Submitted:
12 March 2024
Posted:
18 March 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Method
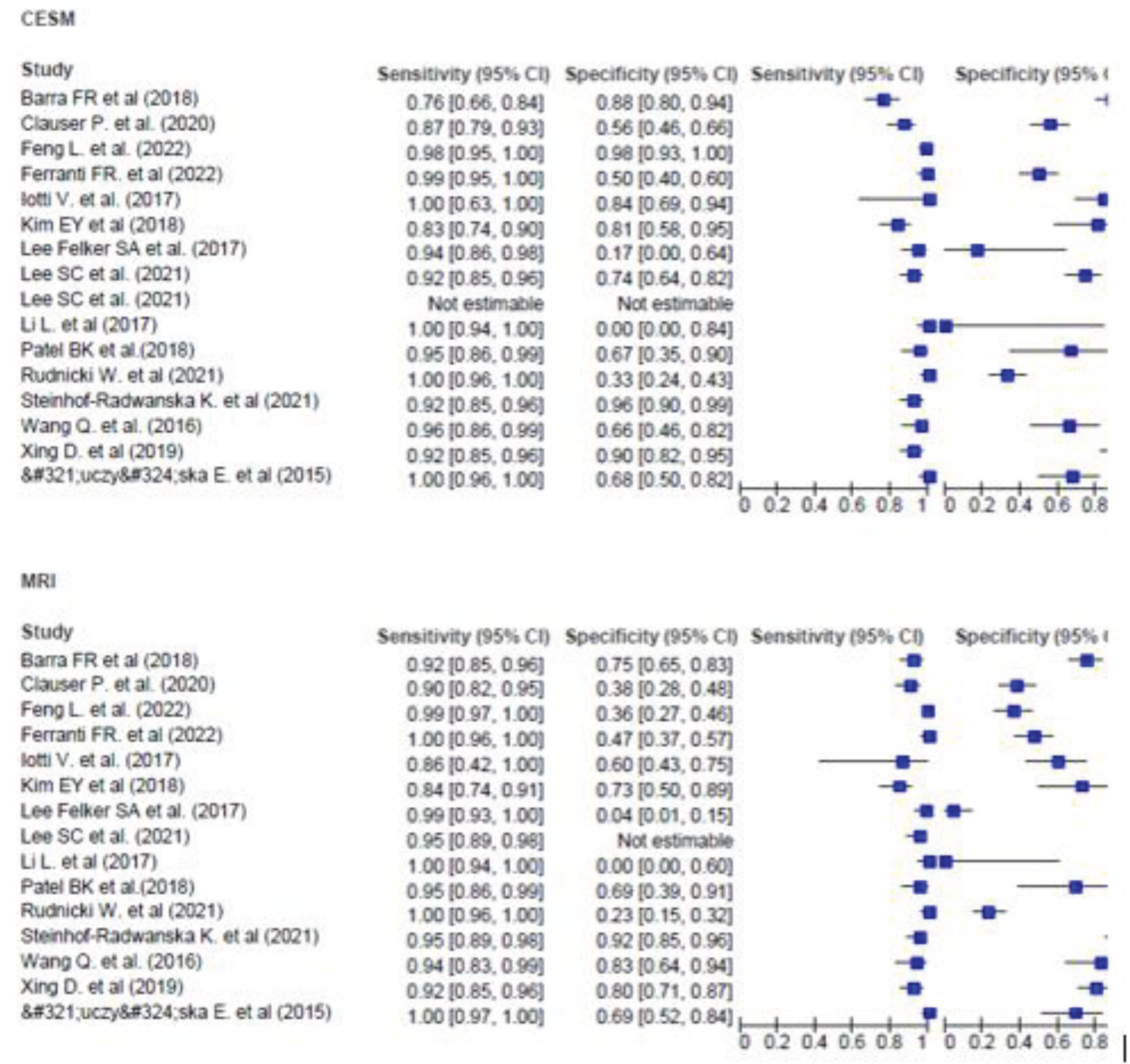
Results
Discussion and Conclusions
Funding
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209-249. [CrossRef]
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003 Apr 26;361(9367):1405-10. [CrossRef]
- Coleman, C. Early Detection and Screening for Breast Cancer. Semin Oncol Nurs. 2017 May;33(2):141-155. [CrossRef]
- Yuan, W.H.; Hsu, H.C.; Chen, Y.Y.; Wu, C.H. Supplemental Breast Cancer-Screening Ultrasonography in Women with Dense Breasts: A Systematic Review and Meta-Analysis. Br. J. Cancer 2020, 123, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Sung JS, Lebron L, Keating D, D’Alessio D, Comstock CE, Lee CH, Pike MC, Ayhan M, Moskowitz CS, Morris EA, Jochelson MS. Performance of Dual-Energy Contrast-enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology. 2019 Oct;293(1):81-88. [CrossRef]
- Sogani, J.; Mango, V.L.; Keating, D.; Sung, J.S.; Jochelson, M.S. Contrast-Enhanced Mammography: Past, Present, and Future. Clin. Imaging 2021, 69, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Thibault F, Balleyguier C, Tardivon A, Dromain C. Contrast enhanced spectral mammography: better than MRI? Eur J Radiol. 2012 Sep;81 Suppl 1:S162-4. [CrossRef]
- Page, M.J., McKenzie, J.E., Bossuyt, P.M. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10, 89 (2021). [CrossRef]
- Łuczyńska E, Heinze-Paluchowska S, Hendrick E, Dyczek S, Ryś J, Herman K, Blecharz P, Jakubowicz J. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit. 2015 ;21:1358-67. 12 May. [CrossRef]
- Wang Q, Li K, Wang L, Zhang J, Zhou Z, Feng Y. Preclinical study of diagnostic performances of contrast-enhanced spectral mammography versus MRI for breast diseases in China. Springerplus. 2016 Jun 17;5(1):763. [CrossRef]
- Li L, Roth R, Germaine P, Ren S, Lee M, Hunter K, Tinney E, Liao L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn Interv Imaging. 2017 Feb;98(2):113-123. [CrossRef]
- Iotti V, Ravaioli S, Vacondio R, Coriani C, Caffarri S, Sghedoni R, Nitrosi A, Ragazzi M, Gasparini E, Masini C, Bisagni G, Falco G, Ferrari G, Braglia L, Del Prato A, Malavolti I, Ginocchi V, Pattacini P. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017 Sep 11;19(1):106. [CrossRef]
- Lee-Felker SA, Tekchandani L, Thomas M, Gupta E, Andrews-Tang D, Roth A, Sayre J, Rahbar G. Newly Diagnosed Breast Cancer: Comparison of Contrast-enhanced Spectral Mammography and Breast MR Imaging in the Evaluation of Extent of Disease. Radiology. 2017 Nov;285(2):389-400. [CrossRef]
- Kim EY, Youn I, Lee KH, Yun JS, Park YL, Park CH, Moon J, Choi SH, Choi YJ, Ham SY, Kook SH. Diagnostic Value of Contrast-Enhanced Digital Mammography versus Contrast-Enhanced Magnetic Resonance Imaging for the Preoperative Evaluation of Breast Cancer. J Breast Cancer. 2018 Dec;21(4):453-462. [CrossRef]
- Patel BK, Hilal T, Covington M, Zhang N, Kosiorek HE, Lobbes M, Northfelt DW, Pockaj BA. Contrast-Enhanced Spectral Mammography is Comparable to MRI in the Assessment of Residual Breast Cancer Following Neoadjuvant Systemic Therapy. Ann Surg Oncol. 2018 May;25(5):1350-1356. [CrossRef]
- Barra FR, Sobrinho AB, Barra RR, et al. Contrast-Enhanced Mammography (CEM) for Detecting Residual Disease after Neoadjuvant Chemotherapy: A Comparison with Breast Magnetic Resonance Imaging (MRI). Biomed Res Int. 2018;2018:8531916. Published 2018 Nov 8. [CrossRef]
- Xing D, Lv Y, Sun B, Xie H, Dong J, Hao C, Chen Q, Chi X. Diagnostic Value of Contrast-Enhanced Spectral Mammography in Comparison to Magnetic Resonance Imaging in Breast Lesions. J Comput Assist Tomogr. 2019 Mar/Apr;43(2):245-251. [CrossRef]
- Clauser P, Baltzer PAT, Kapetas P, Hoernig M, Weber M, Leone F, Bernathova M, Helbich TH. Low-Dose, Contrast-Enhanced Mammography Compared to Contrast-Enhanced Breast MRI: A Feasibility Study. J Magn Reson Imaging. 2020 Aug;52(2):589-595. [CrossRef]
- Rudnicki W, Piegza T, Rozum-Liszewska N, Górski M, Popiela TJ, Basta P, Heinze S, Luczynska E. The effectiveness of contrast-enhanced spectral mammography and magnetic resonance imaging in dense breasts. Pol J Radiol. 2021 Mar 15;86:e159-e164. [CrossRef]
- Steinhof-Radwańska K, Lorek A, Holecki M, Barczyk-Gutkowska A, Grażyńska A, Szczudło-Chraścina J, Bożek O, Habas J, Szyluk K, Niemiec P, Gisterek I. Multifocality and Multicentrality in Breast Cancer: Comparison of the Efficiency of Mammography, Contrast-Enhanced Spectral Mammography, and Magnetic Resonance Imaging in a Group of Patients with Primarily Operable Breast Cancer. Curr Oncol. 2021 Oct 8;28(5):4016-4030. [CrossRef]
- Lee SC, Hovanessian-Larsen L, Stahl D, Cen S, Lei X, Desai B, Yamashita M. Accuracy of contrast-enhanced spectral mammography compared with MRI for invasive breast cancers: Prospective study in population of predominantly underrepresented minorities. Clin Imaging. 2021 Dec;80:364-370. [CrossRef]
- Ferranti FR, Vasselli F, Barba M, Sperati F, Terrenato I, Graziano F, Vici P, Botti C, Vidiri A. Diagnostic Accuracy of Contrast-Enhanced, Spectral Mammography (CESM) and 3T Magnetic Resonance Compared to Full-Field Digital Mammography plus Ultrasound in Breast Lesions: Results of a (Pilot) Open-Label, Single-Centre Prospective Study. Cancers (Basel). 2022 Mar 7;14(5):1351. [CrossRef]
- Feng L, Sheng L, Zhang L, Li N, Xie Y. Comparison of Contrast-Enhanced Spectral Mammography and Contrast-Enhanced MRI in Screening Multifocal and Multicentric Lesions in Breast Cancer Patients. Contrast Media Mol Imaging. 2022 Apr 6;2022:4224701. [CrossRef]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155(8):529-36. [CrossRef]
- Gelardi F, Ragaini EM, Sollini M, Bernardi D, Chiti A. Contrast-Enhanced Mammography versus Breast Magnetic Resonance Imaging: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2022 Aug 4;12(8):1890. [CrossRef]
- Kornecki, A. Current Status of Contrast Enhanced Mammography: A Comprehensive Review. Can Assoc Radiol J. 2022 Feb;73(1):141-156. [CrossRef]
- Patel BK, Gray RJ, Pockaj BA. Potential Cost Savings of Contrast-Enhanced Digital Mammography. AJR Am J Roentgenol. 2017 Jun;208(6):W231-W237. [CrossRef]
- Phillips J, Miller MM, Mehta TS, Fein-Zachary V, Nathanson A, Hori W, Monahan-Earley R, Slanetz PJ. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging. 2017 Mar-Apr;42:193-197. [CrossRef]
- Savaridas SL, Whelehan P, Warwick VR, Vinnicombe SJ, Evans AJ. Contrast-enhanced digital breast tomosythesis and breast MRI to monitor response to neoadjuvant chemotherapy: patient tolerance and preference. Br J Radiol. 2022 Jun 1;95(1134):20210779. [CrossRef]

| Study | Risk of Bias | Applicability Concerns | |||||
|
Patient Selection |
Index Test |
Reference Standard |
Flow and Timing |
Patient Selection |
Index Test |
Reference Standard |
|
| Łuczyńska E. et al (2015) [9] | Low | Low | Low | Low | Low | Low | Low |
| Wang Q. et al. (2016) [10] | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Li L. et al (2017) [11] | Low | Low | Low | Low | Low | Low | Low |
| Iotti V. et al. (2017) [12] | Low | Low | Low | Low | Low | Low | Low |
| Lee Felker SA et al. (2017) [13] | Low | Low | Low | Unclear | Low | Low | Low |
| Kim EY et al (2018) [14] | Unclear | Low | Low | Unclear | Unclear | Low | Low |
|
Patel BK et al. (2018) [15] |
Low | Low | Unclear | Unclear | Low | Low | Low |
| Barra FR et al (2018) [16] | Low | Low | Low | Unclear | Low | Low | Low |
| Xing D. et al (2019) [17] | Low | Low | Low | Unclear | Low | Low | Low |
| Clauser P. et al. (2020) [18] | Low | Low | Low | Unclear | Low | Low | Low |
| Rudnicki W. et al (2021) [19] | Unclear | Low | Low | Unclear | Unclear | Low | Low |
| Steinhof-Radwanska K. et al (2021) [20] | Low | Low | Low | Low | Low | Low | Low |
| Lee SC et al. (2021) [21] | Low | Low | Low | Unclear | Low | Low | Low |
| Ferranti FR. et al (2022) [22] | Low | Low | Low | Low | Low | Low | Low |
| Feng L. et al. (2022) [23] | Low | Low | Low | Unclear | Low | Low | Low |
| Study | Studytype | CESM Contrast | Patients(n) | Detected Lesions | Sensitivity | Specificity | Positive Predictive Value |
Negative Predictive Value |
Accuracy |
| Łuczyńska E. et al (2015) [9] | Prospective | 1.5 ml/kg of body mass of non-ionic contrast agent (Iopromide 370) |
102 | 118 total (81 malignant, 37 benign) MRI: 107 (75 malignant, 32 bening) CESM: 106 (81 malignant, 25 benign) |
MRI: 93% CESM: 100% |
MRI:69% CESM:68% |
MRI: 74% CESM:77% |
MRI:65% CESM; 100% |
MRI: 73% CESM:79% |
| Wang Q. et al. (2016) [10] | Prospective | Omnipaque, 350 mgI/mL; GE Healthcare, Dublin, Ireland) at a 1.5 mL/kg |
68 | 77 (48 malignant, 29 benign) | MRI: 93.8% CESM:95.8% |
MRI:82.8% CESM:65.5% |
MRI: 88.2% CESM:82.1% |
MRI:92.3% CESM: 90.5% |
MRI: 89.6% CESM: 84.4% |
| Li L. et al (2017) [11] | Retrospective | Sieve®370 (Iopamidol injection 76%) | 48 | MRI:66 (62 malignant 4 benign) CESM:64 (62 malignant 2 benign) |
MRI: 100% CESM: 100% |
Not estimable | MRI: 93,9% CESM: 96.9% |
Not estimable | MRI: 93.9% CESM: 96.9% |
| Iotti V. et al. (2017) [12] | Prospective | ioversolo 350 mg/ ml at 1.5 ml/kg |
46 | 46 | MRI: 87% CESM: 100% |
MRI: 60% CESM: 84% |
MRI: 32% CESM: 57% |
MRI:96% CESM: 100% |
MRI:65% CESM:87% |
| Lee Felker SA et al. (2017) [13] | Retrospective | 90 mL of iodinated contrast material (Omnipaque 350, GE Healthcare |
52 | 120 | MRI: 99% CESM: 94% |
MRI: 4% CESM: 17% |
MRI: 60% CESM: 93% |
MRI:67% CESM: 20% |
MRI: 60.17% CESM:87.1% |
| Kim EY et al (2018) [14] | Prospective | Omnipaque 350 (GE Healthcare, Shanghai, China; 1.5 mL/kg of body weight |
84 | 121 | MRI: 83.9% CESM:83.9% |
MRI: 73.6% CESM:81.1% |
MRI:65% CESM:72.2% |
MRI:88.6% CESM:89.6% |
MRI:77.4% CESM:82.1% |
|
Patel BK et al. (2018) [15] |
Propsective | 1.5 mL/kg of iohexol (Omnipaque 350; GE Healthcare) |
65 | 65 | MRI: 95% CESM:95% |
MRI:68.9% CESM: 66.7% |
MRI:57.6% CESM:55.9% |
MRI:96.9% CESM:96.7& |
MRI:85.9% CESM:85.1% |
| Barra FR et al (2018) [16] | Porspective | 1.5 ml/kg of non-ionic contrast medium (Iohexol, 300 mg/ml) |
33 | 33 | MRI: 92% CESM: 76% |
MRI:75% CESM:87.5% |
MRI: 92% CESM:95% |
MRI:75% CESM:86.4% |
MRI: 80.61% CESM: 83.7% |
| Xing D. et al (2019) [17] | Prospective | iohexol at 1.5 mL/kg body | 235 | MRI:258 CESM: 259 |
MRI:91.5% CESM: 91.5% |
MRI: 80.2% CESM: 89.5% |
MRI: 90.5% CESM: 94.7% |
MRI:82.1% CESM:83.7% |
MRI:71.7% CESM:81% |
| Clauser P. et al. (2020) [18] | Prospective | 2 mL/kg body weight of nonionic iodine contrast agent (Iobitridol/Xenetix 350, Guerbet, Villepinte, France |
80 | 93 | MRI: 83.6%-93.4% CESM: 65.6%-90.2% |
MRI: 37.5%-53.1% CESM: 46.9%-96.9% |
MRI: 73.3%-77% CESM: 76.4%-97.6% |
MRI:63%-76.5% CESM:59.6%-71.4% |
MRI:72%-75.3% CESM:75.3%-76.3% |
| Rudnicki W. et al (2021) [19] | Retrospective | Iopromide (1.5 ml/kg of body weight |
121 | MRI: 121 CESM:108 |
MRI 100% CESM: 100% |
MRI:23% CESM: 33% |
MRI:72% CESM: 75% |
MRI: 100% CESM:100% |
MRI:78% CESM:68% |
| Steinhof-Radwa ´ nska K. et al (2021) [20] | Retrospective | 1.5 mL/kg of body mass of non-ionic contrast agent |
60 | MRI:33 CESM:30 |
MRI: 91.18% CESM: 85.29% |
MRI:92.31% CESM:96.15% |
MRI:94% CESM:97% |
MRI:89% CESM:84% |
MRI: 91% CESM: 90% |
| Lee SC et al. (2021) [21] | Prospective | 1.5 ml/kg of iodine contrast |
41 CESM 32 MRI |
41 malignant | MRI:94.74% CESM:92% |
MRI: 0 CESM:74.43% |
MRI:85.71% CESM:95-83% |
MRI:0 CESM: 55.56% |
Not reported |
|
Ferranti FR. et al (2022) [22] |
Prospective Cohort Study | Visipaque 320 | 118 | MRI: 108 CESM:110 |
MRI 99% CESM: 100% |
MRI:47% CESM:50% |
MRI:88% CESM: 92% |
MRI: 90% CEMS.100% |
MRI:88% CESM:93% |
| Feng L. et al. (2022) [23] | Prospective | ioversol injection (1.5 ml/kg) | 54 | 188 177 malignant 11 benign |
MRI: 99.4% CESM:98.3% |
MRI:36.4% CESM:98.3% |
MRI: 96.17% CESM: 97.75% |
MRI: 80% CESM: 70% |
MRI:95.7% CESM:96.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





