Submitted:
18 March 2024
Posted:
19 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
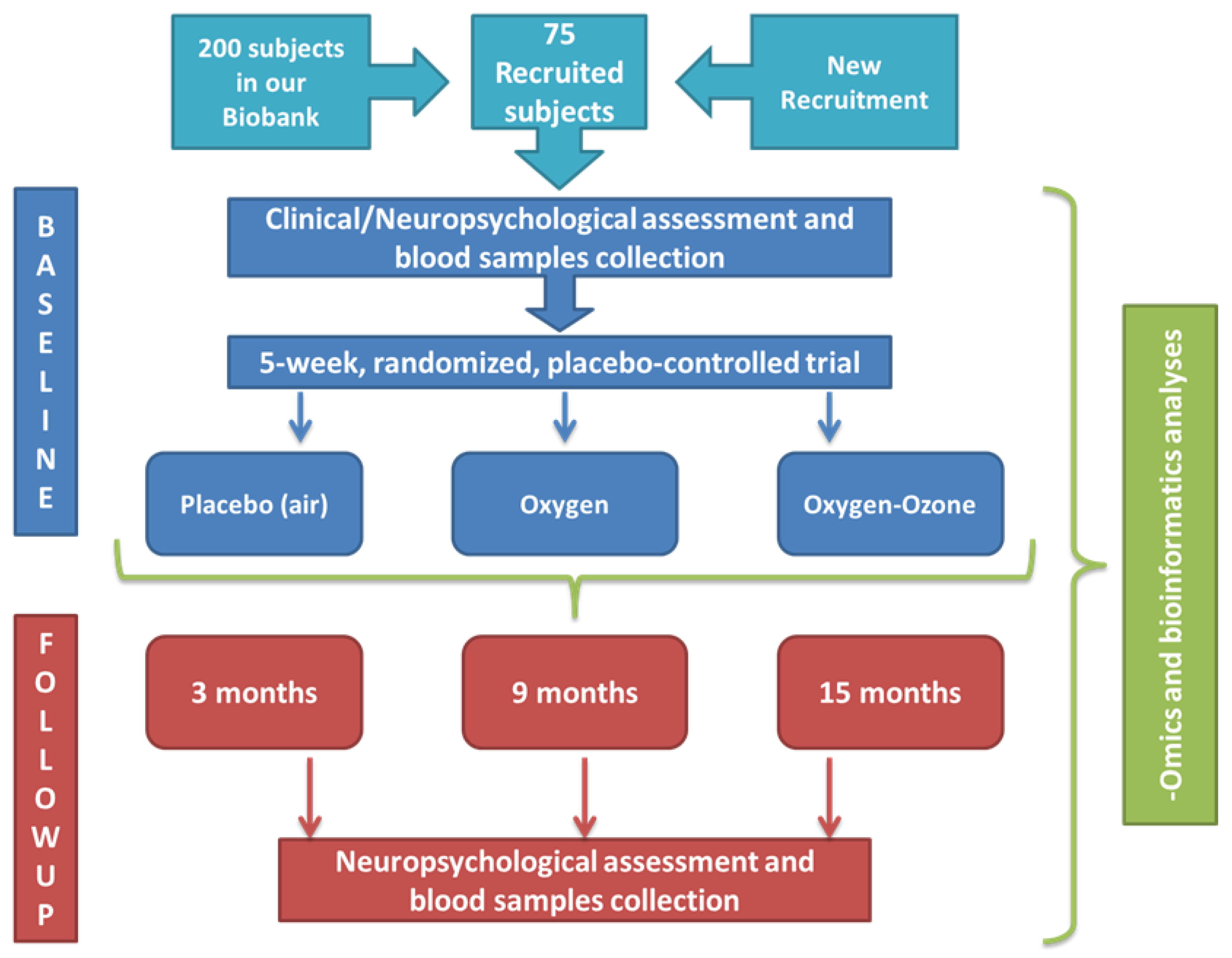
2. Materials and Methods
2.1. Trial Design and Setting
2.2. Ethics Approval
2.3. Inclusion and Exclusion Criteria
2.4. Study Schedule
2.5. Adaptive Randomization
2.6. Compliance and Adverse Effects
2.7. Primary and Secondary Endpoints
2.8. Intervention
2.9. Data Collection
2.10. Data Management
2.11. Data Monitoring
2.12. Statistical Analysis
2.13. Dissemination
3. Results
3.1. Sociodemographic, Anthropometric Features and State of Health
3.2. Clinical and Neuropsychological Features for Frailty Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: an overview. Clin Interv Aging 2014, 9, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Michel, J.; Zekry, D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009, 55, 539–549. [Google Scholar] [CrossRef]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev 2013, 12, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Gan, P.; How, C.H. Approach to frailty in the elderly in primary care and the community. Singapore Med J 2018, 59, 240–245. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Yang, M.; Ge, S.; Cheng, S.; Wang, C.; Zhang, W.; Tian, C.; Mao, J. Apathy co-occurs with subjective cognitive decline among community-dwelling older adults. Geriatr Nurs 2022, 48, 177–182. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Fabricio, D.d.M.; Chagas, M.H.N.; Diniz, B.S. Frailty and cognitive decline. Transl Res 2020, 221, 58–64. [Google Scholar] [CrossRef]
- Yoon, D.H.; Hwang, S.S.; Lee, D.W.; Lee, C.G.; Song, W. Physical Frailty and Cognitive Functioning in Korea Rural Community-Dwelling Older Adults. J Clin Med 2018, 7, 405. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; Nourhashemi, F.; Salva, A.; Robert, P.; Andrieu, S.; Rolland, Y.; Touchon, J.; Fitten, J.L.; Vellas, B. ; IANA/IAGG Cognitive frailty: rational and definition from an (I. A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Rivan, N.F.M.; Shahar, S.; Rajab, N.F.; Singh, D.K.A.; Che Din, N.; Mahadzir, H.; Mohamed Sakian, N.I.; Ishak, W.S.; Abd Rahman, M.H.; Mohammed, Z.; You, Y.X. Incidence and Predictors of Cognitive Frailty Among Older Adults: A Community-based Longitudinal Study. Int J Environ Res Public Health 2020, 17, 1547. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Di Lena, L.; D'Urso, F.; Stallone, R.; Petruzzi, M.; Giannelli, G.; Quaranta, N.; Bellomo, A.; Greco, A.; Daniele, A.; Seripa, D.; Logroscino, G. Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J Alzheimers Dis 2018, 62, 993–1012. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, H.; Du, X.; Yin, L.; Zhang, H.; Zhou, Q. Comparison of the prevalence and associated factors of cognitive frailty between elderly and middle-young patients receiving maintenance hemodialysis. Int Urol Nephrol 2022, 54, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Wilson, R.S.; Tang, Y.; Bennett, D.A. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med 2007, 69, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc 2010, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, G.; Wang, X.; Zheng, L.; Wang, C.; Wang, C.; Chen, L. Prevalence of cognitive frailty among community-dwelling older adults: A systematic review and meta-analysis. Int J Nurs Stud 2022, 125, 104112. [Google Scholar] [CrossRef]
- Sugimoto, T.; Arai, H.; Sakurai, T. An update on cognitive frailty: Its definition, impact, associated factors and underlying mechanisms, and interventions. Geriatr Gerontol Int 2022, 22, 99–109. [Google Scholar] [CrossRef]
- Pothier, K.; Gana, W.; Bailly, N.; Fougere, B. Associations Between Frailty and Inflammation, Physical, and Psycho-Social Health in Older Adults: A Systematic Review. Front Psychol 2022, 13, 805501. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid Med Cell Longev 2020, 2020, 9829176. [Google Scholar] [CrossRef]
- Baranova, I.V.; Bezsmertnyi, Y.A.; Bezsmertnaya, H.V.; Postovitenko, K.P.; Iliuk, I.A.; Gumeniuk, A.F. Analgetic effect of ozone therapy: Myths of reality? Polish Annals of Medicine 2020, 27, 62–67. [Google Scholar]
- Masan, J.; Sramka, M.; Rabarova, D. The possibilities of using the effects of ozone therapy in neurology. Neuro Endocrinol Lett 2021, 42, 13–21. [Google Scholar]
- Galie, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The Role of Nrf2 in the Antioxidant Cellular Response to Medical Ozone Exposure. Int J Mol Sci 2019, 20, 4009. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: an overview of pharmacodynamics, current research, and clinical utility. Med Gas Res 2017, 7, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Haensler, R.; Leon Fernandez, O.S. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int J Mol Sci 2021, 22, 7890. [Google Scholar] [CrossRef]
- Safwat, M.H.; El-Sawalhi, M.M.; Mausouf, M.N.; Shaheen, A.A. Ozone ameliorates age-related oxidative stress changes in rat liver and kidney: effects of pre- and post-ageing administration. Biochemistry (Mosc) 2014, 79, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ma, J.; An, J.; Qian, X.; Wang, Y.; Cope, D.K.; Williams, J.P. Ozone Inhibits APP/Abeta Production and Improves Cognition in an APP/PS1 Transgenic Mouse Model. Neuroscience 2019, 418, 110–121. [Google Scholar] [CrossRef] [PubMed]
- El-Sawalhi, M.M.; Darwish, H.A.; Mausouf, M.N.; Shaheen, A.A. Modulation of age-related changes in oxidative stress markers and energy status in the rat heart and hippocampus: a significant role for ozone therapy. Cell Biochem Funct 2013, 31, 518–525. [Google Scholar] [CrossRef]
- Shehata, N.I.; Abd-Elgawad, H.M.; Mawsouf, M.N.; Shaheen, A.A. The potential role of ozone in ameliorating the age-related biochemical changes in male rat cerebral cortex. Biogerontology 2012, 13, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Abete, P.; Basile, C.; Bulli, G.; Curcio, F.; Liguori, I.; Della-Morte, D.; Gargiulo, G.; Langellotto, A.; Testa, G.; Galizia, G.; Bonaduce, D.; Cacciatore, F. The Italian version of the "frailty index" based on deficits in health: a validation study. Aging Clin Exp Res 2017, 29, 913–926. [Google Scholar] [CrossRef]
- Romero-Ortuno, R. The Frailty Instrument of the Survey of Health, Ageing and Retirement in Europe (SHARE-FI) predicts mortality beyond age, comorbidities, disability, self-rated health, education and depression. Eur Geriatr Med 2011, 2, 323–326. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Petesi, M.; Rosini, S.; Ferrari, C.; Zanetti, O.; Miniussi, C. Anodal tDCS during face-name associations memory training in Alzheimer's patients. Front Aging Neurosci 2014, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- De Renzi, E.; Motti, F.; Nichelli, P. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol 1980, 37, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Arisi, I.; Malimpensa, L.; Manzini, V.; Brandi, R.; Gosetti di Sturmeck, T.; D'Amelio, C.; Crisafulli, S.; Ferrazzano, G.; Belvisi, D.; Malerba, F.; Florio, R.; Pascale, E.; Soreq, H.; Salvetti, M.; Cattaneo, A.; D'Onofrio, M.; Conte, A. Cladribine and ocrelizumab induce differential miRNA profiles in peripheral blood mononucleated cells from relapsing-remitting multiple sclerosis patients. Front Immunol 2023, 14, 1234869. [Google Scholar] [CrossRef] [PubMed]
- Caputo, V.; Pacilli, M.G.; Arisi, I.; Mazza, T.; Brandi, R.; Traversa, A.; Casasanta, G.; Pisa, E.; Sonnessa, M.; Healey, B.; Moggio, L.; D'Onofrio, M.; Alleva, E.; Macri, S. Genomic and physiological resilience in extreme environments are associated with a secure attachment style. Transl Psychiatry 2020, 10, 185–4. [Google Scholar] [CrossRef] [PubMed]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J Nat Sci Biol Med 2011, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Re Lamberto; Malcangi Giuseppe; Martinez-Sanchez Gregorio Medical ozone is now ready for a scientific challenge: Current status and future perspectives. Journal of Experimental and Integrative Medicine 2012, 2, 193–196. [CrossRef]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: a natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res Rev 2020, 63, 101138. [Google Scholar] [CrossRef]
- Bocci Velio OZONE: A new medical drug, Springer Dordrecht: 2010; pp. XXI, 315.
- Schwartz, A. ; Gregorio Martínez Sánchez; Cum, L.; Roberto Quintero; Mccarthy, W.; Moreno, O. MADRID DECLARATION ON OZONE THERAPY (3 rd edition) Official document of ISCO3.
- Facal, D.; Burgo, C.; Spuch, C.; Gaspar, P.; Campos-Magdaleno, M. Cognitive Frailty: An Update. Front Psychol 2021, 12, 813398. [Google Scholar] [CrossRef]
- He, W.; Luo, Y.; Liu, J.; Sun, N.; Guo, D.; Cui, L.; Zheng, P.; Yao, S.; Yang, J.; Wang, H. Trimethylamine N-Oxide, a Gut Microbiota-Dependent Metabolite, is Associated with Frailty in Older Adults with Cardiovascular Disease. Clin Interv Aging 2020, 15, 1809–1820. [Google Scholar] [CrossRef]
- Rietman, M.L.; Hulsegge, G.; Nooyens, A.C.J.; Dolle, M.E.T.; Picavet, H.S.J.; Bakker, S.J.L.; Gansevoort, R.T.; Spijkerman, A.M.W.; Verschuren, W.M.M. Trajectories of (Bio)markers During the Development of Cognitive Frailty in the Doetinchem Cohort Study. Front Neurol 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.R.; Palmer, R.F. ; Alzheimer's Disease Neuroimaging Initiative Blood-based protein mediators of senility with replications across biofluids and cohorts. Brain Commun 2019, 2, fcz036. [Google Scholar] [CrossRef] [PubMed]
- Sargent, L.; Nalls, M.; Amella, E.J.; Slattum, P.W.; Mueller, M.; Bandinelli, S.; Tian, Q.; Swift-Scanlan, T.; Lageman, S.K.; Singleton, A. Shared mechanisms for cognitive impairment and physical frailty: A model for complex systems. Alzheimers Dement (N Y) 2020, 6, e12027. [Google Scholar] [CrossRef] [PubMed]
| Quantitative Variables | F n=50 |
M n=26 |
All n=76 |
χ2 Test |
Comparison gender column proportions |
|---|---|---|---|---|---|
| Demography | |||||
| Age in years [Mean±SD (Min-Max)] | |||||
| 72.4±6.2 (60 - 85) | 71.6±7.1 (60 - 82) | 72.2±6.5 (60 - 85) | ns | ||
| Education [n, %] | |||||
| <9 | 20 (41.7) | 4 (16.0) | 24 (32.9) | 0.03 | |
| ≥9 and <14 | 18 (37.5) | 18 (72.0) | 36 (49.3) | 0.02 | 0.005 |
| ≥14 | 10 (20.8) | 3 (12.0) | 13 (17.8) | ||
| Marital status [n, %] | |||||
| widowed | 12 (27.3) | 4 (16.0) | 16 (23.2) | ||
| not married/single | 6 (13.6) | 4 (16.0) | 10 (14.5) | ns | |
| married | 26 (59.1) | 17 (65.4) | 43 (62.3) | ||
| Living status [n, %] | |||||
| Alone | 25 (50.0) | 9 (33.3) | 34 (44.7) | ns | |
| with family | 25 (50.0) | 17 (65.4) | 42 (55.3) | ||
| C-reactive Protein (CRP) (mg/L) [Mean±SD] | |||||
| 0.0019±0.003 | 0.0011±0.0011 | 0.0017±0.0025 | ns | ||
| Diseases | |||||
| CIRS [n, %] | |||||
| <=6 | 36 (72.0) | 20 (76.9) | 56 (73.7) | ns | |
| >6 | 14 (298.0) | 6 (23.1) | 20 (26.3) | ||
| QTCL [n, %] | |||||
| no | 4 (8.3) | 2 (7.7) | 6 (8.1) | ns | |
| not known | 44 (91.7) | 24 (92.3) | 68 (91.9) | ||
| Numbers medications taken [n, %] | |||||
| <4 | 31 (62.0) | 15 (57.7) | 46 (60.5) | ns | |
| ≥4 | 19 (38.0) | 11 (42.3) | 30 (39.5) | ||
| hypertension [n, %] | |||||
| no | 27 (54.0) | 11 (42.3) | 38 (50.0) | ||
| mild | 2 (4.0) | 0 (0.0) | 2 (2.6) | ns | |
| yes | 21 (42.0) | 15 (57.7) | 36 (47.4) | ||
| angina pectoris [n, %] | |||||
| no | 49 (98.0) | 23 (92.0) | 72 (96.0) | ||
| mild | 1 (2.0) | 1 (4.0) | 2 (2.7) | ns | |
| yes | 0 (0.0) | 1 (4.0) | 1 (1.3) | ||
| heart diseases, pacemaker [n, %] | |||||
| no | 46 (92.0) | 23 (88.5) | 69 (90.8) | ||
| mild | 2 (4.0) | 1 (3.8) | 3 (3.9) | ns | |
| yes | 2 (4.0) | 2 (7.7) | 4 (5.3) | ||
| stroke, cerebral ischemia [n, %] | |||||
| no | 43 (87.8) | 24 (92.3) | 67 (89.3) | ||
| mild | 2 (4.1) | 2 (7.7) | 4 (5.3) | ns | |
| yes | 4 (8.2) | 0 (0.0) | 4 (5.3) | ||
| cancer [n, %] | |||||
| no | 39 (78.0) | 22 (84.6) | 61 (80.3) | ||
| probable | 1 (2.0) | 0 (0.0) | 1 (1.3) | ns | |
| yes | 10 (20.0) | 4 (15.4) | 14 (18.4) | ||
| diabetes [n, %] | |||||
| no | 43 (86.0) | 22 (84.6) | 65 (85.5) | ||
| mild | 1 (2.0) | 1 (3.8) | 2 (2.6) | ns | |
| yes | 6 (12.0) | 3 (11.5) | 9 (11.8) | ||
| osteoarthritis and osteoporosis [n, %] | |||||
| no | 16 (32.0) | 19 (73.1) | 35 (46.1) | 0.001 | |
| probable | 2 (4.0) | 2 (7.7) | 4 (5.3) | 0.001 | |
| yes | 32 (64.0) | 7 (19.2) | 37 (48.7) | <0.001 | |
| chronic bronchitis | |||||
| no | 46 (92.0) | 23 (88.5) | 69 (9.08) | ||
| mild | 3 (6.0) | 1 (3.8) | 4 (5.3) | ns | |
| yes | 1 (2.0) | 2 (7.7) | 3 (3.9) | ||
| thyroid or other endocrine diseases [n, %] | |||||
| no | 40 (80.0) | 25 (96.2) | 65 (85.5) | ||
| mild | 1 (2.0) | 0 (0.0) | 1 (1.3) | ns | |
| yes | 9 (18.0) | 1 (3.8) | 10 (13.2) | ||
| kidney diseases [n, %] | |||||
| no | 49 (98.0) | 24 (92.3) | 73 (96.1) | ||
| mild | 1 (2.0) | 0 (0.0) | 1 (1.3) | ns | |
| yes | 0 (0.0) | 2 (7.7) | 2 (2.6) | ||
| liver/gallbladder disease [n, %] | |||||
| no | 40 (80.0) | 20 (76.9) | 60 (78.9) | ||
| mild | 1 (2.0) | 0 (0.0) | 1 (1.3) | ns | |
| yes | 9 (18.0) | 6 (23.1) | 15 (19.7) | ||
| intestinal diseases [n, %] | |||||
| no | 32 (64.0) | 23 (88.5) | 55 (72.4) | 0.02 | |
| mild | 2 (4.0) | 1 (3.8) | 3 (3.9) | 0.06 | |
| yes | 16 (32.0) | 2 (7.7) | 18 (23.7) | 0.02 | |
| stomach and gullet diseases [n, %] | |||||
| no | 31 (62.0) | 20 (76.9) | 51 (67.1) | ||
| mild | 1 (2.0) | 1 (3.8) | 2 (2.6) | ns | |
| yes | 18 (36.0) | 5 (19.2) | 23 (30.3) | ||
| skin diseases [n, %] | |||||
| no | 48 (96.0) | 23 (88.5) | 71 (93.4) | ||
| probable | 1 (2.0) | 0 (0.0) | 1 (1.3) | ns | |
| yes | 1 (2.0) | 3 (11.5) | 4 (5.1) | ||
| blood diseases [n, %] | |||||
| no | 46 (92.0) | 25 (96.2) | 71 (93.4) | ||
| mild | 0 (0.0) | 1 (3.8) | 1 (1.3) | ns | |
| yes | 4 (8.0) | 0 (0.0) | 4 (5.3) | ||
| vascular diseases [n, %] | |||||
| no | 43 (87.8) | 22 (84.6) | 65 (86.7) | ||
| mild | 1 (2.0) | 0 (0.0) | 1 (1.3) | ns | |
| yes | 5 (10.2) | 4 (15.4) | 9 (12.0) | ||
| eye diseases with low vision [n, %] | |||||
| no | 42 (84.0) | 23 (88.5) | 65 (85.5) | ns | |
| yes | 8 (16.0) | 3 (11.5) | 11 (14.5) | ||
| ear diseases with hearing loss [n, %] | |||||
| no | 38 (76.0) | 11 (42.3) | 49 (64.5) | ||
| mild | 4 (8.0) | 6 (23.1) | 10 (13.2) | 0.01 | |
| yes | 8 (16.0) | 9 (34.6) | 17 (22.4) | ||
| peripheral neuropathies [n, %] | |||||
| no | 47 (94.0) | 26 (100.0) | 73 (96.1) | ns | |
| yes | 3 (6.0) | 0 (0.0) | 3 (3.9) | ||
| psychiatric diseases [n, %] | |||||
| no | 49 (98.0) | 26 (100.0) | 75 (98.7) | ns | |
| yes | 1 (2.0) | 0 (0.0) | 1 (1.3) | ||
| Glucose-6-phosphate dehydrogenase deficiency [n, %] | |||||
| no | 50 (100.0) | 26 (100.0) | 76 (100.0) | ns | |
| uncontrolled hyperthyroidism [n, %] | |||||
| no | 48 (98.0) | 26 (100.0) | 74 (98.7) | ns | |
| yes | 1 (2.0) | 0 (0.0) | 1 (1.3) | ||
| alcohol or drug abuse [n, %] | |||||
| no | 50 (100.0) | 26 (100.0) | 76 (100.0) | ns | |
| Anthropometry and state of health | |||||
| Body mass index (Kg/m2) [Mean±SD (Min-Max)] | |||||
| 27.3±5.5 (18.3 - 41.9) | 27.8±3.7 (19.9 - 35.0) | 27.4±5.0 (18.3 - 41.9) | |||
| Brachial girth (cm) [Mean±SD (Min-Max)] | |||||
| right | 30.2±4.1 (24 - 43) | 30.2±3.5 (24 - 37) | 30.2±3.9 (24 - 43) | ||
| left | 30.0±3.9 (23 - 41) | 29.6±3.5 (24 - 37) | 29.9±3.7 (23 - 41) | ||
| Calf girth (cm) [Mean±SD (Min-Max)] | |||||
| right | 36.7±4.4 (31 - 55) | 38.3±4.6 (32.5 - 52) | 37.3±4.5 (31 - 55) | ||
| left | 36.6±4.7 (30 - 56) | 38.1±3.9 (31.5 - 52) | 37.1±4.5 (30 - 56) | ||
| Hand grip test (Kg) [Mean±SD (Min-Max)] | |||||
| right | 21.2±5.5 (10.1 – 42.3) | 34.8±10.4 (12.0 - 59.5) | 25.9±9.9 (10.1 - 59.5) | <0.001 | |
| left | 19.6±5.2 (9.0 – 35.2) | 32.8±9.0 (15.0 - 50.8) | 24.1±9.2 (9.0 - 50.8) | <0.001 | |
| Peak expiratory flow (L/min) [Mean±SD (Min-Max)] | |||||
| 208.1±76.7 (65 - 355) | 356.2±132.4 (140 - 690) | 259.4±121.5 (65 - 690) | <0.001 | ||
| Timed up-and-go test (s) [Mean±SD (Min-Max)] | |||||
| 3.4±0.9 (2.2 - 6.2) | 2.9±0.5 (2.02 - 4.3) | 3.2±0.8 (2.02 - 6.2) | 0.007 | ||
| Weightlifting 1 (8 Kg females ; 11Kg males) [n, %] | |||||
| not lifted | 1 (2.0) | 2 (7.7) | 3 (3.9) | ns | |
| lifted up | 47 (98.0) | 24 (92.3) | 73 (96.1) | ||
| Weightlifting 2 (9 Kg females ; 12Kg males) [n, %] | |||||
| not lifted | 2 (4.0) | 2 (7.7) | 4 (5.3) | ns | |
| lifted up | 48 (96.0) | 24 (92.3) | 72 (94.7) | ||
| Weightlifting 3 (10 Kg females ; 13Kg males) [n, %] | |||||
| not lifted | 3 (6.3) | 2 (7.7) | 5 (6.6) | ns | |
| lifted up | 45 (93.8) | 24 (92.3) | 71 (93.4) | ||
| Clinical Dementia Rating Scale (CDR) [Mean±SD (Min-Max)] | |||||
| 0.08±0.33 (0.0 – 2.0) | 0.24±1.20 (0.0 – 6.0) | 0.14±0.75 (0.0 – 6.0) | |||
| Tinetti Scale [Mean±SD (Min-Max)] | |||||
| 27.2±1.6 (21 - 28) | 27.3±1.9 (20 - 28) | 27.3±1.7 (20 - 28) | |||
| Barthel Index [Mean±SD (Min-Max)] | |||||
| 0.9±1.5 (0 - 8) | 0.5±0.9 (0 - 2) | 0.8±1.3 (0 - 8) | |||
| Instrumental Activities of Daily living (IADL) [Mean±SD (Min-Max)] | |||||
| 0.04±0.2 (0 - 1) | 0.0±0.0 (0 - 0) | 0.03±0.2 (0 - 1) | |||
| Severity Index (SI-CIRS) [Mean±SD (Min-Max)] | |||||
| 1.4±0.2 (1 - 2.2) | 1.4±0.2 (1 - 1.9) | 1.4±0.2 (1 - 2.2) | |||
| Comorbidity Index (CI-CIRS) [Mean±SD (Min-Max)] | |||||
| 1.8±1.2 (0 - 5) | 1.9±1.4 (0 - 5) | 1.8±1.2 (0 - 5) |
| Quantitative Variables | F n=50 |
M n=26 |
All n=76 |
χ2 Test |
Comparison gender column proportions |
|
|---|---|---|---|---|---|---|
| IFI [Mean±SD (Min-Max)] | ||||||
| 7.17±3.75 (1.75 – 19.50) |
5.80±2.87 (0.50 – 12.75) |
6.70±3.52 (0.50 – 19.50) |
||||
| SHARE-FIt [Mean±SD (Min-Max)] | ||||||
| 0.97±1.51 (-1.63 / 4.41) |
0.54±1.51 (-1.19 / 5.32) |
0.83±1.52 (-1.63 / 5.32) |
||||
| SHARE-FIt_clusters [n, %] | ||||||
| NF | 20 (40.0) | 20 (76.9) | 40 (52.6) | 0.002 | ||
| PF | 18 (36.0) | 4 (15.4) | 22 (28.9) | 0.009 | ||
| F | 12 (24.0) | 2 (7.7) | 14 (18.4) | |||
| CDR [Mean±SD (Min-Max)] | ||||||
| 0.08±0.33 (0.0 – 2.0) |
0.24±1.20 (0.0 – 6.0) |
0.14±0.75 (0.0 – 6.0) | ||||
| Females | Males | All | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| MMSE | 28.75 | 1.34 | 28.46 | 1.84 | 28.65 | 1.52 |
| GDS | 5.85 | 5.51 | 5.04 | 5.28 | 5.58 | 5.41 |
| EMQ | 52.00 | 20.38 | 50.13 | 19.63 | 51.38 | 20.01 |
| CRI-q Total score | 119.71 | 20.51 | 124.00 | 14.94 | 121.14 | 18.84 |
| Raven’s colored progressive matrices | 27.13 | 5.73 | 28.08 | 7.79 | 27.44 | 6.45 |
| Story Recall | 12.60 | 4.04 | 11.45 | 4.15 | 12.22 | 4.08 |
| RAVLT, immediate recall | 44.17 | 11.03 | 39.25 | 8.95 | 42.53 | 10.58 |
| RAVLT, delayed recall | 9.35 | 3.06 | 7.88 | 3.13 | 8.86 | 3.14 |
| FCSRT – Immediate free recall (IFR) | 29.63 | 4.85 | 27.25 | 7.33 | 28.83 | 5.86 |
| FCSRT – Immediate total recall (ITR) | 35.60 | 1.61 | 34.33 | 5.88 | 35.18 | 3.64 |
| FCSRT – delayed free recall (DFR) | 10.48 | 2.19 | 9.63 | 2.39 | 10.19 | 2.28 |
| FCSRT – delayed total recall (DTR) | 11.85 | 0.87 | 11.50 | 2.06 | 11.74 | 1.38 |
| FCSRT – index of sensitivity of cueing (ISC) | 0.96 | 0.10 | 0.91 | 0.19 | 0.94 | 0.14 |
| ROCF - Recall | 13.14 | 5.03 | 16.27 | 7.35 | 14.18 | 6.04 |
| Digit Span Forward | 5.35 | 1.12 | 5.67 | 1.17 | 5.46 | 1.14 |
| Digit Span Backward | 4.19 | 1.04 | 4.33 | 1.20 | 4.24 | 1.09 |
| FNAT (% of correct responses) | 12.48 | 3.44 | 11.88 | 3.07 | 12.28 | 3.31 |
| Token Test | 32.48 | 2.20 | 32.69 | 2.71 | 32.55 | 2.36 |
| FPL | 36.60 | 9.12 | 33.21 | 10.31 | 35.47 | 9.60 |
| FPC | 40.96 | 9.26 | 41.58 | 11.58 | 41.17 | 10.02 |
| B.A.D.A. – Auditory sentence comprehension | 57.42 | 2.47 | 58.50 | 1.72 | 57.78 | 2.30 |
| B.A.D.A. – Action naming | 15.02 | 2.48 | 15.79 | 2.69 | 15.29 | 2.56 |
| B.A.D.A. – Objects naming | 17.09 | 2.28 | 18.00 | 1.35 | 17.40 | 2.05 |
| ROCF - Copy | 31.28 | 3.27 | 31.60 | 3.57 | 31.39 | 3.35 |
| De Renzi test,right upper limb | 68.67 | 3.98 | 68.33 | 3.96 | 68.56 | 3.95 |
| De Renzi test_left upper limb | 69.98 | 3.06 | 70.13 | 2.59 | 70.03 | 2.89 |
| TMT_A (sec) | 46.46 | 22.14 | 40.54 | 12.98 | 44.49 | 19.67 |
| TMT_B (sec) | 162.31 | 98.21 | 161.08 | 143.49 | 161.90 | 114.26 |
| TMT_B-A (sec) | 115.85 | 93.04 | 120.54 | 137.56 | 117.42 | 108.93 |
| TMT_B/A | 3.69 | 1.80 | 3.84 | 2.77 | 3.74 | 2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





