1. Introduction
Worldwide, the protozoan parasite
Neospora caninum causes sporadic, endemic, and epidemic miscarriages in cattle [
1,
2]
, and has also been identified as a cause of abortion in sheep [
3]. The primary method of transmission appears to be endogenous transplacental transmission from dam to calf during pregnancy [
1,
4]. It is also possible for cattle and other animals to be horizontally infected by ingesting oocysts that have been discharged by a canid acting as the final host [
5,
6]. The reproductive disorders caused by
N. caninum have a detrimental economic effect on dairy cow replacement as well as milk production [
7,
8]
. In terms of public health, IgG [
9] or IgM [
10] specific antibodies to
N. caninum have been found in female serum
. Despite the placenta testing negative for this parasite, a recent study found that two samples (1%) out of 201 investigated human umbilical cord blood samples were Nc5 PCR-positive for
N. caninum [
11]. However, there hasn't yet been any proof of a clinical type of neosporosis in humans.
It has been established for dairy cows that milk is a useful substrate to detect antibodies directed against
N. caninum [
12]. The IgG immunoglobulin class is the predominant immunoglobulin class in cow's milk, and the antibodies contained in milk are carried from the serum into the mammary gland in a selective manner [
13]. When compared to milk samples from
N. caninum seronegative cows, those from seropositive cows had a considerably greater IgG level [
14,
15]. Also, a high agreement (95%) between serum and milk antibodies was recorded [
16]
.
In the present study, we aimed at the evaluation of a widely available serum ELISA (ID Screen
® Neospora caninum competition Multispecies ELISA) for detection of antibodies to
N. caninum in milk samples, as ELISAs developed specifically for milk samples are sometimes not widely available and as it would be convenient to test different sample types in the same ELISA. A previous study tested the same commercial serum ELISA for use with whole and skimmed cow’s milk and found a high diagnostic correlation and agreement between serum and milk antibody levels [
17]. However, this study validated the approach via comparison of milk and serum samples obtained from the same animals. We wanted to assess the performance of the serum ELISA on milk samples previously tested with a milk ELISA [
18], and to include milk from different ruminant species, namely cattle, buffaloes, sheep, and goats. Our results provide additional evidence for the utility of a commercially available and widely used serum ELISA test kit in detection of
N. caninum antibody in milk samples.
2. Materials and Methods
2.1. Ethical Statement
This study was performed according to standard procedures identified by the Research Board of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt. The study was approved by the Research Code of Ethics at South Valley University number 36 (RCOE-36).
2.2. Sample Collection and Preparation
Individual milk samples (total number = 149) from Sohag governorate (dairy cows; n = 70), Qena governorate (dairy cows; n = 34), both southern Egypt, and from Dakahlia governorate (dairy buffaloes; n = 16, sheep; n = 18, goats; n = 11), northern Egypt, were collected. An amount of 5 ml of individual milk samples was obtained for laboratory testing. The milk samples were centrifuged at 1000 x g for 10 min. Lactoserum was collected from the layer below the cream layer on the top and stored at -20°C until used [
18].
2.3. Detection of Antibodies to N. caninum in Milk Samples Using a Serum ELISA
Milk samples were tested for antibodies to N. caninum using the ID Screen® Neospora caninum competition Multispecies ELISA (ID. Vet, Grabels, France), marketed for the use in serum and plasma samples, and referred to herein as serum ELISA.
Positive and negative controls provided in the kit and test milk samples were two times diluted (Byrem et al., 2012). Then, plates were incubated at 37o C for 45 min. afterwards, washing and all procedures were done according to manufacturer’s instructions. The ODs obtained (read at 450 nm and measured with an Infinite R© F50/Robotic ELISA reader (Tecan Group Ltd.,Männedorf, Switzerland) were used to calculate the percentage of sample (S) to negative (N) ratio (S/N %) for each of the test samples according to the following formula S/N (%) = OD sample / OD negative control × 100. Samples with an S/P% greater than 60% was considered negative; if the S/P% was between 50% and 60%, the result was considered doubtful and considered positive if the S/P% less than 50%. No doubtful values have been obtained during the test.
2.4. Detection of Antibodies to N. caninum in Milk Samples Using a Milk ELISA
All samples had previously been tested in the Neospora caninum Milk Competitive ELISA (ID. Vet, Grabels, France), a test explicitly developed for use in milk samples and referred to herein as milk ELISA. Testing was performed according to the manufacturer’s instructions and as described previously (Fereig et al., 2022). Briefly, undiluted milk samples, positive and negative control were added to the microplate and incubated at 5°C for 20 h. The ODs obtained were used to calculate the percentage of sample (S) to negative (N) ratio (S/N %) for each of the test samples according to the following formula S/N (%) = OD sample / OD negative control × 100. Samples with an S/P% greater than 50% were considered negative, and considered positive if the S/P% was less than or equal to 50%.
2.5. Statistical Analysis
The 95% confidence intervals (including continuity correction), estimated prevalence, sensitivity, specificity, positive predictive value, negative predictive value, concordance % and kappa value were analyzed using an online statistical website
www.vassarstats.net (accession dates; 01-02 October, 2023) as described previously.
P-values were estimated with GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA). The results were considered significant when the
p-value was < 0.05. Pearson’s correlation coefficient was applied to test the correlation between OD values obtained in serum ELISA and in milk ELISA. Correlation coefficients were calculated using Pearson’s correlation coefficient: |r| = 0.70, strong correlation; 0.5 < |r| < 0.7, moderately strong correlation; and |r| = 0.3–0.5 weak-to-moderate correlation [
18,
19]
.
3. Results and Discussion
The growing human population demands safe and high-quality food including milk and dairy byproducts. Some reports have revealed the presence of
N. caninum DNA in raw milk samples from dairy cows [
18,
20,
21]. This might suggest a potential risk of infection of sucking animals and thus induction of additional economic losses. Another very important point is whether
N. caninum may be infective for humans. This risk seems to be higher in case of consuming the raw milk from an individual animal rather than the consumption of the bulk tank milk in which the parasites would be greatly diluted [
4]
.
The current control strategy for neosporosis relies on a test-and-cull approach. The use of milk samples instead of serum samples for the detection of anti-N. caninum antibodies represents a non-invasive alternative that might gain high importance in control and preventive strategies of N. caninum transmission. . All of these reasons increase the demand to find appropriate diagnostic ELISA tests for detecting and monitoring N. caninum antibodies in milk.
A previous study revealed the adequacy of a commercial serum antibody ELISA in screening
N. caninum antibody in cow milk using the same test against milk and serum sample from the same animal [
17]. However, in the current study, we used a higher variety of animal species milk and tested all samples in a milk antibody ELISA kit (standard) as well as in the serum antibody ELISA kit. We obtained 28.2% as an overall lacto-positive rate (42/149, CI 95%; 21.3–36.2) using milk antibody ELISA, and 17.4% (26/149, 11.9–24.7) using serum antibody ELISA (
Table 1). All 26 samples positive in the serum ELISA were also positive in the milk ELISA. The milk antibody ELISA revealed 29.8% (31/104, CI 95%; 21.4-39.7) in cattle, 18.8% (3/16, CI 95%; 5-46.3) in buffaloes, 33.3% (6/18, CI 95%; 14.4-58.8) in sheep, and 18.2% (2/11, CI 95%; 3.2-52.2) ingoats, respectively as positive rates. When using serum antibody ELISA, positive rates were 21.2% (21/104, CI 95%; 13.2-29.4) in cattle, 6.3% (1/16, CI 95%; 0.3-32.3) in buffaloes, 11.1% (2/18, CI 95%; 2-36) in sheep, and 18.2% (2/11, CI 95%; 3.2-52.2) in goats, respectively (
Table 1).
In case of online software analysis (Vassarstats.net), an estimated prevalence was reported as 28.2 (CI 95%; 21.3–36.2) that was consistent with our manual calculated data of milk antibody ELISA-based testing (
Table 2). The sensitivity, specificity, negative predictive and positive predictive value of serum antibody ELISA compared to milk antibody ELISA for cow milk was found to be 61.4%, 100%, 87%, and 100%, respectively. Furthermore, our test method demonstrated a high concordance (89.9%) and a substantial kappa value (0.7) (
Table 2).
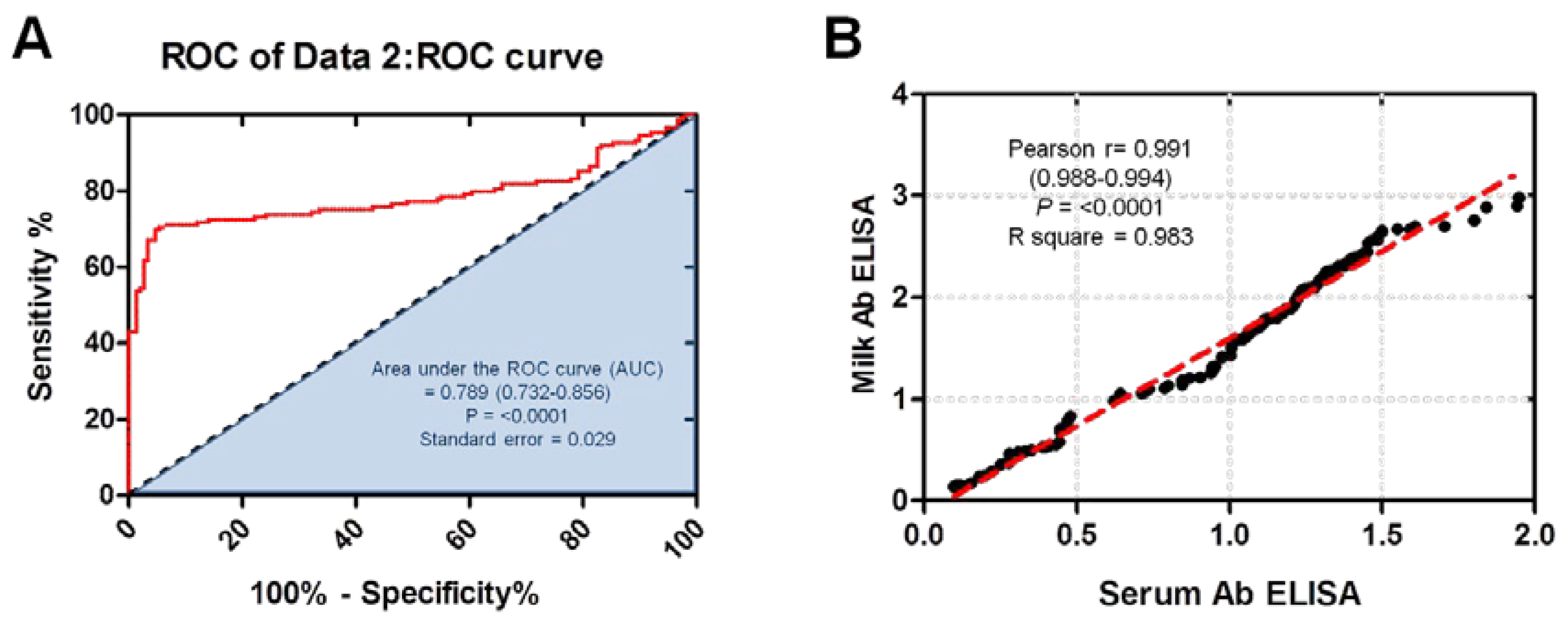
The area under the curve was used to determine the accuracy of the immunoassays for ELISA (
Figure 1A). Area under the receiver operating characteristic curve (AUC) was found to be 0.789 (CI 95%: 0.732–0.856) suggesting high performance of serum antibody ELISA used in milk samples compared to milk antibody ELISA. Similar interpretation was reported in a relevant study using our tested serum antibody ELISA against serum and milk samples from the same group of cow milk [
17]. Correlation between serum antibody ELISA and milk antibody ELISA OD values of tested milk samples was also analyzed. Scatter graphs show the correlation between OD values recorded by serum antibody ELISA and milk antibody ELISA from all tested samples (n = 149). A strong correlation for tested milk samples was observed between the serum antibody ELISA and milk antibody ELISA OD (Pearson’s r = 0.991, P = <0.0001, R square = 0.983) (
Figure 1B) [
19].
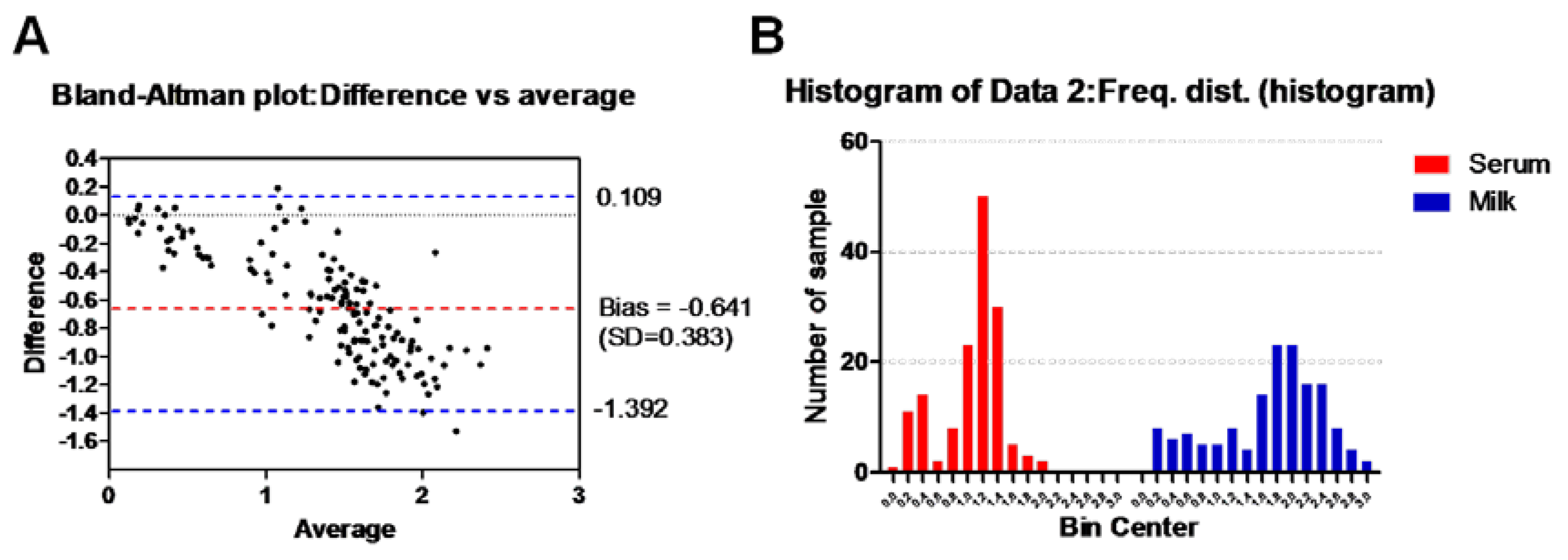
Also
Figure 2A shows Bland–Altman Plot of ELISA testing between the serum antibody ELISA and milk antibody ELISA. Dotted bluish lines between 0.109 and – 1.392 of standard deviation 0.383 from mean (dotted red line). Almost all data points are between ± 1.39 standard deviations (SD) signifying good agreement between methods [
22,
23]. In the same context, histogram of our tested milk samples using both methods showed a good correlation of frequency distribution of obtained data (serum antibody ELISA, 1.1 ± 0.4 SD; milk antibody ELISA, 1.7 ± 0.7 SD) for the total number of values (n = 149) [
23]. However, the number of intervals illustrated at x-axis was higher in case of milk antibody ELISA (n = 15) than that for serum antibody ELISA (11) (
Figure 2B).
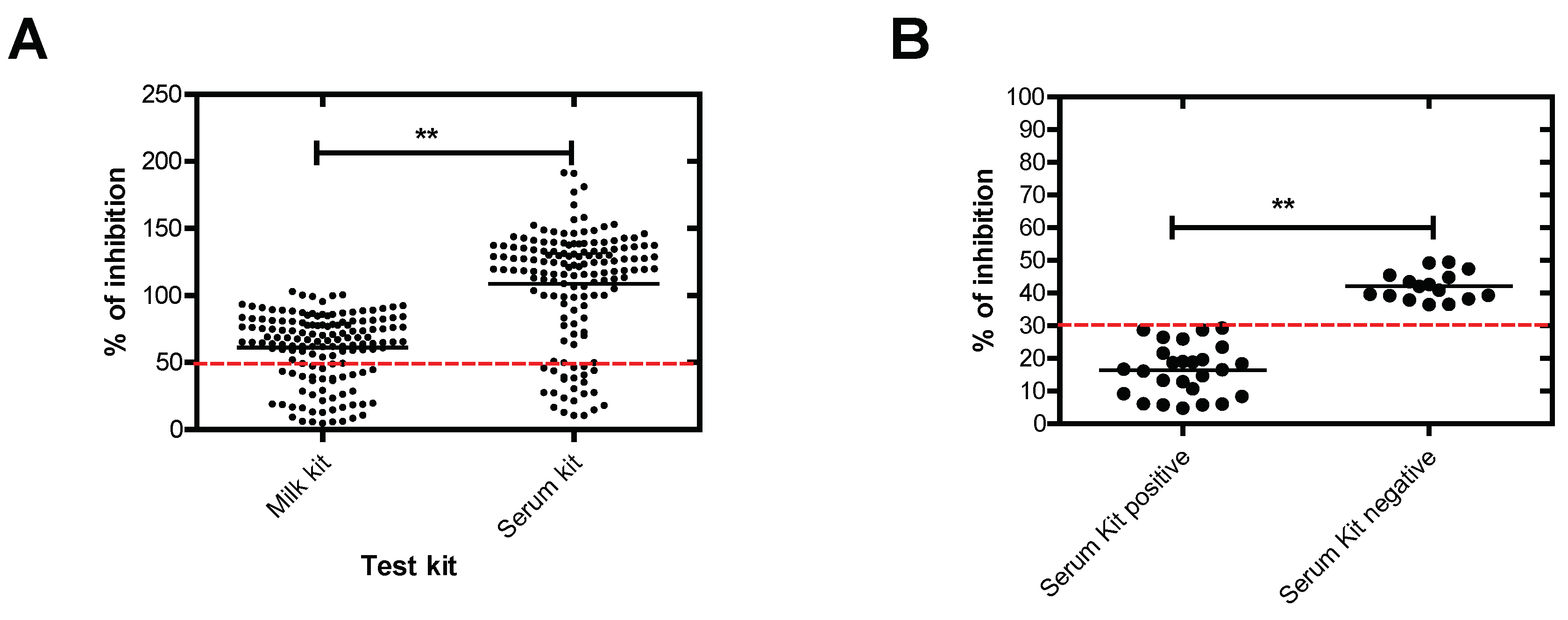
To understand the obtained results, we have assessed the antibody levels to
N. caninum in milk samples via comparing % of inhibition using milk and serum antibody ELISAs. % of inhibition of all samples was significantly lower in case of using milk antibody ELISA (standard method) compared to serum antibody ELISA (test method) (p = < 0.0001). According to the manufacturer’s instructions, the lower % of inhibition indicates the higher positivity and vice versa, where positive values are those of ≤ 50% in both test kits (
Figure 3A). This result indicates the higher proficiency of milk antibody ELISA than serum antibody ELISA. For further analysis, positive and negative serum antibody ELISA milk samples representing all positive samples using milk antibody ELISA was compared also. Significant difference was obtained, where dual positive samples exhibited lower % of inhibition than the positive samples to milk antibody ELISA only (p = < 0.0001) (
Figure 3B).
This result indicates also that serum antibody ELISA could detect mainly samples of strong reactivity that lies at the % of inhibition of ≤ 30% instead of ≤ 50% that reported in case of serum or plasma use. Thus, low sensitivity and high specificity should be considered in case of future use of serum antibody ELISA in testing N. canium antibody in milk instead of serum or plasma. Also, an increase of cut-off value to 60% or considering doubtful samples as positive ones might be another prospect for positive milk sample judgments using serum antibody ELISA.
Collectively, based on a previous study and current data, serum antibody ELISA test kit might be used as an alternative method in detecting N. caninum antibodies in milk samples. The utility of serum antibody ELISA use is highly indicated because of its higher geographical distribution and easier preparation and use.
Author Contributions
Conceptualization and design, R.M.F., and C.F.F.; experiments, R.M.F., formal analysis, investigation, R.M.F., S.A.A and C.F.F.; resources and shared materials, R.M.F., S.A.A., and C.F.F., writing—original draft, R.M.F., S.S.A., and C.F.F; writing—review and editing, R.M.F., and C.F.F. Project administration and funding acquisition; R.M.F., and C.F.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any special fund.
Institutional Review Board Statement
The protocols were approved by the Research Code of Ethics at South Valley University number 36 (RCOE-36).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated and analyzed during this study are included in this published article. Raw data supporting the findings of this study are available from the corresponding author on request.
Acknowledgments
We would like to appreciate the great help of our colleagues at Department of Animal Medicine, Faculty of Veterinary Medicine, South Valley University, Qena for their cooperation and technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubey, J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Schares, G. Neosporosis in animals-the last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Dubey, J.P. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants: An Update. Vet. Clin. North Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Davison, H.C.; Otter, A.; Trees, A.J. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int. J. Parasitol. 1999, 29, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.M.; Dubey, J.P.; Lindsay, D.S.; Jolley, W.R.; Wills, R.A.; McGuire, A.M. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 1998, 28, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Gondim, L.F.; McAllister, M.M.; Pitt, W.C.; Zemlicka, D.E. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J.Parasitol. 2004, 34, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Gottstein, B. Neospora caninum and neosporosis—Recent achievements in host and parasite cell biology andtreatment. Acta Parasitol. 2006, 51, 15–25. [Google Scholar] [CrossRef]
- Moore, D.P.; Cantón, G.J.; Louge Uriarte, E.L. Editorial: Infectious diseases affecting reproduction and the neonatal period incattle. Front. Vet. Sci. 2021, 8, 679007. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Huang, P.; Salem, T.A.; Talaat, R.M.; Nasr, M.I.; Xuan, X.; Nishikawa, Y. Short report: Prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 2009, 80, 263–267. [Google Scholar] [CrossRef]
- Duarte, P.O.; Csordas, B.G.; Oshiro, L.M.; Higa, L.O.S.; Zimmermann, N.P.; Martins, K.R.; Barros, J.C.; Andreotti, R. Serological evaluation of Neospora caninum in pregnant women treated at referral center for prenatal screening in Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e010820. [Google Scholar] [CrossRef]
- Duarte, P.O.; Oshiro, L.M.; Zimmermann, N.P.; Csordas, B.G.; Dourado, D.M.; Barros, J.C.; Andreotti, R. Serological and molecular detection of Neospora caninum and Toxoplasma gondii in human umbilical cord blood and placental tissue samples. Sci. Rep. 2020, 10, 9043. [Google Scholar] [CrossRef] [PubMed]
- Björkman, C.; Johansson, O.; Stenlund, S.; Holmdahl, O.J.; Uggla, A. Neospora species infection in a herd of dairy cattle. J. Am. Vet.Med. Assoc. 1996, 208, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Björkman, C.; Holmdahl, O.J.; Uggla, A. An indirect enzyme-linked immunoassay (ELISA) for demonstration of antibodies to Neospora caninum in serum and milk of cattle. Vet. Parasitol. 1997, 68, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, B.; Cabaj, W.; Pastusiak, K.; Bien, J. The suitability of milk in detection of Neospora caninum infection in cows. Acta Parasitol. 2003, 48, 138–141. [Google Scholar]
- Chanlun, A.; Näslund, K.; Aiumlamai, S.; Björkman, C. Use of bulk milk for detection of Neospora caninum infection in dairyherds in Thailand. Vet. Parasitol. 2002, 110, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Byrem, T.M.; Bartlett, P.C.; Donohue, H.; Voisinet, B.D.; Houseman, J.T. Performance of a commercial serum ELISA for the detection of antibodies to Neospora caninum in whole and skim milk samples. Vet. Parasitol. 2012, 190, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Abdelbaky, H.H.; Mazeed, A.M.; El-Alfy, E.-S.; Saleh, S.; Omar, M.A.; Alsayeqh, A.F.; Frey, C.F. Prevalence of Neospora caninum and Toxoplasma gondii Antibodies and DNA in Raw Milk of Various Ruminants in Egypt. Pathogens 2022, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Abdelbaky, H.H.; Nishikawa, Y. Comparative evaluation of four potent Neospora caninum diagnostic antigens using immunochromatographic assay for detection of specific antibody in cattle. Microorganisms 2021, 9, 2133. [Google Scholar] [CrossRef]
- Moskwa, B.; Pastusiak, K.; Bien, J.; Cabaj, W. The first detection of Neospora caninum DNA in the colostrum of infected cows. Parasitol. Res. 2007, 100, 633–636. [Google Scholar] [CrossRef]
- Gharekhani, J.; Yakhchali, M.; Afshari, A.; Adabi, M. Herd-level contamination of Neospora caninum, Toxoplasma gondii and Brucella in milk of Iranian dairy farms. Food Microbiol. 2021, 100, 103873. [Google Scholar] [CrossRef] [PubMed]
- Raez-Bravo, A.; Granados, J.E.; Serrano, E.; Dellamaria, D.; Casais, R.; Rossi, L.; Puigdemont, A.; Cano-Manuel, F.J.; Fandos, P.; Pérez, J.M.; Espinosa, J.; Soriguer, R.C.; Citterio, C.; López-Olvera, J.R. Evaluation of three enzyme-linked immunosorbent assays for sarcoptic mange diagnosis and assessment in the Iberian ibex, Capra pyrenaica. Parasit Vectors. 2016, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Bogan, J.E., Jr. Analytical and Clinical Evaluation of Two Methods for Measuring Erythrocyte Sedimentation Rate in Eastern Indigo Snakes (Drymarchon couperi). Animals 2023, 13, 464. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).