1. Introduction
“Alternative renewable energy sources such as bioethanol have been gaining popularity because of the escalating energy demands, adverse environmental effects, and energy security concerns associated with conventional energy sources” [
1,
2,
3,
4]. Bioethanol is a biofuel derived from a sustainable lignocellulosic material that possesses the capacity to substantially reduce the release of detrimental pollutants owing to its exceptional combustion efficiency [
5,
6]. Bioethanol production using lignocellulose-based processes can reduce environmental impacts and enhance energy security [
2,
7,
8]. Lignocellulosic biomass has been recognized as a plentiful and sustainable alternative energy resource with the potential to supply environmentally friendly clean energy [
9,
10,
11]. “Lignocellulosic materials refer to plant biomass such as agricultural waste, hardwood, softwood, non-food crop plants, and weed biomass, which are composed of cellulose (30–50%), hemicellulose (20–35%), and lignin (5–25%) [
5,
7].”
“Various lignocellulosic feedstocks, including rice straw, wheat straw, corn stover, sugar cane, and bamboo [
4], as well as weed biomass, such as
Miscanthus × giganteus [
12],
Panicum virgatum [
13] and
Vietnamosasa pusilla” [
14], show bioethanol production potential.
Thysanolaena latifolia, commonly referred to as tiger grass or broom grass, is indigenous to Southeast Asia and belongs to the Poaceae family. It grows in dense clumps and has a lifecycle of up to ten years. It thrives in temperate and subtropical regions and can adapt to various environments, such as hillsides and valleys, among rocks, degraded areas, and wastelands, and is also successfully cultivated in agricultural areas [
15,
16].
Thysanolaena latifolia is used in various ways—its leaves and tips are used as animal feed, “while its bushy, fox-tail-like panicles are utilized for broom-making. However, after cutting the panicles, the stem portion is disposed of by burning, resulting in the release of significant quantities of air pollutants such as carbon monoxide (CO) and nitrogen dioxide (NO
2)” [
17,
18].
Thysanolaena latifolia is synonymous with
T. maxima [
19], which is a common weed in the Northern part of Thailand. “This weed can grow and produce a large amount of biomass even with limited nutrition and water” [
20]. Initial analysis indicates that
T. latifolia’s biomass contains approximately 60% polysaccharide. The substantial polysaccharide content of this biomass suggests that it has considerable potential as a sustainable feedstock for bioethanol production. However, the complex polysaccharide structure significantly affects saccharification, hindering the conversion of biomass into biofuels. Consequently, pretreatment is indispensable for overcoming the intrinsic resistance of lignocellulose to fermentation. “This process improves the enzymatic digestion of the treated substance, contributing to the generation of monomeric sugars that can be subsequently fermented to produce bioethanol” [
4,
5,
21].
“Different approaches, including physical, chemical, physicochemical, and biological reactions, can be utilized for the pretreatment of lignocellulosic materials” [
7,
9]. “Acid-based pretreatment is one of the most commonly used chemical pretreatment methods because it is effective in breaking down the structure of lignocellulosic materials and promoting cellulose conversion” [
9]. “In particular, phosphoric acid (H
3PO
4) has shown great potential as a pretreatment agent because of its ability to break the linkages among lignocellulose components and disrupt the orderly hydrogen bonds in cellulose fibers under mild conditions” [
7,
22,
23]. “Different types of biomasses, including agricultural residues [
24,
25,
26,
27], grasses [
14,
28,
29], hardwood [
30], and softwood [
31], have shown a significant improvement in the yield efficiency of enzymatic hydrolysis following their treatment with H
3PO
4.”
The present study aimed to examine the most effective H3PO4 pretreatment conditions and emphasize the potential of T. latifolia as an environmentally friendly material source for producing alternative bioethanol.
2. Materials and Methods
2.1. Raw Materials
A fresh sample of T. latifolia was collected from the upper ground in the Bo Kluea District, Nan Province, Thailand (19° 19 33.7 N, 101° 11 41.1 E) in August 2022. “The herbarium of the Biology Department of Naresuan University verified the authenticity of the specimen. This specimen was conserved, and the record 05935 was assigned as a reference for the herbarium.”
“The raw biomass was initially washed with water to remove soil particles and dried in the shade for 7 days. It was cut into approximately 5 cm long segments and ground into a powdered form using a milling machine (Retsch, Haan, Germany). The samples were then passed through a 150–300 µm laboratory test sieve before being deposited in containers at 25 °C for subsequent assessment and testing” [
29].
2.2. Chemical Analytical Methods
“The chemical compositions of both raw and treated
T. latifolia feedstocks were analyzed using a standard method developed by the National Renewable Energy Laboratory to evaluate monomer sugars, acid-soluble lignin (ASL), acid-insoluble lignin (AIL), ash, and ethanol extracts” [
32,
33,
34].
“The quantities of each monomer sugar and ethanol were measured using a high-performance liquid chromatography system (HPLC; Agilent 1100; Agilent Technologies, Waldbronn, Germany) with a refractive index detector (RID; G1362A; Agilent Technologies). The RID was maintained at 55 °C. An Aminex HPX-87H column (300 mm × 7.8 mm; Hercules, CA, USA) was used to separate the ethanol and sugar. The column was operated at 60 °C, and 20 µL samples were injected with a mobile phase of 0.005 M sulfuric acid, flowing at 0.6 mL/min” [
26].
2.3. Pretreatment Procedure
“The raw biomass of
T. latifolia was pretreated according to the procedure outlined by Premjet et al.” [
25]. “A 300 mg dry sample was mixed with 24 mL of 70%, 75%, 80%, and 85% H
3PO
4 (v/v) in a 50 mL centrifuge tube. The mixture was stirred thoroughly, and the tubes were covered and heated to 60 °C in a water bath (Grant W28; Grant Instruments, Ltd., Sepreth, UK) for 60 min. The process was completed by adding approximately 20 mL of acetone to the treated sample in tubes and vigorously mixing it with a stirring rod. The liquid mixture was separated using a fixed-angle centrifuge (ScanSpeed 1248; Labogene ApS, DK-3450 Lynge, Denmark) at 7000× g for 10 min, and the supernatant was discarded. This process was repeated thrice. Once completed, the solid fraction was washed with deionized water until the pH reached approximately 6.5–7.”
“Lignin removal (%) and recovery yield (%) were calculated using Equations (1) and (2), respectively:
2.4. Crystallinity and Morphology Analysis
“Analysis was performed according to the procedure suggested by Premjet et al.” [
25]. “Both treated and untreated
T. latifolia biomasses were rinsed three times with acetone and dried overnight at 25 °C. The samples then underwent crushing and screening using a 150 µm sieve. An X-ray diffractometer (PANalytical X’pert Pro, PW 3040/60; Almelo, the Netherlands) was used to examine the crystallinity of the biomass. The sample was scanned between 10° and 40° at a speed of 0.02° s
-1/min.”
“The crystallinity index (CrI) was calculated using the Segal equation [
35]:
“where I002 is the highest intensity of the crystallinity region at 2θ = 22.0°, and Iam corresponds to the lowest intensity of the amorphous region at 2θ = 18.2°.”
“The raw and pretreated
T. latifolia materials were freeze-dried and attached to stubs with a double-sided adhesive for visualization. The biomass was subsequently coated with a fine layer of gold and analyzed using field-emission scanning electron microscopy (SEM) (Apero S; Thermo Fisher Scientific, Waltham, MA, USA)” [
29].
2.5. Enzymatic Hydrolysis
“Enzymatic hydrolysis was performed according to the procedure described by Premjet et al.” [
25]: “Both treated and untreated
T. latifolia samples underwent enzymatic hydrolysis. The reaction was carried out in a 50 mL Erlenmeyer flask containing 0.1 g of biomass (dry weight). The reaction mixture consisted of 0.05 M sodium citrate buffer with a pH of 4.8 and 0.1 mL of a 2% (w/v) sodium azide. The total volume of the reaction mixture was 10 mL. The enzyme mixture contained 30 filter paper units of cellulase (
Trichoderma reesei C2730; Sigma-Aldrich, St. Louis, MO, USA) and 60 units of β-glucosidase (Oriental Yeast Co., Ltd., Tokyo, Japan) per gram of dry biomass. The reaction mixture was incubated for 72 h on a rotary shaker (Innova 4340; New Brunswick Scientific Company, Edison, NJ, USA) at 50 °C and 150 rpm. The hydrolysates (20 µL) were collected at various time intervals (12, 24, 48, and 72 h) for HPLC analysis of monomer sugars.”
“Hydrolysis efficiency (HE) and glucose recovery (GR) were determined using Equations (4) and (5), respectively.
where 0.9 and 1.1 are the conversion factors for glucan to glucose.”
2.6. Yeast Strain
“A yeast strain purchased from the Thailand Institute of Scientific and Technological Research (TISTR),
Saccharomyces cerevisiae TISTR 5339, was used for bioethanol fermentation” [
36].
2.7. Preparation of Seed Culture
“Yeast was propagated as a seed culture in a 10 mL liquid yeast malt medium. The culture was incubated at 30 °C for 18 h at 180 rpm in a rotary shaker incubator (Innova 4340; New Brunswick Scientific)” [
36].
2.8. Preparation of T. latifolia Biomass Hydrolysate
“Thysanolaena latifolia biomass hydrolysate (TBH) was prepared according to the method described by Premjet et al. [
36]. The TBH produced from enzymatic saccharification was subjected to heat treatment at 100 °C for 20 min in a water bath (Grant W28; Grant Instruments, Ltd.). Subsequently, the TBH was precipitated by centrifugation at 12,000× g for 2 h and filtered through a glass microfiber filter to remove plant matter. Then, it was concentrated to obtain approximately 20 g/L glucose, and the pH of the TBH was adjusted to 6 using 1 M NaOH. The TBH was stored at 4 °C for further investigation” [
36].
2.9. Medium for Bioethanol
“Ethanol was produced using control (CT) and TBH media. The TBH medium contained glucose derived from
T. latifolia biomass, whereas commercial glucose was used as the carbon source in the CT medium. Both media contained 20 g/L glucose, 10 g/L yeast extract, 10 g/L peptone, 2 g/L K
2HPO
4, and 2 g/L MgSO
4, and pH was adjusted to 6. Before use, both media were aseptically filtered using a 0.02 µm Millipore filter” [
36].
2.10. Ethanol Production
“Ethanol production by
S. cerevisiae TISTR 5339 was conducted according to the methods described by Premjet et al.” [
36]: “In order to produce ethanol, 50 mL of TBH and the CT medium were mixed with 2% seed culture and placed in a shaker incubator (Innova 4340; New Brunswick Scientific) at 150 rpm and 30 °C. After 3, 6, 9, 12, 15, 18, 21, and 24 h of incubation, the liquid fraction was collected to measure sugar consumption and ethanol production using HPLC.”
“The ethanol yield (%) was calculated using Equation (6):
2.11. Determination of Yeast Cell Growth
“Yeast cell growth was monitored using a UV spectrophotometer (SP-830 Plus; Metertech, Taipei, Taiwan), and the optical density was measured at 600 nm. An optical density of 1.0 (at 600 nm) corresponds to approximately 1.5 × 10
7 cells/mL” [
37].
2.12. Statistical Analysis
“Each experiment was performed in triplicate, and the resulting data were presented in the form of the average and standard deviation (±SD). A Tukey’s test at a 5% level of significance was conducted for the comparison of treatment means and Analysis of Variance using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA)” [
24].
3. Results
3.1. Characterization of T. latifolia Biomass
The composition of the
T. latifolia raw biomass employed in this investigation is listed in
Table 1.
3.2. Effect of H3PO4 Concentrations on Chemical Composition
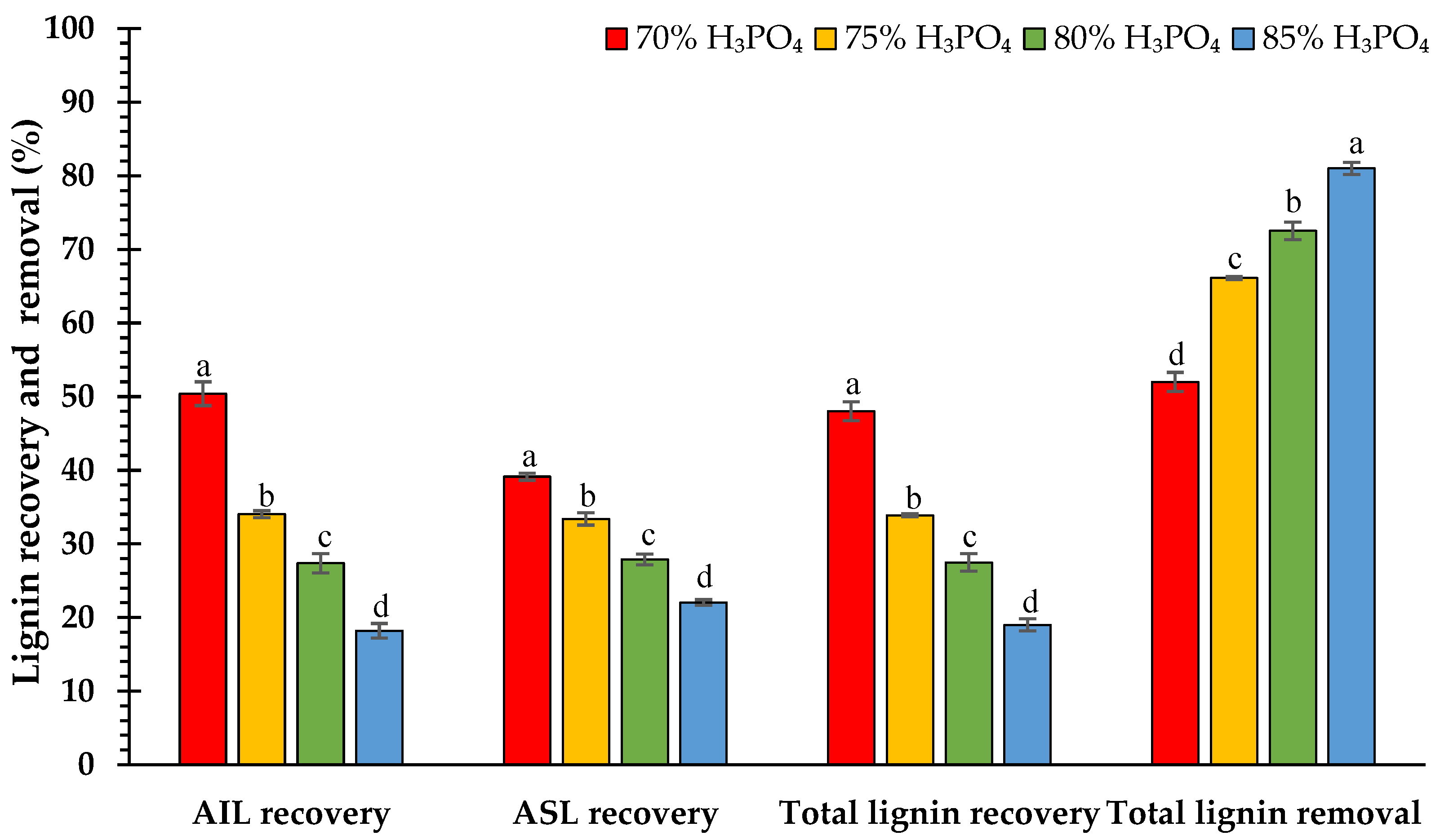
“
Table 2 shows the differences in the chemical structures of the biomass before and after pretreatment under various H
3PO
4 concentrations”. This was done to determine the optimum pretreatment condition of the
T. latifolia feedstock. When the concentration of H
3PO
4 enhanced, both the relative xylan and arabinan contents decreased substantially. The lowest yield of xylan content (8.5 ± 0.1%) was obtained at 85% H
3PO
4, whereas arabinan became undetectable. In contrast, samples treated with 70%, 75%, 80%, and 85% H
3PO
4 exhibited a progressive increase in relative glucan percentage. The maximum relative glucan content (64.4 ± 0.9%) was generated with 85% H
3PO
4 (
Table 2). In addition, an increase in the H
3PO
4 concentration led to a significant (
p < 0.05) decline in the relative amounts of AIL, ASL, and total lignin. Although the relative ASL content was reduced with 70% (3.7 ± 0.0%) and 75% (3.6 ± 0.1%) H
3PO
4, this effect was not statistically significant (
p < 0.05). However, relative ASL decreased significantly with treatment at 80% (3.3 ± 0.1%) and 85% (3.0 ± 0.1%) H
3PO
4. Meanwhile, total lignin removal (81.0 ± 0.8%) was most effective at a concentration of 85% H
3PO
4 (
Figure 1).
“The superscripted characters within rows indicate the statistical significance differences (p < 0.05) between them, n.d. = not determined.”
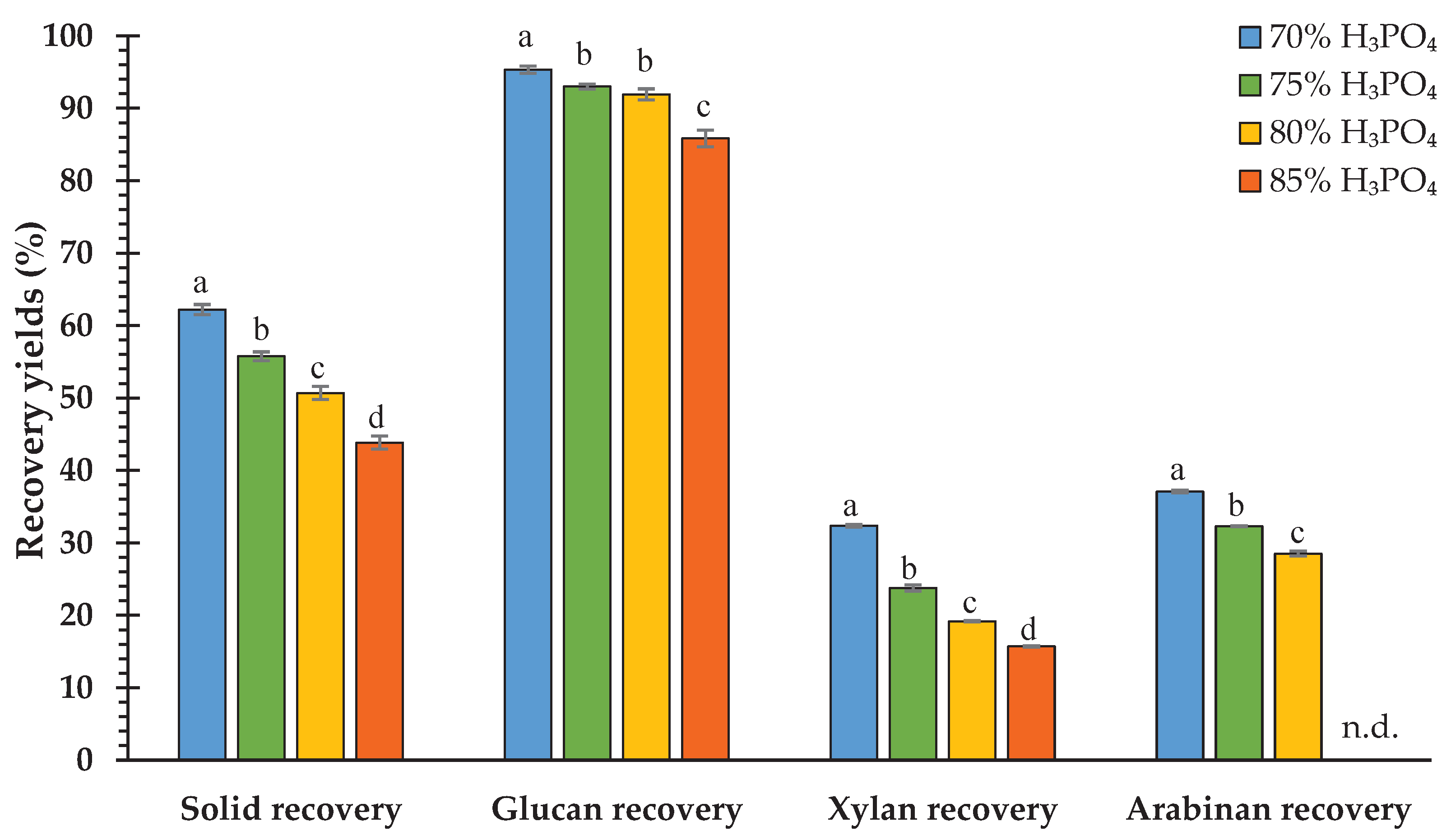
Additionally, substantial declines in the recovery yields of total lignin, glucan, xylan, arabinan, AIL, and ASL were observed as the concentration of H
3PO
4 increased (
Figure 1 and
Figure 2). When the feedstock was treated in 70% H
3PO
4, the highest recovery yields were obtained for solids (62.2 ± 0.7%), glucan (95.3 ± 0.5%), xylan (32.4 ± 0.2%), arabinan (37.1 ± 0.2%), AIL (50.4 ± 1.6%), ASL (39.1 ± 0.5%), and total lignin (48.0 ± 1.3%). Nevertheless, the decline in glucan recovery yield was not significantly different (
p < 0.05) at H
3PO
4 concentrations of 75% (93.0 ± 0.3%) and 80% (91.9 ± 0.8%). However, the sample subjected to treatment with 85% H
3PO
4 resulted in the lowest recovery yields for solids (43.8 ± 0.9%), glucan (85.8 ± 1.2%), xylan (16.2 ± 0.2%), AIL (18.2 ± 1.0%), ASL (22.0 ± 0.4%), and total lignin (19.0 ± 0.8%) whereas arabinan recovery was undetectable (
Figure 1 and
Figure 2).
3.3. Impact of H3PO4 Concentration on Cellulose Crystallinity
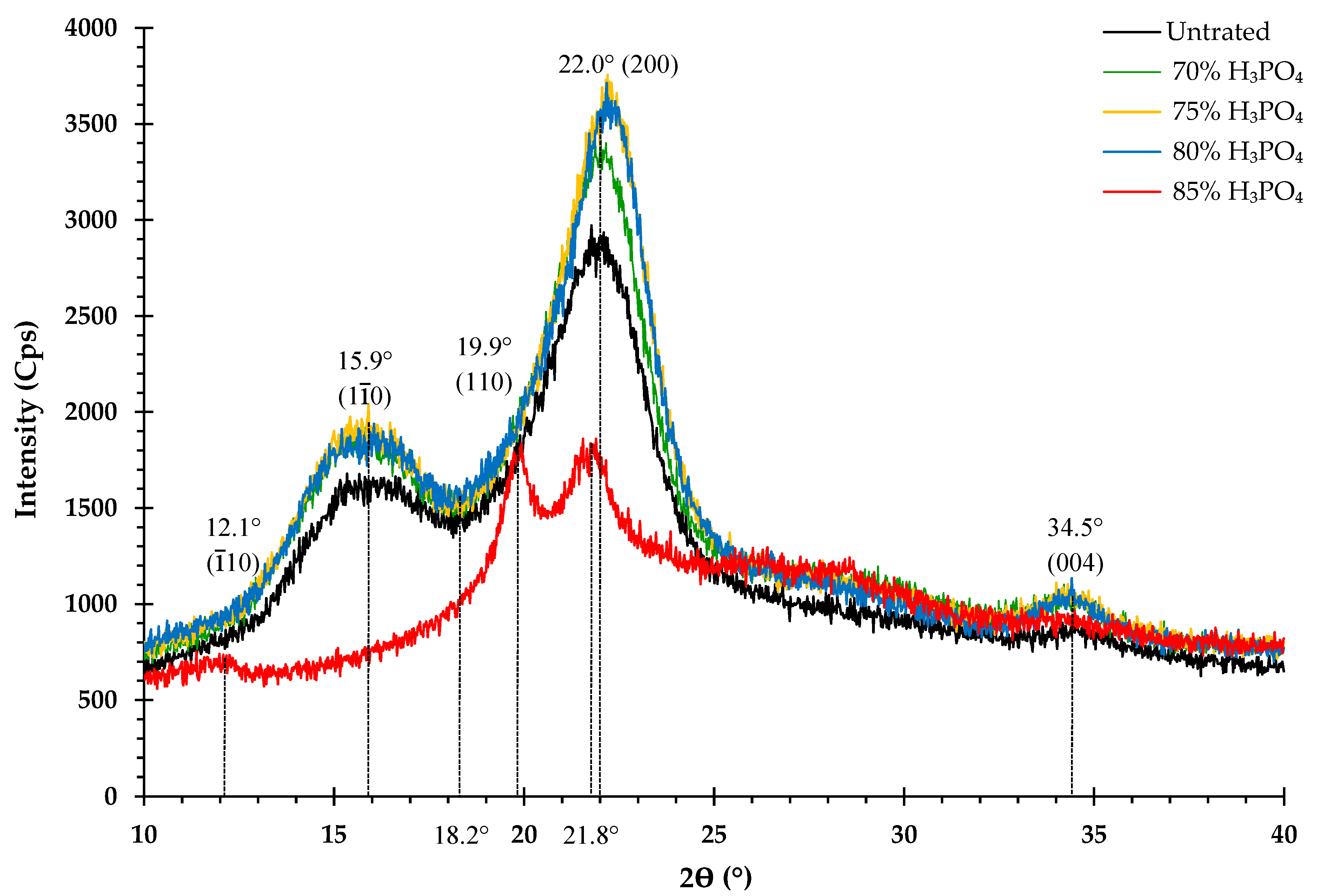
The impact of H
3PO
4 pretreatment on cellulose crystallinity in
T. latifolia feedstock was assessed using X-ray diffraction (XRD). The relative CrIs of both the untreated and treated feedstocks are listed in
Table 3. The XRD patterns and CrI values demonstrated modifications in cellulose crystallinity when the raw material was treated with H
3PO
4 at various concentrations. The XRD pattern of the raw material was classified as cellulose I because it comprised three prominent peaks at 2θ = 15.9°, 22.0°, and 34.5° (
Figure 3), and its relative CrI value was 54.1%. The CrI increased to 57.7% and 61.1% when the sample was treated with 70% and 75% H
3PO
4, respectively. Following treatment with 80% and 85% H
3PO
4, the CrI of this material progressively declined to 59.1% and 49.1%, respectively. Moreover, the XRD patterns of the sample treated with 70%, 75%, and 80% H
3PO
4 remained identical to those of cellulose I crystallinity. After treatment of this biomass with 85% H
3PO
4, the primary peak at 2θ = 22.0° transitioned to an asymmetric peak consisting of a doublet at 2θ = 19.9° and 21.8°, while the broad peak at 2θ = 15.9° disappeared. Additionally, a new smaller peak emerged at about 2θ = 12.1°. These results indicate that cellulose I was transformed into cellulose II.
3.4. Impact of H3PO4 Concentration on Biomass Morphology
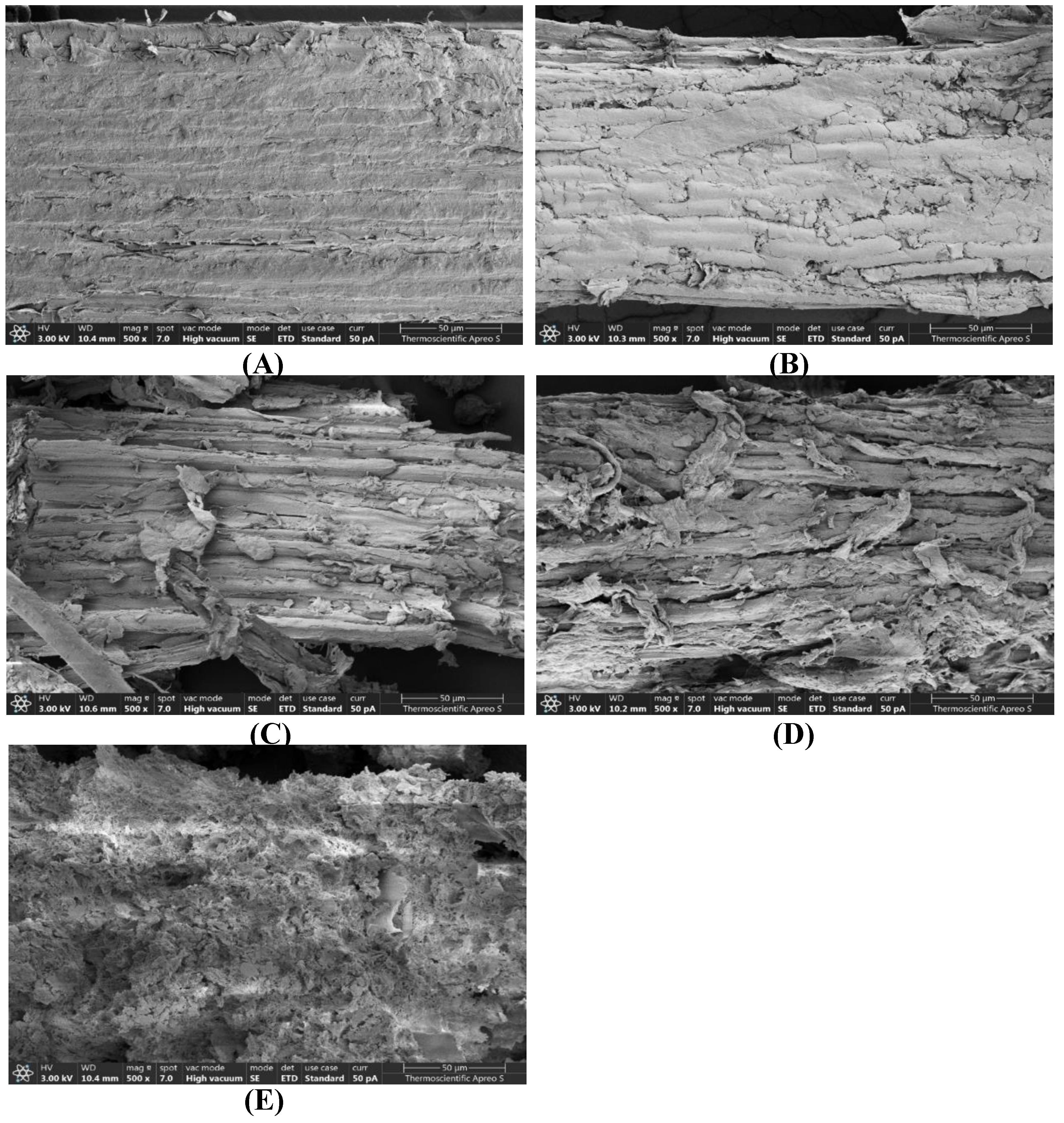
The morphology of untreated and pretreated
T. latifolia was analyzed using SEM to evaluate the physical changes that occurred during pretreatment with various concentrations of H
3PO
4. The SEM images provided a detailed depiction of the untreated
T. latifolia ultrastructure. The morphological surface structure was uniform, intact, and characterized by tightly arranged and inflexible bundles (
Figure 4A). The alteration in the surface structure was observed after the pretreatment of this feedstock with varying concentrations of H
3PO
4. When the sample was exposed to 70% H
3PO
4, the surface structure initially displayed roughening, splitting, expanding, and loosening (
Figure 4B). Samples treated with 75% H
3PO
4 exhibited greater peeling, delamination, loosening, and disintegration on their surfaces (
Figure 4C). An increase in the H
3PO
4 concentration to 80% caused the disintegration of the cellulose fibers and a more pronounced deterioration in their morphological structures (
Figure 4D). The use of 85% H
3PO
4 as a feedstock treatment caused the most severe deterioration and modification of the cellulose fiber structure, resulting in a transformation of the morphological characteristics of the treated material (
Figure 4E).
3.5. Enzymatic Saccharification Yields
To determine the optimal conditions for
T. latifolia biomass pretreatment, both untreated and treated samples were used as substrates for enzymatic hydrolysis. After treating the feedstock with varying concentrations of H
3PO
4, the HE and GR yields of both the untreated and treated samples improved dramatically after 12 h of incubation, increasing progressively after 24, 48, and 72 h (
Figure 5A). The maximum HE and GR yields of both untreated and treated samples were observed within a 72 h incubation period. However, all the treated samples generated greater amounts of HE and GR than the raw material. The untreated
T. latifolia sample generated the least yields of HE (29.5 ± 0.7%) and GR (12.0 ± 0.3%). After treating the sample with 70% and 75% H
3PO
4, the HE yields improved substantially to 65.3 ± 0.6% and 86.9 ± 0.7%, respectively, and increased slightly to 87.2 ± 0.2% and 88.2 ± 0.2%, when treated with 80% and 85% H
3PO
4, respectively (
Figure 5A). However, the HE yields among the specimens treated with 75%, 80%, and 85% H
3PO
4 did not differ significantly (
p < 0.05). In addition, the GR yield improved dramatically to 25.3 ± 0.2%, 32.8 ± 0.3%, and 32.5 ± 0.1% after treatment with 70%, 75%, and 80% H
3PO
4, respectively. Similarly, the GR yields between the two treatment procedures using 75% and 80% H
3PO
4 did not differ significantly (
p < 0.05). Conversely, the GR yield was reduced considerably (30.7 ± 0.1%) by treatment with 85% H
3PO
4 (
Figure 5B).
3.6. Bioethanol Fermentation
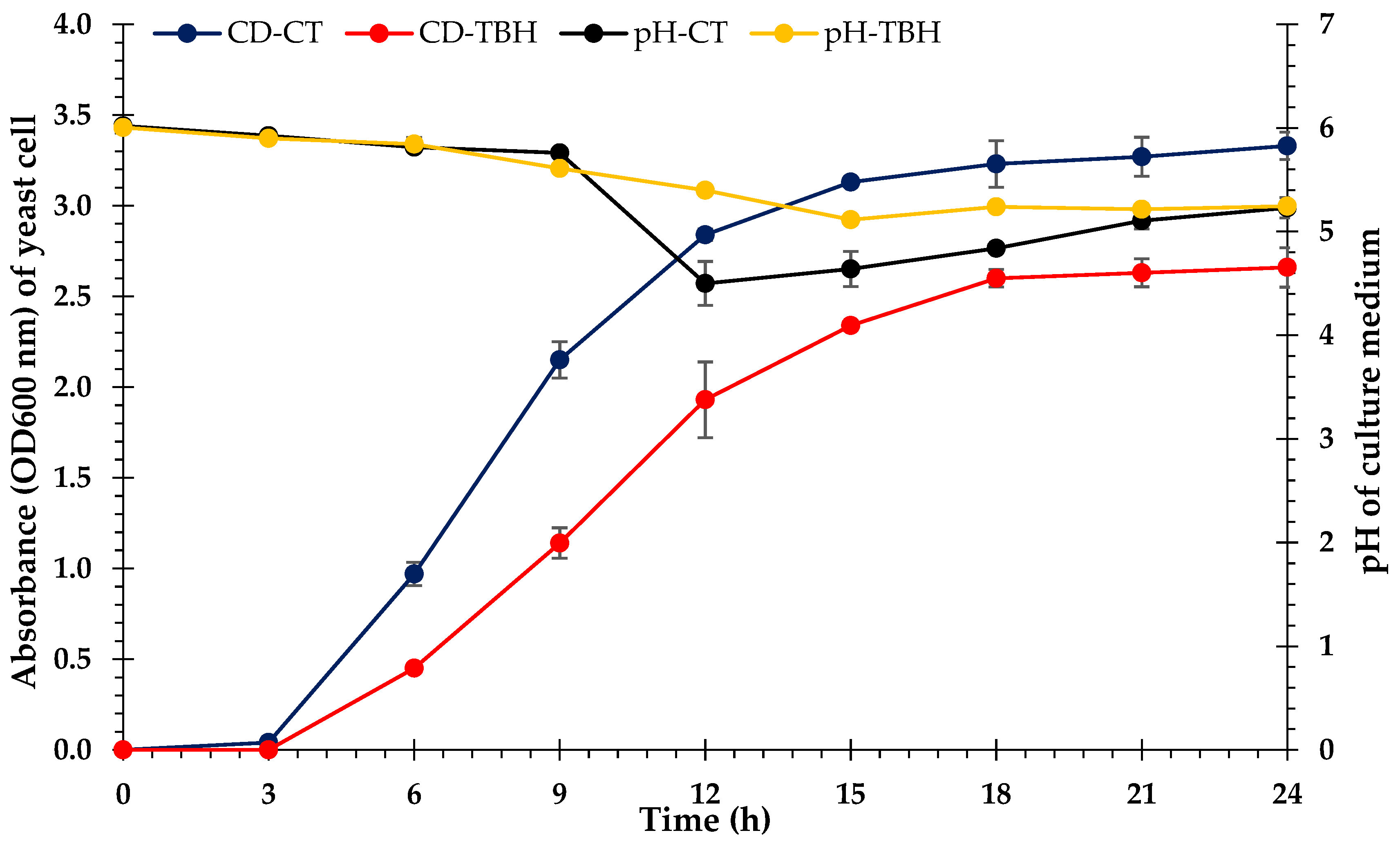
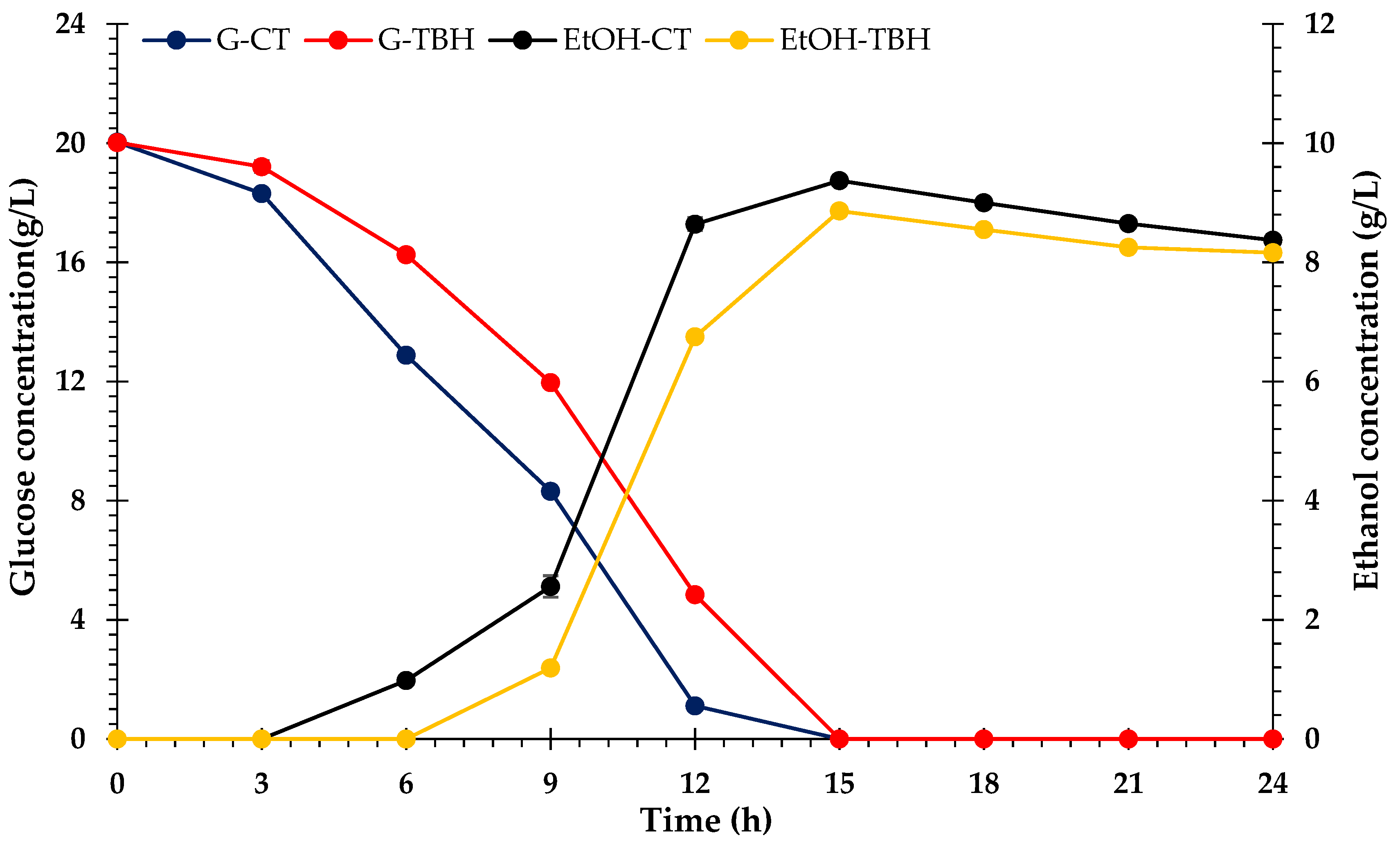
The TBH medium produced by enzymatic hydrolysis without a detoxification process was subjected to fermentation using
S. cerevisiae TISTR 5339 to assess its potential for bioethanol production from lignocellulose biomass. The results of this study indicated that glucose consumption, ethanol synthesis, yeast cell proliferation, and pH levels exhibited similar trends in both the CT and TBH media, as shown in
Figure 6 and
Figure 7. During fermentation, the cell growth profiles of the yeast and the pH observed in both CT and TBH media were slightly different; the CT medium demonstrated rapid growth, reaching the stationary phase at 15 h, while the growth rate in the TBH medium was slower than that in the CT medium (
Figure 6). The pH of the CT and TBH media decreased from 6.0 to 5.2. In addition, the yeast strain
S. cerevisiae TISTR 5339 rapidly utilized the glucose while concurrently producing ethanol. In both CT and TBH media, glucose was entirely consumed in 15 h. The observations of ethanol production revealed that the CT medium produced ethanol (1.0 ± 0.1 g/L or 26.7 ± 1.7% of the ethanol yield) within 6 h, whereas the TBH medium delayed ethanol production (1.2 ± 0.0 g/L or 29.0 ± 0.9% of the ethanol yield) to 9 h. Nevertheless, after 15 h of fermentation, the maximum ethanol yields were produced from both the CT (9.4 ± 0.0 g/L or 91.5 ± 0.3% of the ethanol yield) and TBH (8.9 ± 0.0 g/L or 86.6 ± 0.1% of the ethanol yield) media. Following that, ethanol yields declined steadily in both media (
Figure 7).
4. Discussion
4.1. Characterization of T. latifolia Biomass
The chemical composition of biomass has a substantial effect on the efficacy of bioenergy production [
38]. Consequently, determining the suitability of lignocellulose as an alternative resource necessitates an analysis of its chemical composition. According to the results, lignin, hemicellulose, and cellulose are the three primary building blocks of the
T. latifolia biomass. The total lignin level was substantially higher than that previously reported, whereas the glucan and xylan quantities were comparable [
20]. Variations in the composition of lignocellulosic biomass were attributed to factors such as growing circumstances, diverse sources, harvesting season/stage, and other associated variables [
4,
39,
40]. Nevertheless,
T. latifolia had a lower lignin content than both switchgrass (31.2%) and eucalyptus (29.4%) [
41], and several agricultural wastes, such as sorghum straw (30.4%) [
39], cotton stalk (30.0%), and walnut shell (37.5%) [
1]. Additionally, the total carbohydrates (62.4 ± 0.7%) in the
T. latifolia biomass were higher than those of lignocellulosic materials such as banana pseudo-stems (51.5%) [
42], bean straw (55.0%) [
43], switchgrass (56.8%) [
44], olive tree (57.8%) [
45], and sugar cane (60.7%) [
46], which are promoted as feedstock to produce biofuels. These total carbohydrate percentages demonstrate that
T. latifolia biomass could be utilized to generate cellulosic ethanol.
4.2. Effect of H3PO4 Concentrations on Chemical Composition
The utilization of lignocellulosic biomass exhibits potential as a viable non-edible resource in sugar-centric biorefineries. However, the inherent resistance of lignocellulose to enzymatic degradation is a significant challenge, necessitating a pretreatment procedure to modify the chemical and physical properties of the biomass [
8,
47,
48,
49]. This study demonstrated that an increase in H
3PO
4 concentration led to an increase in the relative glucan content while simultaneously causing a substantial decrease in the relative xylan, arabinan, AIL, ASL, and total lignin contents. “This can be attributed to the natural characteristics of hemicellulose and lignin; hemicellulose has a low degree of polymerization because of its amorphous nature and limited polymerization” [
8,
50]. Meanwhile, “lignin is an amorphous heteropolymer comprising three cinnamyl alcohol precursors (sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol) interconnected by different types of links” [
50,
51]. Consequently, under mild reaction conditions, hemicellulose is more prone to dissolution and removal than lignin [
8,
50]. In contrast, cellulose has a robust and efficiently organized molecular structure characterized by inter- and intra-molecular hydrogen bonding, “which contribute to the formation of a highly organized crystalline region inside cellulose, rendering it more resistant to breakdown under mild reaction conditions” [
1,
5,
23,
48]. The removal of hemicellulose and lignin also increases the relative glucan content in the treated sample [
48,
52]. Furthermore, H
3PO
4 disrupts the glycosidic linkages within the lignin–carbohydrate complex structure of the lignocellulosic biomass during the pretreatment process, “which causes the partial solubilization of hemicellulose, lignin, and cellulose components” [
4,
53,
54].
In addition, the recovery yield of solid, glucan, xylan, arabinan, and total lignin gradually decreased with increasing H
3PO
4 concentration. These modifications indicated that an increase in the H
3PO
4 concentration from 70% to 80% caused a gradual release of xylan, arabinan lignin, and glucan due to the breaking of glycosidic linkages within the lignin–carbohydrate complex structure of
T. latifolia biomass. Consequently, the recovery yields of xylan, arabinan, and total lignin were all <50%. In contrast, the recovery yields of solids and glucans exceeded 50% and 90%, respectively. However, treating the feedstock with the maximum concentration (85% H
3PO
4) can not only damage the structure of the lignin–carbohydrate complex but also disrupt the organized hydrogen bond network that exists in crystalline cellulose. “Because of this structural alteration, the carbohydrate and lignin constituents dissolve more readily, leading to a substantial decrease in the recovery yields of solids, glucan, xylan, and total lignin, whereas arabinan is eliminated entirely” [
23,
25,
53,
54,
55,
56,
57]. These phenomena exhibit parallels to those reported in several studies conducted on different biomass sources, including
Glycyrrhiza glabra [
57],
V. pusilla [
14],
Corchorus capsularis [
58], poplar [
30],
Hibiscus cannabinus [
26],
Durio zibethinus [
24],
Hibiscus sabdariffa [
25], wheat straw [
55], and
Luffa cylindrica [
59].
4.3. Impact of H3PO4 Concentration on Cellulose Crystallinity
Cellulose comprises both intra- and intermolecular hydrogen bonds, leading to exceptional stability in terms of its crystalline structure. This stability, however, has a substantial effect on enzymatic hydrolysis efficiency [
23,
60,
61]. The degradation of cellulose in H
3PO
4 typically corresponds to esterification, which prevents the formation of hydrogen bonds throughout the cellulose chain [
23,
62]. Consequently, H
3PO
4 induces the swelling and disintegration of the crystalline region inside the cellulose structure [
63]. “According to this study, the treatment of
T. latifolia feedstock with 70%, 75%, and 80% H
3PO
4 increased the relative CrI value owing to the partial removal of amorphous fractions, such as hemicellulose and lignin, during the pretreatment process” [
52,
63,
64,
65,
66]. The feedstock of
T. latifolia exhibited a substantial reduction in relative CrI after treatment with 85% H
3PO
4 (
Table 3). This was attributed to the disintegration of cellulose I crystalline sections, which caused the highly structured repeating units to become less rigid, forming cellulose II, also known as amorphous cellulose [
67]. The findings of this study confirmed that the application of 85% H
3PO
4 to
T. latifolia biomass alters the crystal structure of cellulose I to crystalline cellulose II. As several studies reported, crystalline cellulose is transformed into amorphous cellulose through pretreatment with above-critical (˃80.5%) concentrated H
3PO
4 [
14,
67,
68,
69].
4.4. Impact of H3PO4 Concentration on Biomass Morphology
The SEM images demonstrate the recalcitrant nature of the untreated
T. latifolia feedstock, which emerged from a complex of cellulose and hemicellulose biopolymers, together with heteropolymers of lignin [
8,
50,
51]. After treating the sample with H
3PO
4, the images revealed that the morphological structure of
T. latifolia feedstock gradually loosened, delaminated, and fibrillated, resulting in a highly disorganized arrangement. The severity of these modifications was dependent on the concentrations of H
3PO
4, suggesting that the alterations in the resistance structure of
T. latifolia biomass were due to the influence of H
3PO
4, which caused the disruption of the intermolecular connections among cellulose, hemicellulose, and lignin. Consequently, hemicellulose and lignin were separated from the cellulose microfibers [
23,
48,
54,
56,
62]. During the pretreatment process, cellulose swelling occurs as a consequence of the esterification reaction between the hydroxyl group of cellulose and phosphate ions derived from H
3PO
4 to form cellulose phosphate (cellulose-
Օ-PO
3H
2), which extends cellulose chains, also leading to fiber structure disruption [
23,
62,
69,
70]. In addition to modifying the physical structure of the feedstock, H
3PO
4 enhances its surface area and porosity, thereby facilitating enzymatic hydrolysis [
29,
71,
72,
73,
74,
75]. Moreover, the SEM micrographs of
T. latifolia treated with 85% H
3PO
4 revealed the most severe cellulose fiber degradation and structural alterations. This effect is attributable to the critical concentration of H
3PO
4 (80%), which destroys the ordered hydrogen bond network within crystalline cellulose, resulting in a transition from cellulose swelling to cellulose degradation [
23,
48,
69,
70].
The implications of the H
3PO
4 concentration on modifying the morphological structure and cellulose crystalline pattern in this biomass correspond to alterations in the chemical composition, as discussed in
Section 4.2. Furthermore, these effects correspond to those observed in various types of lignocellulosic biomass, including
V. pusilla [
14],
H. cannabinus [
26],
Pennisetum purpureum [
76],
D. zibethinus [
24],
Achyranthes aspera and
Sida acuta [
29], and cotton fibers [
72].
4.5. Enzymatic Saccharification Yields
The current study proved that the raw biomass of
T. latifolia exhibited the lowest HE and GR yields, which can be attributed to the recalcitrance of lignocellulosic biomass, which hinders interactions between enzymes and plant matter [
49,
77]. When the feedstock was treated with 70%, 75%, 80%, and 85% H
3PO
4, the HE and GR yields substantially improved compared with those of the untreated sample. However, the level of improvement varied depending on H
3PO
4 concentration in the pretreatment process. These results suggested that the chemical linkages present in the lignin–carbohydrate complex structure were disrupted when the feedstock was treated with 70–80% H
3PO
4. “Owing to this disintegration, hemicellulose and lignin were partially eliminated during the pretreatment process, indicating that the physical barriers (hemicellulose and lignin) surrounding the cellulose were removed” [
23,
48,
54]. Additionally, these effects increased the pore size and cellulose accessibility surface, leading to improved HE and GR yields for each treated sample [
14,
23,
24,
62,
66]. Consequently, the greatest amounts of HE and GR were achieved through treatment with 75% and 80% H
3PO
4. Pretreatment of the sample with 85% H
3PO
4 facilitated a more rapid degradation of the linkages between lignin and carbohydrates in complex structures, as well as the destruction of the crystalline framework of cellulose under harsh conditions. The cellulose therefore underwent a complete transformation into the amorphous form. This increased the dissolution of cellulose, hemicellulose, and lignin substantially [
23,
63,
69]. The degradation of amorphous cellulose occurs faster and more easily than that of crystalline cellulose [
62,
78]. These modifications significantly reduce the solid and glucan recovery yields of the treated biomass, leading to a substantial decrease in the GR yield following enzymatic hydrolysis [
14,
23,
55,
57,
79,
80].
Although the percentage of HE yields was most likely the maximum when treated with 85% H
3PO
4, the HE yields between the specimens treated with 75%, 80%, and 85% H
3PO
4 had no significant differences (
p < 0.05). The optimal pretreatment conditions are determined by an exceptionally high glucose recovery value that approaches the initial glucan content of the biomass [
14,
80]. Consequently, 75% H
3PO
4 was the most appropriate pretreatment condition for
T. latifolia feedstock because it maximized both the HE (86.9 ± 0.7%) and GR (32.8 ± 0.3%) yields, which were enhanced roughly 2.9 and 2.7 times, respectively, compared with the raw material, as both the solid (55.8 ± 0.6%) and glucan (93.0 ± 0.3%) recovery levels were found to be relatively high. Under these conditions, the 33.9 ± 0.2% lignin retained in the treated biomass demonstrated that removing complete lignin from the biomass is unnecessary. In addition, increasing the percentage of CrI values (61.2%) in the treated sample did not impact the hydrolysis yield. Modifications in the chemical composition, morphological structure, and crystallinity of the treated biomass were correlated with the enhancement of HE and GR yields, and these observed events are similar to those described for other feedstocks, including
A. aspera,
S. acuta [
29],
D. zibethinus Murr [
24],
H. cannabinus [
26],
V. pusilla [
14], and
G. glabra [
57].
4.6. Bioethanol Fermentation
Compared with the CT medium, the proliferation of yeast cells was considerably affected by the non-detoxified TBH medium. Furthermore, the ethanol yield in the CT medium was slightly greater than that in the TBH medium. The findings suggest that the non-detoxified TBH medium could contain inhibitory compounds, such as furfural, 5-hydroxymethyl furfural, levulinic acid, and other aromatic compounds, that were generated during the acid pretreatment of the raw feedstock [
4,
23,
81]. These inhibitors adversely affect microbial growth, leading to a reduction in the rate of sugar uptake, which might subsequently decrease ethanol production [
7,
23]. Hydrolysate detoxification is considered imperative to prevent the inhibitory effects on fermenting organisms before its use as a substrate in bioethanol fermentation [
82,
83]. Despite these challenges, cellulosic ethanol was successfully produced using
S. cerevisiae TISTR 5339 in the TBH medium derived from enzymatic saccharification without a detoxification process. These findings suggest that TBH could potentially be utilized as a sustainable carbon source for cellulosic ethanol production.
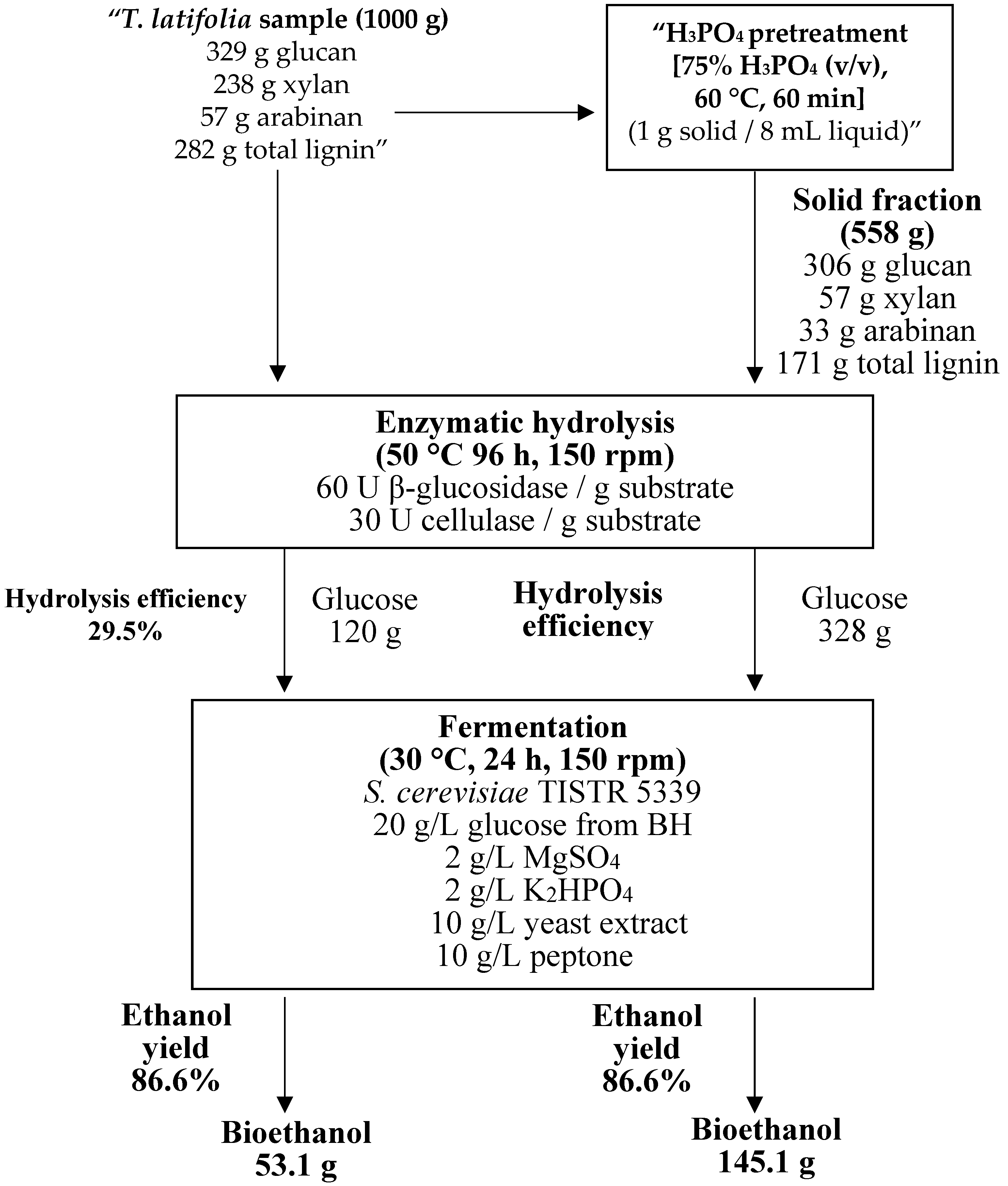
By employing the most effective H
3PO
4 pretreatment strategy for producing cellulosic ethanol, the material balance of
T. latifolia feedstock was assessed, as shown in
Figure 8. The 1000 g of
T. latifolia feedstock pretreated with 75% H
3PO
4 resulted in the recovery of approximately 558 g of the treated material. Subsequently, both the untreated and treated samples were subjected to enzymatic hydrolysis to investigate the influence of pretreatment on enzymatic saccharification, which substantially improved both the HE and GR yields. The treated samples exhibited the highest HE (86.9%) and GR (328 g) yields after 72 h of saccharification. In contrast, untreated samples exhibited the lowest production of HE (29.5%) and GR (120 g). Compared with the untreated material, the HE and GR yields increased by approximately 2.9 and 2.7-fold, respectively. Next, a non-detoxified TBH medium containing 20 g/L glucose from the treated biomass was fermented using
S. cerevisiae TISTR 5339 for ethanol production. The glucose from the untreated and treated feedstocks yielded an estimated 53 and 145 g of cellulosic ethanol, respectively. The ethanol yield from the treated feedstock demonstrated an enhancement of approximately 2.7-fold compared with that of the untreated samples. The results of the current study emphasize the potential utilization of a non-detoxified TBH medium produced from
T. latifolia feedstock as a feasible carbon source for the manufacture of cellulosic ethanol by
S. cerevisiae TISTR 5339.
Overall, our findings provide crucial information and suggest that treatment of T. latifolia biomass with H3PO4 enhances the efficiency of enzymatic hydrolysis and ethanol production. In the future, research should focus on response surface methodology and other similar techniques to analyze the complexities of pretreatment to increase yield and pretreatment efficiency. These experimental designs help assess the effects of critical pretreatment variables, such as acid concentration, temperature, duration, solid and glucan recovery, lignin removal, solid loading in the enzymatic hydrolysis process, and HE and GR yield. Moreover, modification of the genome of the microbial strain is required to enhance its fermentation efficiency and increase its resistance to inhibitors.
5. Conclusions
This study explored the use of T. latifolia feedstock for biochemical conversion, highlighting its potential as a cost-effective alternative energy source. The results showed that T. latifolia feedstock could be utilized as a viable source of lignocellulosic biomass for the production of environmentally friendly biofuels and other bio-based chemicals without affecting global food security. The pretreatment of T. latifolia feedstock with H3PO4 provided substantial advantages as it accelerated the decomposition of hemicellulose and lignin while preserving cellulose. Such modifications could enhance the cellulose surface area, resulting in an increased efficacy of enzymatic hydrolysis and optimal utilization of this biomass. The maximum GR and HE yields were obtained by pretreatment of T. latifolia feedstock with 75% H3PO4 at 60 °C for 60 min. The observed delignification rate of approximately 66% suggests that complete lignin removal may not be necessary. Furthermore, this study demonstrated the potential use of glucose in the TBH medium as a sustainable carbon source for cellulosic ethanol production without additional detoxification, resulting in a considerably higher ethanol yield than that of untreated biomass. Therefore, utilizing T. latifolia feedstock provides an opportunity to enhance the sustainability of bioenergy production and mitigate adverse environmental consequences associated with improper biomass disposal practices. In future studies, the analysis of the effects of pretreatment parameters using response surface methodology will be crucial. Furthermore, bioethanol yields can be increased by enhancing fermentation efficiency through the modification of the microbial strain to improve its efficiency and tolerance to inhibitors.
Author Contributions
Conceptualization, S.P. and D.P.; methodology, S.P., D.P., and S.W.; software, S.P., D.P., and S.W.; validation, S.P. and D.P.; formal analysis, S.P., D.P., and S.W.; investigation, S.P., D.P., and S.W.; resources, S.P. and D.P.; data curation, S.P., D.P., and S.W.; writing—original draft preparation, S.P., D.P., and S.W.; writing—review and editing, S.P. and D.P.; visualization, S.P. and D.P.; supervision, S.P. and D.P.; project administration, S.P.; funding acquisition, S.P. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by Naresuan University (NU) and the National Science, Research, and Innovation Fund (NSRF). 2023; Grant No. 66A107000042.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to extend their sincere appreciation to the Lower Northern Science Park of Naresuan University for their generous provision of scientific instruments utilized in the performance of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Afraz, M.; Muhammad, F.; Nisar, J.; Shah, A.; Munir, S.; Ali, G.; Ahmad, A. Production of value added products from biomass waste by pyrolysis: An updated review. Waste Management Bulletin. 2024, 1, 30–40. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes. 2020, 8. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere. 2020, 242, 125080. [Google Scholar] [CrossRef]
- Bhatia, S.K.J.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Rajesh Banu, J.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment friendly pretreatment approaches for the bioconversion of lignocellulosic biomass into biofuels and value-added products. Environments. 2023, 10. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Chong, C.T.; Atabani, A.E.; Pham, V.V. Acid-based lignocellulosic biomass biorefinery for bioenergy production: Advantages, application constraints, and perspectives. J. Environ. Manage. 2021, 296, 113194. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, X.; Zhao, J.; Qin, Y. Pretreatment strategies to enhance enzymatic hydrolysis and cellulosic ethanol production for biorefinery of corn stover. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Xiao, K.; Li, H.; Liu, L.; Liu, X.; Lian, Y. Quantitative comparison of the delignification performance of lignocellulosic biomass pretreatment technologies for enzymatic saccharification. Environ. Sci. Pollut. Res. Int. 2023, 30, 22929–22940. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; You, T.; Ji, Z.; Zhang, X.; Qin, Y.; Xu, F. Heteropoly acids enhanced neutral deep eutectic solvent pretreatment for enzymatic hydrolysis and ethanol fermentation of Miscanthus x giganteus under mild conditions. Bioresour. Technol. 2019, 293, 122036. [Google Scholar] [CrossRef]
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Switchgrass as an alternative biomass for ethanol production in a biorefinery: Perspectives on technology, economics and environmental sustainability. Renew. Sustain. Energy Rev. 2022, 158, 112115. [Google Scholar] [CrossRef]
- Wongleang, S.; Premjet, D.; Premjet, S. Cellulosic ethanol production from weed biomass hydrolysate of Vietnamosasa pusilla. Polymers. 2023, 15, 1103. [Google Scholar] [CrossRef]
- Sespene, J.; Fetalvero, E.; Faminial, T. Tiger grass industry in Marigondon Norte, San Andres, Romblon: Implications for research and development. Romblon State University Research Journal. 2011, 1, 81–95. [Google Scholar]
- Shrestha, S.; Park, J.H.; Cho, J.G.; Lee, D.Y.; Jeong, R.H.; Song, M.C.; Cho, S.K.; Lee, D.S.; Baek, N.I. Phytochemical constituents from the florets of tiger grass Thysanolaena latifolia from Nepal. J. Asian Nat. Prod. Res. 2016, 18, 206–213. [Google Scholar] [CrossRef]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew. Energy. 2019, 132, 723–741. [Google Scholar] [CrossRef]
- Saikia, D.C.; Goswami, T.; Chaliha, B.P. Paper from Thysanolaena maxima. Bioresour. Technol. 1992, 40, 245–248. [Google Scholar] [CrossRef]
- Flora & fauna, Thysanolaena latifolia. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/5/2512# (accessed on 20 Jan 2024).
- Komolwanich, T.; Prasertwasu, S.; Khumsupan, D.; Tatijarern, P.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Evaluation of highly efficient monomeric sugar yield from Thai Tiger grass (Thysanolaena maxima). Mater. Res. Innov. 2016, 20, 259–267. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H.P. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustainable Energy Fuels. 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Obeng, A.; Premjet, D.; Premjet, S. Fermentable sugar production from the peels of two durian (Durio zibethinus Murr.) cultivars by phosphoric acid pretreatment. Resources. 2018, 7, 60. [Google Scholar] [CrossRef]
- Premjet, S.; Dana, S.; Obeng, A.K.; Premjet, D. Enzymatic response to structural and chemical transformations in Hibiscus sabdariffa var. altissima bark and core during phosphoric acid pretreatment. BioRes. 2018, 13, 6778–6789. [Google Scholar] [CrossRef]
- Premjet, D.; Wongleang, S.; Premjet, S. Enhancing glucose recovery from Hibiscus cannabinus L. through phosphoric acid pretreatment. Energies. 2022, 15, 7573. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, W.; Han, L.; Huang, G. Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour. Technol. 2019, 282, 69–74. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, J.; Yang, H.; Shi, Z.; Zhao, P.; Yang, J. Improving enzymatic saccharification and ethanol production of bamboo residues with sulfomethylation-aided phosphoric acid pretreatment. Ind. Crops Prod. 2021, 170, 113733. [Google Scholar] [CrossRef]
- Siripong, P.; Duangporn, P.; Takata, E.; Tsutsumi, Y. Phosphoric acid pretreatment of Achyranthes aspera and Sida acuta weed biomass to improve enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 303–308. [Google Scholar] [CrossRef]
- Chen, D.; Tang, W.; Wang, H.; Sheng, Y.; Tan, X.; Shi, Y.; Fan, W.; Ge, S. Phosphoric acid pretreatment of poplar to optimize fermentable sugars production based on orthogonal experimental design. Front. Chem. 2023, 11, 1119215. [Google Scholar] [CrossRef]
- Mund, N.K.; Dash, D.; Barik, C.R.; Goud, V.V.; Sahoo, L.; Mishra, P.; Nayak, N.R. Evaluation of efficient glucose release using sodium hydroxide and phosphoric acid as pretreating agents from the biomass of Sesbania grandiflora (L.) pers.: A fast growing tree legume. Bioresour. Technol. 2017, 236, 97–105. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of ash in biomass. In Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory (National Renewable Energy Laboratory, Office of Energy Efficiency and Renewable Energy): Golden, CO, USA, 2008; pp. 1–8. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory (National Renewable Energy Laboratory, Office of Energy Efficiency and Renewable Energy): Golden, CO, USA, 2012; pp. 1–15. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass. In Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory (National Renewable Energy Laboratory, Office of Energy Efficiency and Renewable Energy): Golden, CO, USA, 2008; pp. 1–12. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Siripong, P.; Doungporn, P.; Yoo, H.Y.; Kim, S.W. Improvement of sugar recovery from Sida acuta (Thailand weed) by NaOH pretreatment and application to bioethanol production. Korean J. Chem. Eng. 2018, 35, 2413–2420. [Google Scholar] [CrossRef]
- Wongleang, S.; Premjet, D.; Premjet, S. Physicochemical pretreatment of Vietnamosasa pusilla for bioethanol and xylitol production. Polymers. 2023, 15, 3990. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef]
- Khan, M.A.; Dharmalingam, B.; Chuetor, S.; Cheng, Y.-S.; Sriariyanun, M. Comprehensive review on effective conversion of lignocellulosic biomass to levulinic acid. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic digestion of lignocellulosic biomass: Substrate characteristics (challenge) and innovation. Fermentation. 2023, 9. [Google Scholar] [CrossRef]
- Li, H.Y.; Chen, X.; Wang, C.Z.; Sun, S.N.; Sun, R.C. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels. 2016, 9, 166. [Google Scholar] [CrossRef]
- Pan, S.; Chi, Y.; Zhou, L.; Li, Z.; Du, L.; Wei, Y. Evaluation of squeezing pretreatment for improving methane production from fresh banana pseudo-stems. Waste Manag. 2020, 102, 900–908. [Google Scholar] [CrossRef]
- Montoya-Rosales, J.J.; Peces, M.; González-Rodríguez, L.M.; Alatriste-Mondragón, F.; Villa-Gómez, D.K. A broad overview comparing a fungal, thermal and acid pre-treatment of bean straw in terms of substrate and anaerobic digestion effect. Biomass Bioenergy. 2020, 142, 105775. [Google Scholar] [CrossRef]
- Bonfiglio, F.; Cagno, M.; Yamakawa, C.K.; Mussatto, S.I. Production of xylitol and carotenoids from Switchgrass and Eucalyptus globulus hydrolysates obtained by intensified steam explosion pretreatment. Ind. Crops Prod. 2021, 170, 113800. [Google Scholar] [CrossRef]
- Fonseca, B.G.; Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Bioconversion in batch bioreactor of olive-tree pruning biomass optimizing treatments for ethanol production. Biochem. Eng. J. 2020, 164, 107793. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Tao, S.; Hu, L.; Zhang, X.; Lin, X. Process optimization for deep eutectic solvent pretreatment and enzymatic hydrolysis of sugar cane bagasse for cellulosic ethanol fermentation. Renew. Energy. 2021, 177, 259–267. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules. 2022, 27. [Google Scholar] [CrossRef]
- Li, T.; Fang, Q.; Chen, H.; Qi, F.; Ou, X.; Zhao, X.; Liu, D. Solvent-based delignification and decrystallization of wheat straw for efficient enzymatic hydrolysis of cellulose and ethanol production with low cellulase loadings. RSC Adv. 2017, 7, 10609–10617. [Google Scholar] [CrossRef]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and fermentation of lignocellulosic biomass: Reaction mechanisms and process engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Kundu, C.; Samudrala, S.P.; Kibria, M.A.; Bhattacharya, S. One-step peracetic acid pretreatment of hardwood and softwood biomass for platform chemicals production. Sci. Rep. 2021, 11, 11183. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ding, S.Y.; Mielenz, J.R.; Cui, J.B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef]
- Prasad, S.; Malav, M.K.; Kumar, S.; Singh, A.; Pant, D.; Radhakrishnan, S. Enhancement of bio-ethanol production potential of wheat straw by reducing furfural and 5-hydroxymethylfurfural (HMF). Bioresour. Technol. Rep. 2018, 4, 50–56. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Zhang, Y.H.P. Cellulose solvent- and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour. Technol. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Xie, X.; Chernyshev, V.M.; Liu, Y.; Qi, W. Effects of phosphoric acid/hydrogen peroxide, ammonia/hydrogen peroxide and deep eutectic solvent pretreatments on component separation and enzymatic saccharification of Glycyrrhiza residue. Ind. Crops Prod. 2023, 196, 116525. [Google Scholar] [CrossRef]
- Wongleang, S.; Dana, S.; Premjet, D.; Premjet, S. Phosphoric acid pretreatment of Corchorus capsularis L. biomass for enhancing glucose recovery. NU. Int. J. Sci. 2023, 20, 1–13. [Google Scholar]
- Wang, Q.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Hu, Y.; Zhang, J.; Song, C.; Zeng, Y. Pretreating Luffa sponge (Luffa cylindrica L.) with concentrated phosphoric acid and subsequent enzymatic saccharification. BioResources. 2016, 11, 14. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Hossain, A.; Rahaman, M.S.; Lee, D.; Phung, T.K.; Canlas, C.G.; Simmons, B.A.; Renneckar, S.; Reynolds, W.; George, A.; Tulaphol, S.; et al. Enhanced softwood cellulose accessibility by H3PO4 pretreatment: High sugar yield without compromising lignin integrity. Ind. Eng. Chem. Res. 2020, 59, 1010–1024. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Hu, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Fates of hemicellulose, lignin and cellulose in concentrated phosphoric acid with hydrogen peroxide (PHP) pretreatment. RSC Adv. 2018, 8, 12714–12723. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Chen, C.; Wang, Y.; Hu, S.; Cui, C.; Qin, P.; Tan, T. Effect of chemical pretreatments on corn stalk bagasse as immobilizing carrier of Clostridium acetobutylicum in the performance of a fermentation-pervaporation coupled system. Bioresour. Technol. 2016, 220, 68–75. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Improved glucose recovery from durian peel by alkaline-catalyzed steam pretreatment. PeerJ. 2021, 9, e12026. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Du, X.; Lucia, L.A.; Ghiladi, R.A. Development of a highly efficient pretreatment sequence for the enzymatic saccharification of Loblolly pine wood. ACS Sustainable Chem. Eng. 2016, 4, 3669–3678. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Wi, S.; Zhang, Y.H. Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol. Bioeng. 2011, 108, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Cui, J.; Lynd, L.R.; Kuang, L.R. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules. 2006, 7, 644–648. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of microcrystalline cellulose in phosphoric acid—Molecular changes and kinetics. Molecules. 2009, 14, 5027–5041. [Google Scholar] [CrossRef]
- Gabhane, J.; Prince William, S.P.M.; Vaidya, A.N.; Mahapatra, K.; Chakrabarti, T. Influence of heating source on the efficacy of lignocellulosic pretreatment—A cellulosic ethanol perspective. Biomass Bioenergy. 2011, 35, 96–102. [Google Scholar] [CrossRef]
- Gourlay, K.; Arantes, V.; Saddler, J.N. Use of substructure-specific carbohydrate binding modules to track changes in cellulose accessibility and surface morphology during the amorphogenesis step of enzymatic hydrolysis. Biotechnol. Biofuels. 2012, 5, 51. [Google Scholar] [CrossRef]
- Jackson de Moraes Rocha, G.; Martin, C.; Soares, I.B.; Souto Maior, A.M.; Baudel, H.M.; Moraes de Abreu, C.A. Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy. 2011, 35, 663–670. [Google Scholar] [CrossRef]
- Tong, D.; Zhan, P.; Zhang, W.; Zhou, Y.; Huang, Y.; Qing, Y.; Chen, J. Surfactant-assisted dilute phosphoric acid plus steam explosion of poplar for fermentable sugar production. ChemistrySelect. 2022, 7, e202200423. [Google Scholar] [CrossRef]
- Tong, W.; Fang, H.; Song, K.; Xie, X.; Wang, J.; Jin, Y.; Wu, S.; Hu, J.; Chu, Q. Modified acid pretreatment to alter physicochemical properties of biomass for full cellulose/hemicellulose utilization. Carbohydr. Polym. 2023, 299, 120182. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Thermochemical pretreatment enhanced bioconversion of Elephant grass (Pennisetum purpureum): Insight on the production of sugars and lignin. Biomass Convers. Biorefin. 2022, 12, 1125–1138. [Google Scholar] [CrossRef]
- Shiva, R.-J., R. M.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; López-Sandin, I.; Morais, A.R.C.; Ruiz, H.A. Enzymatic hydrolysis, kinetic modeling of hemicellulose fraction, and energy efficiency of autohydrolysis pretreatment using agave bagasse. Bioenergy Res. 2023, 16, 75–87. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.-H.P. New lignocellulose pretreatments using cellulose solvents: A review. J. Chem. Technol. Biotechnol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Lee, J.H.; Kim, D.S.; Lee, J.H.; Lee, S.K.; Lee, S.J.; Park, C.; Kim, S.W. Enhancement of glucose yield from canola agricultural residue by alkali pretreatment based on multi-regression models. J. Ind. Eng. Chem. 2017, 51, 303–311. [Google Scholar] [CrossRef]
- Arisht, S.N.; Abdul, P.M.; Liu, C.-M.; Lin, S.-K.; Maaroff, R.M.; Wu, S.-Y.; Jahim, J.M. Biotoxicity assessment and lignocellulosic structural changes of phosphoric acid pre-treated young coconut husk hydrolysate for biohydrogen production. Int. J. Hydrog. Energy. 2019, 44, 5830–5843. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting non-conventional yeasts for ethanol production from rice straw hydrolysate and their inhibitor tolerance. Renew. Energy. 2020, 147, 1694–1703. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).