Submitted:
15 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis
2.3. Preparation of PIMs and Stability Test
2.4. Transport Studies
2.5. Characteristics of Developed Polymer Inclusion Membranes (PIMs)
2.5.1. Morphology
2.5.2. Analysis of Thermal Properties: Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA) of PIMs
2.5.3. Analysis of Structure: Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) of PIMs
3. Results and Discussion
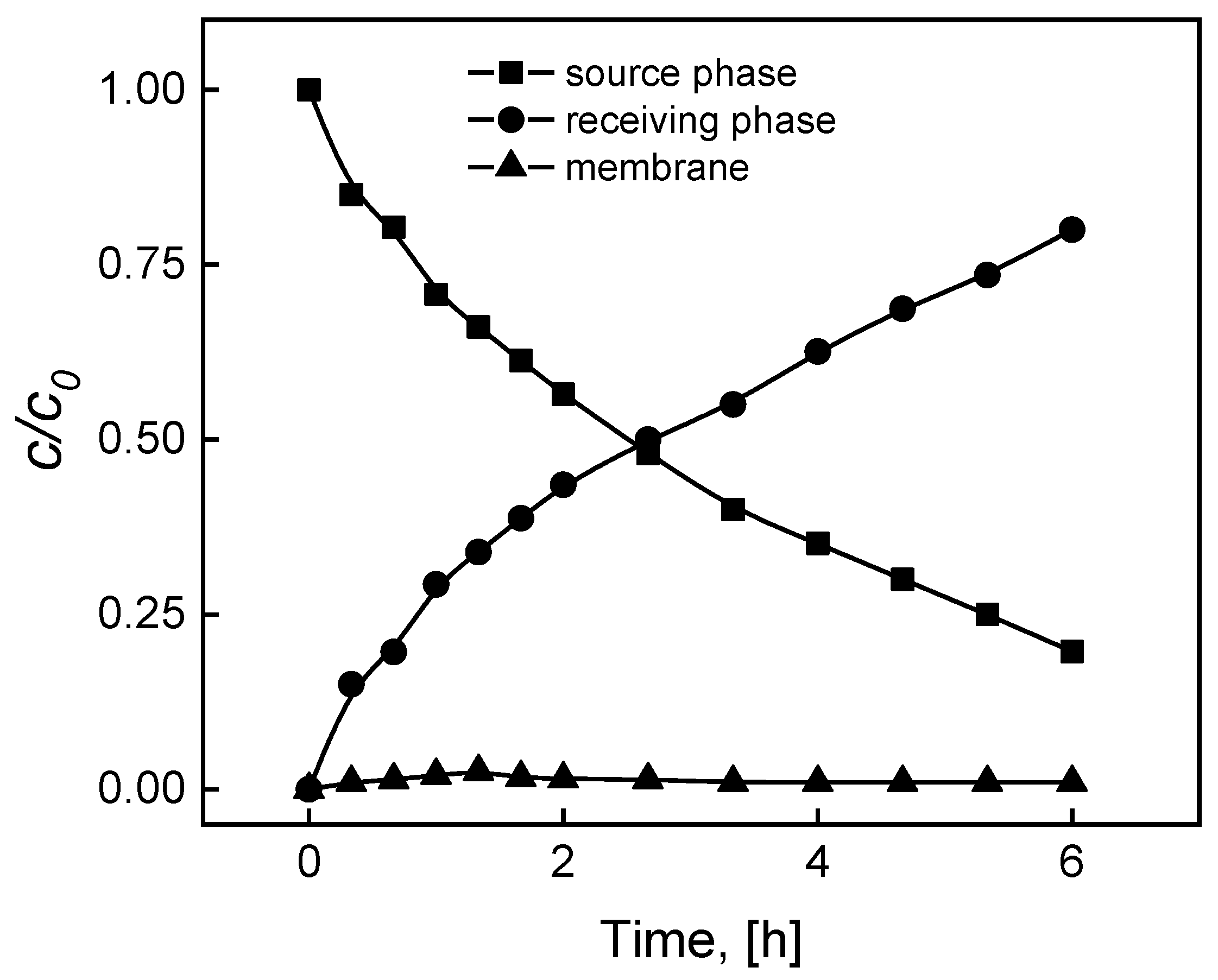
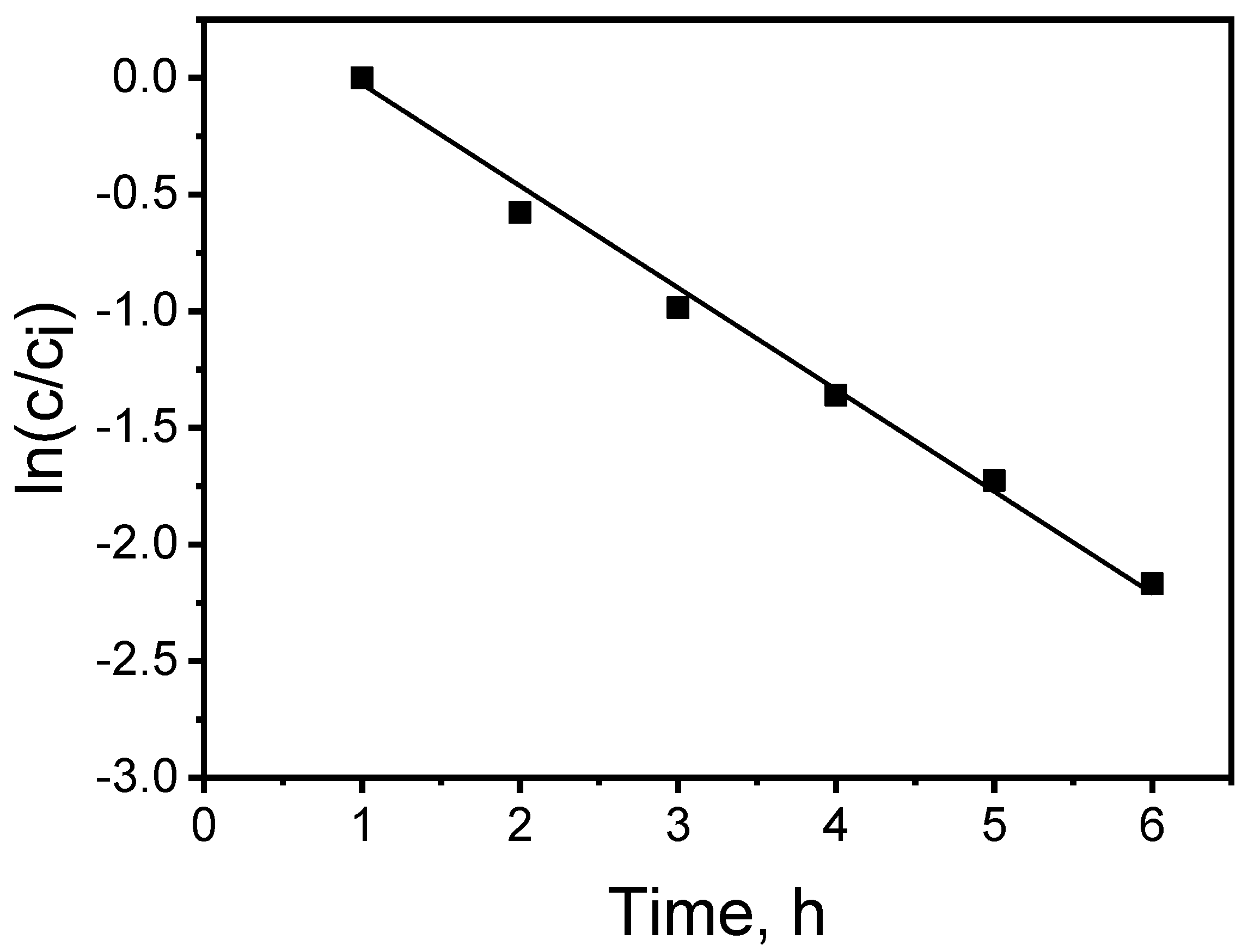
3.1. Kinetics and Repeatability of the Methylene Blue (MB) Dye Transport across Polymer Inclusion Membranes (PIMs)
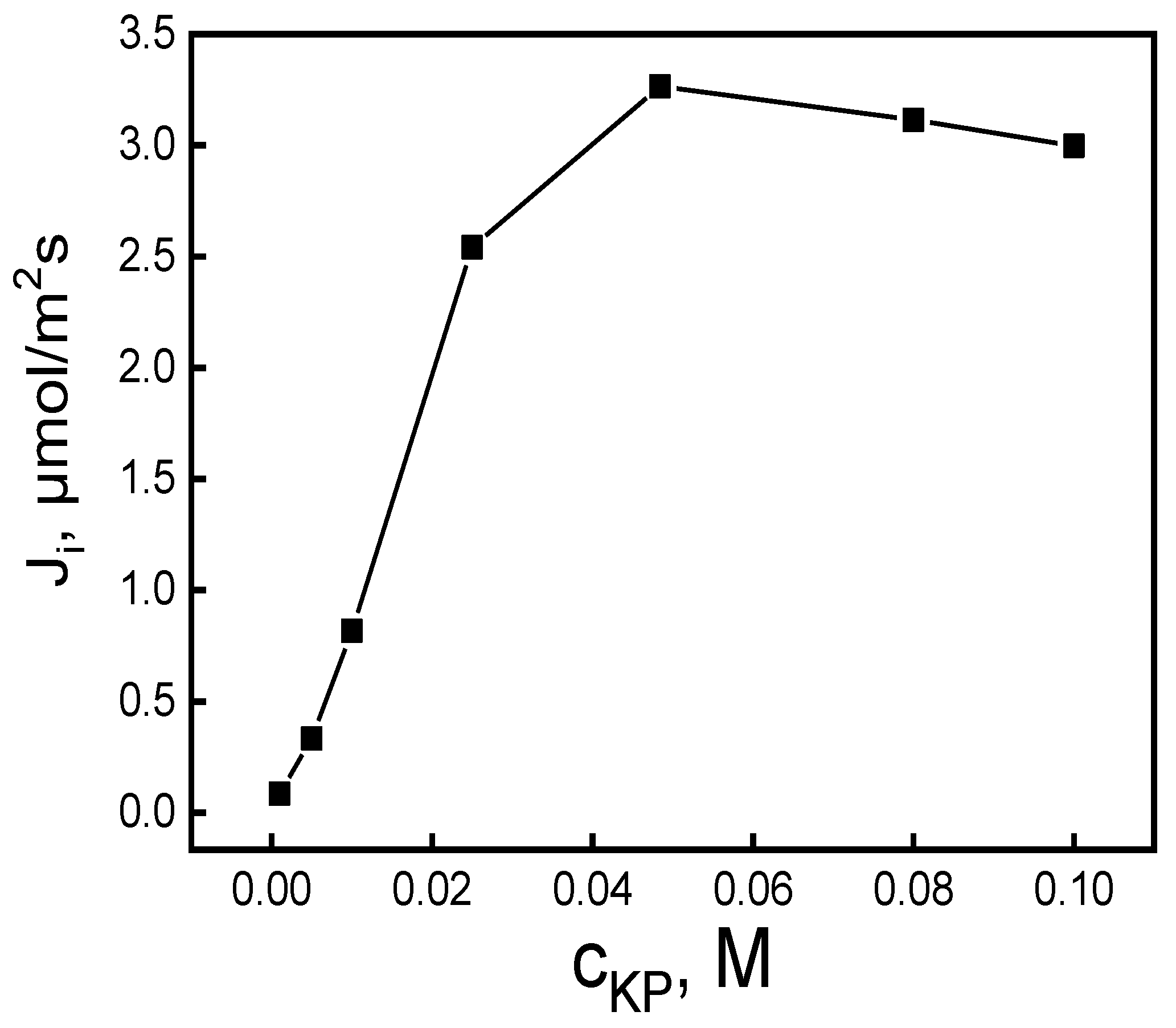
3.2. The Effect of Carrier Concentration
3.3. Modification of Source Phase Composition
3.4. Modification of Receiving Phase Composition
3.5. Membrane Reusability and Proposed Transport Mechanism
3.6. Physical-Chemical Characterization of the Developed PIMs
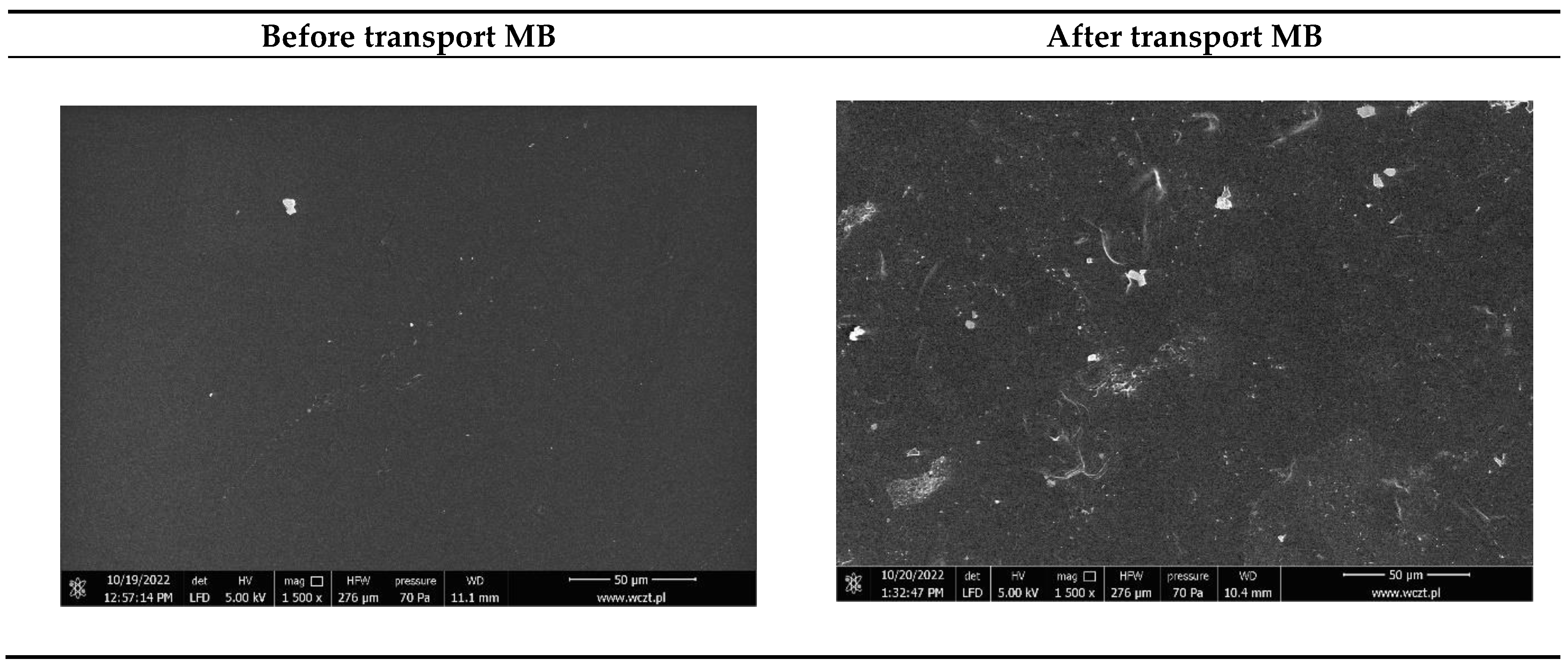
3.6.1. Morphology of the Developed PIMs
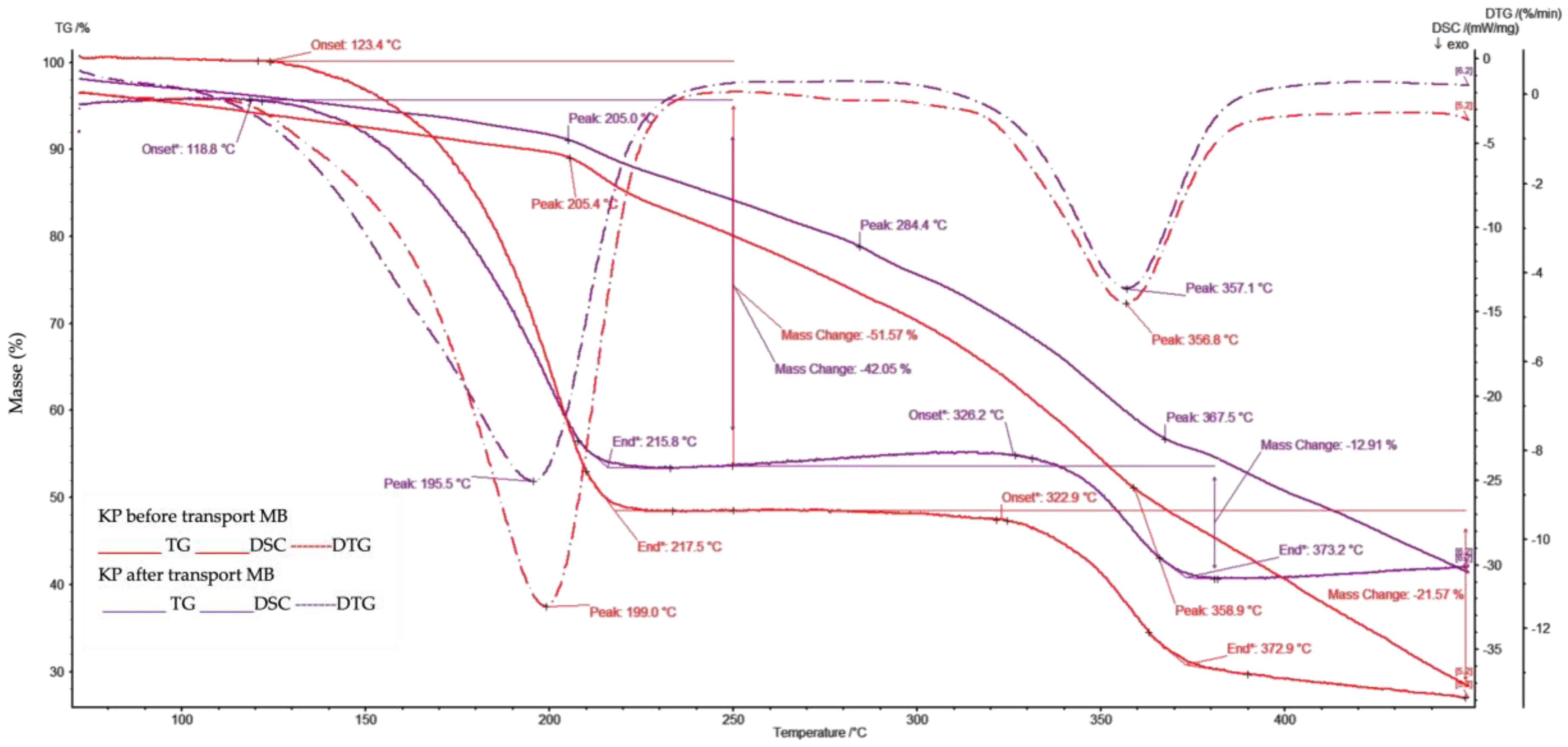
3.6.2. Thermal Properties Analysis Results
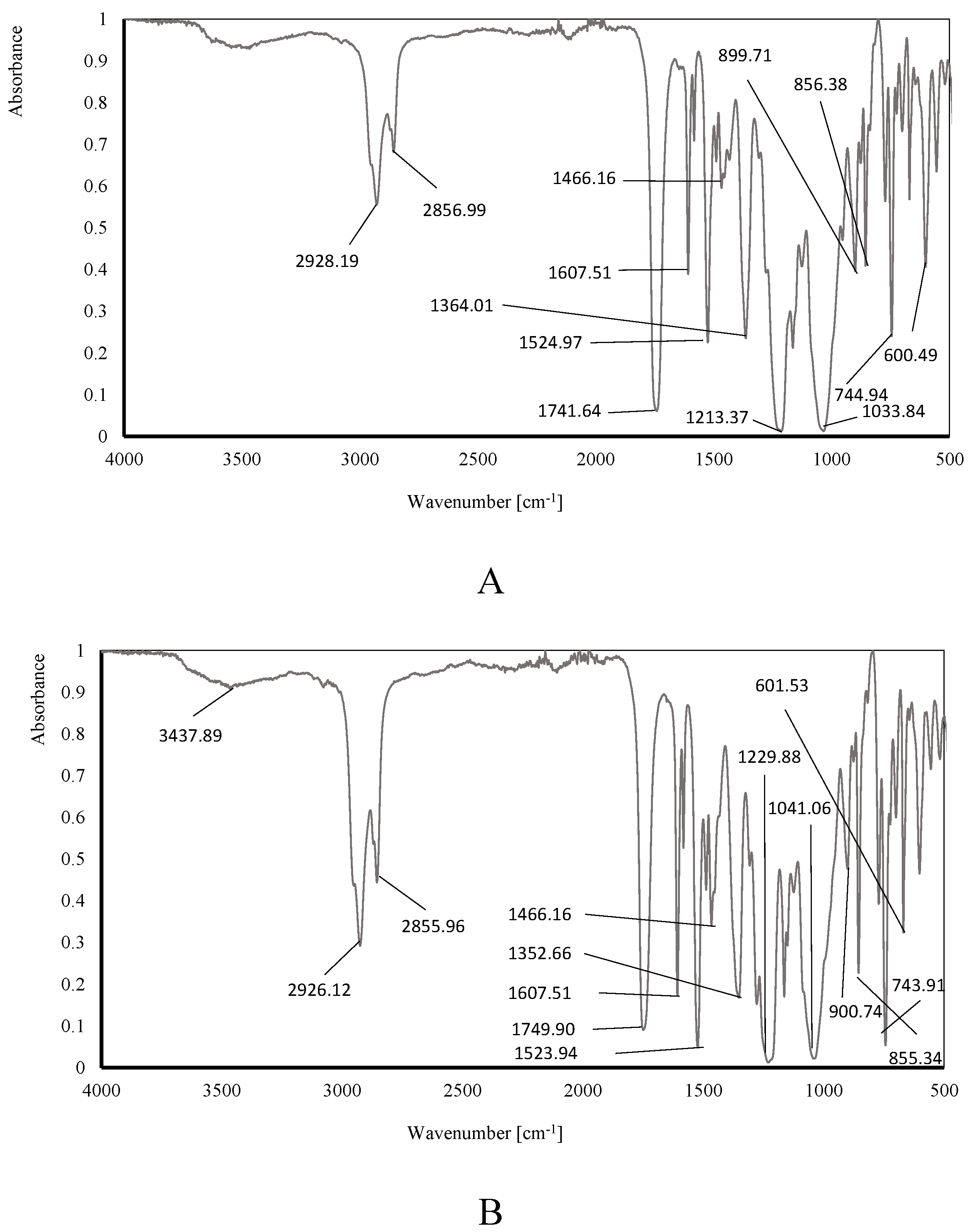
3.6.3. ATR-FTIR
4. Conclusions
Author Contributions
References
- Sivakumar, R.; Lee, N.Y. Adsorptive removal of organic pollutant methylene blue using polysaccharide-based composite hydrogels. Chemosphere 2022, 286, 131890. [CrossRef]
- Khan, I.; Khan, I.; Usman, M.; Imran, M.; Saeed, K. Nanoclay-mediated photocatalytic activity enhancement of copper oxide nanoparticles for enhanced methyl orange photodegradation. J. Mater. Sci. Mater. Electron. 2020, 31, 8971–8985. [CrossRef]
- Alencar, L.V.T.D.; Passos, L.M.S.; Soares, C.M.F.; Lima, A.S.; Souza, R.L. Efficiency Method for Methylene Blue Recovery Using Aqueous Two-Phase Systems Based on Cholinium-Ionic Liquids. J. Fash. Technol. Text. Eng. 2020, 6, 13–20. [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [CrossRef]
- Fong, W.M.; Affam, A.C.; Chung, W.C. Synthesis of Ag/Fe/CAC for colour and COD removal from methylene blue dye wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 3485–3494. [CrossRef]
- Benosmane, N.; Boutemeur, B.; Hamdi, S.M.; Hamdi, M. Removal of methylene blue dye from aqueous solutions using polymer inclusion membrane technology. Appl. Water Sci. 2022, 12, 1–11. [CrossRef]
- Derakhshan, Z.; Baghapour, M.A.; Ranjbar, M.; Faramarzian, M. Adsorption of Methylene Blue Dye from Aqueous Solutions by Modified Pumice Stone: Kinetics and Equilibrium Studies. Heal. Scope 2013, 2, 136–144. [CrossRef]
- Allouche, F.-N.; Yassaa, N. Potential adsorption of methylene blue from aqueous solution using green macroalgaePosidonia oceanica.. IOP Conf. Series: Mater. Sci. Eng. 2018, 323, 012006. [CrossRef]
- Han, T.H.; Khan, M.M.; Kalathil, S.; Lee, J.; Cho, M.H. Simultaneous Enhancement of Methylene Blue Degradation and Power Generation in a Microbial Fuel Cell by Gold Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 8174–8181. [CrossRef]
- Ashraf, M.W. Removal of methylene blue dye from wastewaters by using supported liquid membrane technology. Pol. J. Chem. Technol. 2016, 18, 26-30.
- Konczyk, J.; Nowik-Zajac, A.; Kozlowski, C.A. Calixarene-based extractants for heavy metal ions removal from aqueous solutions. Sep. Sci. Technol. 2016, 51, 2394–2410. [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [CrossRef]
- Benosmane, N.; Boutemeur, B.; Hamdi, S.; Hamdi, M. Citric acid removal from aqueous solutions using a polymer inclusion membrane based on a mixture of CTA and CA. Desalination Water Treat. 2018, 114, 163–168. [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective Transport of Ag(I) through a Polymer Inclusion Membrane Containing a Calix[4]pyrrole Derivative from Nitrate Aqueous Solutions. Int. J. Mol. Sci. 2020, 21, 5348. [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Lagiewka, J.; Malina, G. Separation of Mercury(II) from Industrial Wastewater through Polymer Inclusion Membranes with Calix[4]pyrrole Derivative. Membranes 2022, 12, 492. [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C.A. Removal of Pb(II) Ions Using Polymer Inclusion Membranes Containing Calix[4]resorcinarene Derivative as Ion Carrier. Polymers 2019, 11, 2111. [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Malina, G. Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes. Minerals 2020, 10, 909. [CrossRef]
- Minhas, M.A.; Rauf, A.; Rauf, S.; Minhas, F.T.; Memon, N.; Jabbar, A.; Bhanger, M.I.; Malik, M.I. Selective and efficient extraction of cationic dyes from industrial effluents through polymer inclusion membrane. Sep. Purif. Technol. 2021, 272, 118883. [CrossRef]
- Lagiewka, J.; Nowik-Zajac, A.; Pajdak, A.; Zawierucha, I. A novel multifunctional β-cyclodextrin polymer as a promising sorbent for rapid removal of methylene blue from aqueous solutions. Carbohydr. Polym. 2023, 307, 120615. [CrossRef]
- Gale P.A.; Sessler J.L.; Kràl, V. Calixpyrroles. Chem. Commun. 1998, 1-8.
- Gale, P.A.; Sessler, J.L.; Král, V.; Lynch, V. Calix[4]pyrroles: Old Yet New Anion-Binding Agents. J. Am. Chem. Soc. 1996, 118, 5140–5141. [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C. Application of Cr(VI) transport across the polymer inclusion membrane with calixresorcin [4]arene derivative as ion carrier. Sep. Sci. Technol. 2020, 55, 2204–2210. [CrossRef]
- Danesi, P.R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19, 857–894. [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective removal of silver(i) using polymer inclusion membranes containing calixpyrroles. RSC Adv. 2019, 9, 31122–31132. [CrossRef]
- Kebiche-Senhadji, O.;Mansouri, S.; Tingry, P.; Seta, M.; Benamor, M. Faciliated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (D2EHPA) metal carriers. J. Membr. Sci. 2008, 310, 438-445. [CrossRef]
- Scott, K. Handbook of Industrial Membranes; Elsevier BV: Amsterdam, NX, Netherlands, 1995; ISBN: 9781856172332.
- Cox, M. Solvent Extraction in Hydrometallurgy, in: Solvent Extraction. Principles and Practice, Eds. J. Rydberg; M. Cox; C. Musikas, C.R. Choppin; M. Dekker, 2004.
- Rodríguez de San Miguel, E.; Aquilar, J.C.; De Gyves, J. Structural effects on metal ion migration across polymer inclusion membranes: Dependence of transport profiles on nature of active plasticizer. J. Membr. Sci. 2008, 307, 105-116.
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: a review. Environ. Int. 2004, 30, 953–971. [CrossRef]
- Cho, Y.; Xu, C.; Cattrall, R.W.; Kolev, S.D. A polymer inclusion membrane for extracting thiocyanate from weakly alkine solutions. J. Membr. Sci. 2011, 367, 85. [CrossRef]
- Sabzi, N.E.; Kiasat, A.R. β-Cyclodextrin Based Nanosponge as a Biodegradable Porous Three-Dimensional Nanocatalyst in the One-Pot Synthesis of N- Containing Organic Scaffolds. Catal. Lett. 2018, 148, 2654–2664. [CrossRef]
- Benosmane, N.; Guedioura, B.; Hamdi, S.M.; Hamdi, M.; Boutemeur, B. Preparation, characterization and thermal studies of polymer inclusion cellulose acetate membrane with calix[4]resorcinarenes as carriers. Mater. Sci. Eng. C 2010, 30, 860–867. [CrossRef]
- Arous, O.; Amara, M.; Kerdjoudi, H. Selective transport of metal ions using polymer inclusion membranes containing crown-ether and cryptands. Arab. J. Sci. Eng. 2010, 35, 79-93.
- Mohapatra, P.; Lakshmi, D.; Bhattacharyya, A.; Manchanda, V. Evaluation of polymer inclusion membranes containing crown ethers for selective cesium separation from nuclear waste solution. J. Hazard. Mater. 2009, 169, 472–479. [CrossRef]



| pH of source phase | Receiving phase | % Removal of MB |
|---|---|---|
| 3 | 0.1 M HCl | 5.48 |
| 6 | 18.43 | |
| 9 | 74.57 | |
| 10 | 93.10 | |
| 11 | 92.12 | |
| 12 | 91.89 |
| Concentration of HCl in receiving phase | % Removal of MB |
|---|---|
| 0.1 M | 93.10 |
| 0.2 M | 89.75 |
| 0.3 M | 88.48 |
| 0.5 M | 70.58 |
| 1.0 M | 65.54 |
| Bond | Membrane before transport process (A) | Membrane after transport process (B) | Typical absorption range [cm-1]* |
|---|---|---|---|
| Wavenumber [cm-1] | |||
| R-X alkyl halides | 600.50 | 601.53 | 500-680 |
| C-H aromatics | 744.94 | 743.91 | 705-745 |
| C-H aromatics | 856.37 | 855.34 | 862 |
| N-H amines groups | 899.71 | 900.74 | 665-910 |
| RCO-OH carboxylic groups RCOOR’ C-O |
1033.84 | 1041.06 | 1000-1320 |
| Ar-O-R ethers groups | 1213.37 | 1229.88 | 1220-1260 |
| C-H alkanes | 1364.01 | 1352.66 | 1360, second 723 |
| RCH2CH3 alkanes CH2. CH3 |
1466.16 | 1466.16 | 1460 |
| N-O nitro groups | 1524.97 | 1523.94 | 1520, second 1350 |
| C=C alkenes | 1607.51 | 1607.51 | 1611 |
| RCOOR’ esters groups | 1741.64 | 1749.90 | 1735 |
| -CH2- alkanes C=C CO-OH carboxylic groups dimer OH |
2856.99 | 2855.96 | 2850 2800-3400 |
| -CH2- alkanes | 2928.19 | 2926.12 | 2925 |
| RCONHR’ | 3437.89 | 3437.89 | 3440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





