Bioavailability And Anti-Inflammatory Activities. Antioxidants, 12, 724.
- Kotha, Raghavendhar R, Fakir Shahidullah Tareq, Elif Yildiz, and Devanand L Luthria. “Oxidative Stress and Antioxidants—a Critical Review on in Vitro Antioxidant Assays.” Antioxidants 11, no. 12 (2022): 2388.
- Kunnumakkara, Ajaikumar B, Preetha Anand, and Bharat B Aggarwal. “Curcumin Inhibits Proliferation, Invasion, Angiogenesis and Metastasis of Different Cancers through Interaction with Multiple Cell Signaling Proteins.” Cancer letters 269, no. 2 (2008): 199-225.
- Kunnumakkara, Ajaikumar B, Devivasha Bordoloi, Choudhary Harsha, Kishore Banik, Subash C Gupta, and Bharat B Aggarwal. “Curcumin Mediates Anticancer Effects by Modulating Multiple Cell Signaling Pathways.” Clinical science 131, no. 15 (2017): 1781-99.
- Luo, Hui, Shengjie Yang, Da Hong, Wei Xue, and Pu Xie. “Synthesis and in Vitro Antitumor Activity of (1e,4e)-1-Aryl-5-(2-((Quinazolin-4-Yl)Oxy)Phenyl)-1,4-Pentadien-3-One Derivatives.” Chemistry Central Journal 11, no. 1 (2017/03/15 2017): 23.
- Lien, Jin-Cherng, Chao-Ming Hung, Yi-Jing Lin, Hui-Chang Lin, Ting-Chia Ko, Li-Chung Tseng, Sheng-Chu Kuo, et al. “Pculin02h, a Curcumin Derivative, Inhibits Proliferation and Clinical Drug Resistance of Her2-Overexpressing Cancer Cells.” Chemico-Biological Interactions 235 (2015): 17-26.
- Liu, Siyu, Jie Liu, Lan He, Liu Liu, Bo Cheng, Fangliang Zhou, Deliang Cao, and Yingchun He. “A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health.” Molecules 27, no. 14 (2022): 4400.
- Lopes-Rodrigues, Vanessa, Ana Oliveira, Marta Correia-da-Silva, Madalena Pinto, Raquel T Lima, Emilia Sousa, and M Helena Vasconcelos. “A Novel Curcumin Derivative Which Inhibits P-Glycoprotein, Arrests Cell Cycle and Induces Apoptosis in Multidrug Resistance Cells.” Bioorganic & Medicinal Chemistry 25, no. 2 (2017): 581-96.
- Lu, Ko-Hsiu, Peace Wun-Ang Lu, Eric Wun-Hao Lu, Chiao-Wen Lin, and Shun-Fa Yang. “Curcumin and Its Analogs and Carriers: Potential Therapeutic Strategies for Human Osteosarcoma.” International Journal of Biological Sciences 19, no. 4 (2023): 1241.
- Khan, M. A., El-Khatib, R., Rainsford, K. & Whitehouse, M. 2012. Synthesis And Anti-Inflammatory Properties Of Some Aromatic And Heterocyclic Aromatic Curcuminoids. Bioorganic Chemistry, 40, 30-38.
- Konno, H., Endo, H., Ise, S., Miyazaki, K., Aoki, H., Sanjoh, A., Kobayashi, K., Hattori, Y. & Akaji, K. 2014. Synthesis And Evaluation Of Curcumin Derivatives Toward An Inhibitor Of Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1. Bioorganic & Medicinal Chemistry Letters, 24, 685-690.
- Koohpar, Z. K., Entezari, M., Movafagh, A. & Hashemi, M. 2015. Anticancer Activity Of Curcumin On Human Breast Adenocarcinoma: Role Of Mcl-1 Gene. Iranian Journal Of Cancer Prevention, 8.
- Kuptniratsaikul, V., Dajpratham, P., Taechaarpornkul, W., Buntragulpoontawee, M., Lukkanapichonchut, P., Chootip, C., Saengsuwan, J., Tantayakom, K. & Laongpech, S. 2014. Efficacy And Safety Of Curcuma Domestica Extracts Compared With Ibuprofen In Patients With Knee Osteoarthritis: A Multicenter Study. Clinical Interventions In Aging, 451-458.
- Li, J., Wang, Y., Yang, C., Wang, P., Oelschlager, D. K., Zheng, Y., Tian, D.-A., Grizzle, W. E., Buchsbaum, D. J. & Wan, M. 2009. Polyethylene Glycosylated Curcumin Conjugate Inhibits Pancreatic Cancer Cell Growth Through Inactivation Of Jab1. Molecular Pharmacology, 76, 81-90.
- Li, L., Braiteh, F. S. & Kurzrock, R. 2005. Liposome-Encapsulated Curcumin: In Vitro And In Vivo Effects On Proliferation, Apoptosis, Signaling, And Angiogenesis. Cancer: Interdisciplinary International Journal Of The American Cancer Society, 104, 1322-1331.
- Liu, H.-T. & Ho, Y.-S. 2018. Anticancer Effect Of Curcumin On Breast Cancer And Stem Cells. Food Science And Human Wellness, 7, 134-137.
- Manju, S. & Sreenivasan, K. 2011. Synthesis And Characterization Of A Cytotoxic Cationic Polyvinylpyrrolidone–Curcumin Conjugate. Journal Of Pharmaceutical Sciences, 100, 504-511.
- Mary, C. P. V., Vijayakumar, S. & Shankar, R. 2018. Metal Chelating Ability And Antioxidant Properties Of Curcumin-Metal Complexes–A Dft Approach. Journal Of Molecular Graphics And Modelling, 79, 1-14.
- Mbese, Z., Khwaza, V. & Aderibigbe, B. A. 2019. Curcumin And Its Derivatives As Potential Therapeutic Agents In Prostate, Colon And Breast Cancers. Molecules, 24, 4386.
- Menon, Venugopal P, and Adluri Ram Sudheer. “Antioxidant and Anti-Inflammatory Properties of Curcumin.” The molecular targets and therapeutic uses of curcumin in health and disease (2007): 105-25.
- Nagahama, K., Utsumi, T., Kumano, T., Maekawa, S., Oyama, N. & Kawakami, J. 2016. Discovery Of A New Function Of Curcumin Which Enhances Its Anticancer Therapeutic Potency. Scientific Reports, 6, 1-14.
- Nakagawa-Goto, K., Yamada, K., Nakamura, S., Chen, T.-H., Chiang, P.-C., Bastow, K. F., Wang, S.-C., Spohn, B., Hung, M.-C. & Lee, F.-Y. 2007. Antitumor Agents. 258. Syntheses And Evaluation Of Dietary Antioxidant—Taxoid Conjugates As Novel Cytotoxic Agents. Bioorganic & Medicinal Chemistry Letters, 17, 5204-5209.
- Noureddin, S. A., El-Shishtawy, R. M. & Al-Footy, K. O. 2019. Curcumin Analogues And Their Hybrid Molecules As Multifunctional Drugs. European Journal Of Medicinal Chemistry, 182, 111631.
- Olanlokun, John O., Wisdom Oshireku Abiodun, Oluwakemi Ebenezer, Neil A. Koorbanally, and Olufunso Olabode Olorunsogo. “Curcumin Modulates Multiple Cell Death, Matrix Metalloproteinase Activation and Cardiac Protein Release in Susceptible and Resistant Plasmodium Berghei-Infected Mice.” Biomedicine & Pharmacotherapy 146 (2022/02/01/ 2022): 112454.
- Panahi, Y., Sahebkar, A., Amiri, M., Davoudi, S. M., Beiraghdar, F., Hoseininejad, S. L. & Kolivand, M. 2012. Improvement Of Sulphur Mustard-Induced Chronic Pruritus, Quality Of Life And Antioxidant Status By Curcumin: Results Of A Randomised, Double-Blind, Placebo-Controlled Trial. British Journal Of Nutrition, 108, 1272-1279.
- Patwardhan, Raghavendra S., Rahul Checker, Deepak Sharma, Vineet Kohli, K. I. Priyadarsini, and Santosh K. Sandur. “Dimethoxycurcumin, a Metabolically Stable Analogue of Curcumin, Exhibits Anti-Inflammatory Activities in Murine and Human Lymphocytes.” Biochemical Pharmacology 82, no. 6 (2011/09/15/ 2011): 642-57.
- Peng, Ying, Mingyue Ao, Baohua Dong, Yunxiu Jiang, Lingying Yu, Zhimin Chen, Changjiang Hu, and Runchun Xu. “Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures.” Drug Design, Development and Therapy 15, no. null (2021/11/02 2021): 4503-25.
- Pandey, M. K., Kumar, S., Thimmulappa, R. K., Parmar, V. S., Biswal, S. & Watterson, A. C. 2011. Design, Synthesis And Evaluation Of Novel Pegylated Curcumin Analogs As Potent Nrf2 Activators In Human Bronchial Epithelial Cells. European Journal Of Pharmaceutical Sciences, 43, 16-24.
- Priya, R., Balachandran, S., Daisy, J. & Mohanan, P. 2015. Reactive Centers Of Curcumin And The Possible Role Of Metal Complexes Of Curcumin As Antioxidants. Univ J Phys Appl, 3, 6-16.
- Priyadarsini, K. I. 2014. The Chemistry Of Curcumin: From Extraction To Therapeutic Agent. Molecules, 19, 20091-20112.
- Qadir, M. I., Naqvi, S. T. Q., Muhammad, S. A., Qadir, M. & Naqvi, S. T. 2016. Curcumin: A Polyphenol With Molecular Targets For Cancer Control. Asian Pacific Journal Of Cancer Prevention, 17, 2735-2739. 2739.
- Ravipati, A. S., Zhang, L., Koyyalamudi, S. R., Jeong, S. C., Reddy, N., Bartlett, J., Smith, P. T., Shanmugam, K., Münch, G. & Wu, M. J. 2012. Antioxidant And Anti-Inflammatory Activities Of Selected Chinese Medicinal Plants And Their Relation With Antioxidant Content. Bmc Complementary And Alternative Medicine, 12, 173.
- Reddy, C. A., Somepalli, V., Golakoti, T., Kanugula, A. K., Karnewar, S., Rajendiran, K., Vasagiri, N., Prabhakar, S., Kuppusamy, P. & Kotamraju, S. 2014. Mitochondrial-Targeted Curcuminoids: A Strategy To Enhance Bioavailability And Anticancer Efficacy Of Curcumin. Plos One, 9, E89351.
- Radkar, Pranav, Prabhu Shankar Lakshmanan, Jenet Jemila Mary, Sunil Chaudhary, and Sathish Kumar Durairaj. “A Novel Multi-Ingredient Supplement Reduces Inflammation of the Eye and Improves Production and Quality of Tears in Humans.” Ophthalmology and Therapy 10, no. 3 (2021): 581-99.
- Raghavan, Saiharish, Prasath Manogaran, Krishna Kumari Gadepalli Narasimha, Balasubramanian Kalpattu Kuppusami, Palanivelu Mariyappan, Anjana Gopalakrishnan, and Ganesh Venkatraman. “Synthesis and Anticancer Activity of Novel Curcumin–Quinolone Hybrids.” Bioorganic & Medicinal Chemistry Letters 25, no. 17 (2015): 3601-05.
- Ravindran, Jayaraj, Gottumukkala V Subbaraju, Modukuri V Ramani, Bokyung Sung, and Bharat B Aggarwal. “Bisdemethylcurcumin and Structurally Related Hispolon Analogues of Curcumin Exhibit Enhanced Prooxidant, Anti-Proliferative and Anti-Inflammatory Activities in Vitro.” Biochemical pharmacology 79, no. 11 (2010): 1658-66.
- Rišiaňová, Lucia, Eva Fischer-Fodor, Jindra Valentová, Corina Tatomir, Nicoleta Corina Decea, Piroska Virag, Iveta Pechová, Ferdinand Devínsky, and Natalia Miklášová. “Synthesis, Structural Characterization and Biological Activity of Novel Knoevenagel Condensates on Dld-1 Human Colon Carcinoma.” Bioorganic & Medicinal Chemistry Letters 27, no. 11 (2017): 2345-49.
- Rizvi, Syed A. A., Soheila Kashanian, and Mehran Alavi. “Demothoxycurcumin as a Curcumin Analogue with Anticancer, Antimicrobial, Anti-Inflammatory, and Neuroprotective Activities: Micro and Nanosystems.” Nano Micro Biosystems 2, no. 4 (2023): 7-14.
- Rodrigues, Fiona C, NV Anil Kumar, and Goutam Thakur. “Developments in the Anticancer Activity of Structurally Modified Curcumin: An up-to-Date Review.” European journal of medicinal chemistry 177 (2019): 76-104.
- Salehi, B., Stojanović-Radić, Z., Matejić, J., Sharifi-Rad, M., Kumar, N. V. A., Martins, N. & Sharifi-Rad, J. 2019. The Therapeutic Potential Of Curcumin: A Review Of Clinical Trials. European Journal Of Medicinal Chemistry, 163, 527-545.
- Setthacheewakul, S., Mahattanadul, S., Phadoongsombut, N., Pichayakorn, W. & Wiwattanapatapee, R. 2010. Development And Evaluation Of Self-Microemulsifying Liquid And Pellet Formulations Of Curcumin, And Absorption Studies In Rats. European Journal Of Pharmaceutics And Biopharmaceutics, 76, 475-485.
- Shanmugam, M. K., Rane, G., Kanchi, M. M., Arfuso, F., Chinnathambi, A., Zayed, M., Alharbi, S. A., Tan, B. K., Kumar, A. P. & Sethi, G. 2015. The Multifaceted Role Of Curcumin In Cancer Prevention And Treatment. Molecules, 20, 2728-2769.
- Shehzad, A., Khan, S., Shehzad, O. & Lee, Y. 2010. Curcumin Therapeutic Promises And Bioavailability In Colorectal Cancer. Drugs Of Today, 46, 523.
- Shen, L. & Ji, H.-F. 2007. Theoretical Study On Physicochemical Properties Of Curcumin. Spectrochimica Acta Part A: Molecular And Biomolecular Spectroscopy, 67, 619-623.
- Simmler, C., Hajirahimkhan, A., Lankin, D. C., Bolton, J. L., Jones, T., Soejarto, D. D., Chen, S.-N. & Pauli, G. F. 2013. Dynamic Residual Complexity Of The Isoliquiritigenin–Liquiritigenin Interconversion During Bioassay. Journal Of Agricultural And Food Chemistry, 61, 2146-2157.
- Somers-Edgar, T. J., Taurin, S., Larsen, L., Chandramouli, A., Nelson, M. A. & Rosengren, R. J. 2011. Mechanisms For The Activity Of Heterocyclic Cyclohexanone Curcumin Derivatives In Estrogen Receptor Negative Human Breast Cancer Cell Lines. Investigational New Drugs, 29, 87-97.
- Srivastava, S., Mishra, S., Surolia, A. & Panda, D. 2016. C1, A Highly Potent Novel Curcumin Derivative, Binds To Tubulin, Disrupts Microtubule Network And Induces Apoptosis. Bioscience Reports, 36.
- Subhan, M., Alam, K., Rahaman, M., Rahman, M. & Awal, R. 2014. Synthesis And Characterization Of Metal Complexes Containing Curcumin (C 21 H 20 O 6) And Study Of Their Anti-Microbial Activities And Dna-Binding Properties. Journal Of Scientific Research, 6, 97-109.
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A. & Bray, F. 2021. Global Cancer Statistics 2020: Globocan Estimates Of Incidence And Mortality Worldwide For 36 Cancers In 185 Countries. Ca: A Cancer Journal For Clinicians, 71, 209-249.
- Sohail, Muhammad, Wenna Guo, Hui Xu, and Feng Zhao. “A Promising Anticancer Agent Dimethoxycurcumin: Aspects of Pharmacokinetics, Efficacy, Mechanism, and Nanoformulation for Drug Delivery.” Frontiers in Pharmacology 12 (2021): 665387.
- Srivastava, Shalini, Satyendra Mishra, Avadhesha Surolia, and Dulal Panda. “C1, a Highly Potent Novel Curcumin Derivative, Binds to Tubulin, Disrupts Microtubule Network and Induces Apoptosis.” Bioscience reports 36, no. 2 (2016): e00323.
- Tiekou Lorinczova, Helena, Gulshanara Begum, Lina Temouri, Derek Renshaw, and Mohammed Gulrez Zariwala. “Co-Administration of Iron and Bioavailable Curcumin Reduces Levels of Systemic Markers of Inflammation and Oxidative Stress in a Placebo-Controlled Randomised Study.” Nutrients 14, no. 3 (2022): 712.
- Tu, Zhi-Shan, Qi Wang, Dan-Dan Sun, Fang Dai, and Bo Zhou. “Design, Synthesis, and Evaluation of Curcumin Derivatives as Nrf2 Activators and Cytoprotectors against Oxidative Death.” European journal of medicinal chemistry 134 (2017): 72-85.
- Tang, H., Murphy, C. J., Zhang, B., Shen, Y., Van Kirk, E. A., Murdoch, W. J. & Radosz, M. 2010. Curcumin Polymers As Anticancer Conjugates. Biomaterials, 31, 7139-7149.
- Tennesen, H. & Karlsen, J. 1985. Studies On Curcumin And Curcuminoids (Vi. Kinetics Of Curcumin Degradation In Aqueous Solution). Z. Lebensm.-Unters. Forsch, 180, 402-404.
- Teymouri, M., Barati, N., Pirro, M. & Sahebkar, A. 2018. Biological And Pharmacological Evaluation Of Dimethoxycurcumin: A Metabolically Stable Curcumin Analogue With A Promising Therapeutic Potential. Journal Of Cellular Physiology, 233, 124-140.
- Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L. & Byrne, D. H. 2006. Comparison Of Abts, Dpph, Frap, And Orac Assays For Estimating Antioxidant Activity From Guava Fruit Extracts. Journal Of Food Composition And Analysis, 19, 669-675.
- Tomren, M., Masson, M., Loftsson, T. & Tønnesen, H. H. 2007. Studies On Curcumin And Curcuminoids: Xxxi. Symmetric And Asymmetric Curcuminoids: Stability, Activity And Complexation With Cyclodextrin. International Journal Of Pharmaceutics, 338, 27-34.
- Tønnesen, H. H. 1989. Studies On Curcumin And Curcuminoids. Xvi. Effect Of Curcumin Analogs On Hyaluronic Acid Degradation In Vitro. International Journal Of Pharmaceutics, 51, 259-261.
- Tripathi, A. & Misra, K. 2016. Designing And Development Of Novel Curcumin Analogues/Congeners As Inhibitors Of Breast Cancer Stem Cells Growth. Chemical Engineering Transactions, 49, 79-84.
- Tsukamoto, M., Kuroda, K., Ramamoorthy, A. & Yasuhara, K. 2014. Modulation Of Raft Domains In A Lipid Bilayer By Boundary-Active Curcumin. Chemical Communications, 50, 3427-3430.
- Usharani, P., Mateen, A., Naidu, M., Raju, Y. & Chandra, N. 2008. Effect Of Ncb-02, Atorvastatin And Placebo On Endothelial Function, Oxidative Stress And Inflammatory Markers In Patients With Type 2 Diabetes Mellitus: A Randomized, Parallel-Group, Placebo-Controlled, 8-Week Study. Drugs In R & D, 9, 243-250.
- Vyas, A., Dandawate, P., Padhye, S., Ahmad, A. & Sarkar, F. 2013. Perspectives On New Synthetic Curcumin Analogs And Their Potential Anticancer Properties. Current Pharmaceutical Design, 19, 2047-2069.
- Wang, Y., Yu, J., Cui, R., Lin, J. & Ding, X. 2016. Curcumin In Treating Breast Cancer: A Review. Journal Of Laboratory Automation, 21, 723-731.
- Wang, Y.-J., Pan, M.-H., Cheng, A.-L., Lin, L.-I., Ho, Y.-S., Hsieh, C.-Y. & Lin, J.-K. 1997. Stability Of Curcumin In Buffer Solutions And Characterization Of Its Degradation Products. Journal Of Pharmaceutical And Biomedical Analysis, 15, 1867-1876.
- Wang, Z., Leung, M. H., Kee, T. W. & English, D. S. 2010. The Role Of Charge In The Surfactant-Assisted Stabilization Of The Natural Product Curcumin. Langmuir, 26, 5520-5526.
- Wu, Lixian, Jing Yu, Ruijia Chen, Yang Liu, Liguang Lou, Ying Wu, Lisen Huang, et al. “Dual Inhibition of Bcr-Abl and Hsp90 by C086 Potently Inhibits the Proliferation of Imatinib-Resistant Cml Cells.” Clinical cancer research 21, no. 4 (2015): 833-43.
- Xu, G., Wei, D., Wang, J., Jiang, B., Wang, M., Xue, X., Zhou, S., Wu, B. & Jiang, M. 2014. Crystal Structure, Optical Properties And Biological Imaging Of Two Curcumin Derivatives. Dyes And Pigments, 101, 312-317.
- Yin, Ying, Yan Tan, Xueni Wei, Xiaoshun Li, Hongfei Chen, Zehua Yang, Guotao Tang, et al. “Recent Advances of Curcumin Derivatives in Breast Cancer.” Chemistry & Biodiversity 19, no. 10 (2022): e202200485.
- Yadav, V. R., Prasad, S., Kannappan, R., Ravindran, J., Chaturvedi, M. M., Vaahtera, L., Parkkinen, J. & Aggarwal, B. B. 2010. Retracted: Cyclodextrin-Complexed Curcumin Exhibits Anti-Inflammatory And Antiproliferative Activities Superior To Those Of Curcumin Through Higher Cellular Uptake. Elsevier.
- Yallapu, M. M., Jaggi, M. & Chauhan, S. C. 2010. Poly (Β-Cyclodextrin)/Curcumin Self-Assembly: A Novel Approach To Improve Curcumin Delivery And Its Therapeutic Efficacy In Prostate Cancer Cells. Macromolecular Bioscience, 10, 1141-1151.
- Zhang, Q., Li, D., Liu, Y., Wang, H., Zhang, C., Huang, H., He, Y., Chen, X., Du, Z. & Zheng, X. 2016. Potential Anticancer Activity Of Curcumin Analogs Containing Sulfone On Human Cancer Cells. Archives Of Biological Sciences, 68, 125-133.
- Zhao, S., Pi, C., Ye, Y., Zhao, L. & Wei, Y. 2019. Recent Advances Of Analogues Of Curcumin For Treatment Of Cancer. European Journal Of Medicinal Chemistry, 180, 524-535.
- Zarei, Amin, Leila Khazdooz, Anahita Khojastegi, Ataf Ali Altaf, and Alireza Abbaspourrad. “Oil Soluble Iron: Curcumin Derivatives and Their Complex.” Food Chemistry 431 (2024): 137085.
- Zhang, Mei, Xiaolin Zhang, Taoran Tian, Qi Zhang, Yuting Wen, Junyao Zhu, Dexuan Xiao, Weitong Cui, and Yunfeng Lin. “Anti-Inflammatory Activity of Curcumin-Loaded Tetrahedral Framework Nucleic Acids on Acute Gouty Arthritis.” Bioactive Materials 8 (2022/02/01/ 2022): 368-80.
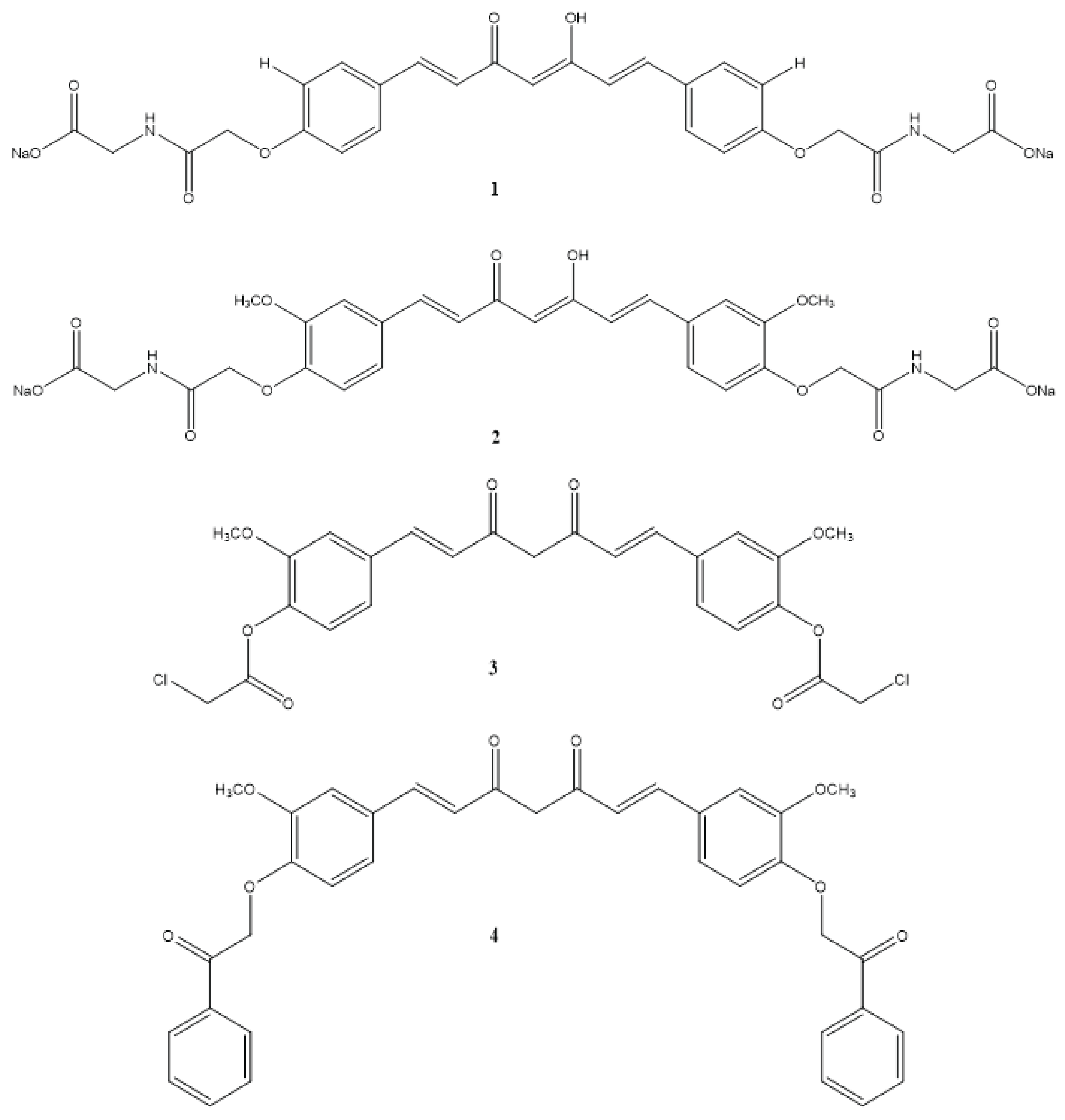
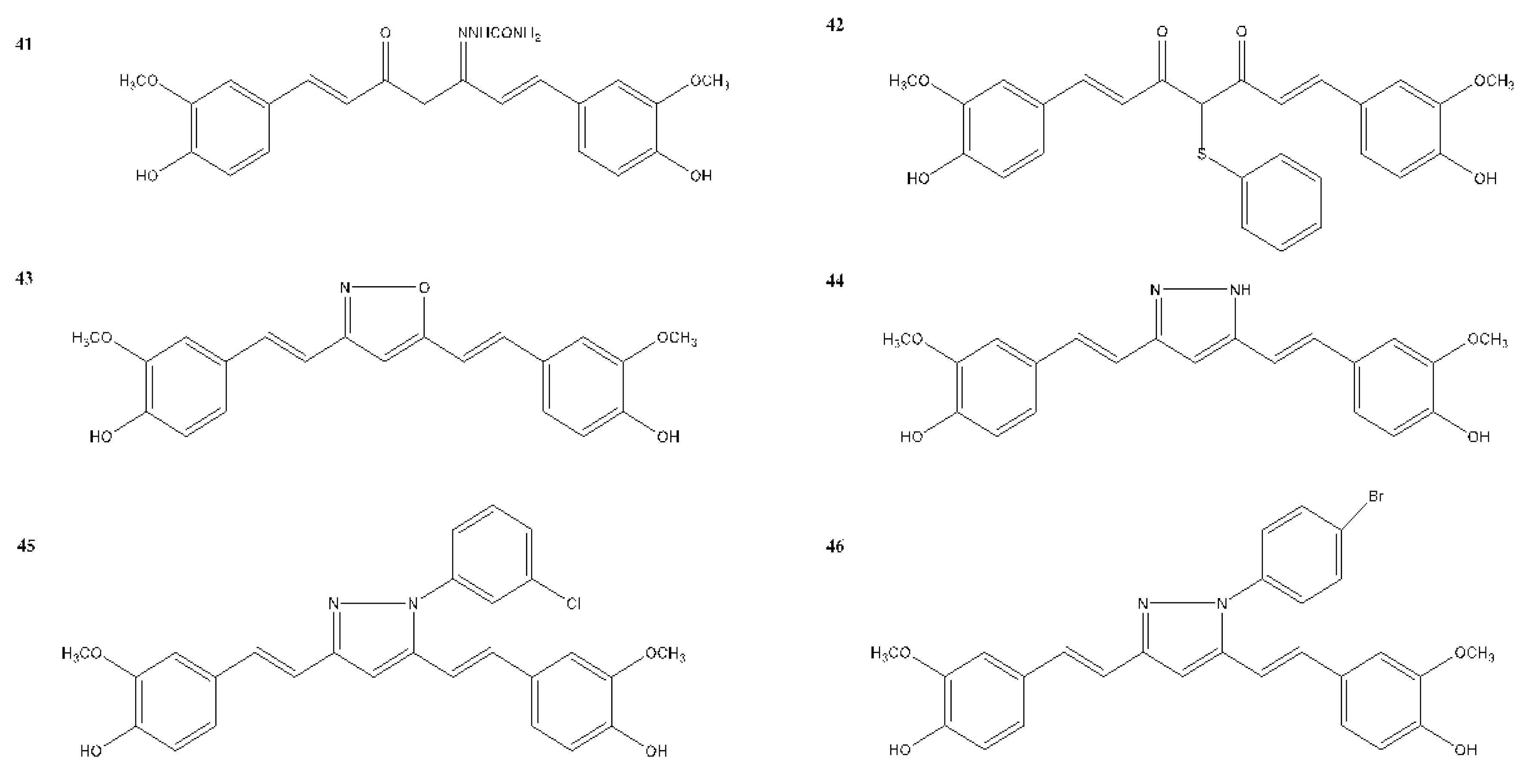
| Compound(s) | Biological Activity | Cell line tested (IC50 value - uM) | References | ||
|---|---|---|---|---|---|
| Antioxidant | Anticancer | Anti-inflammatory | |||
| 1 | – | + | – | HeLa - 0.5 ± 0.003 | Banuppriya et al.,2018 |
| 2 | – | + | – | HeLa - 0.5 ± 0.005 | Banuppriya et al.,2018 |
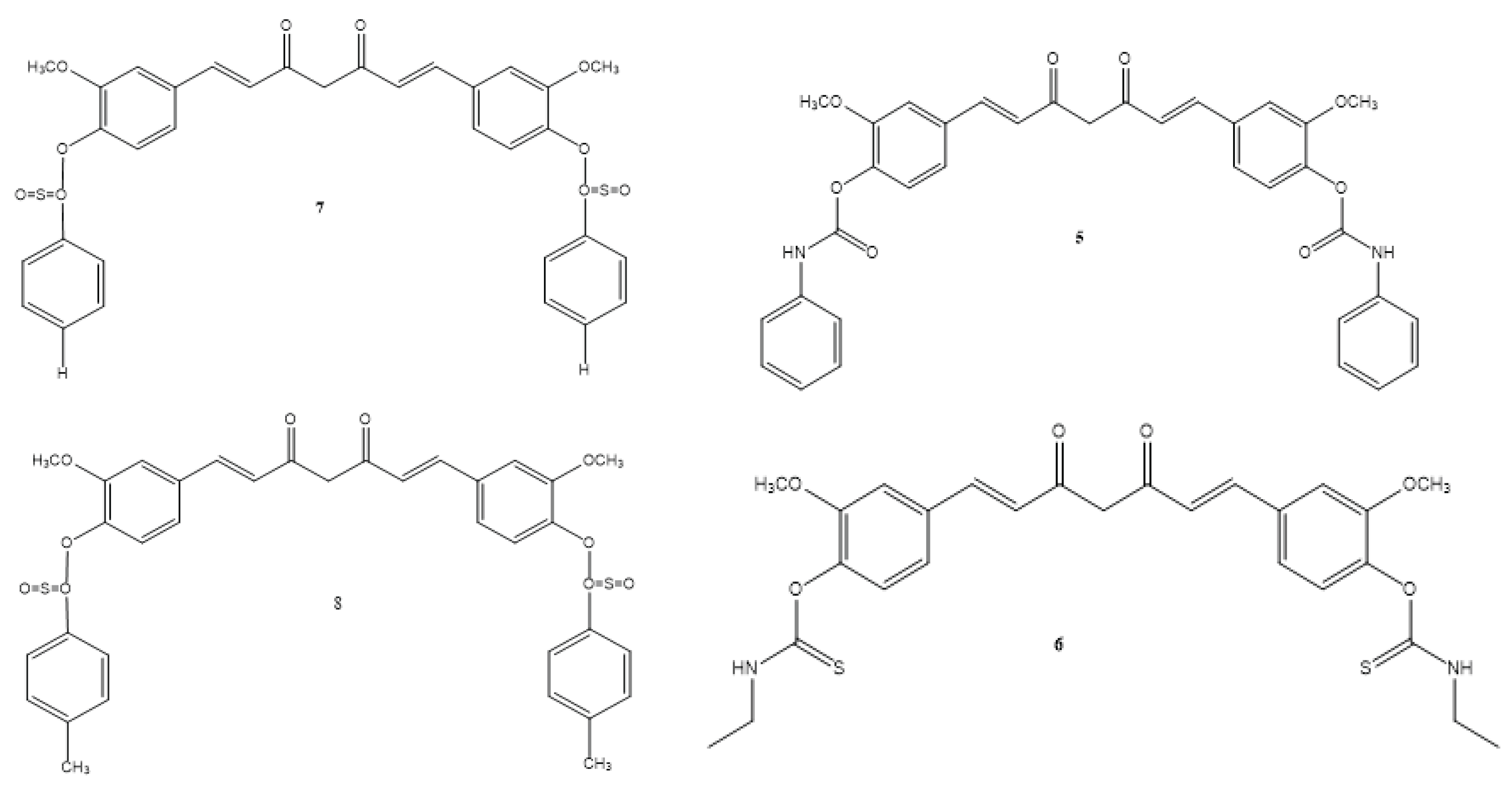
| 3 | + | – | + | Not reported | El-Gazzar et al., 2016 |
| 4 | + | – | + | Not reported | El-Gazzar et al., 2016 |
| 5 | + | – | + | Not reported | El-Gazzar et al., 2016 |
| 6 | + | – | + | Not reported | El-Gazzar et al., 2016 |
| 7 | + | – | + | Not reported | El-Gazzar et al., 2016 |
| 8 | + | – | + | Not reported | El-Gazzar et al., 2016 |
| 9 | – | + | + | MDA-MB-231 - 2.67 ± 0.18 HCT-116 - 3.91 ± 0.27 PC-3 - 3.90 ±0.08 |
Hsieh et al., 2017 |
| 10 | – | + | – | A549 - 23.9 ± 2.5 MCF-7 - 36.2 ± 1.99 SKOV3 - 12.8 ± 0.21 H460 - 21.75 ± 0.55 |
Raghavan et al., 2015 |
| 11 | + | + | – | SKOV3 - 5.58 ± 2.0 | Ciochina et al., 2014 |
| 12 | + | + | – | SKOV3 - 3.51 ± 0.74 | Ciochina et al., 2014 |
| 13 | + | + | – | H460 - 3.4 ± 0.84 RH460 - 2.6 ± 0.25 K562 - 6.3 ± 0.95 K562 Doxorubicin - 2.73 ± 0.61 |
Rodrigues et al., 2017 |
| 14 | – | + | – | Hep G2 - 0.31 LX-2 - 0.62 SMMC-7721 - 0.81 MDA-MB-231 - 0.52 |
Cao et al., 2014 |
| 15 | – | + | – | HT29 - 41.56 | Rao et al., 2014 |
| 16 | – | + | – | MCF-7 - 0.51 Hep G2 - 0.58 LX-2 - 0.63 3T3 - 0.79 |
Feng et al., 2015 |
| 17 | – | + | – | MCF-7 | Kanwar et al., 2011 |
| 18 | – | + | – | Not reported | Lien et al., 2015 |
| 19 | – | + | – | Hep G2 - 23 | Borik et al., 2018 |
| 20 | – | + | – | MCF-7 | Borik et al., 2018 |
| 21 | – | + | – | Caco-2 - 7.8 HT-29 - 4 EA.hy926 - 3.3 |
Vreese et al., 2016 |
| 22 | – | + | – | GBM - 0.87 GBM2 - 1.43 GBM3 - 1.45 GBM4 - 1.26 GBM5 - 0.92 GBM6 - 2.32 U373 MG - >5 U87 MG - 0.38 U251 MG - 0.33 |
Hacker et al., 2016 |
| 23 | – | + | – | Not reported | Tu et al., 2017 |
| 24 | – | + | – | K562 | Fan et al., 2017 |
| 25 | – | – | – | Not reported | Wu et al., 2015 |
| 26 | – | + | – | DLD-1 - 5.063 ± 0.09 | Rišiaňová et al., 2017 |
| 27 | – | + | – | DLD-1 - 5.101 ± 0.11 | Rišiaňová et al., 2017 |
| 28 | – | + | – | DLD-1 - 5.064 ± 0.12 | Rišiaňová et al., 2017 |
| 29 | – | + | – | MCF-7 - 1.5 ± 0.7 | Sirvastava et al., 2016 |
| 30 | – | + | – | MDA-MB-231 - 5.37 MDA-MB-231 - 2.67 Doxorubicin-resistant MDA-MB-231 - 5.70 |
Chui et al., 2021 |
| 31 | – | + | – | Not reported | Lu et al., 2023 |
| 32 | – | + | – | 22RV1 cells 48 h - 8.791 72 h - 8.516 |
Gong et al., 2024 |
| 33 | + | – | – | Not reported | Zarei et al., 2024 |
| 34 | + | – | – | Not reported | Zarei et al., 2024 |
| 35 | + | – | – | 1.92 (%) | Zarei et al., 2024 |
| 36 | – | + | – | SUM149 - 11.20 MDA-MB-231 - 18.00 |
Yin et al., 2022 |
| 37 | – | + | – | MDA-MB-231 - 0.52 | Yin et al., 2022 |
| 38 | – | + | – | MCF-7 - 73.4 | Yin et al., 2022 |
| 39 | – | + | – | MDA-MB-231 - EC50 - 0.42 | Yin et al., 2022 |
| 40 | – | + | – | MDA-MB-231 - EC50 - 0.78 | Yin et al., 2022 |
| 41 | – | + | – | MCF-7 | Yin et al., 2022 |
| 42 | – | + | – | SUM149 - 13.50 MDA-MB-231 - 15.00 |
Yin et al., 2022 |
| 43 | – | + | – | MCF-7 - 13.10 MCF-7R - 12.00 |
Yin et al., 2022 |
| 44 | – | + | – | MCF-7 - 2.56 MDA-MB-231 - 3.37 |
Yin et al., 2022 |
| 45 | – | + | – | MCF-7 - 34.99 | Yin et al., 2022 |
| 46 | – | + | – | MCF-7 - 5.80 | Yin et al., 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





