Submitted:
15 March 2024
Posted:
18 March 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Normal LV Filling Dynamics
2.1. Interpreting e’ and E/c’ in Diastolic Dynamics
3. Abnormal LV Filling Patterns
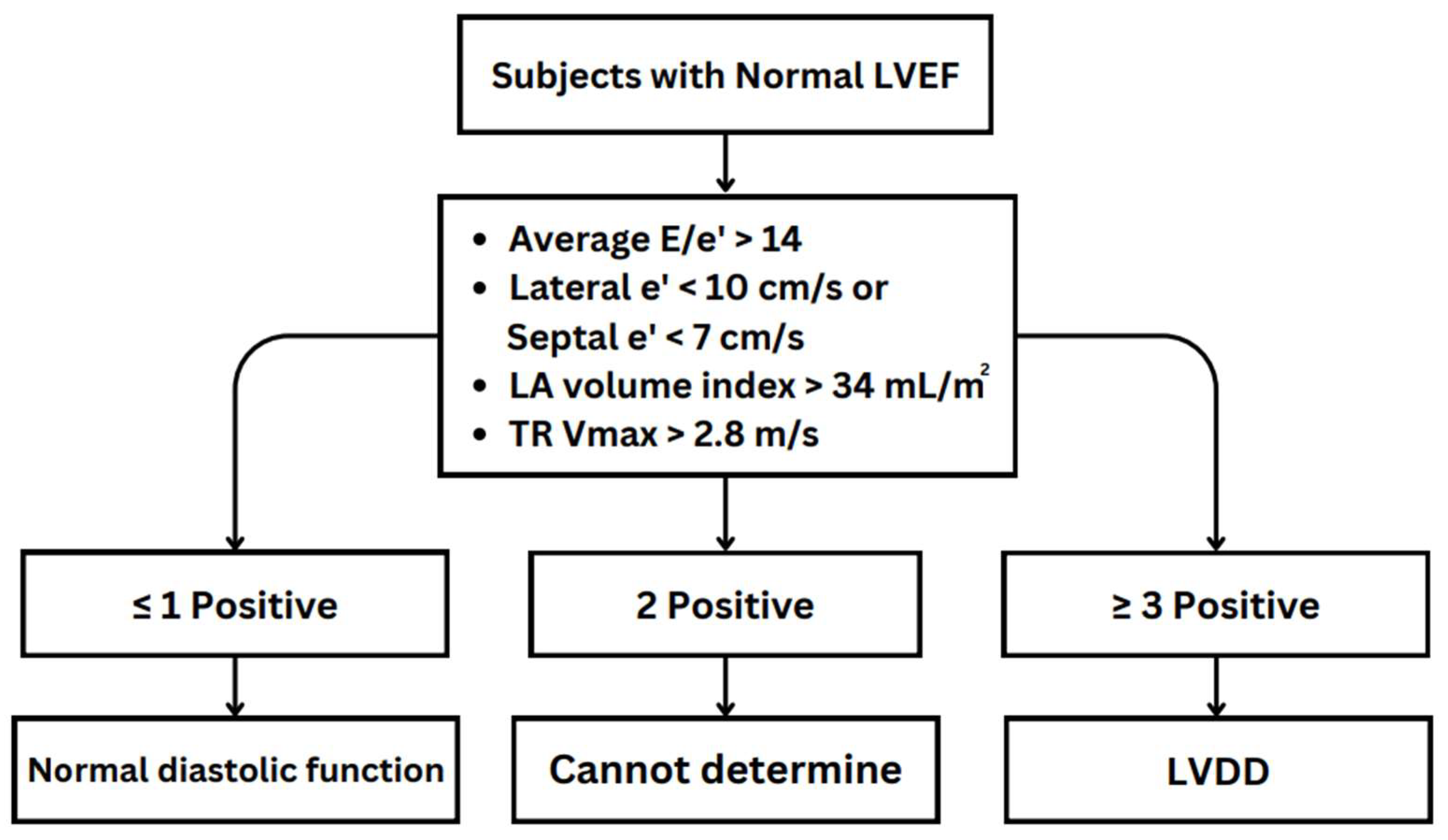
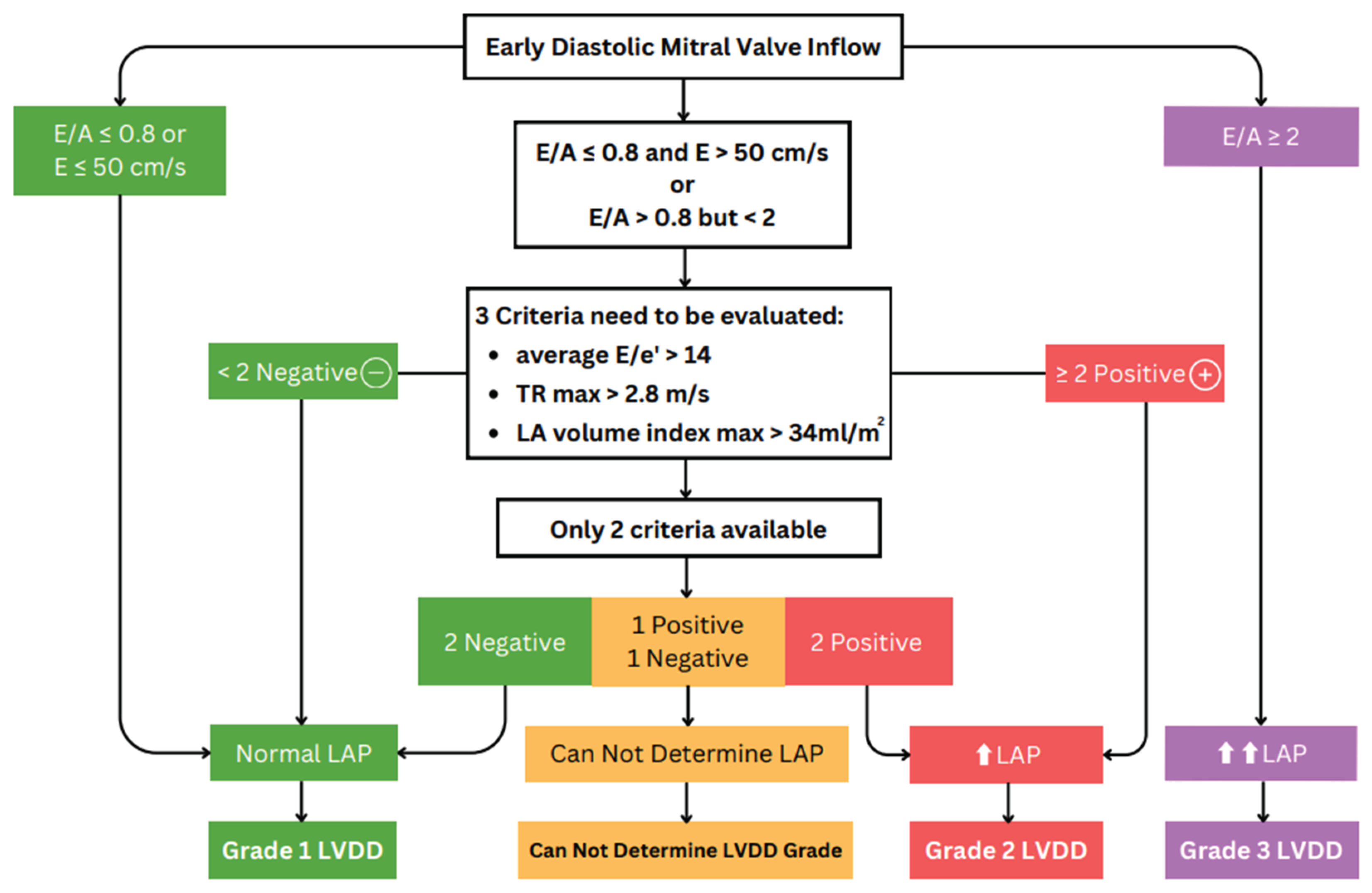
3.1. Echocardiography Parameters and Evaluation Algorithms for Diastolic Dysfunction
4. Stress Echocardiography Testing for Normal and Abnormal Diastolic Function
4.1. Stress Echocardiography during Relaxation in Healthy Individuals
4.2. Stress Echocardiography in Patients with Left Ventricular Diastolic Dysfunction
5. Other Advanced Echocardiography Techniques for Evaluating LV Diastolic Function
5.1. Strain Imaging
5.1.1. LV Strain and Strain Rate
5.1.2. LAS
5.2. Integrating Artificial Intelligence (AI)
6. Clinical Implications
6.1. Heart Failure with Preserved Ejection Fraction
6.2. Hypertensive Heart Disease
7. Future Perspectives
8. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Playford, D.; Strange, G.; Celermajer, D.S.; Evans, G.; Scalia, G.M.; Stewart, S.; Prior, D. Diastolic dysfunction and mortality in 436 360 men and women: the National Echo Database Australia (NEDA). Eur. Heart J. Cardiovasc. Imaging 2021, 22, 505–515. [Google Scholar] [CrossRef]
- Ghio, S.; Carluccio, E.; Scardovi, A.B.; Dini, F.L.; Rossi, A.; Falletta, C.; Scelsi, L.; Greco, A.; Temporelli, P.L. Prognostic relevance of Doppler echocardiographic re-assessment in HFrEF patients. Int. J. Cardiol. 2021, 327, 111–116. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Nabi, F.; Chang, S.M.; Al-Mallah, M.; Shah, D.J.; Bhimaraj, A. Imaging for implementation of heart failure guidelines. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1283–1292. [Google Scholar] [CrossRef]
- Lam, C.S.; Roger, V.L.; Rodeheffer, R.J.; Borlaug, B.A.; Enders, F.T.; Redfield, M.M. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J. Am. Coll. Cardiol. 2009, 53, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Anthony, C.; Akintoye, E.; Wang, T.; Klein, A. Echo doppler parameters of diastolic function. Curr. Cardiol. Rep. 2023, 25, 235–247. [Google Scholar] [CrossRef]
- Hodzic, A.; Garcia, D.; Saloux, E.; Ribeiro, P.A.B.; Ethier, A.; Thomas, J.D.; Milliez, P.; Normand, H.; Tournoux, F. Echocardiographic evidence of left ventricular untwisting-filling interplay. Cardiovasc. Ultrasound 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Remme, E.W.; Opdahl, A.; Smiseth, O.A. Mechanics of left ventricular relaxation, early diastolic lengthening, and suction investigated in a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1678–1687. [Google Scholar] [CrossRef]
- Opitz, C.A.; Kulke, M.; Leake, M.C.; Neagoe, C.; Hinssen, H.; Hajjar, R.J.; Linke, W.A. Damped elastic recoil of the titin spring in myofibrils of human myocardium. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 12688–12693. [Google Scholar] [CrossRef]
- Takagi, S.; Yokota, M.; Iwase, M.; Yoshida, J.; Hayashi, H.; Sotobata, I.; Koide, M.; Saito, H. The important role of left ventricular relaxation and left atrial pressure in the left ventricular filling velocity profile. Am. Heart J. 1989, 118, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Opdahl, A.; Remme, E.W.; Helle-Valle, T.; Edvardsen, T.; Smiseth, O.A. Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate. Circulation 2012, 126, 1441–1451. [Google Scholar] [CrossRef]
- Wang, J.; Khoury, D.S.; Thohan, V.; Torre-Amione, G.; Nagueh, S.F. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation 2007, 115, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Uchinoumi, H.; Bers, D.M. Increasing SERCA function promotes initiation of calcium sparks and breakup of calcium waves. J. Physiol. 2021, 599, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Lipskaia, L.; Chemaly, E.R.; Hadri, L.; Lompre, A.M.; Hajjar, R.J. Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin. Biol. Ther. 2010, 10, 29–41. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxen, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F. Left ventricular diastolic function: Understanding pathophysiology, diagnosis, and prognosis with echocardiography. JACC Cardiovasc. Imaging 2020, 13, 228–244. [Google Scholar] [CrossRef]
- Ho, C.Y.; Solomon, S.D. A clinician's guide to tissue Doppler imaging. Circulation 2006, 113, e396–398. [Google Scholar] [CrossRef]
- Park, J.H.; Marwick, T.H. Use and limitations of E/e' to assess left ventricular filling pressure by echocardiography. J. Cardiovasc. Ultrasound 2011, 19, 169–173. [Google Scholar] [CrossRef]
- Chao, C.J.; Kato, N.; Scott, C.G.; Lopez-Jimenez, F.; Lin, G.; Kane, G.C.; Pellikka, P.A. Unsupervised machine learning for assessment of left ventricular diastolic function and risk stratification. J. Am. Soc. Echocardiogr. 2022, 35, 1214–1225. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Zile, M.R. Left ventricular diastolic dysfunction and diastolic heart failure. Annu. Rev. Med. 2004, 55, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Deshpande, S.; Akam-Venkata, J.; Shakti, D.; Moskowitz, W.; Lipshultz, S.E. Heart failure with preserved ejection fraction in children. Pediatr. Cardiol. 2023, 44, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Kane, G.C.; Melenovsky, V.; Olson, T.P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2016, 37, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Little, W.C.; Oh, J.K. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation 2009, 120, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Tschope, C.; Paulus, W.J. Is echocardiographic evaluation of diastolic function useful in determining clinical care? Doppler echocardiography yields dubious estimates of left ventricular diastolic pressures. Circulation 2009, 120, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Little, W.C.; Ohno, M.; Brucks, S.; Morimoto, A.; Cheng, H.J.; Cheng, C.P. Diastolic mitral annular velocity during the development of heart failure. J. Am. Coll. Cardiol. 2003, 41, 1590–1597. [Google Scholar] [CrossRef]
- Ommen, S.R.; Nishimura, R.A.; Appleton, C.P.; Miller, F.A.; Oh, J.K.; Redfield, M.M.; Tajik, A.J. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000, 102, 1788–1794. [Google Scholar] [CrossRef]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of pulmonary artery pressure by echocardiography—A comprehensive review. Int. J. Cardiol. Heart Vasc. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J.; et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: Importance of left atrial strain. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Silva, M.R.; Sampaio, F.; Braga, J.; Ribeiro, J.; Fontes-Carvalho, R. Left atrial strain evaluation to assess left ventricle diastolic dysfunction and heart failure with preserved ejection fraction: a guide to clinical practice: Left atrial strain and diastolic function. Int. J. Cardiovasc. Imaging 2023, 39, 1083–1096. [Google Scholar] [CrossRef]
- Kurt, M.; Wang, J.; Torre-Amione, G.; Nagueh, S.F. Left atrial function in diastolic heart failure. Circ. Cardiovasc. Imaging 2009, 2, 10–15. [Google Scholar] [CrossRef]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef]
- Obokata, M.; Negishi, K.; Kurosawa, K.; Arima, H.; Tateno, R.; Ui, G.; Tange, S.; Arai, M.; Kurabayashi, M. Incremental diagnostic value of LA strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc. Imaging 2013, 6, 749–758. [Google Scholar] [CrossRef]
- Badesch, D.B.; Champion, H.C.; Gomez Sanchez, M.A.; Hoeper, M.M.; Loyd, J.E.; Manes, A.; McGoon, M.; Naeije, R.; Olschewski, H.; Oudiz, R.J.; et al. Diagnosis and assessment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54, S55–S66. [Google Scholar] [CrossRef]
- Gillebert, T.C. Prediction of filling pressures and outcome in heart failure: can we improve E/e'? Eur. Heart J. Cardiovasc. Imaging 2019, 20, 655–657. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Merli, E.; Ciampi, Q.; Scali, M.C.; Zagatina, A.; Merlo, P.M.; Arbucci, R.; Daros, C.B.; de Castro e Silva Pretto, J.L.; Amor, M.; Salame, M.F.; et al. Pulmonary congestion during exercise stress echocardiography in ischemic and heart failure patients. Circ. Cardiovasc. Imaging 2022, 15, e013558. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Andersen, O.S.; Smiseth, O.A. Diastolic stress test: Invasive and noninvasive testing. JACC Cardiovasc. Imaging 2020, 13, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Ciampi, Q.; Arbucci, R.; Cortigiani, L.; Zagatina, A.; Celutkiene, J.; Bartolacelli, Y.; Kane, G.C.; Lowenstein, J.; Pellikka, P. Stress Echo 2030: the new ABCDE protocol defining the future of cardiac imaging. Eur. Heart J. Suppl. 2023, 25, C63–C67. [Google Scholar] [CrossRef]
- Gibby, C.; Wiktor, D.M.; Burgess, M.; Kusunose, K.; Marwick, T.H. Quantitation of the diastolic stress test: filling pressure vs. diastolic reserve. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Kim, K.H.; Kane, G.C.; Luong, C.L.; Oh, J.K. Echocardiographic diastolic stress testing: What does it add? Curr. Cardiol. Rep. 2019, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Studer Bruengger, A.A.; Kaufmann, B.A.; Buser, M.; Hoffmann, M.; Bader, F.; Bernheim, A.M. Diastolic stress echocardiography in the young: a study in nonathletic and endurance-trained healthy subjects. J. Am. Soc. Echocardiogr. 2014, 27, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Oh, J.K.; Pellikka, P.A.; Ommen, S.R.; Stussy, V.L.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J. Am. Soc. Echocardiogr. 2005, 18, 63–68. [Google Scholar] [CrossRef]
- Ha, J.W.; Lulic, F.; Bailey, K.R.; Pellikka, P.A.; Seward, J.B.; Tajik, A.J.; Oh, J.K. Effects of treadmill exercise on mitral inflow and annular velocities in healthy adults. Am. J. Cardiol. 2003, 91, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Al-Gburi, A.J.J. Left ventricular diastolic reserve by exercise stress echocardiography in prediabetes. Tzu Chi Med. J. 2023, 35, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-W.; Choi, D.; Park, S.; Choi, E.-Y.; Shim, C.-Y.; Kim, J.-M.; Ahn, J.-A.; Lee, S.-W.; Oh, J.K.; Chung, N. Left ventricular diastolic functional reserve during exercise in patients with impaired myocardial relaxation at rest. Heart 2009, 95, 399–404. [Google Scholar] [CrossRef]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: A simultaneous invasive-echocardiographic study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A test in context: E/A and E/e' to assess diastolic dysfunction and LV filling pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef]
- Pavlin, E.G.; VanNimwegan, D.; Hornbein, T.F. Failure of a high-compliance low-pressure cuff to prevent aspiration. Anesthesiology 1975, 42, 216–219. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Gharedaghi, M.H.; Zubair, M.; Kohanchi, D.; Aghajani, K.; Candido, K. Speckle- tracking echocardiography for the staging of diastolic dysfunction: The correlation between strain-based indices and the severity of left ventricular diastolic dysfunction. J. Cardiothorac. Vasc. Anesth. 2021, 35, 216–221. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Gharedaghi, M.H.; Shafaroodi, H.; Ghasemi, M.; Aghajani, K.; Candido, K. Left ventricular strain rate for intraoperative evaluation of cardiac diastolic function by transesophageal echocardiography: The correlation between late diastolic peak longitudinal strain rate and the severity of diastolic dysfunction. J. Cardiothorac. Vasc. Anesth. 2022, 36, 178–183. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Gharedaghi, M.H.; Petrossian, V.; Kohanchi, D. Intraoperative assessment of coronary artery stenosis by 2D speckle-tracking echocardiography: The correlation between peak strain rate during early diastole and the severity of coronary artery stenosis in patients undergoing coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.; MacLaren, G.; Chen, R.; Connelly, K.A. Perioperative applications of deformation (myocardial strain) imaging with speckle-tracking echocardiography. J. Cardiothorac. Vasc. Anesth. 2014, 28, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. Measurement of strain and strain rate by echocardiography: ready for prime time? J. Am. Coll. Cardiol. 2006, 47, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Zhang, D.; Huang, R.; Zhang, F.; Wang, Q.; Guo, S. Association of left atrial myocardial function with left ventricular diastolic dysfunction in subjects with preserved systolic function: a strain rate imaging study. Clin. Cardiol. 2010, 33, 643–649. [Google Scholar] [CrossRef]
- Kornev, M.; Caglayan, H.A.; Kudryavtsev, A.V.; Malyutina, S.; Ryabikov, A.; Schirmer, H.; Rosner, A. Influence of hypertension on systolic and diastolic left ventricular function including segmental strain and strain rate. Echocardiography 2023, 40, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Maragiannis, D.; Nagueh, S.F. Echocardiographic evaluation of left ventricular diastolic function: an update. Curr. Cardiol. Rep. 2015, 17, 3. [Google Scholar] [CrossRef]
- Kato, T.; Noda, A.; Izawa, H.; Nishizawa, T.; Somura, F.; Yamada, A.; Nagata, K.; Iwase, M.; Nakao, A.; Yokota, M. Myocardial velocity gradient as a noninvasively determined index of left ventricular diastolic dysfunction in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003, 42, 278–285. [Google Scholar] [CrossRef]
- Kong, L.Y.; Gao, X.; Ding, X.Y.; Wang, G.; Liu, F. Left ventricular end-diastolic strain rate recovered in hypothyroidism following levothyroxine replacement therapy: A strain rate imaging study. Echocardiography 2019, 36, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Kasner, M.; Gaub, R.; Sinning, D.; Westermann, D.; Steendijk, P.; Hoffmann, W.; Schultheiss, H.P.; Tschope, C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure-volume loop analysis. Eur. J. Echocardiogr. 2010, 11, 743–751. [Google Scholar] [CrossRef]
- Hayashi, T.; Yamada, S.; Iwano, H.; Nakabachi, M.; Sakakibara, M.; Okada, K.; Murai, D.; Nishino, H.; Kusunose, K.; Watanabe, K.; et al. Left ventricular global strain for estimating relaxation and filling pressure: A multicenter study. Circ. J. 2016, 80, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Cardiol. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Kosmala, W.; Marwick, T.H. Asymptomatic left ventricular diastolic dysfunction: Predicting progression to symptomatic heart failure. JACC Cardiovasc. Imaging 2020, 13, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Jarasunas, J.; Aidietis, A.; Aidietiene, S. Left atrial strain - an early marker of left ventricular diastolic dysfunction in patients with hypertension and paroxysmal atrial fibrillation. Cardiovasc. Ultrasound 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: have we finally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef]
- Cameli, M.; Sparla, S.; Losito, M.; Righini, F.M.; Menci, D.; Lisi, M.; D'Ascenzi, F.; Focardi, M.; Favilli, R.; Pierli, C.; et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography 2016, 33, 398–405. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Khan, S.U. Left atrial strain for assessment of left ventricular diastolic function: Focus on populations with normal LVEF. JACC Cardiovasc. Imaging 2023, 16, 691–707. [Google Scholar] [CrossRef]
- Hewing, B.; Theres, L.; Spethmann, S.; Stangl, K.; Dreger, H.; Knebel, F. Left atrial strain predicts hemodynamic parameters in cardiovascular patients. Echocardiography 2017, 34, 1170–1178. [Google Scholar] [CrossRef]
- Yeung, D.F.; Abolmaesumi, P.; Tsang, T.S.M. Artificial intelligence for left ventricular diastolic function assessment: A new paradigm on the horizon. J. Am. Soc. Echocardiogr. 2023, 36, 1079–1082. [Google Scholar] [CrossRef]
- Othman, F.; Abushahba, G.; Salustri, A. Adherence to the American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations for the evaluation of left ventricular diastolic function by echocardiography: A quality improvement project. J. Am. Soc. Echocardiogr. 2019, 32, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Park, J.J.; Ali, T.; Lee, S. Artificial intelligence for the diagnosis of heart failure. NPJ Digit. Med. 2020, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Salem Omar, A.M.; Shameer, K.; Narula, S.; Abdel Rahman, M.A.; Rifaie, O.; Narula, J.; Dudley, J.T.; Sengupta, P.P. Artificial intelligence-based assessment of left ventricular filling pressures from 2-dimensional cardiac ultrasound images. JACC Cardiovasc. Imaging 2018, 11, 509–510. [Google Scholar] [CrossRef]
- Pandey, A.; Kagiyama, N.; Yanamala, N.; Segar, M.W.; Cho, J.S.; Tokodi, M.; Sengupta, P.P. Deep-learning models for the echocardiographic assessment of diastolic dysfunction. JACC Cardiovasc. Imaging 2021, 14, 1887–1900. [Google Scholar] [CrossRef]
- Chiou, Y.A.; Hung, C.L.; Lin, S.F. AI-assisted echocardiographic prescreening of heart failure with preserved ejection fraction on the basis of intrabeat dynamics. JACC Cardiovasc. Imaging 2021, 14, 2091–2104. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F.; Zhang, P.; Lin, X.; Wang, W.; Pu, H.; Chen, X.; Chen, Y.; Yu, L.; Deng, Y.; et al. Artificial intelligence-assisted left ventricular diastolic function assessment and grading: Multiview versus single view. J. Am. Soc. Echocardiogr. 2023, 36, 1064–1078. [Google Scholar] [CrossRef]
- Beladan, C.C.; Botezatu, S.; Popescu, B.A. Reversible left ventricular diastolic dysfunction—Overview and clinical implications. Echocardiography 2020, 37, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Matyal, R.; Skubas, N.J.; Shernan, S.K.; Mahmood, F. Perioperative assessment of diastolic dysfunction. Anesth. Analg. 2011, 113, 449–472. [Google Scholar] [CrossRef]
- Huttin, O.; Fraser, A.G.; Lund, L.H.; Donal, E.; Linde, C.; Kobayashi, M.; Erdei, T.; Machu, J.L.; Duarte, K.; Rossignol, P.; et al. Risk stratification with echocardiographic biomarkers in heart failure with preserved ejection fraction: the media echo score. ESC Heart Fail. 2021, 8, 1827–1839. [Google Scholar] [CrossRef]
- Vasan, R.S.; Xanthakis, V.; Lyass, A.; Andersson, C.; Tsao, C.; Cheng, S.; Aragam, J.; Benjamin, E.J.; Larson, M.G. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham study: An echocardiographic study over 3 decades. JACC Cardiovasc. Imaging 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Morris, D.A.; Ma, X.X.; Belyavskiy, E.; Aravind Kumar, R.; Kropf, M.; Kraft, R.; Frydas, A.; Osmanoglou, E.; Marquez, E.; Donal, E.; et al. Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: a meta-analysis. Open Heart 2017, 4, e000630. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, S.; Wolsk, E.; Granton, D.; Azevedo, L.; Valle, F.H.; Gustafsson, F.; Mak, S. Pulmonary arterial wedge pressure at rest and during exercise in healthy adults: A systematic review and meta-analysis. J. Card. Fail. 2019, 25, 114–122. [Google Scholar] [CrossRef]
- Malik, A.; Brito, D.; Vaqar, S.; Chhabra, L. Congestive heart failure. In StatPearls; Treasure Island (FL), 2023.
- Saha, S.K.; Kiotsekoglou, A.; Nanda, N.C. Echocardiography 2020: Toward deciphering the "Rosetta stone" of left ventricular diastolic function. Echocardiography 2020, 37, 1886–1889. [Google Scholar] [CrossRef]
- Shah, A.M.; Cikes, M.; Prasad, N.; Li, G.; Getchevski, S.; Claggett, B.; Rizkala, A.; Lukashevich, I.; O'Meara, E.; Ryan, J.J.; et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 2019, 74, 2858–2873. [Google Scholar] [CrossRef]
- Chetrit, M.; Cremer, P.C.; Klein, A.L. Imaging of diastolic dysfunction in community-based epidemiological studies and randomized controlled trials of HFpEF. JACC Cardiovasc. Imaging 2020, 13, 310–326. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Borlaug, B.A. Diastolic dysfunction and heart failure with preserved ejection fraction: Understanding mechanisms by using noninvasive methods. JACC Cardiovasc. Imaging 2020, 13, 245–257. [Google Scholar] [CrossRef]
- Harada, T.; Kagami, K.; Kato, T.; Obokata, M. Echocardiography in the diagnostic evaluation and phenotyping of heart failure with preserved ejection fraction. J. Cardiol. 2022, 79, 679–690. [Google Scholar] [CrossRef]
- Lundberg, A.; Johnson, J.; Hage, C.; Back, M.; Merkely, B.; Venkateshvaran, A.; Lund, L.H.; Nagy, A.I.; Manouras, A. Left atrial strain improves estimation of filling pressures in heart failure: a simultaneous echocardiographic and invasive haemodynamic study. Clin. Res. Cardiol. 2019, 108, 703–715. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e34–e61. [Google Scholar] [CrossRef]
- Belyavskiy, E.; Morris, D.A.; Url-Michitsch, M.; Verheyen, N.; Meinitzer, A.; Radhakrishnan, A.K.; Kropf, M.; Frydas, A.; Ovchinnikov, A.G.; Schmidt, A.; et al. Diastolic stress test echocardiography in patients with suspected heart failure with preserved ejection fraction: a pilot study. ESC Heart Fail. 2019, 6, 146–153. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Mazur, W.; Truong, V.T.; Nagueh, S.F.; Fowler, J.A.; Shelton, K.; Joshi, V.M.; Ness, K.K.; Srivastava, D.K.; Robison, L.L. Prevalence of diastolic dysfunction in adult survivors of childhood cancer: a report from SJLIFE cohort. Cardiooncology 2023, 5, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; O'Meara, E.; Desai, A.S.; Heitner, J.F.; Li, G.; Fang, J.; et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ. Heart Fail. 2014, 7, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; Garcia-Izquierdo, E.; Chetrit, M.; Monivas-Palomero, V.; Mingo-Santos, S.; Andersen, O.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef]
- Singh, A.; Medvedofsky, D.; Mediratta, A.; Balaney, B.; Kruse, E.; Ciszek, B.; Shah, A.P.; Blair, J.E.; Maffessanti, F.; Addetia, K.; et al. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int. J. Cardiovasc. Imaging 2019, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Telles, F.; Nanayakkara, S.; Evans, S.; Patel, H.C.; Mariani, J.A.; Vizi, D.; William, J.; Marwick, T.H.; Kaye, D.M. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Kohncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Fontes-Carvalho, R.; Vilela, E.M.; Bettencourt, P.; Leite-Moreira, A.; Azevedo, A. Diastolic function is impaired in patients with prehypertension: Data from the EPIPorto study. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Park, J.H. Role of strain echocardiography in patients with hypertension. Clin. Hypertens. 2022, 28, 6. [Google Scholar] [CrossRef]
- Wan, S.-H.; Vogel, M.W.; Chen, H.H. Pre-clinical diastolic dysfunction. J. Am. Coll. Cardiol. 2014, 63, 407–416. [Google Scholar] [CrossRef]
- Cameli, M.; Lembo, M.; Sciaccaluga, C.; Bandera, F.; Ciccone, M.M.; D'Andrea, A.; D'Ascenzi, F.; Esposito, R.; Evola, V.; Liga, R.; et al. Identification of cardiac organ damage in arterial hypertension: insights by echocardiography for a comprehensive assessment. J. Hypertens. 2020, 38, 588–598. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Thijs, L.; Knez, J.; Herbots, L.; Zhang, Z.; Staessen, J.A. Prognostic value of left ventricular diastolic dysfunction in a general population. J. Am. Heart Assoc. 2014, 3, e000789. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Marwick, T.H. Phenotyping the hypertensive heart. Eur. Heart J. 2022, 43, 3794–3810. [Google Scholar] [CrossRef]
- Cuspidi, C.; Meani, S.; Fusi, V.; Valerio, C.; Catini, E.; Sala, C.; Sampieri, L.; Magrini, F.; Zanchetti, A. Prevalence and correlates of left atrial enlargement in essential hypertension: role of ventricular geometry and the metabolic syndrome: the Evaluation of Target Organ Damage in Hypertension study. J. Hypertens. 2005, 23, 875–882. [Google Scholar] [CrossRef]
- Wu, V.C.; Takeuchi, M.; Kuwaki, H.; Iwataki, M.; Nagata, Y.; Otani, K.; Haruki, N.; Yoshitani, H.; Tamura, M.; Abe, H.; et al. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc. Imaging 2013, 6, 1025–1035. [Google Scholar] [CrossRef]
- Astarita, C.; Palinkas, A.; Nicolai, E.; Maresca, F.S.; Varga, A.; Picano, E. Dipyridamole-atropine stress echocardiography versus exercise SPECT scintigraphy for detection of coronary artery disease in hypertensives with positive exercise test. J. Hypertens. 2001, 19, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Fazlinezhad, A.; Jalalyazdi, M.; Gharaee, A.; Hosseini, L.; Andalibi, M.S.S. Diastolic function changes during stress echocardiography in hypertensive patients. Razavi Int. J. Med. 2017, 5, e42876. [Google Scholar] [CrossRef]
- Peteiro, J.; Pazos, P.; Bouzas, A.; Pinon, P.; Estevez, R.; Castro-Beiras, A. Assessment of diastolic function during exercise echocardiography: annulus mitral velocity or transmitral flow pattern? J. Am. Soc. Echocardiogr. 2008, 21, 178–184. [Google Scholar] [CrossRef]
- Dini, F.L.; Galderisi, M.; Nistri, S.; Buralli, S.; Ballo, P.; Mele, D.; Badano, L.P.; Faggiano, P.; De Gregorio, C.; Rosa, G.M. Abnormal left ventricular longitudinal function assessed by echocardiographic and tissue Doppler imaging is a powerful predictor of diastolic dysfunction in hypertensive patients: the SPHERE study. Int. J. Cardiol. 2013, 168, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ni, C.F.; Yang, C.; Yan, Z.N.; Fan, L. Assessment of subclinical left atrial myocardial dysfunction in essential hypertension patients with normal left ventricle function by two-dimensional strain and volume-derived variables. J. Clin. Ultrasound 2021, 49, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, T.; Ilic, A.; Milovancev, A.; Bjelobrk, M.; Stefanovic, M.; Stojsic-Milosavljevic, A.; Tadic, S.; Golubovic, M.; Popov, T.; Petrovic, M. Left atrial strain as a predictor of left ventricular diastolic dysfunction in patients with arterial hypertension. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef]
- Carluccio, E.; Cameli, M.; Rossi, A.; Dini, F.L.; Biagioli, P.; Mengoni, A.; Jacoangeli, F.; Mandoli, G.E.; Pastore, M.C.; Maffeis, C. Left atrial strain in the assessment of diastolic function in heart failure: a machine learning approach. Circ. Cardiovasc. Imaging 2023, 16, e014605. [Google Scholar] [CrossRef]
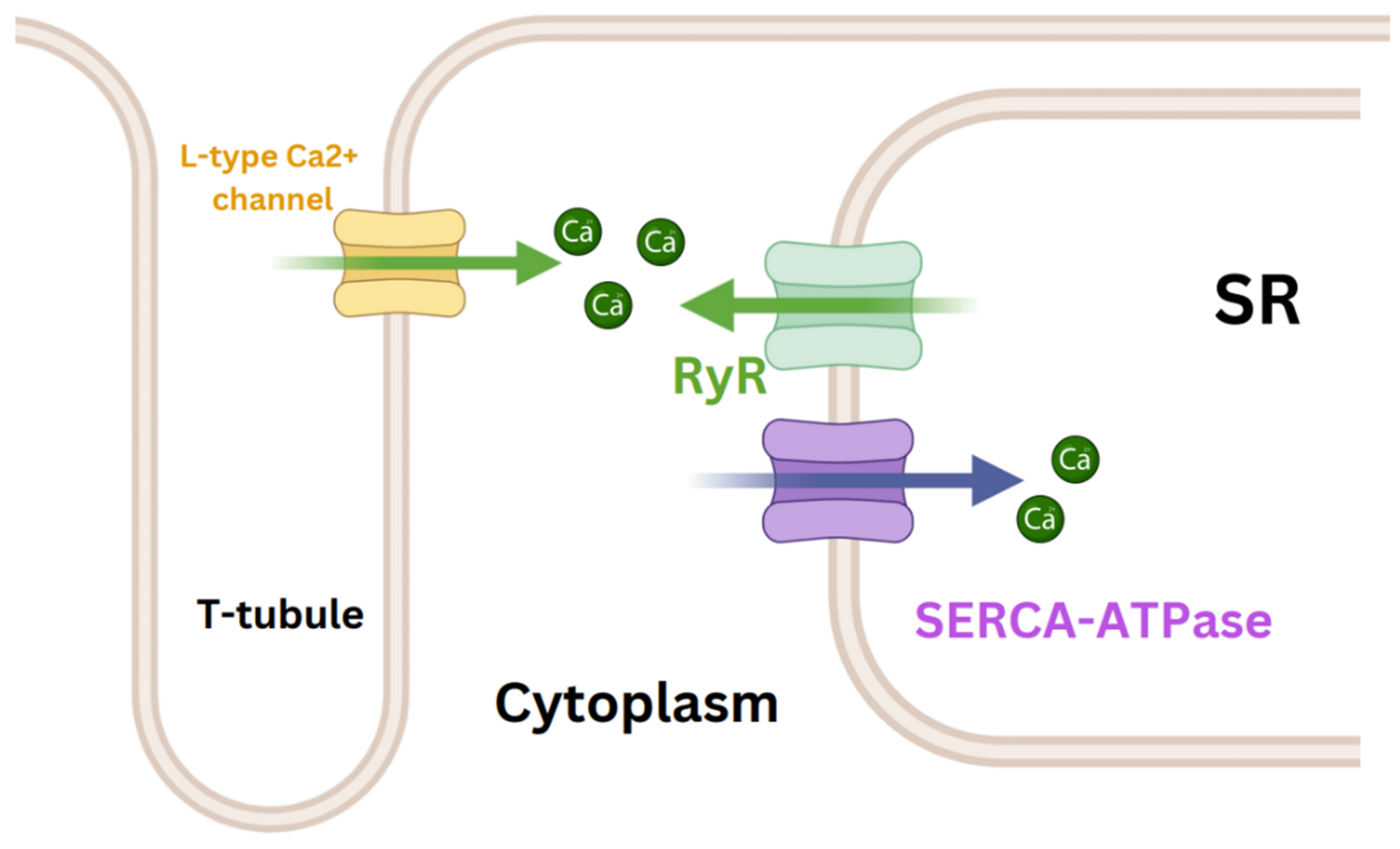
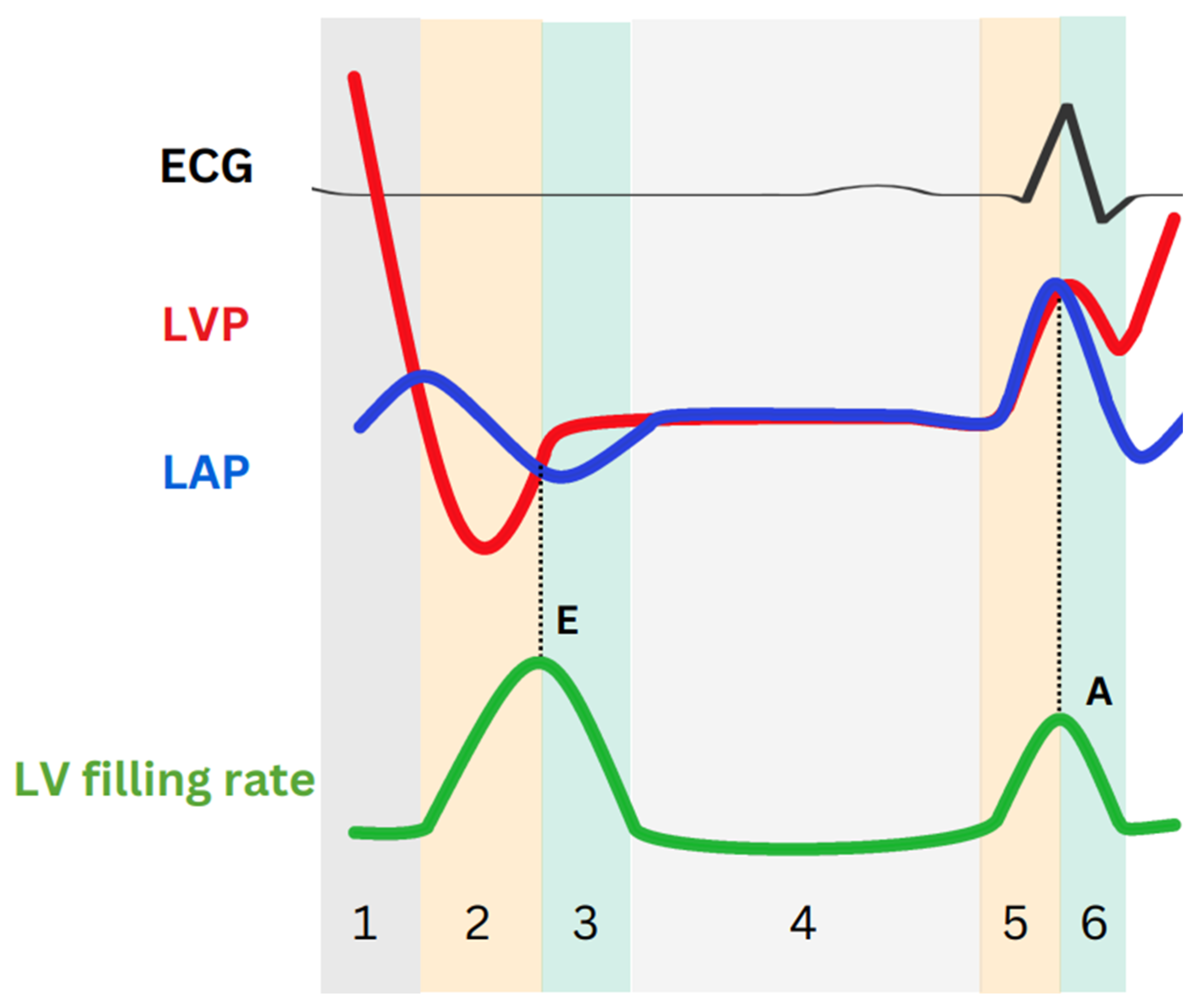
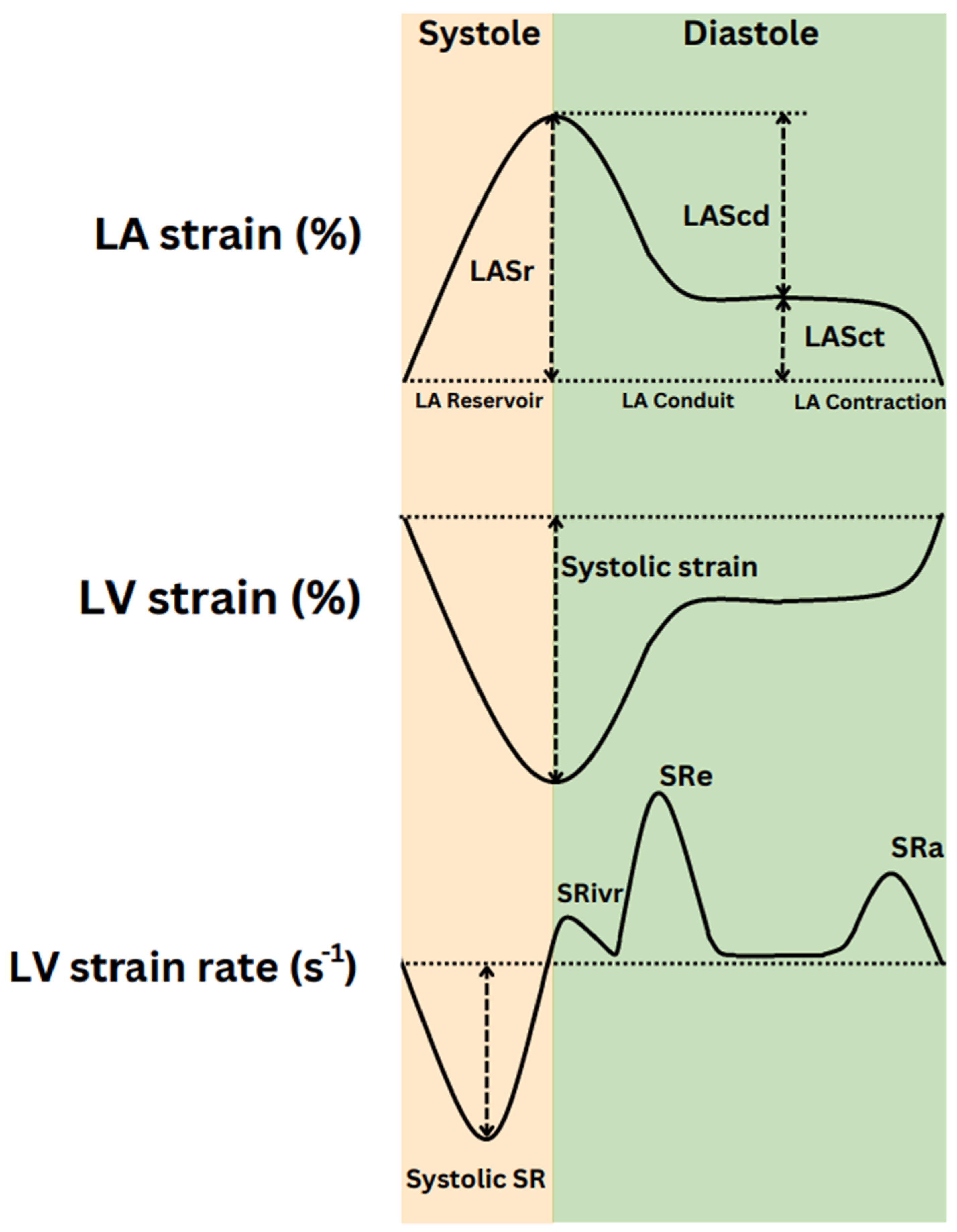
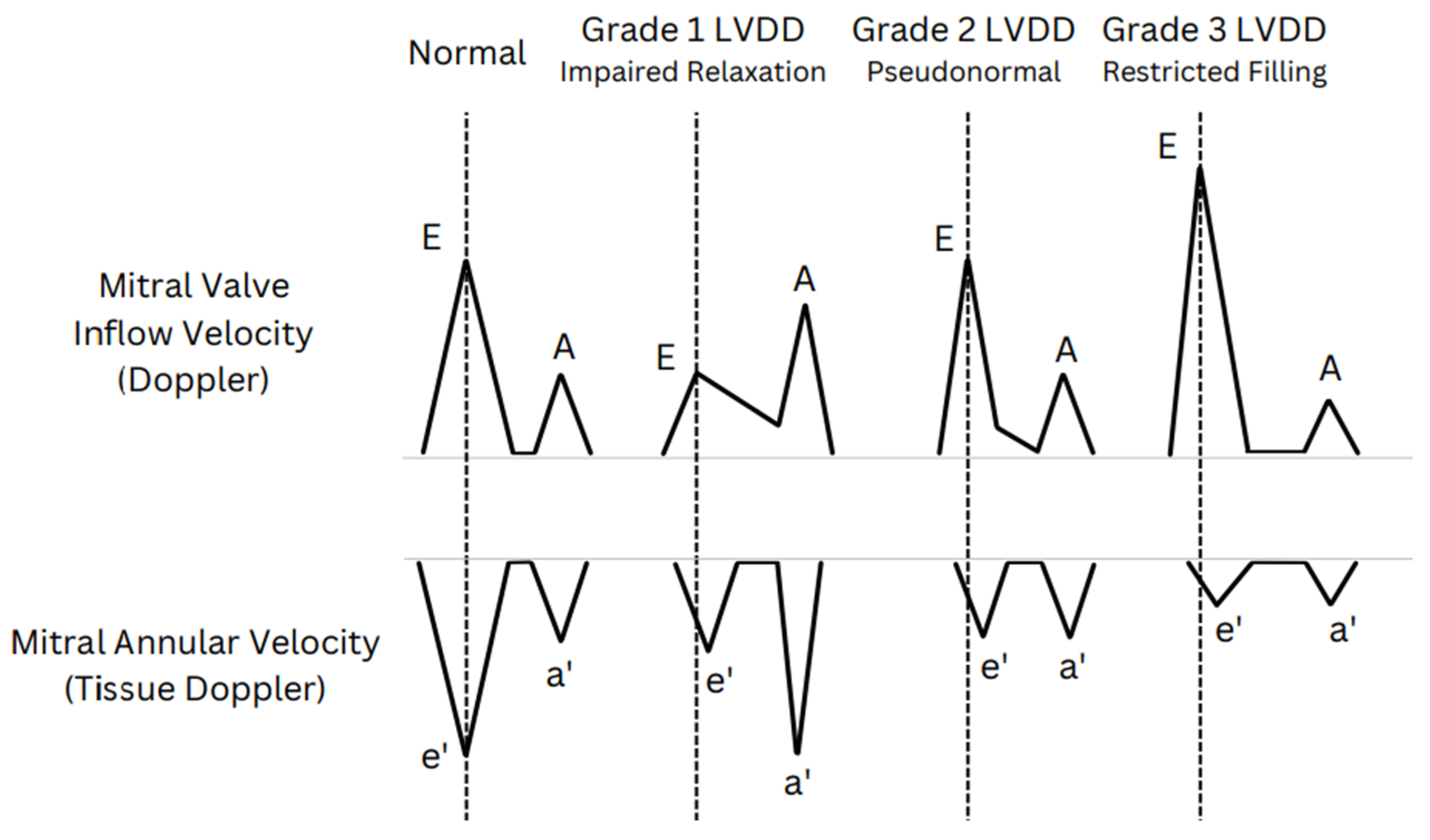
| In Figure 2 | Cardiac phase | Factors affecting | Parameter |
|---|---|---|---|
| 1 | Isovolumetric relaxation (LAP < LVP) |
Active relaxation (primary) induced by SR uptake of Ca2+ |
|
| 2 | Early rapid filling (LAP > LVP) |
|
|
| 3 | Inflow deceleration (LAP < LVP) | LV stiffness | E wave deceleration time |
| 4 | Diastasis (LAP = LVP) |
|
|
| 5 | LA contraction (LAP > LVP) | LAP | A wave peak |
| 6 | LA relaxation | LA stiffness | E/e′/LAS |
| E Wave (early filling) |
e' Wave (relaxation rate) |
E/e’ Ratio (filling pressure) |
|
|---|---|---|---|
| Normal diastolic function | ↑ | ↑ | N |
| Diastolic dysfunction | ↑ | Slight↑/N | ↑↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





