1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 2019 (COVID-19), has led to significant morbidity and mortality globally [
1]. The epidemic waves and the emergence of different SARS-CoV-2 variants have progressed at varying paces and volumes in different countries [
2]. As of December 2022, more than 640 million confirmed cases and over 6.6 million deaths worldwide had been reported, with Kenya representing 341,636 cases and 5,684 deaths [
3].
COVID-19 surveillance, especially in low- and middle-income countries, tends to underestimate the true extent of exposure and immunity in the community as PCR testing for active SARS-CoV-2 infections often falls short due to asymptomatic and pre-symptomatic cases, which go unnoticed as PCR testing is usually carried out as a confirmation for symptomatic individuals [
4,
5]. Seroprevalence studies conducted worldwide have demonstrated significant variations in SARS-CoV-2 exposure across populations. Various studies on the seroprevalence of COVID-19 across different countries have suggested that the reported cases have significantly underestimated the actual scope of the infection [
6,
7].
By September 2021, the global seroprevalence from infection or vaccination was estimated to be 59.2%, with notable variations between regions [
6]. COVID-19 vaccinations in Kenya commenced in March 2021, with priority being given to frontline workers and the elderly [
8]. Vaccinations were progressively extended to the rest of the population such that by July 2022, 23.4% of the population had received at least one dose of COVID-19 vaccine [
3]. In Kenya, several seroprevalence studies have been conducted to assess the true burden of SARS-CoV-2 and the development of population immunity [
9]. These studies have revealed diverse transmission patterns nationwide, with varying seroprevalence rates in different regions and sub-populations. A national sero-surveillance study of Kenyan blood donors found an adjusted seroprevalence of 48.5% in early 2021, with higher rates observed in the capital city, Nairobi, compared to rural areas [
10].
The advent of the Omicron variant led to a rapid increase in infections and a subsequent rise in seropositivity in various regions across the globe. A serosurveillance study in Switzerland conducted post-Omicron reported an estimated seroprevalence of 93.8%, with 72.4% attributed to natural infection [
11]. A similar study conducted in Kilifi and Nairobi in 2022 revealed a seroprevalence of 70% and 90%, respectively [
12].
While seroprevalence studies have been conducted in Kenya, studies focused specifically on the Rift Valley region remain limited. This geographic area presents a unique context for understanding the impact of COVID-19 in Kenya. Factors such as population density, healthcare access, and specific socioeconomic features of the Rift Valley can influence the pattern of disease transmission and immunity within this region.
Estimating the burden of SARS-CoV-2 infection in a population is crucial for public health planning, resource allocation and understanding the dynamics of the pandemic. In this study, we aimed to determine the seroprevalence of antibodies and antibody levels against SARS-CoV-2 among patients visiting hospital-based sentinel sites in the Rift Valley following the Omicron wave to understand the region's immune landscape.
2. Methodology
2.1. Study Area
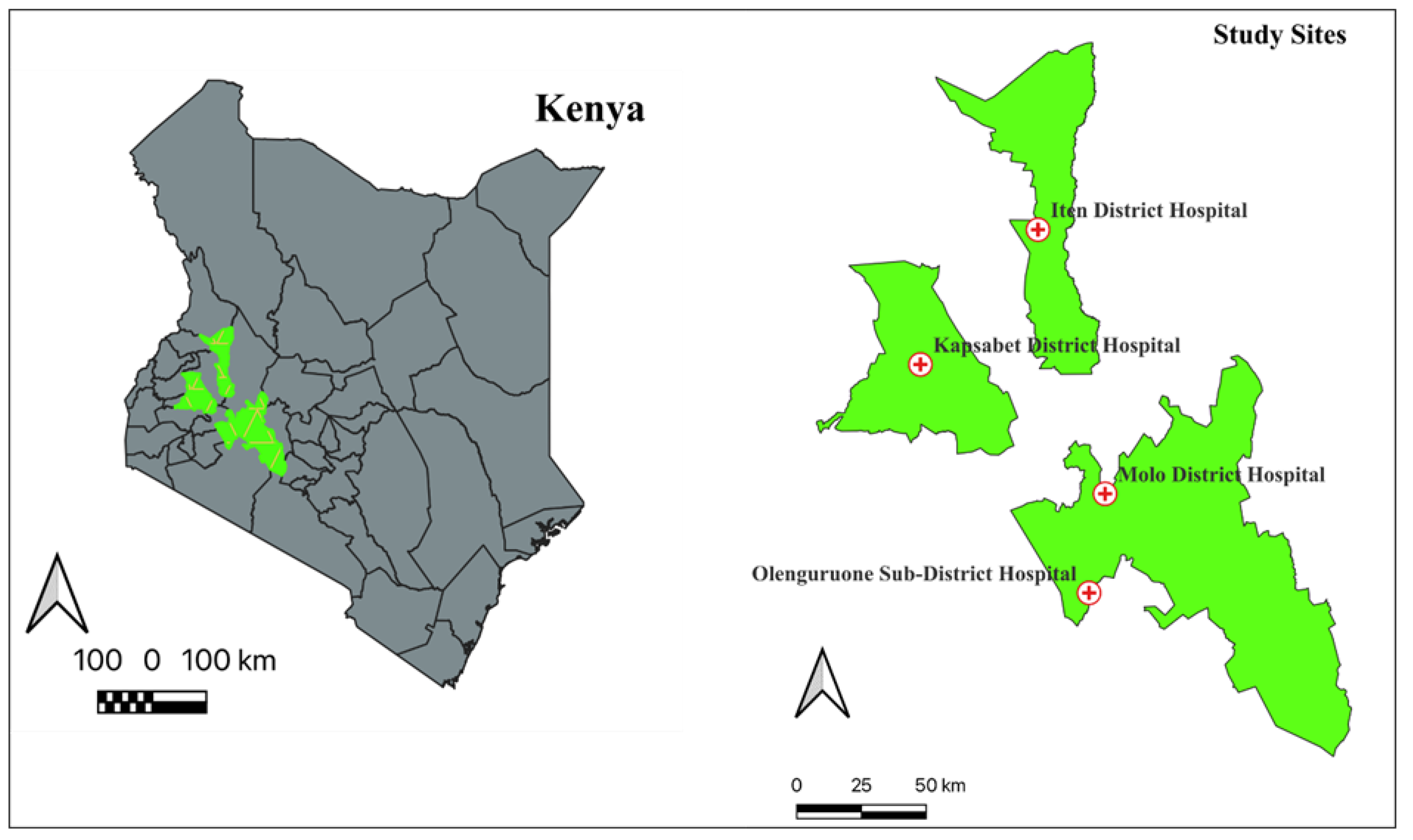
This study was conducted in four hospital-based sites established for ARI sentinel surveillance. They include Olenguruone Level 4 Hospital (Nakuru County), Molo Level 4 Hospital (Nakuru County), Iten County Referral Hospital (Elgeyo Marakwet County) and Kapsabet County Referral Hospital (Nandi County)(
Figure 1).
2.2. Study Design and Sample Collection
We used a cross-sectional study design. Patients aged six months or older visiting the sentinel sites between January 2022 and December 2022, presenting with acute respiratory infection symptoms, were enrolled in the study. A written informed consent in either English, Kiswahili, or the local dialect was sought from each participant; for minors, parents or guardians supplied the written consent. Socio-epidemiological data from each patient was recorded using a standardized questionnaire, including a unique identifier and demographics. Blood samples were drawn from the patients into anticoagulant-free vacutainers and allowed to clot by leaving them standing at room temperature for 2 hours. Then centrifugation was done at 1500xg for 10 min to the serum. The serum samples were transported in a cold chain to the Sample Management and Receiving Facility (SMRF) at the Kenya Medical Research Institute (KEMRI), where they were stored at -80°C until ready for processing.
2.3. Case Definition
An ARI case was defined as an individual with one or more of the following symptoms: fever, cough, sore throat or respiratory distress within the last seven days.
2.4. Laboratory Analysis
We used Invitrogen Human SARS-CoV-2 spike IgG ELISA kit (Thermo Fisher Scientific, Waltham, USA) to determine the presence and quantify immunoglobulin G (IgG) antibodies against SARS-CoV-2 S protein in human serum. The assay uses microtiter plate wells pre-coated with a trimerized Spike protein. The test was done according to the manufacturer's protocols. Briefly, the reagents and serum samples were brought to room temperature, and all the controls and buffers were reconstituted accordingly. A two-fold serial dilution of the high positive control was used to prepare assay standards, with a range from 15,000 units/ml to 234 units/ml. The wells were washed twice and 90 μl of assay buffer was added, followed by 10μl of standards, controls or samples. The microtiter plate was covered with an adhesive strip and then incubated for 30 minutes at 37 °C. The plates were washed three times, after which 100μl of HRP conjugate solution was added to each well and incubated for 15 minutes at 37 °C. The plates were washed three times, and 100μl substrate solution was added and incubated at room temperature for 15 minutes. Finally, 100 μl of stop solution was added, and the optical density (OD) was measured at 450 nm on a microplate reader (Multiskan EX, Lab systems, Finland). The cut-off for positivity was determined by calculating the ratio of the patient sample's absorbance versus the calibrator's absorbance. The results were interpreted according to the manufacturer's instructions, where a ratio of <1.0 is considered negative, ≥1.0 to <1.3 is deemed indeterminate, and ≥1.3 is considered positive. Indeterminate results were retested. We utilized a linear regression model with OD values and corresponding antibody concentrations from serial dilutions to determine the Binding Antibody Units (BAU)/ml (BAU/ml) for the positive samples.
2.5. Data Analysis
The socio-epidemiological data were exported to Microsoft Excel (Office 365) and merged with the laboratory results. The overall seroprevalence of anti-SARS-CoV-2 IgG was calculated. Participants were stratified into age cohorts with a range of 10-year intervals, except for individuals aged between 0–18 years. Subsequently, descriptive statistical methods were applied to assess data distribution across four facilities, accounting for demographic attributes such as age, gender, prior SARS-CoV-2 infections, and vaccination status. A binary logistic regression analysis was performed to identify factors influencing SARS-CoV-2 seropositivity. Covariates considered in the regression model included previous infection history, vaccination status, and demographic characteristics. A p-value of <0.05 was used to denote significant differences in the variants. All statistical computations and analyses were conducted using Stata software (version 18; StataCorp, 2023).
3. Results
This study enrolled 557 participants, with a higher proportion of females (n=339, 60.9%) than males (n=218, 39.1%). The majority of participants were aged between 19 and 30 years (n=127) or above 61 (n=103). The analysis of seropositivity revealed a remarkably high overall seroprevalence rate of 97.8% (n= 545), with only 2.2% (n= 12) of participants testing negative for SARS-CoV-2 antibodies. Overall, 55.3% (n=308) of the study participants were vaccinated. While more females were vaccinated overall, analysis within each gender group reveals a slightly higher vaccination coverage among enrolled males (n=127, 58.3%) than enrolled females (n=181, 53.4%). There was a relatively low, self-reported prior COVID-19 infection rate (7.0%, n=39). Of those reporting prior infection, males had a slightly higher proportion of reported prior infection rate (7.8%) than females (6.5%). The demographic profile is summarized in
Table 1 below.
Across the different sites, Iten County Referral Hospital had the highest enrolment (n= 237), followed by Molo Level 4 Hospital (n= 126), Kapsabet County Referral Hospital (n= 103) and Olenguruone Level 4 Hospital (n= 91) (
Table 2). The distribution of study participants across the different sites by age group exhibited heterogeneity, providing a good representation of persons of all ages in the population. Iten County Referral Hospital primarily enrolled older adults (<61 years), while Molo Level 4 Hospital and Kapsabet County Referral Hospital mainly enrolled younger adults (19-30 years). Olenguruone Level 4 Hospital, in contrast, predominantly enrolled patients in the middle-aged bracket (41-50 years).
There were significant site-specific differences in vaccination coverage across the different sites. Molo Level 4 Hospital had notably high vaccination rates, with 73.8% of all the study participants vaccinated, while Olenguruone Level 4 Hospital had the lowest vaccination rates, at about 31.9% (
Table 3). Gender disparities were apparent at Iten County Referral Hospital (32.5%) and Olenguruone Level 4 Hospital (44%), where the proportion of unvaccinated females was approximately double that of unvaccinated males.
There were remarkably low self-reported prior COVID-19 infection rates across all the health facilities (
Table 4). A notable absence of self-reported prior COVID-19 infections was observed among males at the Iten County Referral Hospital and among both males and females at Olenguruone Level 4 Hospital. Kapsabet County Referral Hospital had marginally higher rates of self-reported prior infections among both genders compared to other health facilities. The striking divergence with the observed high seroprevalence in the study indicates significant underreporting of past infections.
3.1. Seropositivity across Age Groups
A high COVID-19 seropositivity rate was observed across all age groups, with prevalence ranging from 96.9% to 99.2%.% (Supplementary
Table 1). The highest seropositivity rate was observed in the 19-30 age group (99.2%), while the lowest rate was observed in the 51-60 age group (96.9%). The overall COVID-19 seropositivity across all age groups was 97.8%. The high seroprevalence likely reflects both widespread prior exposure to SARS-CoV-2 and the impact of vaccination efforts.
Analysis of individual sites revealed disparities in seropositivity rates (
Table 5). Kapsabet County Referral Hospital exhibited the highest rates, with 100% seropositivity across all age groups. Notably, study participants aged 0-18 years, three out of the four sites reported a seropositivity rate of 100%, while Iten County Referral Hospital reported a slightly lower rate of 96.4%.
3.2. Predictors of SARS-CoV-2 Antibody Seroprevalence
The odds of a positive outcome between males as a reference and females are 0.76 times lower; however, their association is not significant. Moreover, vaccination demonstrated a marked effect, with vaccinated individuals showing 3.33 times higher odds of SARS-CoV-2 antibody seroprevalence than unvaccinated individuals. Prior SARS-CoV-2 infection conferred a substantial increase in seropositivity odds, indicating a robust immune response following vaccination or natural exposure. Seropositivity rates remained largely consistent across age groups, except for individuals over 61 demonstrating significantly higher odds. This suggests an increased likelihood of seropositivity within this older demographic. Additionally, the Kapsabet County Referral Hospital exhibited the highest odds ratio, implying that a greater proportion of seropositive patients originated from this site than the other three locations (
Table 6).
4. Discussion
Understanding the true extent of SARS-CoV-2 exposure within a population is crucial for guiding public health strategies [
7]. While routine testing identifies active infections, seroprevalence studies utilizing anti-SARS-CoV-2 antibodies offer a broader picture of past exposure, including asymptomatic and undetected cases [
13,
14]. In this study conducted between January and December 2022, we investigated the seroprevalence of SARS-CoV-2 IgG antibodies in symptomatic patients.
The study observed a high seroprevalence of SARS-CoV-2 antibodies among patients who visited the hospital-based sentinel sites. This is consistent with similar studies worldwide highlighting the widespread prevalence of SARS-CoV-2 [
14]. Meta-analyses of standardized population-based studies show that the global seroprevalence of SARS-CoV-2 has risen considerably over time, with regional variation exceeding reported case numbers [
6,
15]. In a rural Ugandan cohort study, seropositivity surged after the Omicron wave, with most previously uninfected, unvaccinated individuals becoming infected for the first time and over half of those with prior exposure experiencing likely reinfection [
16].
Despite the high seroprevalence, the study participants reported relatively low rates of prior COVID-19 infection. This likely reflects under-estimation, a common phenomenon in disease surveillance where many cases go undetected because they are asymptomatic or do not seek healthcare [
17]. This is further compounded by limited testing resources and healthcare infrastructure in Africa [
18,
19]. A similar study in the USA revealed COVID-19 infections likely three to twenty times higher than reported cases [
20].
The study identified variations in vaccination coverage and self-reported infection rates across different study sites. While natural infection played a role in generating immunity among the study population, vaccination also likely contributed significantly. Contrasting patterns in vaccination highlight potential inequalities in vaccine access, availability, and uptake. Studies have linked lower vaccination rates in some communities to vaccine hesitancy fuelled by misinformation or distrust of public health authorities [
21,
22,
23]. Olenguruone, the most rural of the sentinel sites, exhibited the lowest vaccine coverage. The lower vaccination rates in this rural area reflect a broader pattern of vaccine inequity observed in other studies. Rural or remote communities frequently face logistical barriers, including distribution challenges, limited cold-chain storage capacity, or healthcare provider shortages, hindering access to essential vaccines [
24,
25].
Vaccination played a crucial role, with vaccinated individuals showing significantly higher odds of having antibodies against the virus. A longitudinal study in the UK highlights the effectiveness of vaccination in protecting populations, demonstrating that higher antibody levels resulting from vaccination significantly reduce the risk of future COVID-19 infection [
26]. This highlights the effectiveness of vaccination in protecting populations. Individuals over 61 demonstrated significantly higher odds of SARS-CoV-2 seropositivity compared to other age groups. This is likely due to the age-prioritized vaccination strategy, where older adults were among the first to receive vaccines [
27].
Enrolment disparities between sites and distinct age-group preferences likely reflect differences in demographic engagement, service specialization, and other access-related factors. Level 4 facilities, often located in rural areas, serve a localized population, while referral hospitals, typically found in urban centres, draw patients from a wider region and focus on providing specialized care. Access-related factors can create significant barriers for individuals from lower-income communities or specific age groups due to limited resources and transportation challenges [
28,
29]. Furthermore, the type of healthcare facility can influence enrolment patterns [
30,
31].
5. Conclusion
The high seroprevalence observed in this study starkly contrasts with reported COVID-19 cases, suggesting a significant underestimation of infections. Variations in vaccination coverage and infection rates across study sites highlight potential disparities in vaccine access and uptake, underscoring the need for targeted interventions to address barriers to vaccination in rural and remote areas. Vaccination remains crucial in conferring immunity, but the observed disparities across facilities emphasize the need for equitable healthcare and vaccination distribution. Targeted interventions are crucial to address vaccine hesitancy and accessibility barriers, promoting equitable vaccination coverage and mitigating future COVID-19 transmission.
This study is subject to a few limitations. First, our recruitment strategy of enrolling only symptomatic individuals visiting hospital-based sentinel sites could introduce bias into the study findings, potentially skewing the seroprevalence estimates. Furthermore, reliance on self-reported vaccination status and prior COVID-19 infection introduces the possibility of recall bias. Moreover, the study's geographic scope within the Rift Valley limits the extrapolation of findings to other regions.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU) under the study protocol KEMRI/SERU/CVR/012/4126. All study participants provided informed written consent prior to sample collection.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Vincent Ruttoh, Ibrahim Ndungu, Samwel Symekher and Samson Nzou; Data curation, Brian Kipkoech, Ronald Tonui and Ruth John; Formal analysis, Brian Kipkoech, Ronald Tonui, Ruth John and Sally Kamau; Funding acquisition, Vincent Ruttoh and Samson Nzou; Investigation, Vincent Ruttoh, Ruth Gicho, Sally Kamau, Maureen Njihia, Samwel Symekher, Timothy Mwanzia, James Gikunda, Matthew Munyao and Caroline Njoroge; Methodology, Vincent Ruttoh, Ibrahim Ndungu, Maureen Njihia, Samwel Symekher, Timothy Mwanzia, James Gikunda, Matthew Munyao and Caroline Njoroge; Project administration, Vincent Ruttoh, Ruth Gicho and Samwel Symekher; Resources, Vincent Ruttoh and Samson Nzou; Software, Brian Kipkoech and Ronald Tonui; Supervision, Vincent Ruttoh, Ibrahim Ndungu and Samson Nzou; Validation, Sally Kamau, Maureen Njihia, Timothy Mwanzia, James Gikunda, Matthew Munyao and Caroline Njoroge; Visualization, Ronald Tonui, Ruth John and Ruth Gicho; Writing – original draft, Vincent Ruttoh and Brian Kipkoech; Writing – review & editing, Ronald Tonui, Ruth John, Ibrahim Ndungu, Ruth Gicho, Sally Kamau, Maureen Njihia, Samwel Symekher, Timothy Mwanzia, James Gikunda, Matthew Munyao, Caroline Njoroge and Samson Nzou.
Funding
This work is supported by a grant from Pfizer Global Medical Grants, Grant ID: 61773471. The funder had no role in the study design, implementation, decision to publish, or manuscript preparation.
Data availability statement
The data used to support the findings of this study will be made available on request from the corresponding author through the KEMRI Data Governance Committee.
Acknowledgments
We thank the study participants and sentinel site staff who supported this work. We also thank the county public health departments of the respective counties. This manuscript is published with the approval of the Director, KEMRI.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Gupta, A., Marzook, H. & Ahmad, F. Comorbidities and clinical complications associated with SARS-CoV-2 infection: an overview. Clinical and Experimental Medicine 2022 23:2 23, 313–331 (2022). [CrossRef]
- Deepanshi, Budhiraja, I., Garg, D., Kumar, N. & Sharma, R. A comprehensive review on variants of SARS-CoVs-2: Challenges, solutions and open issues. Comput Commun 197, 34–51 (2023). [CrossRef]
- Mathieu, E. et al. Coronavirus Pandemic (COVID-19). Our World in Data (2020).
- Mercer, T. R. & Salit, M. Testing at scale during the COVID-19 pandemic. Nature Reviews Genetics 2021 22:7 22, 415–426 (2021). [CrossRef]
- Usuf, E. & Roca, A. Seroprevalence surveys in sub-Saharan Africa: what do they tell us? Lancet Glob Health 9, e724–e725 (2021). [CrossRef]
- Bergeri, I. et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med 19, (2022). [CrossRef]
- True extent of SARS-CoV-2 Infection through seroprevalence studies. https://www.who.int/news/item/03-02-2022-true-extent-of-sars-cov-2-infection-through-seroprevalence-studies.
- Kenya completes its first round of COVID-19 vaccinations | Gavi, the Vaccine Alliance. https://www.gavi.org/vaccineswork/kenya-completes-its-first-round-covid-19-vaccinations.
- Mulabbi, E. N. et al. Seroprevalence of human coronaviruses among patients visiting hospital-based sentinel sites in Uganda. BMC Infect Dis 21, 1–8 (2021). [CrossRef]
- Uyoga, S. et al. Prevalence of SARS-CoV-2 Antibodies From a National Serosurveillance of Kenyan Blood Donors, January-March 2021. JAMA 326, 1436–1438 (2021).
- Zaballa, M. E. et al. Seroprevalence of anti-SARS-CoV-2 antibodies and cross-variant neutralization capacity after the Omicron BA.2 wave in Geneva, Switzerland: a population-based study. The Lancet Regional Health - Europe 24, 100547 (2023).
- Kagucia, E. W. et al. SARS-CoV-2 seroprevalence and implications for population immunity: Evidence from two Health and Demographic Surveillance System sites in Kenya, February-December 2022. Influenza Other Respir Viruses 17, (2023).
- Han, D., Li, R., Han, Y., Zhang, R. & Li, J. COVID-19: Insight into the asymptomatic SARS-COV-2 infection and transmission. Int J Biol Sci 16, 2803 (2020). [CrossRef]
- Rostami, A. et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect 27, 331–340 (2021). [CrossRef]
- Bobrovitz, N. et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLoS One 16, e0252617 (2021). [CrossRef]
- Briggs, J. et al. Seroprevalence of Antibodies to SARS-CoV-2 in Rural Households in Eastern Uganda, 2020-2022. JAMA Netw Open 6, e2255978–e2255978 (2023). [CrossRef]
- Gibbons, C. L. et al. Measuring underreporting and under-ascertainment in infectious disease datasets: A comparison of methods. BMC Public Health 14, 1–17 (2014). [CrossRef]
- Adebisi, Y. A. et al. SARS-CoV-2 diagnostic testing in Africa: needs and challenges. Pan Afr Med J 35, 4 (2020). [CrossRef]
- Dzinamarira, T., Dzobo, M. & Chitungo, I. COVID-19: A perspective on Africa’s capacity and response. J Med Virol 92, 2465–2472 (2020). [CrossRef]
- Wu, S. L. et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nature Communications 2020 11:1 11, 1–10 (2020). [CrossRef]
- Sewpaul, R. et al. Vaccine hesitancy and related factors among South African adults in 2021: unpacking uncertainty versus unwillingness. Front Public Health 11, (2023). [CrossRef]
- Troiano, G. & Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 194, 245–251 (2021). [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, Vol. 9, Page 160 9, 160 (2021). [CrossRef]
- Sinnei, D. K., Karimi, P. N., Maru, S. M., Karengera, S. & Bizimana, T. Evaluation of vaccine storage and distribution practices in rural healthcare facilities in Kenya. J Pharm Policy Pract 16, 1–7 (2023). [CrossRef]
- Ortiz, J. R. et al. The potential effects of deploying SARS-Cov-2 vaccines on cold storage capacity and immunization workload in countries of the WHO African Region. Vaccine 39, 2165–2176 (2021). [CrossRef]
- Cheetham, N. J. et al. Antibody levels following vaccination against SARS-CoV-2: associations with post-vaccination infection and risk factors in two UK longitudinal studies. Elife 12, (2023).
- Rajshekhar, N. et al. Original research: Exploring COVID-19 vaccine hesitancy and uptake in Nairobi’s urban informal settlements: an unsupervised machine learning analysis of a longitudinal prospective cohort study from 2021 to 2022. BMJ Open 13, (2023).
- Bakibinga, P. et al. Demand and supply-side barriers and opportunities to enhance access to healthcare for urban poor populations in Kenya: a qualitative study. BMJ Open 12, e057484 (2022). [CrossRef]
- Otieno, P. O. et al. Access to primary healthcare services and associated factors in urban slums in Nairobi-Kenya. BMC Public Health 20, 1–9 (2020). [CrossRef]
- Kirkland, E. et al. Patient Demographics and Clinic Type Are Associated With Patient Engagement Within a Remote Monitoring Program. Telemedicine Journal and e-Health 27, 843 (2021). [CrossRef]
- Lewis, D. J. & Longley, P. A. Patterns of Patient Registration with Primary Health Care in the UK National Health Service. Annals of the Association of American Geographers 102, 1135–1145 (2012). [CrossRef]
Footnotes
| 1 |
ICRH – Iten County Referral Hospital. |
| 2 |
KCRH - Kapsabet County Referral Hospital. |
| 3 |
ICRH – Iten County Referral Hospital. |
| 4 |
KCRH - Kapsabet County Referral Hospital. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).