Submitted:
19 March 2024
Posted:
20 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Method
2.1. Extraction Procedure Method
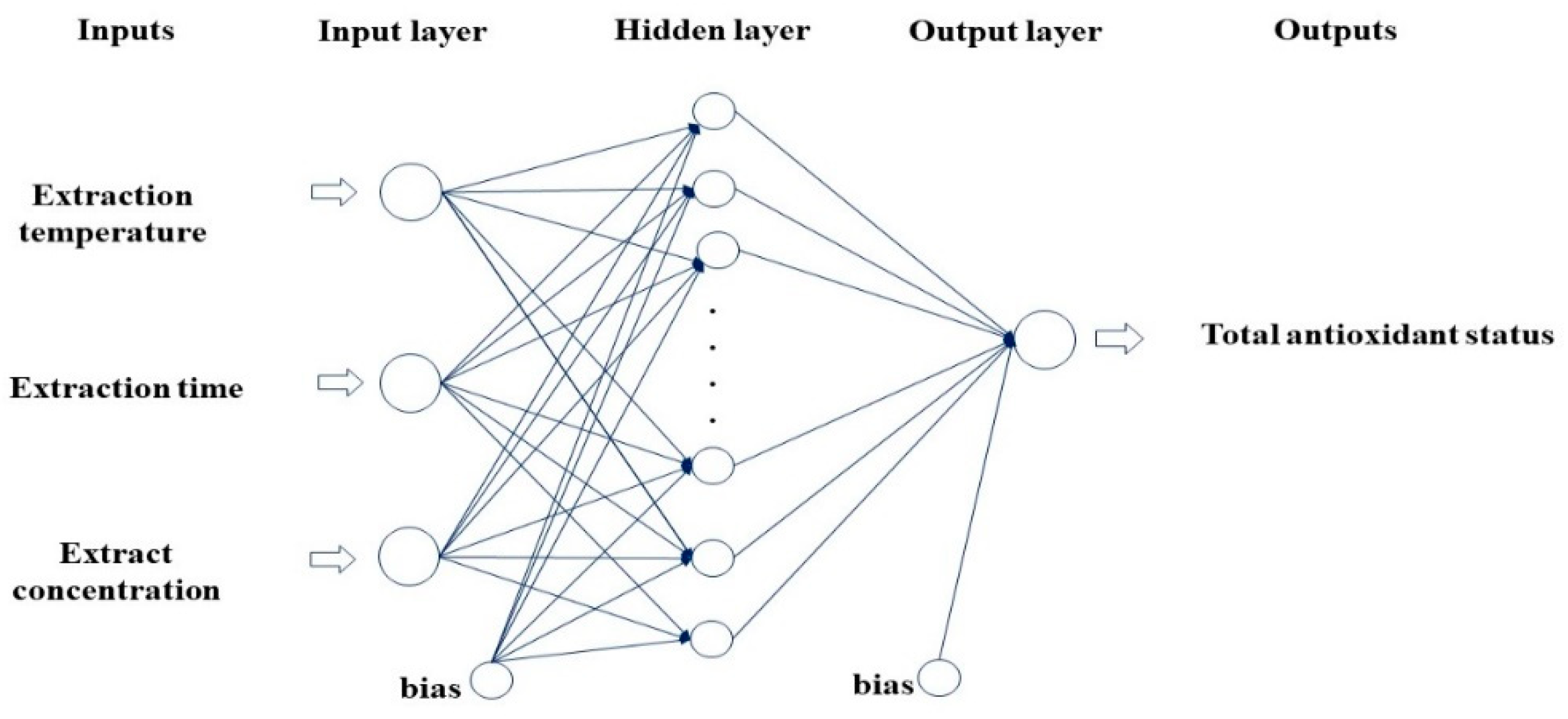
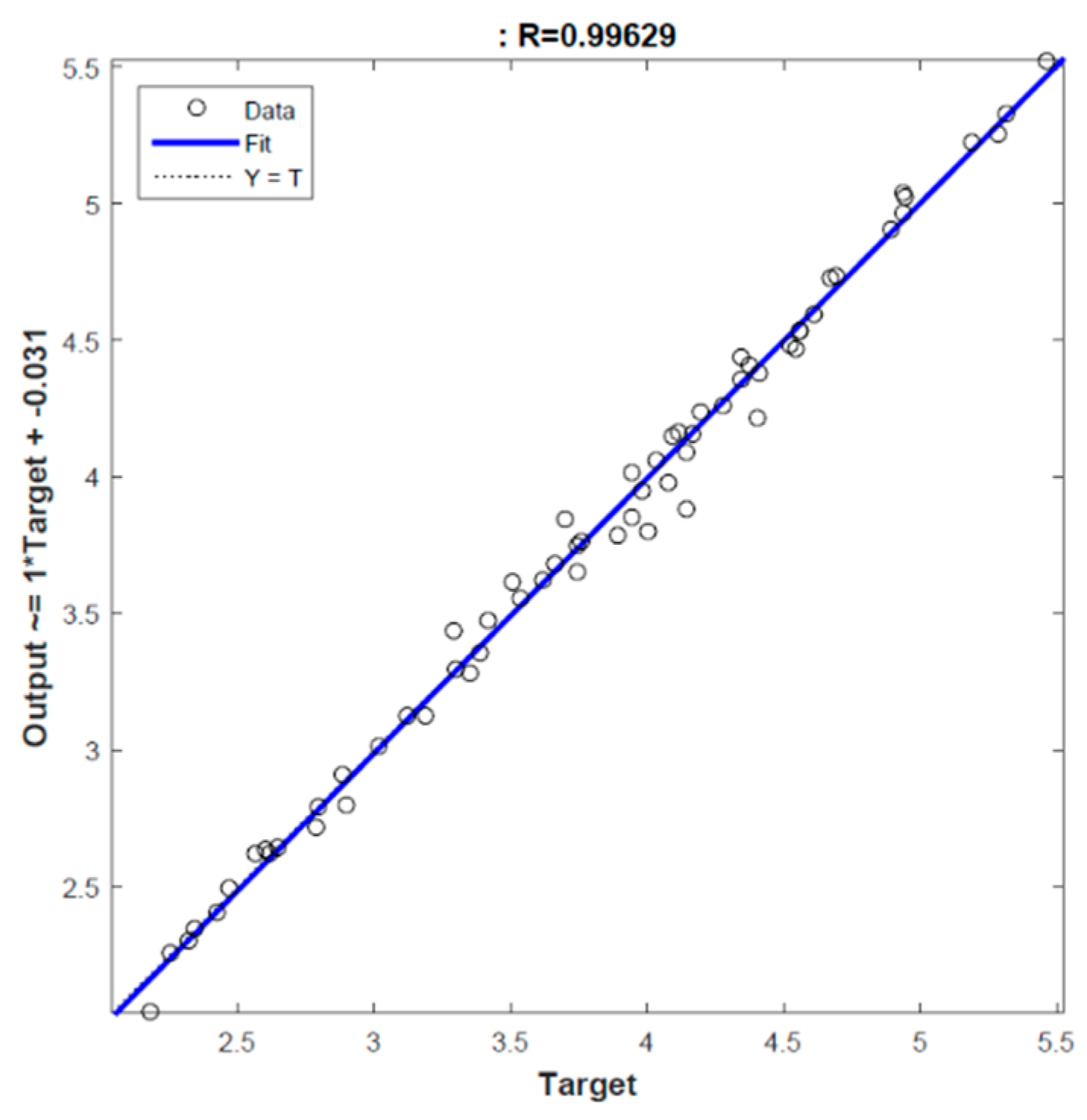
2.2. Modelling
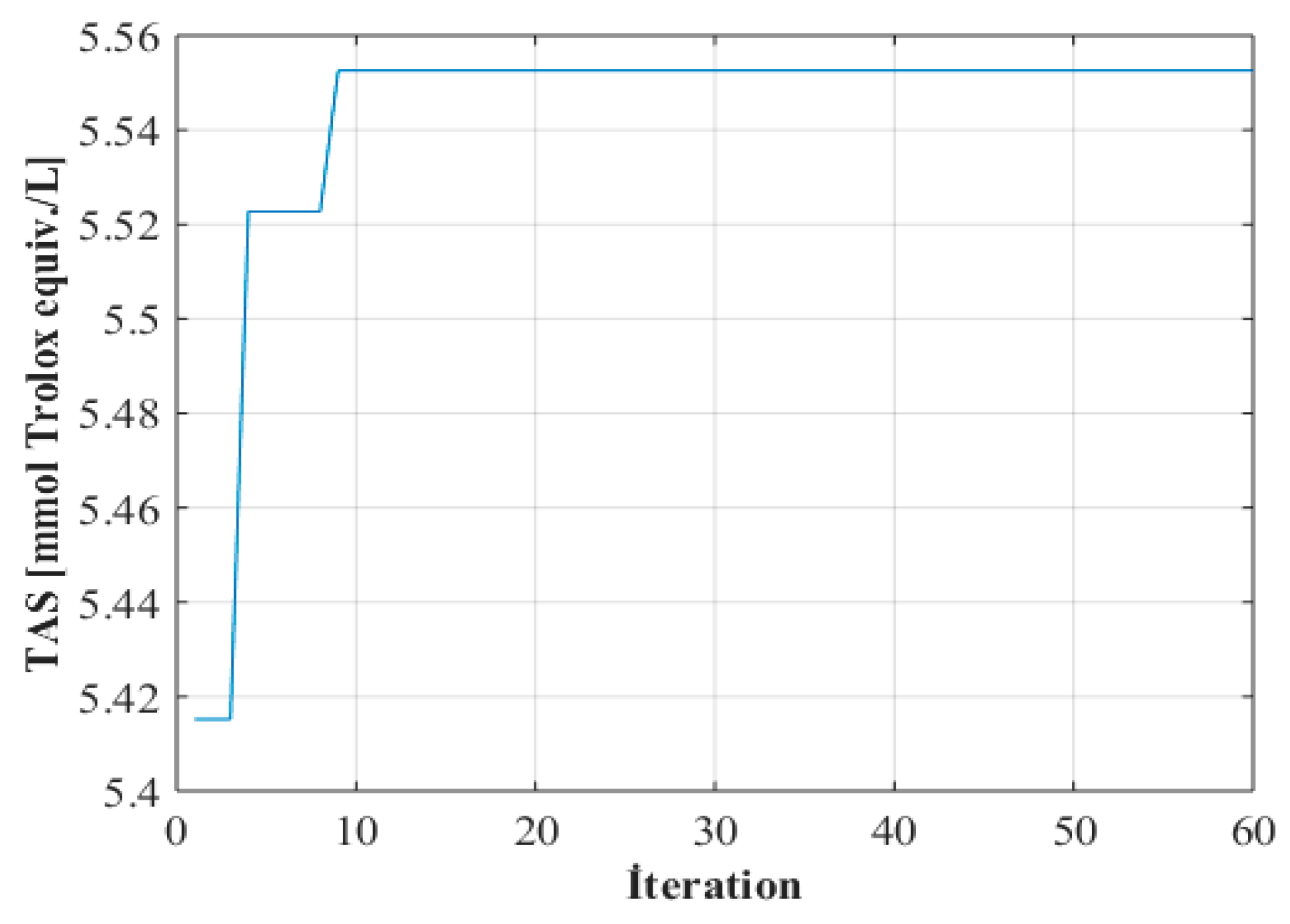
2.3. Optimization
2.4. Extraction Processes
2.5. MTT Analysis
2.6. Anticholinesterase Analysis
2.7. Antimicrobial Analysis
2.8. Total Phenolic Analysis
2.9. Antioxidant Analysis
2.9.1. Total Antioxidant and Oxidant Analysis
2.9.2. DPPH Free Radical Scavenging Activity
2.9.3. Ferric Reducing Antioxidant Power Assay
2.10. Phenolic Analysis
2.11. Statistical Analysis
3. Results and Discussions
3.1. Optimization of Extraction Conditions
3.2. Antiproliferative Activity
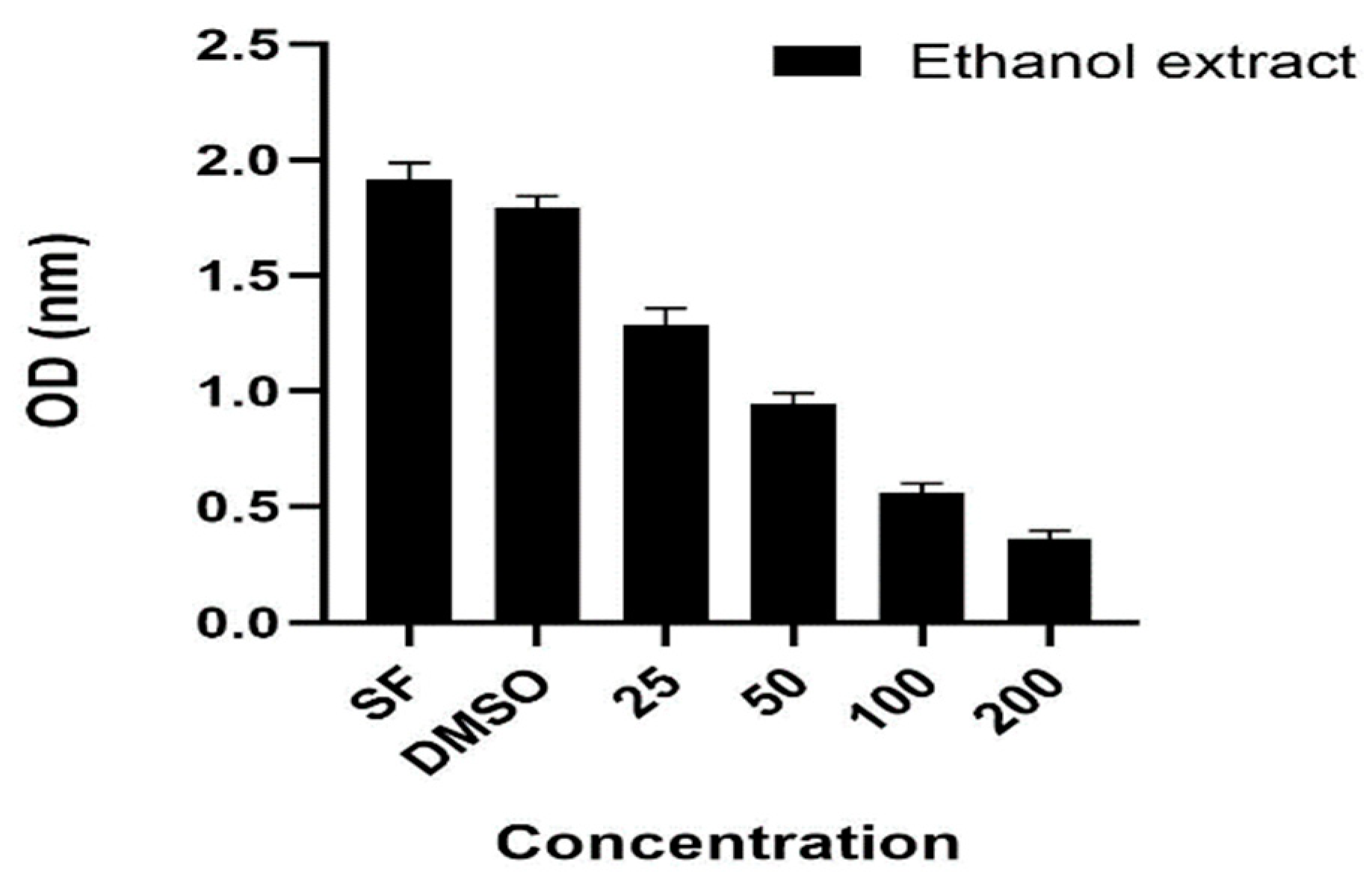
3.3. Antialzeihmer Activity
3.4. Antimicrobial Potential
3.5. Total Phenolic Contents
3.6. Antioxidant Activity
3.7. Phenolic Contents
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ivanova, T.; Krupodorova, T.; Barshteyn, V.; Artamonova, A.; Shlyakhovenko, V. Anticancer substances of mushroom origin. Exp. Oncol. 2014, 36, 58–66. [Google Scholar]
- Lazur, J.; Hnatyk, K.; Kała, K.; Sułkowska-Ziaja, K.; Muszyńska, B. Discovering the Potential Mechanisms of Medicinal Mushrooms Antidepressant Activity: A Review. Antioxidants 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Z.; Song, K.; Li, L.; Chen, M. Medicinal value of edible mushroom polysaccharides: A review. J. Future Foods 2023, 3, 16–23. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-volatile taste components of several speciality mushrooms. Food Chem. 2001, 73, 461–466. [Google Scholar] [CrossRef]
- Bal, C.; Akgul, H.; Sevindik, M.; Akata, I.; Yumrutas, O. Determination of the anti-oxidative activities of six mushrooms. Fresenius Environ. Bull. 2017, 26, 6246–6252. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Hindler, J.; Hochstein, L.; Howell, A. Preparation of routine media and reagents used in antimicrobial sensitivity testing. Part 1. McFarland standards. In Clinical Microbiology Procedures Handbook, Isenberg, H.D., Ed.; Clinical microbiology procedures handbook; American Society for Microbiology: Washington, DC, USA, 1992; pp. 5.19.11–15.19.16. [Google Scholar]
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Sevindik, M.; Dogan, M.; Akgül, H. Antioxidant, antimicrobial activities and heavy metal contents of some Myxomycetes. Fresenius Environ. Bull. 2020, 29, 7840–7846. [Google Scholar]
- Bal, C.; Eraslan, E.C.; Sevindik, M. Antioxidant, Antimicrobial Activities, Total Phenolic and Element Contents of Wild Edible Mushroom Bovista nigrescens. Prospect. Pharm. Sci. 2023, 21, 37–41. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Sevindik, M. The novel biological tests on various extracts of Cerioporus varius. Fresenius Environ. Bull. 2019, 28, 3713–3717. [Google Scholar]
- Kalkan, M.; Aygan, A.; Çömlekçioglu, N.; Çömlekçioğlu, U. Investigation of Some Bioactive Properties and Antimicrobial Activity of Olea Europaea Leaves. Turk. J. Agric. -Food Sci. Technol. 2023, 11, 496–504. [Google Scholar]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Sevindik, M. Anticancer, antimicrobial, antioxidant and DNA protective potential of mushroom Leucopaxillus gentianeus (Quél.) Kotl. Indian J. Exp. Biol. (IJEB) 2021, 59, 310–315. [Google Scholar]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef]
- Gençalp Rüstem, D.; Aydin, H.H.; Kalmis, E.; Kayalar, H.; Ak, H. The effects of Hericium erinaceus extracts on cell viability and telomerase activity in MCF-7 cells. Turk. J. Biochem. 2023, 48, 298–302. [Google Scholar] [CrossRef]
- Konrath, E.L.; Passos, C.d.S.; Klein-Júnior, L.C.; Henriques, A.T. Alkaloids as a source of potential anticholinesterase inhibitors for the treatment of Alzheimer's disease. J. Pharm. Pharmacol. 2013, 65, 1701–1725. [Google Scholar] [CrossRef]
- Tel, G.; Ozturk, M.; Duru, M.E.; Turkoglu, A. Antioxidant and anticholinesterase activities of five wild mushroom species with total bioactive contents. Pharm. Biol. 2015, 53, 824–830. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Im, K.H.; Choi, J.; Shin, P.G.; Lee, T.S. Evaluation of antioxidant, anti-cholinesterase, and anti-inflammatory effects of culinary mushroom Pleurotus pulmonarius. Mycobiology 2016, 44, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Garrab, M.; Edziri, H.; El Mokni, R.; Mastouri, M.; Mabrouk, H.; Douki, W. Phenolic composition, antioxidant and anticholinesterase properties of the three mushrooms Agaricus silvaticus Schaeff., Hydnum rufescens Pers. and Meripilus giganteus (Pers.) Karst. in Tunisia. South Afr. J. Bot. 2019, 124, 359–363. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef]
- Sevindik, M.; Bal, C.; Eraslan, E.C.; Uysal, I.; Mohammed, F.S. Medicinal mushrooms: a comprehensive study on their antiviral potential. Prospect. Pharm. Sci. 2023, 21, 42–56. [Google Scholar] [CrossRef]
- Kim, M.-U.; Lee, E.-H.; Jung, H.-Y.; Lee, S.-Y.; Cho, Y.-J. Inhibitory activity against biological activities and antimicrobial activity against pathogenic bacteria of extracts from Hericium erinaceus. J. Appl. Biol. Chem. 2019, 62, 173–179. [Google Scholar] [CrossRef]
- Alkin, M.; Söğüt, E.; Seydim, A.C. Determination of bioactive properties of different edible mushrooms from Turkey. J. Food Meas. Charact. 2021, 15, 3608–3617. [Google Scholar] [CrossRef]
- Li, H.; Park, S.; Moon, B.; Yoo, Y.-B.; Lee, Y.-W.; Lee, C. Targeted phenolic analysis in Hericium erinaceum and its antioxidant activities. Food Sci. Biotechnol. 2012, 21, 881–888. [Google Scholar] [CrossRef]
- Kopylchuk, H.; Voloshchuk, O.; Pasailiuk, M. Comparison of total amino acid compositions, total phenolic compounds, total flavonoid content, β-carotene content and hydroxyl radical scavenging activity in four wild edible mushrooms. Ital. J. Mycol. 2023, 52, 112–125. [Google Scholar]
- Eraslan, E.C.; Altuntas, D.; Hayri, B.; Celal, B.; Akgül, H.; Akata, I.; Sevindik, M. Some biological activities and element contents of ethanol extract of wild edible mushroom Morchella esculenta. Sigma J. Eng. Nat. Sci. 2021, 39, 24–28. [Google Scholar]
- Krupodorova, T.; Sevindik, M. Antioxidant potential and some mineral contents of wild edible mushroom Ramaria stricta. AgroLife Sci. J. 2020, 9. [Google Scholar]
- Korkmaz, A.İ.; Bal, C.; Eraslan, E.C.; Sevindik, M.; Akgul, H. Biological activities of Agrocybe praecox (spring fieldcap mushroom). Prospect. Pharm. Sci. 2023, 21, 33–39. [Google Scholar] [CrossRef]
- Karaltı, İ.; Eraslan, E.C.; Sarıdoğan, B.G.Ö.; Akata, I.; Sevindik, M. Total Antioxidant, Antimicrobial, Antiproliferative Potentials and Element Contents of Wild Mushroom Candolleomyces candolleanus (Agaricomycetes) from Turkey. Int. J. Med. Mushrooms 2022, 24, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bal, C.; Baba, H.; Akata, I.; Sevindik, M.; Selamoglu, Z.; Akgül, H. Biological Activities of Wild Poisonous Mushroom Entoloma sinuatum (Bull.) P. Kumm (Boletales). KSU J. Agric. Nat 2022, 25, 83–87. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. The Addition of Reishi and Lion’s Mane Mushroom Powder to Pasta Influences the Content of Bioactive Compounds and the Antioxidant, Potential Anti-Inflammatory, and Anticancer Properties of Pasta. Antioxidants 2023, 12, 738. [Google Scholar] [CrossRef]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Antibiofilm activity and bioactive phenolic compounds of ethanol extract from the Hericium erinaceus basidiome. J. Adv. Pharm. Technol. Res. 2022, 13, 111. [Google Scholar] [CrossRef]
| Extraction temperature ˚C |
Extraction time (h) |
Extract concentration (mg/mL) | TAS (mmol trolox equivalent/L) |
| 40 | 3 | 0.25 | 2.799±0.233 |
| 0.5 | 3.127±0.121 | ||
| 1 | 3.556±0.074 | ||
| 2 | 4.011±0.173 | ||
| 5 | 0.25 | 3.297±0.106 | |
| 0.5 | 3.683±0.115 | ||
| 1 | 4.149±0.119 | ||
| 2 | 4.465±0.212 | ||
| 7 | 0.25 | 4.159±0.186 | |
| 0.5 | 4.480±0.141 | ||
| 1 | 4.965±0.076 | ||
| 2 | 5.325±0.125 | ||
| 9 | 0.25 | 3.355±0.139 | |
| 0.5 | 3.759±0.115 | ||
| 1 | 3.979±0.086 | ||
| 2 | 4.356±0.205 | ||
| 50 | 3 | 0.25 | 3.433±0.088 |
| 0.5 | 3.612±0.059 | ||
| 1 | 3.746±0.066 | ||
| 2 | 3.803±0.080 | ||
| 5 | 0.25 | 3.624±0.033 | |
| 0.5 | 3.782±0.073 | ||
| 1 | 4.234±0.066 | ||
| 2 | 4.535±0.070 | ||
| 7 | 0.25 | 4.260±0.072 | |
| 0.5 | 4.530±0.119 | ||
| 1 | 4.907±0.064 | ||
| 2 | 5.254±0.122 | ||
| 9 | 0.25 | 3.475±0.049 | |
| 0.5 | 3.846±0.103 | ||
| 1 | 4.058±0.084 | ||
| 2 | 4.374±0.063 | ||
| 60 | 3 | 0.25 | 3.651±0.118 |
| 0.5 | 3.851±0.089 | ||
| 1 | 4.086±0.142 | ||
| 2 | 4.439±0.105 | ||
| 5 | 0.25 | 3.882±0.098 | |
| 0.5 | 4.214±0.168 | ||
| 1 | 4.725±0.158 | ||
| 2 | 5.020±0.113 | ||
| 7 | 0.25 | 4.731±0.091 | |
| 0.5 | 5.035±0.061 | ||
| 1 | 5.221±0.093 | ||
| 2 | 5.323±0.109 | ||
| 9 | 0.25 | 3.950±0.102 | |
| 0.5 | 4.152±0.094 | ||
| 1 | 4.404±0.046 | ||
| 2 | 4.594±0.090 | ||
| 70 | 3 | 0.25 | 2.038±0.096 |
| 0.5 | 2.301±0.107 | ||
| 1 | 2.496±0.059 | ||
| 2 | 2.623±0.118 | ||
| 5 | 0.25 | 2.344±0.127 | |
| 0.5 | 2.619±0.089 | ||
| 1 | 2.791±0.059 | ||
| 2 | 3.010±0.119 | ||
| 7 | 0.25 | 2.643±0.063 | |
| 0.5 | 2.906±0.095 | ||
| 1 | 3.128±0.051 | ||
| 2 | 3.282±0.074 | ||
| 9 | 0.25 | 2.256±0.059 | |
| 0.5 | 2.407±0.064 | ||
| 1 | 2.638±0.092 | ||
| 2 | 2.717±0.110 |
| AChE (μg/mL) | BChE (μg/mL) | |
| Ethanol | 13.85±0.94b | 28.00±0.89b |
| Galantamine | 6.21±0.62a | 25.01±0.43a |
| S. aureus | S. aureus MRSA | E. faecalis | E. coli | P. aeruginosa | A. baumannii | C. glabrata | C. albicans | C. krusei | |
|---|---|---|---|---|---|---|---|---|---|
| Ethanol extract | 25 | 25 | 50 | 100 | 100 | 100 | 100 | 50 | 100 |
| Ampicillin | 1.56 | 3.12 | 1.56 | 3.12 | 3.12 | - | - | - | - |
| Amikacin | - | - | - | 1.56 | 3.12 | 3.12 | - | - | - |
| Ciprofloksasin | 1.56 | 3.12 | 1.56 | 1.56 | 3.12 | 3.12 | - | - | - |
| Flukanazol | - | - | - | - | - | - | 3.12 | 3.12 | - |
| Amfoterisin B | - | - | - | - | - | - | 3.12 | 3.12 | 3.12 |
| TAS (mmol/L) | 5.426±0.123 |
| TOS (µmol/L) | 6.621±0.197 |
| OSI (TOS/(TAS*10)) | 0.122±0.003 |
| TPC (mg/g) | 59.75±1.82 |
| DPPH (mg Trolox Equi/g) | 73.36±2.04 |
| FRAP (mg Trolox Equi/g) | 107.66±2.41 |
| Phenolic compounds | Values (ppb) |
|---|---|
| Acetohydroxamic acid | 2464.15 |
| Catechinhyrate | 17546.53 |
| Vanillic acid | none |
| Syringic acid | None |
| Thymoquinone | None |
| Resveratrol | 9459.3 |
| Myricetin | 1361 |
| Kaempferol | None |
| Fumaric acid | 9393.41 |
| Gallic acid | 92003.93 |
| Protocatechuic acid | 3848.8 |
| 4-hydroxybenzoic acid | 408.74 |
| Caffeic acid | None |
| Salicylic acid | None |
| Phloridzindyhrate | 230.83 |
| 2-hydoxycinamic acid | 940.18 |
| Oleuropein | None |
| 2-hyroxy1,4 naphthaquinone | None |
| Naringenin | 426.01 |
| Silymarin | None |
| Quercetin | 2342.81 |
| Luteolin | 444.83 |
| Alizarin | None |
| Curmin | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





