Submitted:
15 March 2024
Posted:
15 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
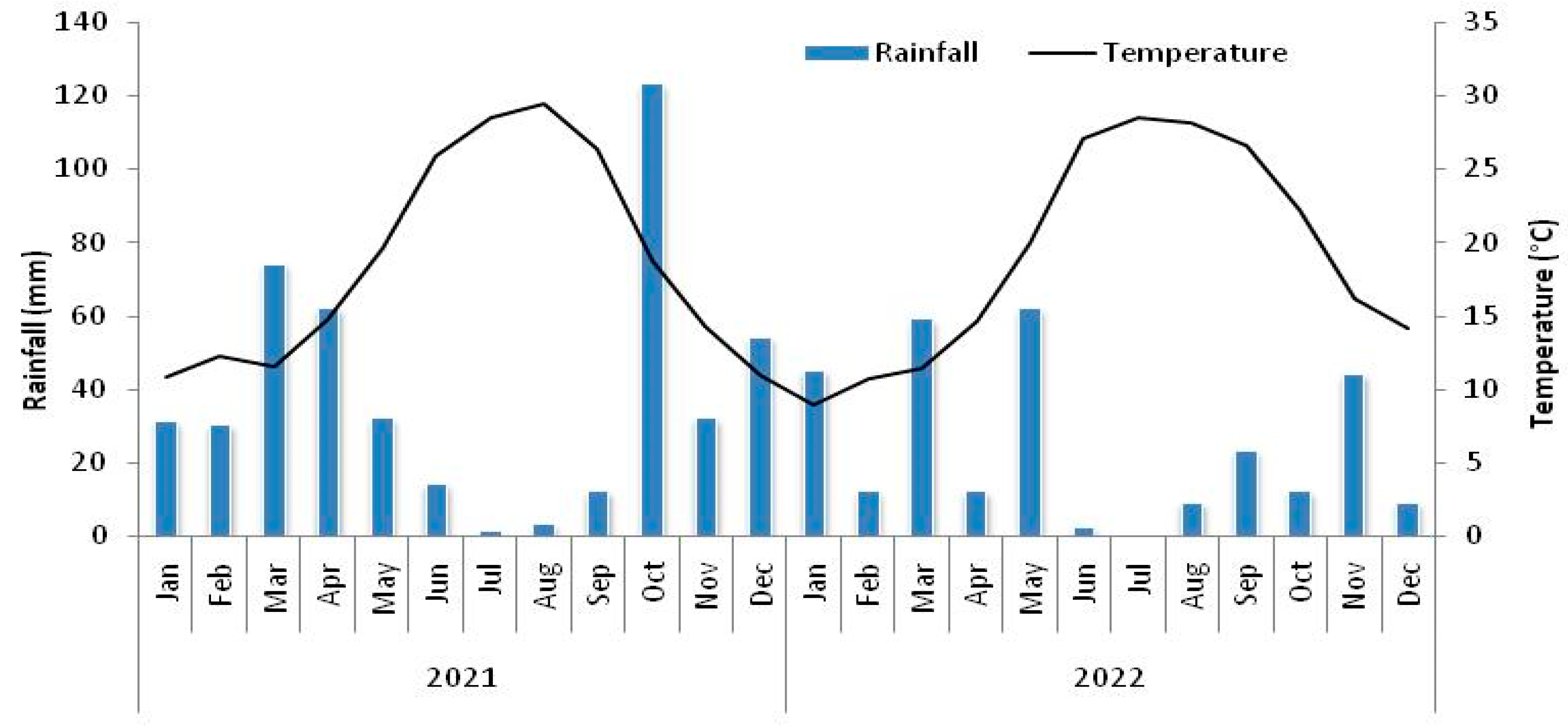
2.1. Description of the Study Area and Experimental Design
2.2. Experimental Design
2.3. Cover Crops Sampling and Analysis
2.4. Soil Sampling and Analysis
2.5. Soil Functional Quality
2.5.1. Enzymatic Activity
2.5.2. Microbial Diversity
2.6. Statistical Analysis
3. Results
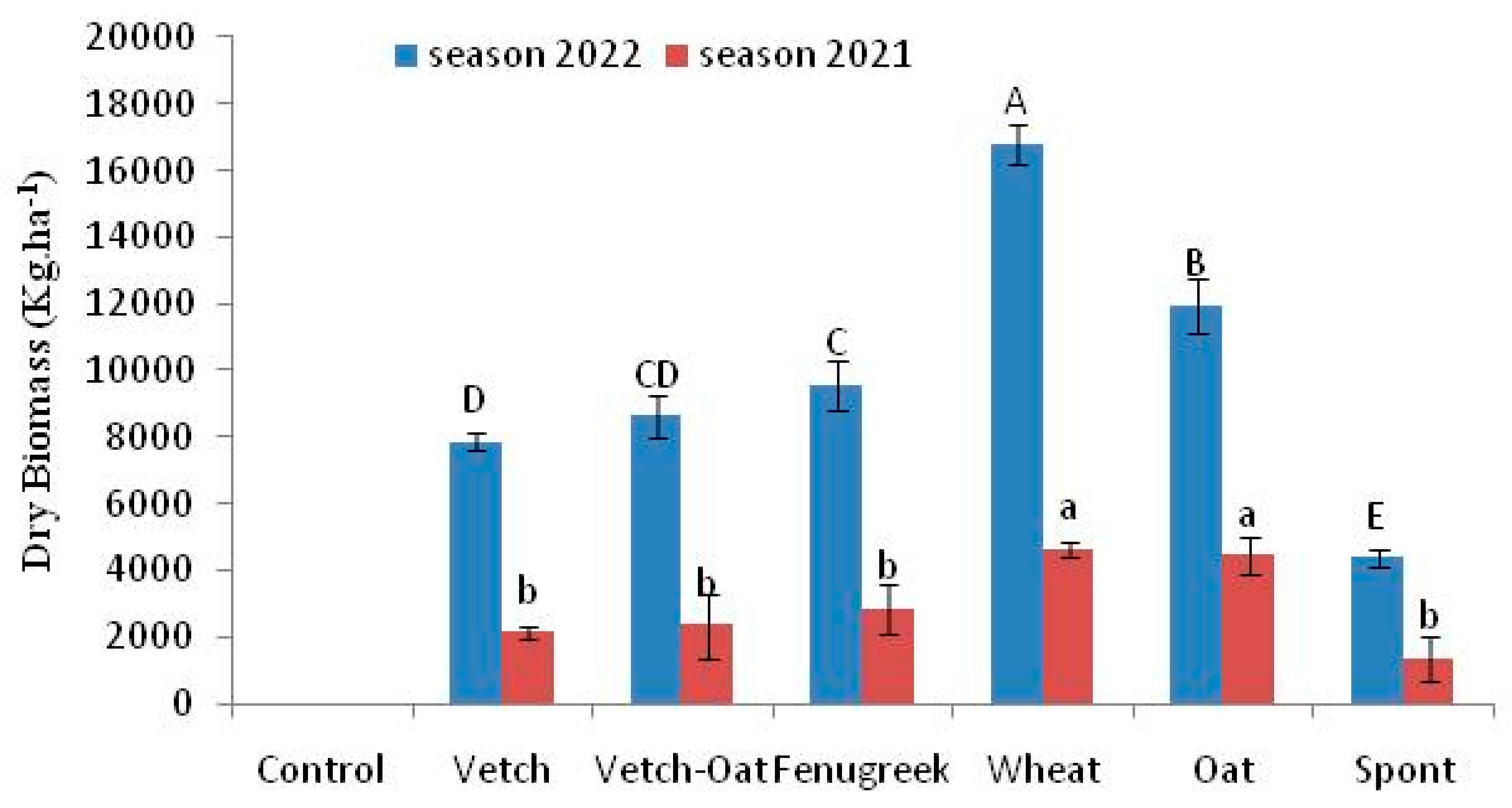
3.1. Cover Crops and Residues Dry Biomass Incorporated into the Soil
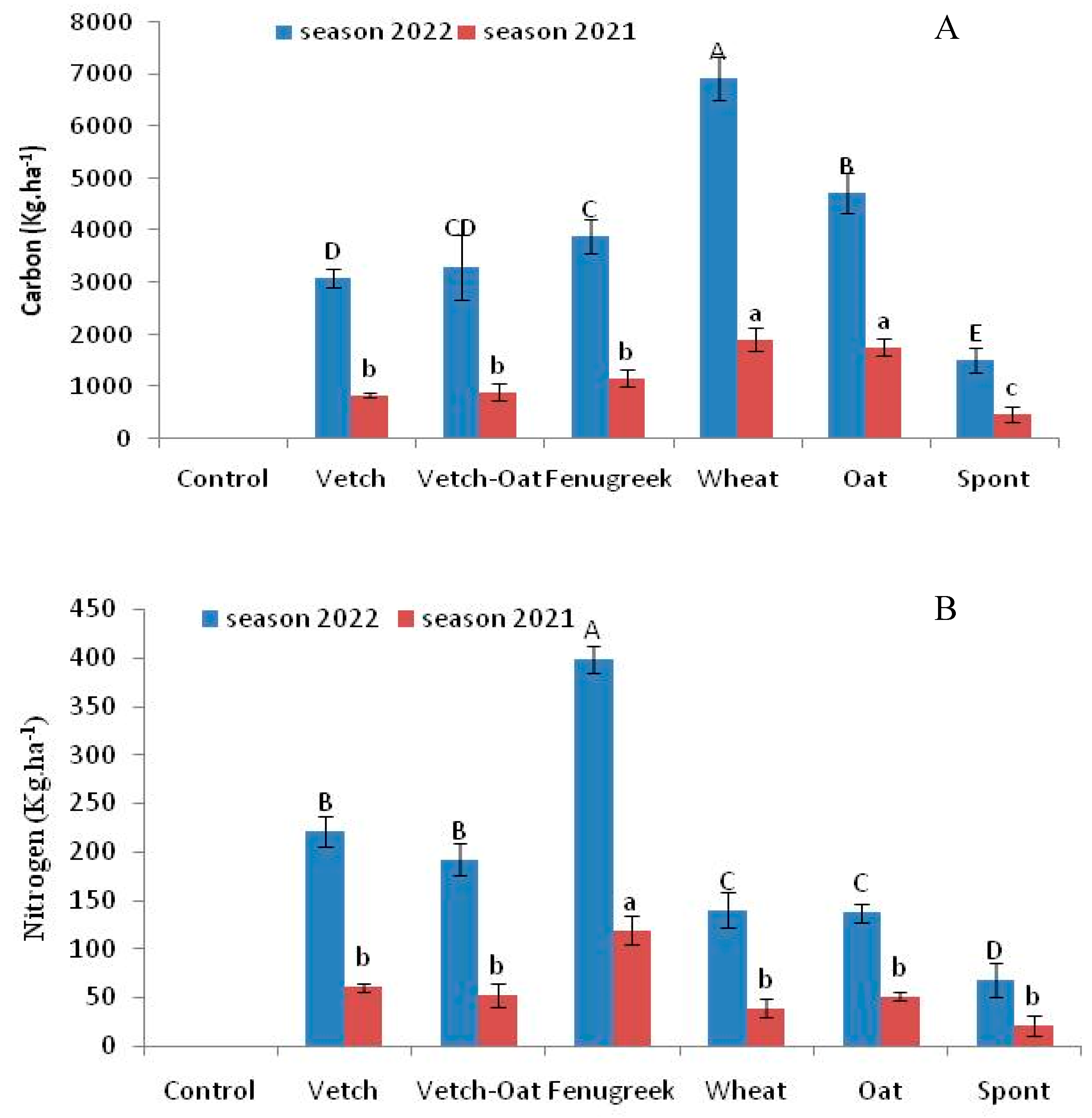
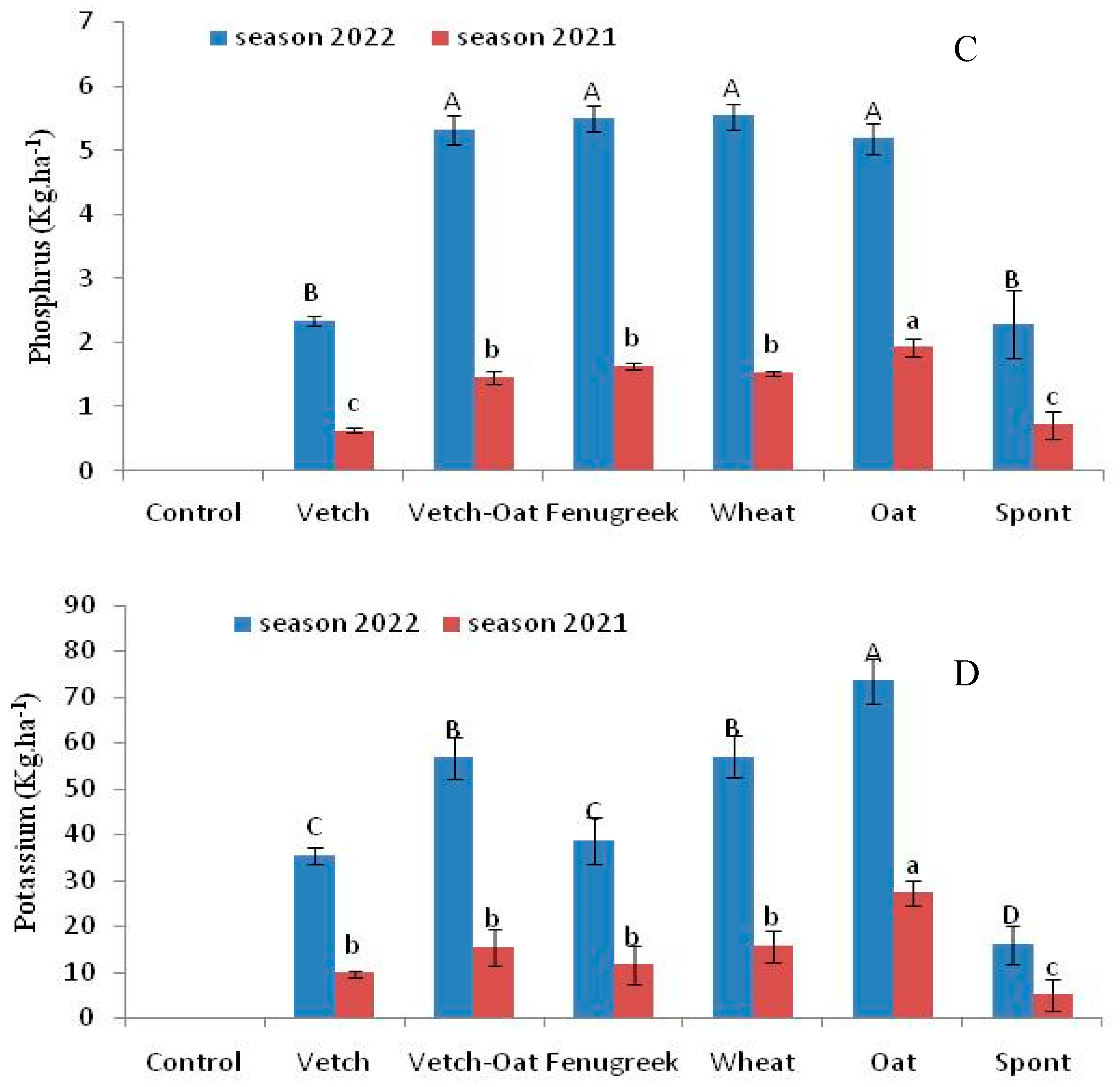
3.2. Nutrients and Carbon Retention by Cover Crops and Residues Incorporated in the Soil
3.3. Soil Organic Matter and Macronutrients Availabilities after Residues and Cover Crops Incorporation
3.4. Soil Functional Quality
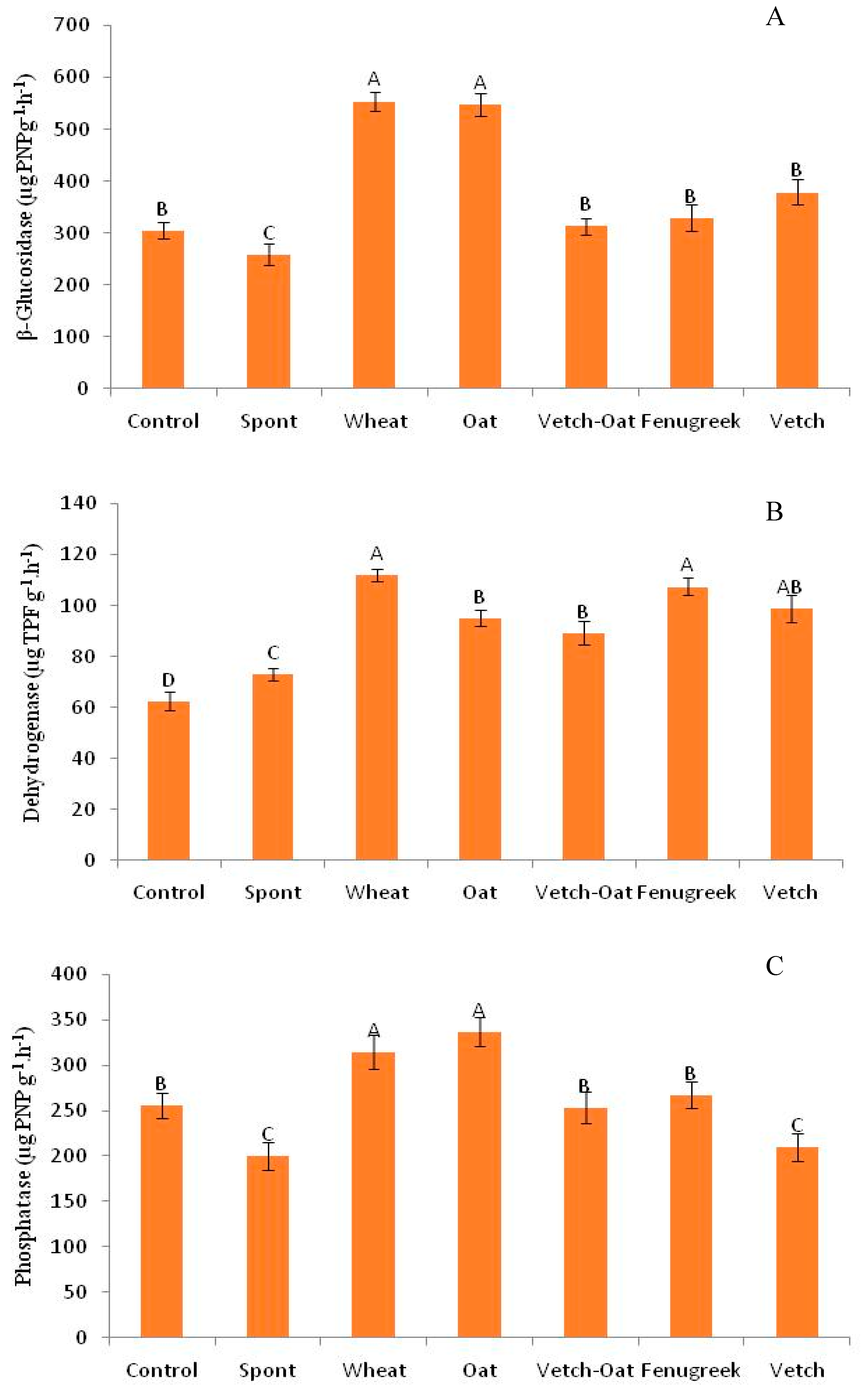
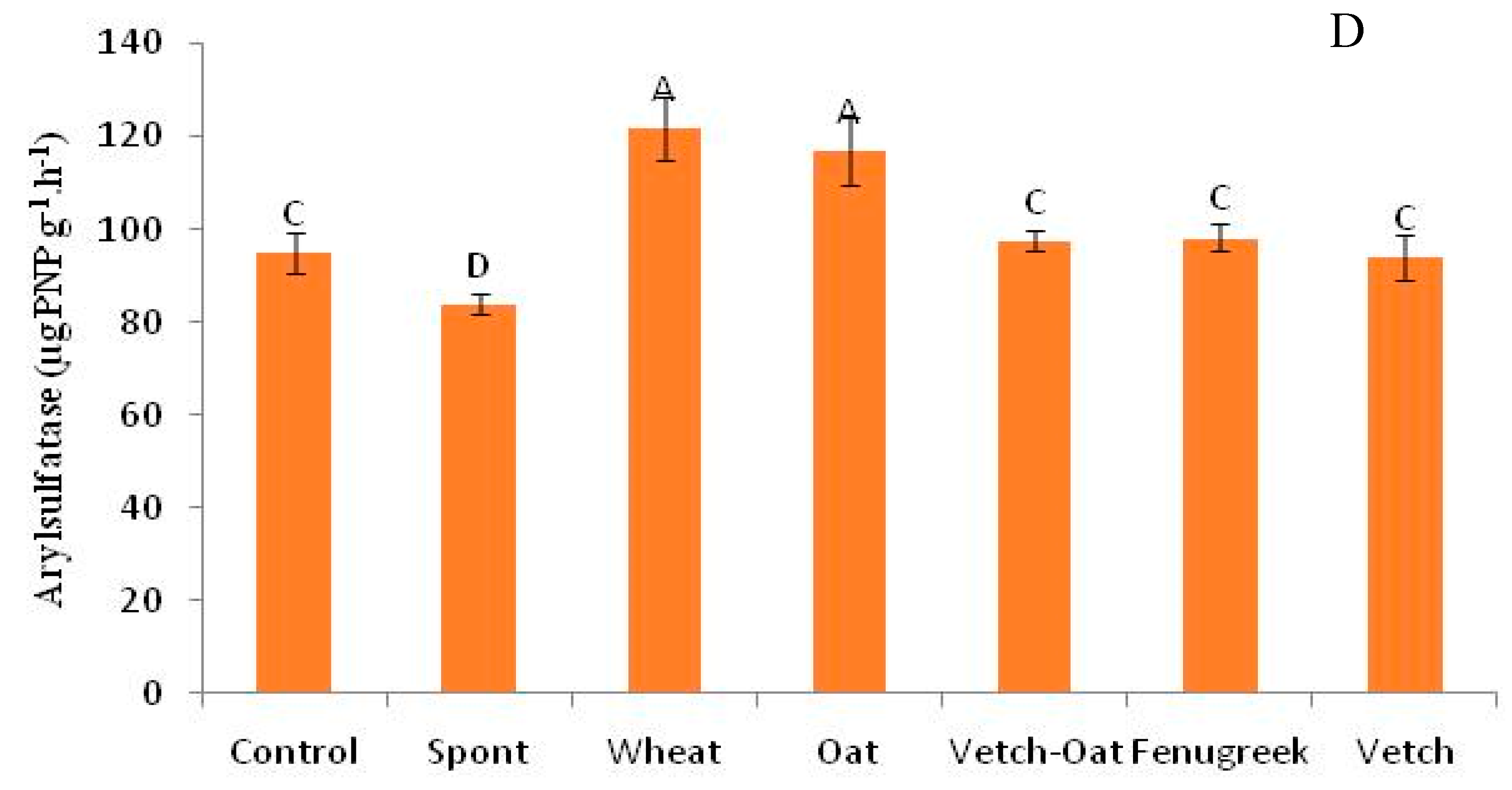
3.4.1. Soil Enzymatic Activities
3.4.2. Carbon Utilization Sources
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OLIVAE. Official journal of the international olive council.2017, (n.d.). www.internationaloliveoil.
- Ben Abdallah, S.; Elfkih, S.; Suárez-Rey, E. M.; Parra-López, C.; Romero-Gámez, M. Evaluation of the environmental sustainability in the olive growing systems in Tunisia. Journal of Cleaner Production. 2021, 282, 124526. [Google Scholar] [CrossRef]
- Elfkih, S.; Hadiji, O.; Ben Abdallah, S.; Boussadia, O. Water Accounting for Food Security: Virtual Water and Water Productivity in the Case of Tunisian Olive Oil Value Chain. Agriculture, 2023, 13, 13061205. [Google Scholar] [CrossRef]
- DGPA. Document de la Direction Générale de la Production Agricole en Tunisie. 2021.
- ONH. Rapport de l’Office National de l’Huile en Tunisie. 2023.
- Gargouri, K.; Mhiri, A. Relalionship between soil fertility, phosphorus and potassium nutrition on the olive in Tunisia. Opt. Mediterranean’s. 2003, 50, 199–204. [Google Scholar]
- Chehab, H.; Tekaya, M.; Ouhibi, M.; Gouiaa, M.; Zakhama, H.; Mahjoub, Z.; Laamari, S.; Sfina, H.; Chihaoui, B.; Boujnah, D.; Mechri, B. Effects of compost, olive mill wastewater and legume cover cropson soil characteristics, tree performance and oil quality of olive trees cv.Chemlali grown under organic farming system. Scientia Horticulturae. 2019, 253, 163–171. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Brahmi, F.; Dabbou, S.; Ben Hassine, K.; Taamali, A.; Chehab, H.; Ellouz, M.; Zarrouk, M.; Hammami, M. Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chemistry. 2010, 119, 220–225. [Google Scholar] [CrossRef]
- Ben Youssef, N.; Zarrouk, W.; Carrasco-Pancorbo, A.; Zarrouk, M. Effect of olive ripeness on chemical properties and phenolic composition of Chétoui virgin olive oil. Journal of The Science of Food and Agriculture. 2010, 90, 199–204. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Kamran, M.; Chen, X.; Tan, H.; Long, M. Effects of Grass Inter-Planting on Soil Nutrients, Enzyme Activity, and Bacterial Community Diversity in an Apple Orchard. Frontiers in Plant Science. 2022, 13, 901143. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Patterson, J. How can science policy help to deliver the global goals. The Guardian, Science Policy Blog. 2015, 10, 17–25. [Google Scholar]
- Koudahe, K.; Allen, S. C.; Djaman, K. Critical review of the impact of cover crops on soil properties. International Soil and Water Conservation Research. 2022, 10, 343–354. [Google Scholar] [CrossRef]
- Alessandro, Piccolo. The Nature of Soil Organic Matter and Innovative Soil Managements to Fight Global Changes and Maintain Agricultural Productivity. Carbonn Sequestration in Agricultural Soils 2012, 1–19.
- Blanco-Canqui, H.; Mikha, M. M.; Presley, D. R.; Claassen, M. M. Addition of Cover Crops Enhances No-Till Potential for Improving Soil Physical Properties. Soil Science Society of America Journal. 2011, 75, 1471–1482. [Google Scholar] [CrossRef]
- Beniaich, A.; Guimarães, V.D.; Avanzi, J.C.; Silva, B.M.; Salvador, F.A.G.; Santos, W.P.; Silva, M.L.N. Spontaneous vegetation as an alternative to cover crops in olive orchards reduces water erosion and improves soil physical properties under tropical conditions. Agricultural Water Management. 2023, 279, 108186. [Google Scholar] [CrossRef]
- Fernandez, O.R.; Ruibérriz de Torres, M.A.R.; Garcia, J.M., Garcia, M.M., Bojollo, R.M.C.; Legumes used as cover crops to reduce fertilization problems improving soil nitrate in an organic orchard. European Journal of Agronomy. 2018, 95, 1–13. [CrossRef]
- Huertas, A. J.; Cuartero, J.; Ros, M.; Pascual, J. A.; Parras-Alcántara, L.; González-Rosado, M.; Özbolat, O.; Zornoza, R.; Egea-Cortines, M.; Hurtado-Navarro, M.; & Lozano-García, B. How binomial (traditional rainfed olive grove-Crocus sativus) crops impact the soil bacterial community and enhance microbial capacities. Journal of Environmental Management, 2023, 3023, 345.
- Adetunji, A. T.; Ncube, B.; Mulidzi, R.; & Lewu, F. B. Management impact and benefit of cover crops on soil quality: A review. Soil and Tillage Research. 2020, 204.
- Sofo, A.; Palese, A.; Maria, C.; Teresa, C.; Giuseppe, R.P.; Curci, M.; Crecchio, C.; Xiloyannis; C.; Genetic, Functional, and Metabolic Responses of Soil Microbiota in a Sustainable Olive Orchard. Soil Science 2010, 175.
- Sofo, A.; Ciarfaglia, A.; Scopa, A.; Camele, I.; Curci, M.; Crecchio, C.; Xiloyannis, C.; Palese, A. M. Soil microbial diversity and activity in a Mediterranean olive orchard using sustainable agricultural practices. Soil Use and Management. 2014, 30, 160–167. [Google Scholar] [CrossRef]
- Montes-Borrego, M.; Metsis, M.; Blanca B Landa, B.B. Arbuscular Mycorhizal Fungi Associated with the Olive Crop across the Andalusian Landscape: Factors Driving Community Differentiation. PLoS ONE, 2014, 9, e96397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, W.; Xue, J.; Jing, Z.; Qiu, L.; Chen, X.; Hu, F.; Kardol, P.; Liu, M. Leveraging functional traits of cover crops to coordinate crop productivity and soil health. Journal of Applied Ecology 2022, 59, 2627–2641. [Google Scholar] [CrossRef]
- Samuel, I.; Haruna, N. N. Influence of Cover Crop, Tillage, and Crop Rotation Management on Soil Nutrients. Soil Biology & Biochemistry 2020, 25, 142–149. [Google Scholar]
- Bernnan, B.E.; Veronica, A.M. . Soil microbial biomass and enzyme data after six years of cover crop and compost treatments in organic vegetable production. Soil.Sci.Soc.Am.J. 2019, 83, 624–637. [Google Scholar] [CrossRef]
- Herencia, J. F. Enzymatic activities under different cover crop management in a Mediterranean olive orchard. Biological Agriculture and Horticulture. 2015, 31, 45–52. [Google Scholar] [CrossRef]
- Henriquez, C.; Uribe, L.; Valenciano, A.; Nogales, R. Soil enzyme activity -dehidrogenase, ß-glucosidase, Phosphatase and urease-under different crops. Agron. Costarricense. 2014, 38, 43–54. [Google Scholar]
- Weber K, Legge R.; One-dimensional metric for tracking bacterial community divergence using sole carbon source utilization pat -terns. J Microbiol Methods. 2009, 79, 55–61. [CrossRef]
- Tabatabai, M.A.; Soil enzymes. Methods of Soil Analysis: Microbiological and Biochemical Properties. Part 2. SSSA Book Ser, Madison. 1994, 775–833.
- Grzadiel, J.; Furtak, K.; Galazka, A. Community-Level physiological profiles of microorganism from different types of soil that are characteristic to Poland-a long-term microplot experiment. Sustainability. 2019, 11, 56–62. [Google Scholar] [CrossRef]
- Ben zineb, A.; Barkaoui, K.; Karray, F.; Mhiri, N.; Sayadi, S.; Mliki, A.; Gargouri, M. Olive agroforestry shapes rhizosphere microbiome networks associated with annual crops and impacts the biomass production under low-rainfed conditions. Frontiers in Microbiology. 2022, 13, 977797. [Google Scholar] [CrossRef]
- Amassaghrou, A.; Barkaoui, K.; Bouaziz, A.; Alaoui, S. B.; Fatemi, Z. E. A.; Daoui, K. Yield and related traits of three legume crops grown in olive-based agroforestry under an intense drought in the South Mediterranean. Saudi Journal of Biological Sciences. 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Temani, F.; Bouaziz, A.; Daoui, K.; Wery, J.; Barkaoui, K. Olive agroforestry can improve land productivity even under low water availability in the South Mediterranean. Agriculture, Ecosystems and Environment. 2021, 307, 107234. [Google Scholar] [CrossRef]
- Torrús-Castillo, M.; Domouso, P.; Herrera-Rodríguez, J. M.; Calero, J.; García-Ruiz, R. Aboveground Carbon Fixation and Nutrient Retention in Temporary Spontaneous Cover Crops in Olive Groves of Andalusia. Frontiers in Environmental Science. 2022, 10, 868410. [Google Scholar] [CrossRef]
- Arruda, B. Manipulation of the soil microbiome regulates the colonization of plants by arbuscular mycorrhizal fungi. Mycorrhiza. 2021, 31, 31–545. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M. Â.; Dimande, P.; Pereira, E. L.; Ferreira, I. Q.; Freitas, S.; Correia, C. M.; Moutinho-Pereira, J.; Arrobas, M. Early-maturing annual legumes: an option for cover cropping in rainfed olive orchards. Nutrient Cycling in Agroecosystems 2015, 103, 153–166. [Google Scholar] [CrossRef]
- Soriano, M.; Cabezas, J.M.; Gomez, A.J. Field evaluation of selected autochthonous herbaceous species for cover crops in Mediterranean woody crops. European Journal of Agronomy 2023, 143, 126723. [Google Scholar] [CrossRef]
- Tul, S.; Manolikaki, I.; Digalaki, N.; Psarras, G.; Koufakis, I.; Kalaitzaki, A.; Sergentani, C.; Koubouris, G. Contribution of a Seeded Cover Crop Mixture on Biomass Production and Nutrition Status Compared to Natural Vegetation in a Mediterranean Olive Grove. International Journal of Plant Biology. 2022, 13, 235–244. [Google Scholar] [CrossRef]
- Rodrigues, Â.; Correia, C.M.; Claro, A.M.; Ferreira, I.Q.; Barbosa, J.C.; Moutinho-Pereira, J M.; Bacelar, E.A.; Fernandes-Silva, A.A.; Arrobas, M. Soil nitrogen availability in olive orchards after mulching legume cover crop residues. Scientia Horticulturae. 2014, 158, 45–51. [Google Scholar] [CrossRef]
- Ferreira, I. Q.; Rodrigues, M. Â.; Claro, A. M.; Arrobas, M. Management of Nitrogen-Rich Legume Cover Crops as Mulch in Traditional Olive Orchards. Communications in Soil Science and Plant Analysis. 2015, 46, 1881–1894. [Google Scholar] [CrossRef]
- Stein, S.; Hartung, J.; Perkons, U.; Möller, K.; Zikeli, S. Plant and soil N of different winter cover crops as green manure for subsequent organic white cabbage. Nutrient Cycling in Agroecosystems. 2023, 10306–10309. [Google Scholar] [CrossRef]
- Lee, A.; Neuberger, P.; Omokanye, A.; Hernandez-Ramirez, G.; Kim, K.; Gorzelak, A.; Arbuscular mycorrhizal fungi in oat-pea intercropping. Sci Rep. 2023, 13, 390. [CrossRef] [PubMed]
- Afzal, A.; Bano, A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Ttiticum aestivum). International Journal of Agricultural and Biology. 2010, 1814, 9596. [Google Scholar]
- Rodrigues, M.; Withers, P. J. A.; Soltangheisi, A.; Vargas, V.; Holzschuh, M.; Pavinato, P. S. Tillage systems and cover crops affecting soil phosphorus bioavailability in Brazilian Cerrado Oxisols. Soil and Tillage Research. 2021, 205, 104770. [Google Scholar] [CrossRef]
- Tul, S.; Manolikaki, I.; Digalaki, N.; Psarras, G.; Koufakis, I.; Kalaitzaki, A.; Sergentani, C.; Koubouris, G. Contribution of a Seeded Cover Crop Mixture on Biomass Production and Nutrition Status Compared to Natural Vegetation in a Mediterranean Olive Grove. International Journal of Plant Biology. 2022, 13, 235–244. [Google Scholar] [CrossRef]
- Fernandes, G.; Marques, A.; César, R.; Ribeiro, B. R.; Cardoso, Paula, S. Potassium Uptake Kinetics In Native Forage Grass Species From Pampa Biome. Soil science. Cienc. Rural. 2022, 52, 8478cr2020.
- Guesmi, H.; Aichi, H.; Bendhafer, G.; Fouad, Y.; Menasseri, S.; Chaar, H. Assessment of radial variation of soil properties in an olive tree-barley/common vetch agroforestry system under low-input conditions in a Tunisian semi-arid climate after three cropping seasons s. Journal of Research in Environmental and Earth Sciences. 2022, 11, 330–339. [Google Scholar]
- Porwollik, V.; Rolinski, S.; Heinke, J.; Von Bloh, W.; Schaphoff, S.; Müller, C. The role of cover crops for cropland soil carbon, nitrogen leaching, and agricultural yields -A global simulation study with LPJmL (V. 5.0-tillage-cc). Biogeosciences. 2022, 19, 957–977. [Google Scholar] [CrossRef]
- Rattan, Lal. Regenerative agriculture for food and climate. Journal of Soil and Water Conservation. Journal of Soil and Water Conservation 2020, 79, 0620A. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Gao, H.; Zhang, R.; Yang, L.; Guo, Y.; Li, H.; Awasthi, M. K.; Li, G. Long-term cover crops improved soil phosphorus availability in a rain-fed apple orchard. Chemosphere. 2021, 275, 13009. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Gómez-Muñoz, B.; Oberson, A.; Magid, J. Differences in cover crop contributions to phosphorus uptake by ryegrass in two soils with low and moderate P status. Geoderma. 2022, 426, 116075. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Jose´, M.P.; Carlos, C.; Angelo, R.M. Olive response to potassium applications under different water regimes and cultivars Isabel Q. Nutr Cycl Agroecosyst. 2018, 112, 387–401. [Google Scholar] [CrossRef]
- Chavarría, D.; Romina, A.; Verdenelli Dannae, L.; Silvina, S.; Gil, V.; et al. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. European Journal of Soil Biology. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Martín, M.P.R.; Fernández-Ondoño, E.; Ortiz-Bernad, I.; Abreu, M.M. Influence of Intensive and Super-Intensive Olive Grove Management on Soil Quality—Nutrients Content and Enzyme Activities. Plants. 2023, 12, 12152779. [Google Scholar]
- Feng, H.; Sekaran, U.; Wang, T.; Kumar, S. On-farm assessment of cover cropping effects on soil C and N pools, enzyme activities, and microbial community structure. Journal of Agricultural Science. 2021, 159, 216–226. [Google Scholar] [CrossRef]
- Stegarescu, G.; Reintam, E.; Tõnutare, T. Cover crop residues effect on soil structural stability and phosphatase activity. Acta Agriculturae Scandinavica Section B: Soil and Plant Science. 2021, 71, 992–1005. [Google Scholar] [CrossRef]
- Peregrina, F.; Pérez-Álvarez, E. P.; & García-Escudero, E. The short term influence of aboveground biomass cover crops on C sequestration and β-glucosidase in a vineyard ground under semiarid conditions. Spanish Journal of Agricultural Research 2014, 12, 1000–1007.
- Navas, M.; Benito, M.; Rodríguez, I.; Masaguer, A. Effect of five forage legume covers on soil quality at the Eastern plains of Venezuela. Applied Soil Ecology. 2011, 49, 242–249. [Google Scholar] [CrossRef]
- Roper, M.M.; Ophel-Keller, K.M.; Soil microflora as bio-indicators of soil health. In: Pankhurst CE, Doube BM,Gupta VVSR (eds) Biological Indicators of soil health.CAB International. 1997, New York.
- Marais, A.; Labuschagne, J.; Booyse, M. Influence of oats cover crop preceding dryland lucerne establishment on some aspects of soil microbial ecology. South African Journal of Plant and Soil. 2020, 37, 87–89. [Google Scholar] [CrossRef]
- Gomez, M.B.; Pittroff, S.M.; De Neergaard, A.; Jensen, L.S.; Nicolaisen, M.H.; et al. Penicillium bilaii effects on maize growth and P uptake from soil and localized sewage sludge in a rhizobox experiment. Biol Fert Soil. 2017, 53, 23–35. [Google Scholar] [CrossRef]
- Lucas, M.M. The Symbiosome: Legume and Rhizobia Co-evolution toward a Nitrogen-Fixing Organelle. Front Plant Sci. 2018, 22, 22–2229. [Google Scholar]



| Treatments | OM (%) | N (%) | P (µg.g-1) | K (mg.g-1) | ||||
|---|---|---|---|---|---|---|---|---|
| Season | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 |
| Control | 1.85±0.034b | 2.07±0.043c | 0.88±0.007b | 1.00±0.016b | 2.68±0.03b | 2.62±0.04c | 2.85±0.03b | 2.48±0.07b |
| Spont | 3.03±0.029a | 3.50±0.084a | 1.57±0.039a | 1.54±0.023a | 3.82±0.02a | 4.08±0.09b | 3.41±0.034a | 3.01±0.03a |
| Wheat | 2.84±0.048a | 3.72±0.043a | 1.43±0.021a | 1.63±0.062a | 3.73±0.12a | 5.88±0.07a | 3.51±0.089a | 2.87±0.07a |
| Oat | 2.13±0.017b | 4.12±0.015a | 1.00±0.02b | 1.68±0.02a | 3.13±0.06b | 4.55±0.04b | 4.00±0.098a | 2.80±0.04a |
| Vetch-Oat | 3.24±0.054a | 3.31±0.04b | 1.57±0.031a | 1.68±0.031a | 3.71±0.09a | 4.56±0.06b | 4.30±0.075a | 3.22±0.03a |
| Fenugreek | 3.04±0.048a | 3.52±0.026a | 1.43±0.04a | 1.33±0.016a | 4.04±0.11a | 4.13±0.09b | 3.50±0.087a | 3.31±0.05a |
| Vetch | 3.07±0.017a | 3.38±0.036a | 1.50±0.017a | 1.43±0.04a | 3.81±0.05a | 4.60±0.07b | 2.91±0.073b | 3.56±0.05a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





