1. Introduction
Cephalopods are a popular seafood worldwide. As protein accounts for approximately 70–80% of their total weight, they are a rich protein source in the human diet [
1]. Various cephalopods species are farmed on an industrial scale [
2] but the choice of feed for the various squid and octopus species is mainly based on research into the digestive physiology of the Mexican four-eyed octopus and the common octopus [
3]. The lack of similar knowledge specific to squid species is a major obstacle to the growth of squid farming.
The bigfin reef squid (
Sepioteuthis lessoniana) has become a major commercial species in several countries [
4]. In Thailand,
S. lessoniana is found in both the Gulf of Thailand and the Andaman Sea [
4]. The
S. lessoniana presents great potential for aquaculture due to its popularity among consumers. Moreover, the digestive physiology of this squid has already been studied [
5]. In the squid digestive system, several degrading enzymes exist to hydrolyze main nutrients, but the types and functions of enzymes vary in different species. In our previous study of
Sepioteuthis lessoniana, a chymotrypsin-like serine proteinase was detected in viscera that was highly proteolytic which could be of importance for the development of feeding strategies in bigfin reef squid farming. However, more detailed information on its digestive physiology and enzyme characteristics is needed.
Chymotrypsin is a serine proteinase which cleaves peptide bond on carbonyl groups of hydrophobic amino acid residues. Chymotrypsin is also crucial for the digestion of proteins. [
6]. It is synthesized in the form of chymotrypsinogen in the pancreases of vertebrates and in the digestive glands of invertebrates [
7]. As reported previously, chymotrypsins have been extracted and biochemically characterized from the digestive organs of several species of fish and some marine invertebrates, such as the viscera of white shrimp [
8], the pyloric caecum of Monterey sardine [
9] and the hepatopancreas of cuttlefish [
6]. To determine the potential viability of farming bigfin reef squid, we purified chymotrypsin from the digestive organs of the species and then studied the molecular and biochemical characteristics of the purified product.
2. Materials and Methods
2.1. Chemicals
Sephacryl S-200 and DEAE-Sepharose were obtained from GE Healthcare Bio-Science AB (Uppsala, Sweden). All chemicals with analytical grade were acquired from Fluka Chemicals (Buchs, Switzerland).
2.2. Preparation of Squid and Crude Enzyme Extract
Fresh bigfin reef squid were acquired from the fishing port in Satun, Thailand. With a sample to ice ratio of 1:3 (w/w), all samples were kept in ice and delivered in less than three hours to Faculty of Agro and Bio Industry, Thaksin University, Phatthalung. The digestive organs were separated, collected, defatted [
10], and prepared as a powder for the isolation of chymotrypsin.
The extraction of crude chymotrypsin from the powder followed the procedure developed by Poonsin et al. [
11]. In brief, the defatted digestive organ powder was suspended in starting buffer (SB) comprising 10 mM Tris-HCl, pH 7.0 and 20 mM NaCl, at 1:9 (w/v) ratio. After 30 min of continuous stirring at 4°C, the mixture was centrifuged at 18,000 × g at 4°C for 30 min. The obtained supernatant was designated as “crude enzyme extract”.
2.3. Chymotrypsin Purification
The crude enzyme extract was precipitated with ammonium sulfate (0-40% saturation). The obtained pellets were collected, solubilized in a small amount of SB before being dialyzed overnight with three changes of 15 volumes of SB in a dialysis bag (MW cut-off: 14,000 Da). The dialysate was then loaded for purification by size exclusion chromatography in a Sephacryl S-200 column (2.5 × 100 cm) that had been equilibrated with SB. Three milliliters of fractions were taken at a flow rate of 0.5 ml min‒1 during the elution process, which was carried out with SB. Each eluted fraction was tested for protein concentration and chymotrypsin activity. The enzyme-active fractions were mixed and concentrated using Vivaspin Turbo 15 ultrafiltration (Goettingen, Germany). The concentrated fractions were further put onto a DEAE-Sepharose (Whatman, U.K.) anion exchange column (5 × 20 cm) equilibrated with SB at a flow rate of 0.5 ml min‒1. Afterwards, SB was used to wash the column until the A280 value was below 0.05. A linear gradient of NaCl (0.0 to 0.5 M) was used for the elution, with a flow rate of 0.3 ml min‒1. Three milliliter fractions were collected and the ones with chymotrypsin activity were analyzed for enzymological characteristics.
2.4. Chymotrypsin Activity Assay
Chymotrypsin activity was determined using the modified technique outlined by Rungruangsak-Torrissen [
12]. In brief, chymotrypsin solution (200 µL) was combined with 350 µl of 0.3 mM N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilid (SAPNA) as a substrate in 0.2 M McIlvaine’s buffer (0.1 M sodium citrate and 0.2 M sodium phosphate), pH 6.0 and the mixture was incubated for 60 min at 45°C. To stop the reaction, 400 µl of 30% acetic acid was applied. The synthesis of p-nitroanilide was measured spectrophotometrically at 410 nm. One unit of chymotrypsin activity was expressed as 1 μmol of p-nitroanilide min
-1.
2.5. Protein Concentration Measurement
The protein concentration of samples was evaluated by Lowry’s procedure with bovine serum albumin as a standard [
13]. During purification steps, the absorbance at 280 nm was applied to measure the protein concentration of eluted samples.
2.6. Determination of Optimal Activity and Stability of Chymotrypsin
2.6.1. Optimum pH and Temperature
Determination of optimal pH of chymotrypsin purified from bigfin reef squid was carried out over a pH range of 2.0‒11.0 (0.2 M McIlvaine’s buffer for pH 2.0‒7.5 and 0.1 M glycine-NaOH for pH 8.0‒11.0) at 45°C for 60 min and SAPNA was used as a substrate to evaluate the enzyme activity. Determination of optimal temperature of purified chymotrypsin was carried out at varying temperatures (25-80°C) in a water bath (Memmert, Schwabach, Germany) for 60 min at pH 7.0, and the chymotrypsin activity was assessed using SAPNA as the substrate. Relative chymotrypsin activity (%) was determined by considering the maximum observed chymotrypsin activity as 100%.
2.6.2. pH and Thermal Stabilities
The stability of purified chymotrypsin was assessed by determining chymotrypsin activity after 1 h of enzyme incubation at different pH values (2.0-7.5) at the ambient temperature. To study thermal stability of purified chymotrypsin, chymotrypsin activity was examined after 1 hour of enzyme incubation at temperatures ranging from 0 to 80°C. Afterwards, enzymes were cooled in iced water before determination of chymotrypsin activity. The residual chymotrypsin activity was tested by SAPNA as a substrate at pH 7.0, 55°C, and the findings were presented as the relative activity (%) compared to the initial activity.
2.7. Determination of Inhibition Against Chymotrypsin
The influences of different protease inhibitors on the inhibition of chymotrypsin activity were ascertained using the procedures outlined by Poonsin et al. [
11]. Briefly, 0.1 mM for 1-(L-trans-epoxysuccinyl-leucylamino)-4-guanidinobutane (E-64), 1 mM for N-ethylmaleimide, 1.0 g l
‒1 for soybean trypsin inhibitor (SBTI), 5 mM for N-p-tosyl-L-Lys-chloromethyl ketone (TLCK), 5 mM for N-tosyl-L-phechloromethyl ketone (TPCK), 0.01 mM for pepstatin A, 5 mM for benzamidine, 1 mM for phenylmethylsulfonyl fluoride (PMSF) and 2 mM for ethylenediaminetetraacetic acid (EDTA) were the final concentrations of inhibitor solution that were incubated with the purified chymotrypsin. Incubation was carried out for 30 min at ambient temperature (26‒28°C), and the residual activity was evaluated using SAPNA as substrate at pH 7.0, 55°C to calculate the percent inhibition.
2.8. Determination of Kinetics of Chymotrypsin
The activity of purified chymotrypsin from bigfin reef squid was investigated with SAPNA at final concentrations ranging from 0.1–0.5 M, where the final concentration of the purified chymotrypsin was 0.1 µg/ml. The Lineweaver and Burk [
14] kinetic models were used to create various kinetic parameters, including apparent Michaelis-Menten constant (K
m), the maximum velocity (V
max) and the catalytic efficiencies (K
cat/K
m). Values of turnover number (K
cat) were also determined using the following equation: V
max/[E] = K
cat, where [E] represents the concentration of purified chymotrypsin.
2.9. SDS-PAGE and Native-PAGE
SDS-PAGE was applied to assess the purity and molecular weight of purified chymotrypsin, as documented by Laemmli [
15]. Samples were combined in a 1:1 ratio with 10% βME in sample buffer (0.125 M Tris-HCl, pH 6.8, containing 4% SDS and 20% glycerol), and then boiled and loaded onto the gel at the protein concentration of 15 µg (4% stacking and 12% separating). Electrophoresis was carried out using a Mini-Protein II Cell equipment with a continuous current of 15 mA per gel. Afterwards, the gel was stained and de-stained.
Native PAGE was also performed using similar procedures for SDS-PAGE, but without heating the sample or adding SDS and reducing agents (βME) in the sample and the gel.
2.10. Molecular Weight Measurement
Using gel filtration chromatography on a Sephacryl S-200 column, the molecular weight (MW) of purified chymotrypsin was determined. The available partition coefficient (Kav) of the chymotrypsin separated on gel filtration chromatography was plotted against the logarithm of the MW of the protein standards to determine its MW. The elution volume (Ve) of each protein standard and chymotrypsin was determined. The elution volume of blue dextran was used to calculate the void volume (Vo) (MW 2,000,000). The standards utilized were aprotinin (MW 6,500), trypsinogen (MW 24,000), bovine serum albumin (MW 66,000), and catalase (MW 232,000) (Sigma Chemical Co., St. Louis, MO, USA.).
2.11. Measurement of N-Terminal Amino Acid Sequence
The sequence of N-terminal amino acids of purified chymotrypsin was measured using the procedure outlined by Klomklao et al. [
10]. The SDS-PAGE gel containing purified chymotrypsin band was moved to a polyvinylidene difluoride (PVDF) membrane. The protein bands were excised after being stained with Coomassie Brillian Blue R-250 and assessed with a Shimadzu PPSQ-33A protein sequencer (Kyoto, Japan).
2.12. Statistical Analysis
A completely randomized design was employed in this investigation. Three duplicates of the tests were conducted. Data were subjected to analysis of variance, and mean comparison was performed by Duncan’s Multiple Range Test, [
16] and the analyses were carried out using SPSS version 14 (SPSS Inc., Chicago, USA).
3. Results and Discussion
3.1. Purification of Chymotrypsin
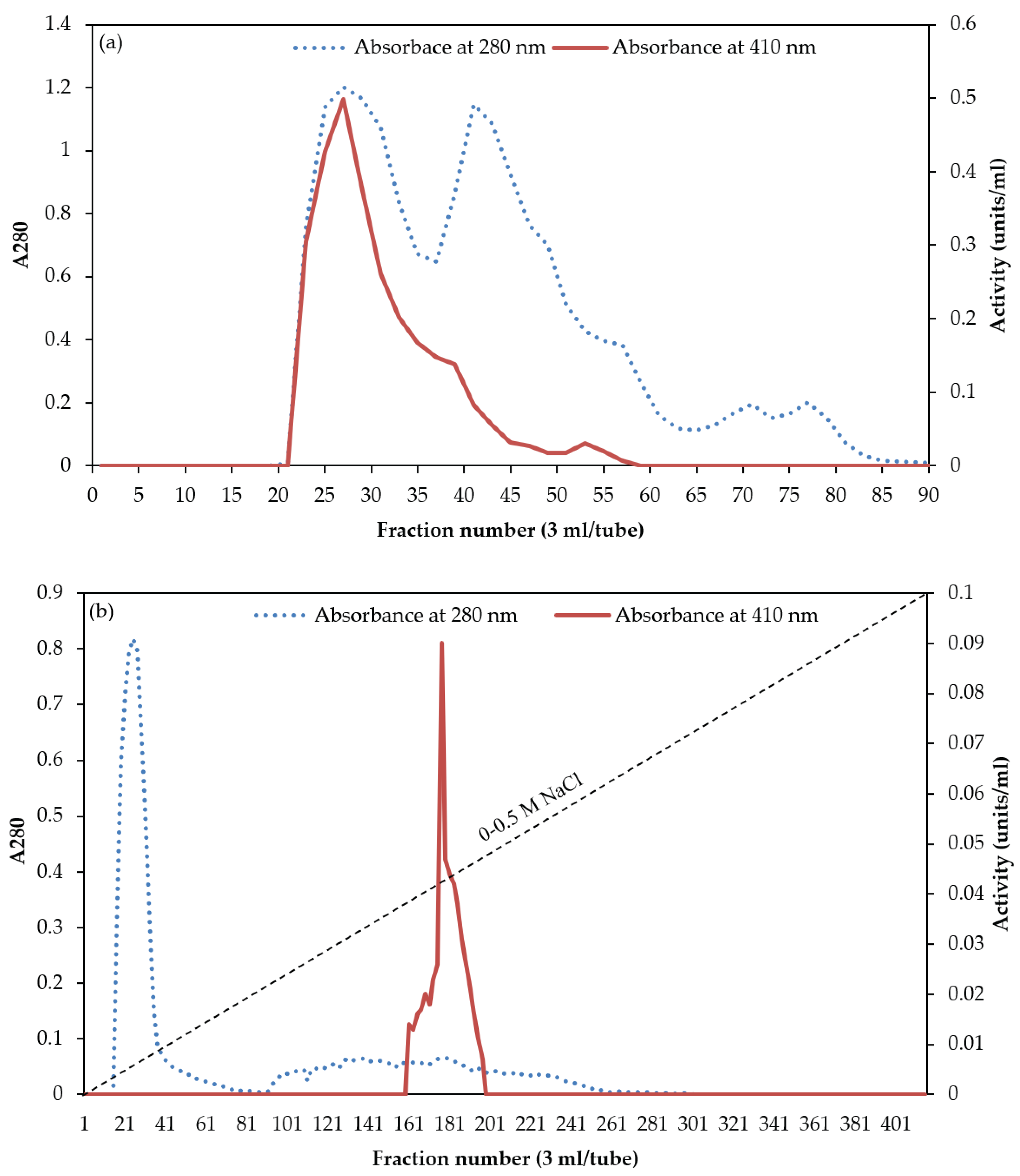
The total and specific activity, total protein, yield and purity of chymotrypsin from the digestive organs of bigfin reef squid are presented in
Table 1. Ammonium sulfate fractionation has been the first step used in the removal and separation of several proteins from a crude enzyme extract [
11]. In this study, ammonium sulfate fractionation at saturations of 0–40% (w/v) obtained a chymotrypsin specific activity at 7.69 U/mg protein. The elution profiles of chymotrypsin using chromatographic columns are shown in
Figure 1. The purification profile (
Table 1) shows that, after purification by Sephacryl S-200 gel filtration column, the obtained purity increased by 12.93-fold, indicating that a large number of ‘junk proteins’ was eliminated. After isolation by DEAE-Sepharose anion exchange column, the purity of the chymotrypsin was 41.27 fold the purity of the crude enzyme, with a yield of 5.74%.
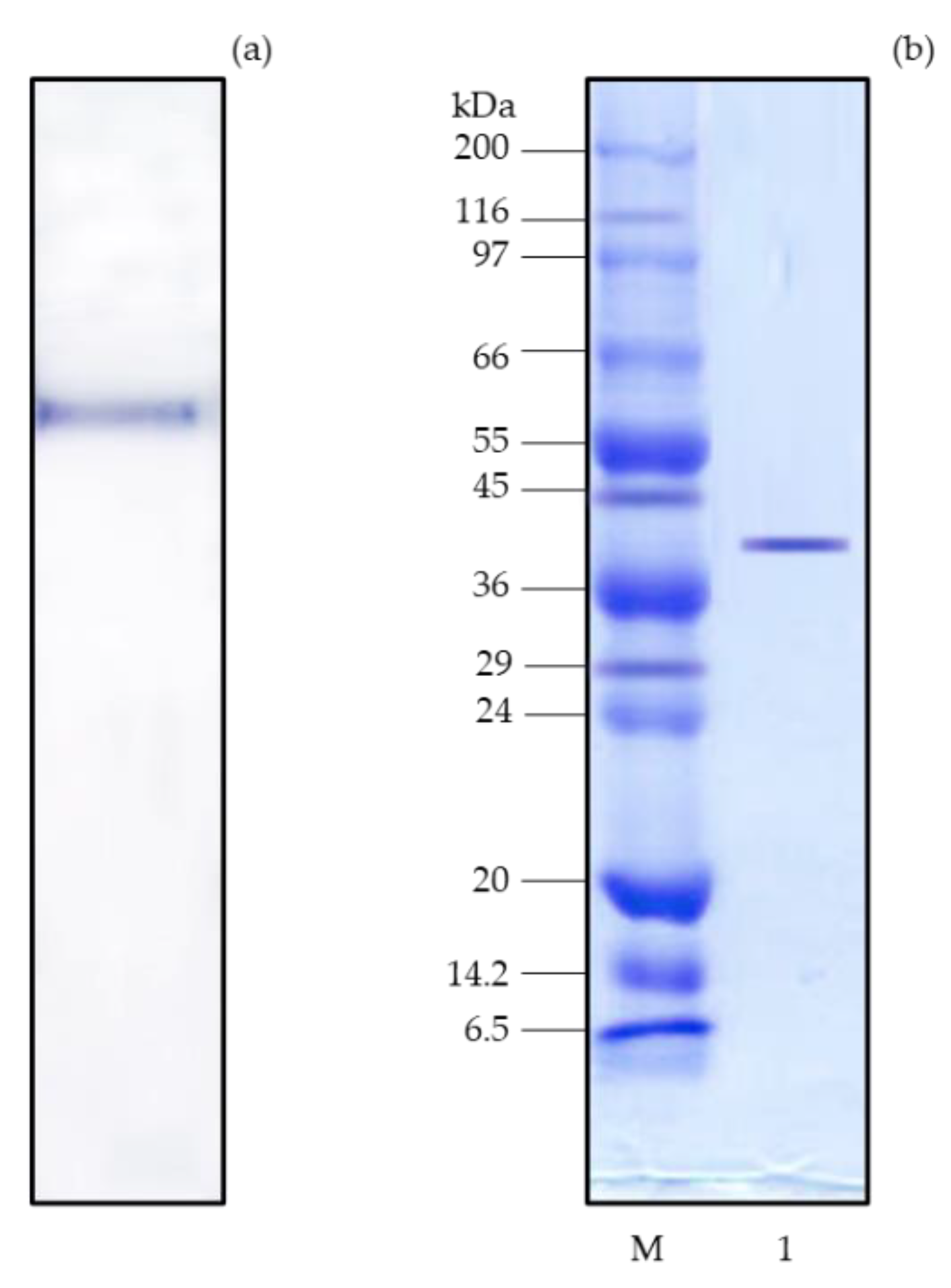
3.2. Native and SDS-PAGE
Purify of chymotrypsin purified from digestive organs of bigfin reef squid was estimated using native-PAGE (
Figure 2a). Only one protein band was seen on the gel, demonstrating that chymotrypsin was homogeneous after the purification procedure. Previous research reported similar results on the native-PAGE gel from chymotrypsin isolated from red abalone, Haliotis rufescens [
17], and cuttlefish [
6].
The purified chymotrypsin analyzed using SDS-PAGE was presented in
Figure 2b. The gel displayed a one band with chymotrypsin molecular weight (MW) of 43 kDa, which corresponded to the value obtained using gel filtration chromatography on Sephacryl S-200 (data not illustrated). This finding revealed that the purified chymotrypsin from bigfin reef squid digestive organs is a monomeric protein with a MW of 43 kDa. In general, chymotrypsin enzymes from aquatic animals have MWs from 22 to 35 kDa. Reported examples include MWs of 27 kDa in cod [
18], 25.5 kDa in Monterey sardine [
9], 31 kDa in jumbo squid [
19], and 35.7 kDa in yellowleg shrimp, Penaeus californiensis [
7].
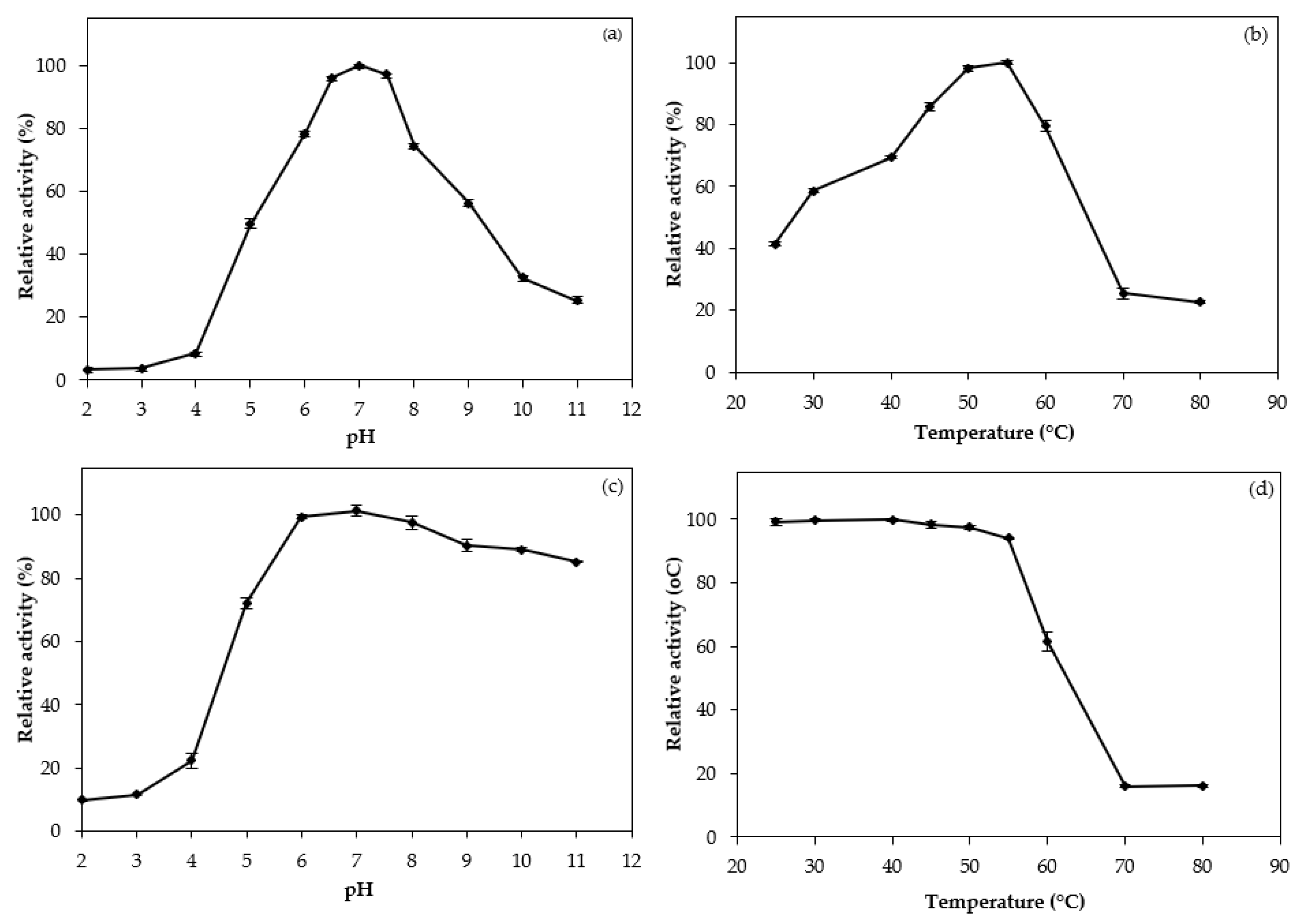
3.3. pH and Temperature Profiles
The purified chymotrypsin from bigfin reef squid digestive organs displayed the maximum activity at pH 7.0 (
Figure 3a). At pH 6.0–8.0, relative chymotrypsin activity was still approximately 75%. As reported previously, the optimum pH values of chymotrypsin derived from marine invertebrates are in the neutral to alkaline range [
6]. Similar to the present finding, Marquez-Rios et al. [
19] found an optimum pH of 7.0 for chymotrypsin from the hepatopancreas of the jumbo squid, and Bibo-Verdugo et al. [
20] found an optimum pH between 7.0 and 8.0 for chymotrypsin from the viscera of the California spiny lobster.
The optimal temperature profile of purified chymotrypsin is illustrated in
Figure 3b. The highest chymotrypsin activity was observed at 55°C. Above 60°C, chymotrypsin activity rapidly declined. The purified chymotrypsin from bigfin reef squid was generally active at relatively high temperatures from 40–60°C, probably because the bigfin reef squid has a habitat in relatively warm seas [
10,
19]. It was therefore concluded that the obtained chymotrypsin had an optimum conformation at relatively high temperatures. These profiles were similar to those of chymotrypsin enzymes extracted and purified from the cuttlefish [
6], the scallop Pecten maximus [
21] and the rainbow trout Oncorhynchus mykiss [
22]. In addition, the chymotrypsin activity decreased at high temperatures because of the thermal denaturation of enzyme protein.
3.4. pH and Thermal Stabilities
As shown in
Figure 3c, at pH values below 5, the purified chymotrypsin quickly became unstable, but from pH 6.0 to 11.0, the enzyme was very stable with relative activities of over 80%. Thus, bigfin reef squid chymotrypsin was stable at a relatively wide pH range from weak acidic to moderate alkali conditions. Chymotrypsin derived from cuttlefish [
6], jumbo squid [
19] and yellow-leg shrimp [
7] showed similar trends of stability with regard to pH variation. In general, the pH instability of an enzyme is correlated with the change in its net charge, which usually occurs when an enzyme is at a pH lower than its pI [
23].
As shown in
Figure 3d, the purified chymotrypsin was thermally stable at temperatures under 55°C. However, the chymotrypsin quickly lost its stability above 60°C, and was mostly inactive from 70 to 80°C (
Figure 3d) due to denaturation and loss of conformational structure [
24]. The thermal stability profile of chymotrypsin in this study was unlike some fish-derived chymotrypsin enzymes whose stability was optimal at approximately 30°C and which became unstable above 55°C [
19]. The purified chymotrypsin in the present investigation remained stable at temperatures up to 55°C. This degree of thermal stability is similar to that of chymotrypsin purified from the hepatopancreas of cuttlefish and white shrimp which respectively showed 75% and 80% relative activity at 50°C [
12]. Navarrete-del-Toro et al. [
7] found that chymotrypsin isolated from yellow shrimp showed 90% of its original activity at 50°C. The thermal stability of enzymes is linked to the interactions that stabilize enzyme protein structures. These include hydrophobic interactions, disulfide linkages, and the strong hydrophobic interactions within protein structures [
11].
3.5. Influence of Protease Inhibitors
The influence of a variety of inhibitors of protease on bigfin reef squid chymotrypsin is described in
Table 2. The purified enzyme was greatly inhibited by PMSF (9.05% relative activity), a serine proteinase inhibitor, and TPCK (27.59% relative activity), a specific chymotrypsin inhibitor. SBTI, a serine proteinase inhibitor, exhibited minimal inhibition against the purified chymotrypsin, which retained 89.23% of its original activity. Other studied inhibitors that are effective against cysteine (E-64), metallo (EDTA) and aspartic (pepstatin A) proteinases and trypsin (TLCK), barely inhibited our purified chymotrypsin. The findings of this study were similar to the findings of a study of chymotrypsin isolated from jumbo squid [
19] that was strongly inhibited by TPCK (6% of original activity) and PMSF (0% of original activity) Moreover, Balti et al. [
6] found that chymotrypsin obtained from the hepatopancreas of cuttlefish was fully inactivated by PMSF and SBTI. The inhibition study indicated that the purified enzyme was chymotrypsin.
3.6. Kinetics Studies
Using Lineveaver-Burk plots, the kinetic constants (K
m and K
cat) of the bigfin reef squid chymotrypsin were measured. The K
m, K
cat and catalytic efficiency (K
cat/K
m) of purified chymotrypsin were 1.33 mM, 31.46 s
‒1 and 23.65 s
‒1 mM
‒1, respectively. The K
m value of the studied chymotrypsin was lower than that of chymotrypsin from the whiteleg shrimp [
8], which suggested that bigfin reef squid chymotrypsin had a higher affinity. The K
cat value of the studied chymotrypsin was similar to the K
cat values of chymotrypsin isolated from cattle (Bos taurus) [
25]. The catalytic efficiency (K
cat/K
m) of the studied chymotrypsin showed a higher value than chymotrypsin from the carp Cyprinus carpio [
26] and whiteleg shrimp [
8]. The results suggested that chymotrypsin from bigfin reef squid digestive organs was more efficient in catalysis than some of its counterparts.
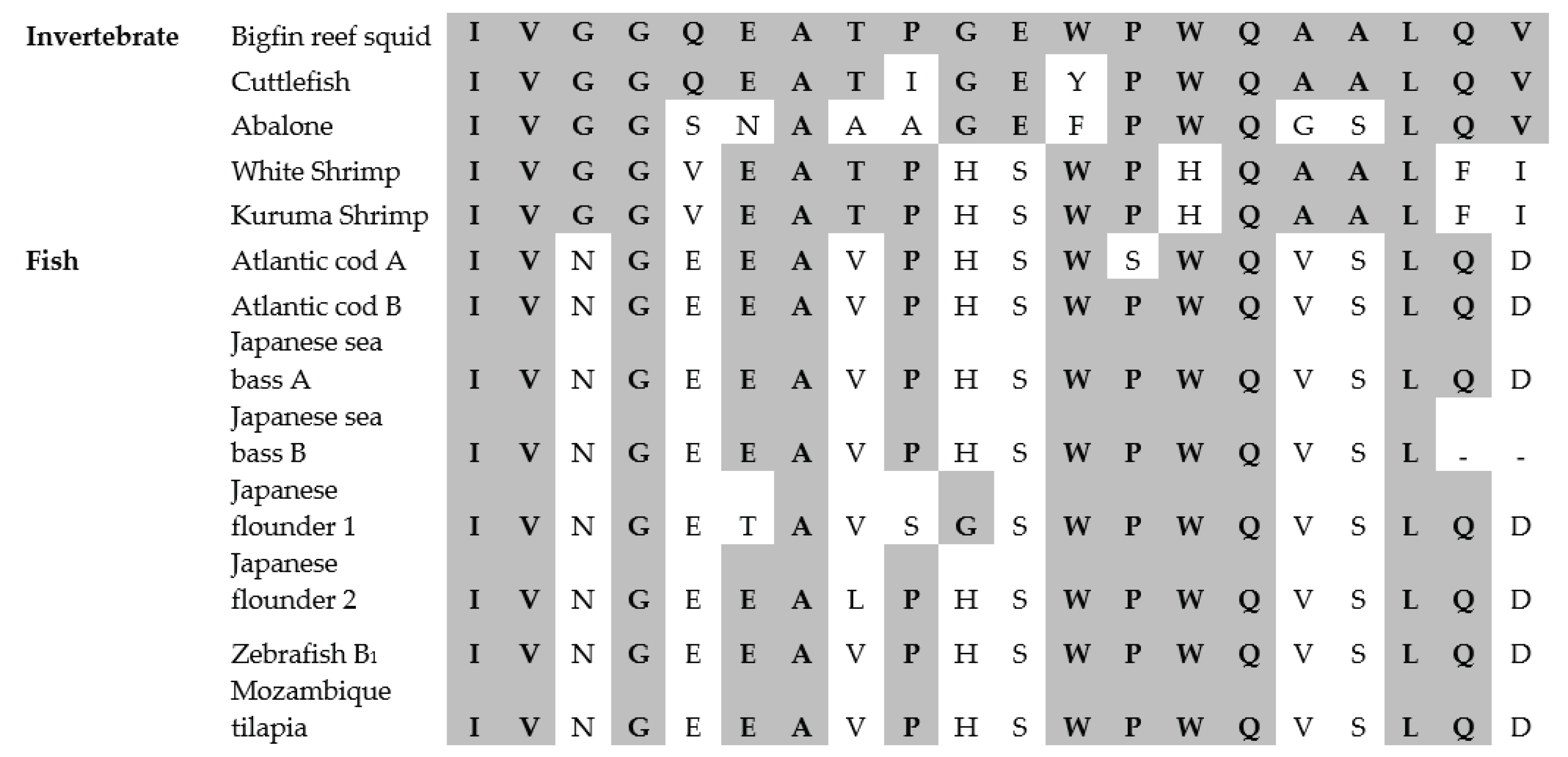
3.7. N-Terminal Amino Acid Sequence
Figure 4 illustrates the twenty amino acids sequence on the N-terminal of the purified chymotrypsin from the bigfin reef squid digestive organ and a comparison with the N-terminal sequences of chymotrypsin enzymes from other species. The N-terminal sequence of the purified chymotrypsin was IVGGQEATPGEWPWQAALQV. The sequence was uniform, suggesting that the enzyme was in a pure form. According to the comparison, the amino acid residues such as I1, V2, G4, A7, Q15 and L18 were found in all samples of purified chymotrypsin (
Figure 4). The studied chymotrypsin exhibited high similarity to cuttlefish chymotrypsin [
6]. There was difference of only two amino acid residues between the two enzymes. Chymotrypsin from white shrimp [
27] and kuruma shrimp [
28] exhibited moderate similarity (six different residues) to the proposed chymotrypsin.
4. Conclusions
A new chymotrypsin was isolated to homogeneity from bigfin reef squid digestive organs, with an optimal temperature of 55°C and pH of 7.0. PMSF and TPCK strongly inhibited the purified chymotrypsin. The kinetics parameters Km, Kcat and Kcat/Km were 1.33 mM, 31.46 s‒1 and 23.65 s‒1 mM‒1, respectively. Its N-terminal amino acid sequence was IVGGQEATPGEWPWQAALQV that displayed a high homology with cuttlefish chymotrypsin. This first report of the isolation and biochemical characteristics of chymotrypsin from the bigfin reef squid produced useful findings that could inform research into the physiological and nutritional responses of the species for future farming development and have potential applications in the food industry.
Author Contributions
Conceptualization, S.K. and K.T.; methodology, J.S. and S.K.; software, Y.Z.; validation, S.K., Y.Z. and K.T.; writing—original draft preparation, J.S.; writing—review and editing, S.K., Y.Z. and K.T.; supervision, S.K. and K.T.; funding acquisition, J.S., S.K. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Thailand Research Fund under the Royal Golden Jubilee Ph.D. Programme to Jirapan Satjarak, Grant Number: PHD/0038/2559. This work was also supported by National Higher Education, Science, Research and Innovation Policy Council, Thaksin University (Research project grant) Fiscal Year 2023.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This study was financially supported by the Thailand Research Fund under the Royal Golden Jubilee Ph.D. Programme to Jirapan Satjarak, Grant Number: PHD/0038/2559. This work was also supported by National Higher Education, Science, Research and Innovation Policy Council, Thaksin University (Research project grant) Fiscal Year 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, P.G.; Turk, P.E.; Yang, W.T.; Hanlon, R.T. Biological characteristics and biomedical applications of the squid Sepioteuthis lessoniana cultured through multiple generations. Biol. Bull. 1994, 186, 328–341. [Google Scholar] [CrossRef]
- Ibarra-García, L.E.; Tovar-Romírez, D.; Rosas, C.; Campa-Córdova, Á.I.; Mazón-Suástegui, J.M. Digestive enzymes of the California two-spot octopus, Octopus bimaculoides (Pickford and McConnaughey, 1994). Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2018, 215, 10–18. [Google Scholar] [CrossRef]
- Bastos, P.; Fracalossi, D.M.; Chimal, M.E.; Sánchez, A.; Rosas, C. Digestive enzymes and timing of digestion in Octopus vulgaris type II. Aquac. Rep. 2020, 16, 100262. [Google Scholar] [CrossRef]
- Jivaluk, J.; Nabhitabhata, J.; Nateewathana, A.; Watprasit, P. Description of Thai type of bigfin reef squid, Sepioteuthis lessoniana, hatchling with note on comparison to Japanese types. Phuket Marine Biological Center Research Bulletin. 2005, 66, 117–126. [Google Scholar]
- Semmens, J.M. Changes in the digestive gland of the loliginid squid Sepioteuthis lessoniana (Lesson 1830) associated with feeding. J. Exp. Mar. Bio. Ecol. 2002, 274, 19–39. [Google Scholar] [CrossRef]
- Balti, R.; Bougherra, F.; Bougatef, A.; Hayet, B.K.; Nedjar-Arroume, N.; Dhulster, P.; Guillochon, D.; Nasri, M. Chymotrypsin from the hepatopancreas of cuttlefish (Sepia officinalis) with high activity in the hydrolysis of long chain peptide substrates: purification and biochemical characterization. Food Chem. 2012, 130, 475–484. [Google Scholar] [CrossRef]
- Navarrete-del-Toro, M.A.; García-Carreño, F.L.; Hernández-Cortés, P.; Molnár, T.; Gráf, L. Biochemical characterisation of chymotrypsin from the midgut gland of yellowleg shrimp, Penaeus californiensis. Food Chemi. 2015, 173, 147–155. [Google Scholar] [CrossRef]
- Hernández-Cortés, P.; Whitaker, J.R.; García-Carreño, F.L. Purification and characterization of chymotrypsin from Penaeus vannamei (Crustacea: decapoda). J. Food Biochem. 1997, 21, 497–514. [Google Scholar] [CrossRef]
- Castillo-Yañez, F.J.; Pacheco-Aguilar, R.; Lugo-Sanchez, M.E.; Garcia-Sanchez, G.; Quintero-Reyes, I.E. Biochemical characterization of an isoform of chymotrypsin from the viscera of Monterey sardine (Sardinops sagax caerulea), and comparison with bovine chymotrypsin. Food Chem. 2009, 112, 634–639. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B.K.; Saeki, H. Trypsins from yellowfin tuna (Thunnus albocores) spleen: Purification and characterization. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2006, 144, 47–56. [Google Scholar] [CrossRef]
- Poonsin, T.; Simpson, B.K.; Benjakul, S.; Visessanguan, W.; Yoshida, A.; Osatomi, K.; Klomklao, S. Anionic trypsin from the spleen of albacore tuna (Thunnus alalunga): purification, biochemical properties and its application for proteolytic degradation of fish muscle. Int. J. Biol. Macromol. 2019, 133, 971–979. [Google Scholar] [CrossRef]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.; Waagbø, R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.G.D.; Torrie, J.H. Principle and procedures of statistics: a biometrical approach. New York: McGraw-Hill. 1980.
- Groppe, J.C.; Morse, D.E. Molluscan chymotrypsin-like protease; structure, localization and substrate specificity. Arch. Biochem. Biophys. 1993, 305, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Leth-Larsen, R.; Ásgeirsson, B.; Thórólfsson, M.; Nørregaard-Madsen, M.; Højrup, P. Structure of chymotrypsin variant B from Atlantic cod, Gadus morhua. Biochim. Biophys. Acta. 1996, 1297, 49–56. [Google Scholar] [CrossRef]
- Marquez-Rios, E.; Coto-Arriola, O.; Villalba-Villalba, A.G.; Ezquerra-Brauer, J.M.; Ocaño-Higuera, V.M.; Lopez-Corona, B.E.; Torres-Arreola, W. Chymotrypsin isolation from jumbo squid (Dosidicus gigas) hepatopancreas: Partial characterization and effect on muscle collagen. Food Sci. Biotechnol. 2016, 25, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Bibo-Verdugo, B.; Rogo-Arreola, L.; Navarrete-del-Toro, M.A.; García-Carreño, F. A chymotrypsin from the digestive tract of California spiny lobster, Panulirus interruptus: Purification and biochemical characterization. Mar. Biotechnol. 2015, 17, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Le Chevalier, P.; Sellos, D.; Van Wormhoudt, A. Purification and partial characterization of chymotrypsin-like proteases from the digestive gland of the scallop Pecten maximus. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1995, 110, 777–784. [Google Scholar] [CrossRef]
- Kristjánsson, M.M.; Nielsen, H.H. Purification and characterization of two chymotrypsin-like proteases from the pyloric caeca of rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1992, 101, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsson, M.M.; Nielsen, H.H. Purification and characterization of two chymotrypsin-like proteases from the pyloric caeca of rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1992, 101, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Poonsin, T.; Simpson, B.K.; Visessanguan, W.; Yoshida, A.; Klomklao, S. Optimal immobilization of trypsin from the spleen of albacore tuna (Thunnus alalunga) and its characterization. Int. J. Biol. Macromol. 2020, 143, 462–471. [Google Scholar] [CrossRef] [PubMed]
- DelMar, E.G.; Largman, C.; Brodrick, J.W.; Geokas, M.C. A sensitive new substrate for chymotrypsin. Anal. Biochem. 1979, 99, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Gertler, A.; Birk, Y. Pancreatic proteolytic enzymes from carp (Cyprinus carpio) I. purification and physical properties of trypsin, chymotrypsin, elastase and carboxypeptidase B. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1981, 69, 639–646. [Google Scholar] [CrossRef]
- Sellos, D.; Van Wormhoudt, A. Molecular cloning of a cDNA that encodes a serine protease with chymotryptic and collagenolytic activities in the hepatopancreas of the shrimp Penaeus vannamei (Crustacea, Decapoda). FEBS Letters, 1992, 309, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Danwattananusorn, T.; Kondo, H.; Aoki, T.; Hirono, I. Molecular cloning, characterization and expression analysis of a chymotrypsin-like serine protease from kuruma shrimp Marsupenaeus japonicas. Fish Sci. 2009, 75, 1231–1238. [Google Scholar] [CrossRef]
- Jiang, Y.K.; Sun, L.C.; Cai, Q.F.; Liu, G.M.; Yoshida, A.; Osatomi, K.; Cao, M.J. Biochemical characterization of chymotrypsins from the hepatopancreas of Japanese sea bass (Lateolabrax japonicus). J. Agric. Food. Chem. 2010, 58, 8069–8076. [Google Scholar] [CrossRef]
- Suzuki, T.; Srivastava, A.S.; Kurokawa, T. cDNA cloning and phylogenetic analysis of pancreatic serine proteases from Japanese flounder, Paralichthys olivaceus. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2002, 131, 63–70. [Google Scholar] [CrossRef]
- Lo, M.J.; Weng, C.F. Developmental regulation of gastric pepsin and pancreatic serine protease in larvae of the euryhaline teleost, Oreochromis mossambicus. Aquaculture. 2006, 261, 1403–1412. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).