Submitted:
26 February 2024
Posted:
12 March 2024
You are already at the latest version
Abstract
Keywords:
Impact Statement
Introduction
Materials and methods
Bacterial strains
- (i)
- WHO reference panel
- (ii)
- Clinical isolates
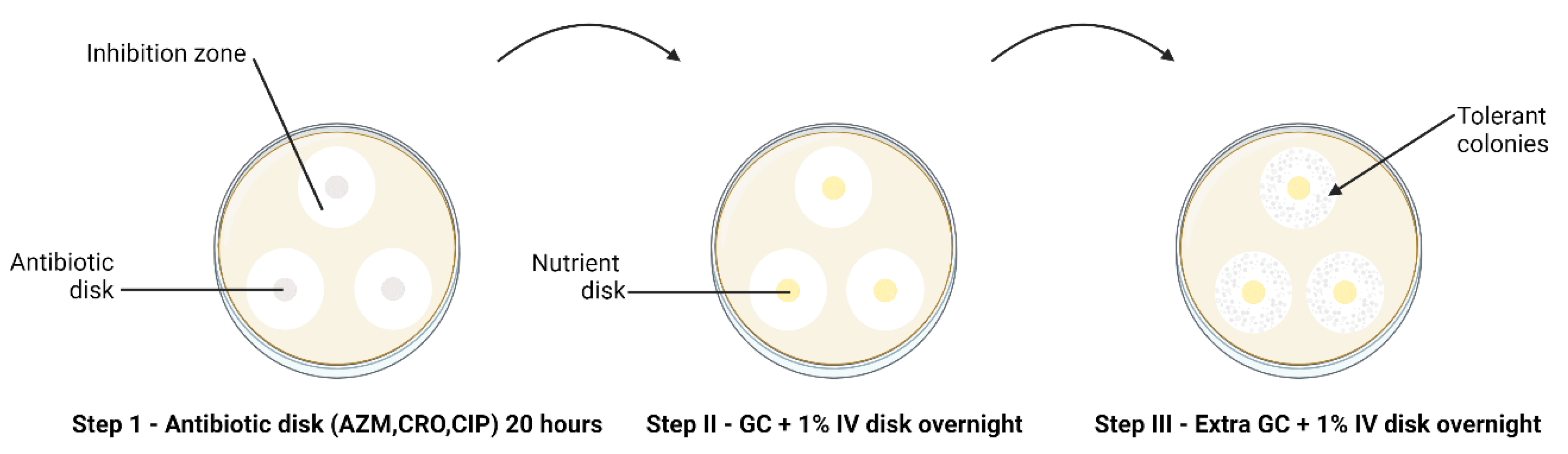
Tolerance Detection
Antimicrobial Susceptibility Testing
Statistical Analysis
Results
Detection of Tolerance and Heterotolerance in WHO Reference Strains
Differences in Azithromycin and Ceftriaxone Tolerance Across Infection Sites
No Difference in Tolerance to Ciprofloxacin between the Infection Sites
Heterotolerance and Variability in Azithromycin, Ciprofloxacin and Ceftriaxone Tolerance among Clinical N. gonorrhoeae Isolates
No Decreased Susceptibility to Azithromycin, Ceftriaxone, and Ciprofloxacin in Tolerant Colonies
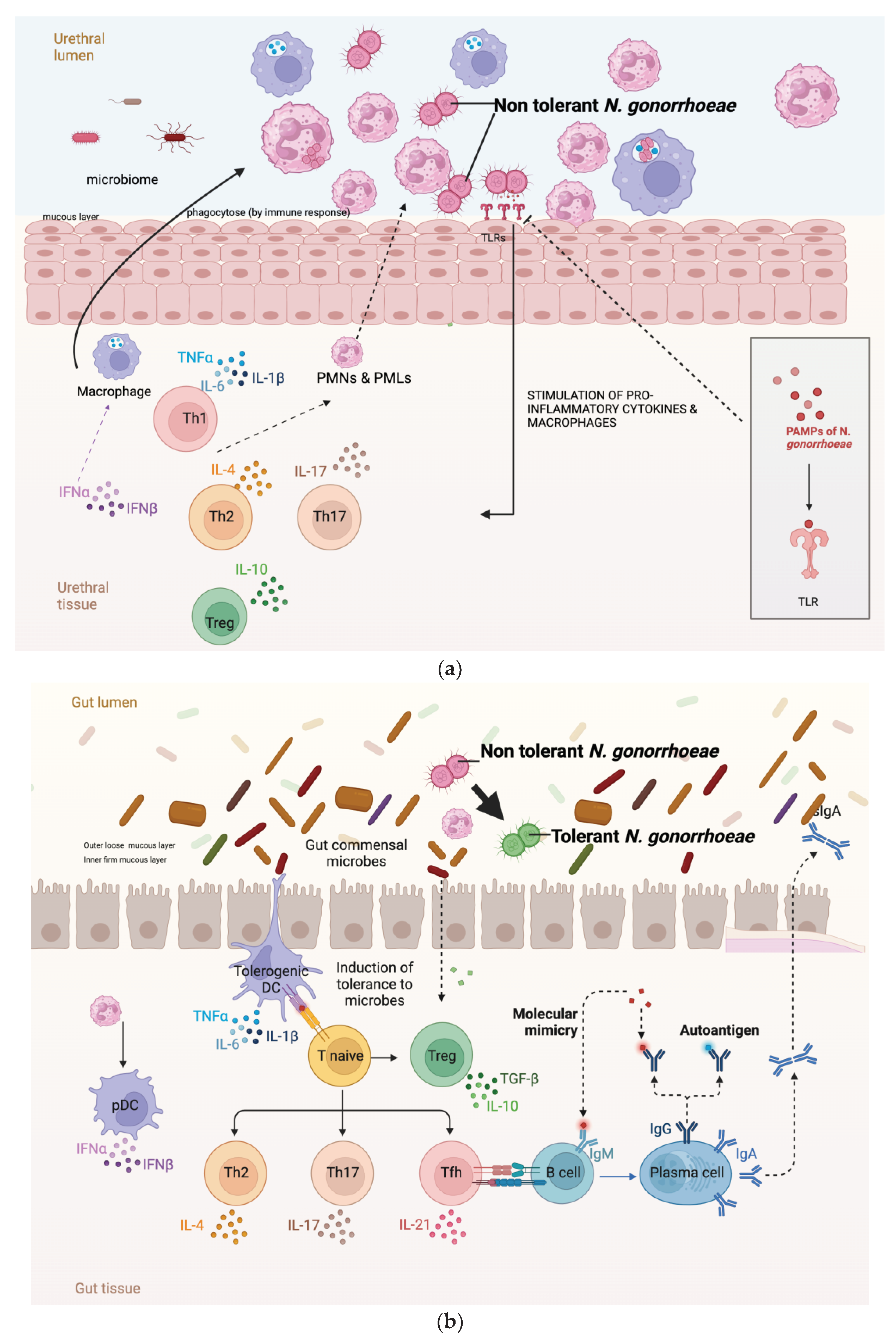
Discussion
Funding
Conflicts of Interest
References
- Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nature reviews. Microbiology 2016, 14, 320–330.
- Sulaiman, J.E.; Lam, H. Evolution of Bacterial Tolerance Under Antibiotic Treatment and Its Implications on the Development of Resistance. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Handwerger, S.; Tomasz, A. Antibiotic Tolerance Among Clinical Isolates of Bacteria. Clin. Infect. Dis. 1985, 7, 368–386. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. https://www.science.org (2017). [CrossRef]
- Gefen, O.; Chekol, B.; Strahilevitz, J.; Balaban, N.Q. TDtest: easy detection of bacterial tolerance and persistence in clinical isolates by a modified disk-diffusion assay. Sci. Rep. 2017, 7, srep41284. [Google Scholar] [CrossRef]
- Kotková, H.; Cabrnochová, M.; Lichá, I.; Tkadlec, J.; Fila, L.; Bartošová, J.; Melter, O. Evaluation of TD test for analysis of persistence or tolerance in clinical isolates of Staphylococcus aureus. J. Microbiol. Methods 2019, 167, 105705. [Google Scholar] [CrossRef]
- Hill, S.A.; Masters, T.L.; Wachter, J. Gonorrhea – an evolving disease of the new millennium. Microb. Cell 2016, 3, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Piszczek J, St. Jean R, Khaliq Y. Gonorrhea: Treatment update for an increasingly resistant organism. Can Pharm J / Rev des Pharm du Canada 2015, 148, 82–89. [Google Scholar]
- Unemo M, Seifert HS, Hook EW, Hawkes S, Ndowa F, et al. Gonorrhoea. Nat Rev Dis Prim 2019, 5, 79.
- world Health Organization, Prudden HJ. WHO preferred product characteristics for gonococcal vaccines. 2020.
- World Health Organisation. WHO guidelines for the treatment of Neisseria gonorrhoeae. https://apps.who.int/iris/bitstream/handle/10665/246114/9789241549691-eng.pdf?sequence=1 (2016, accessed 23 May 2023).
- Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerging infectious diseases 2011, 17, 148–149.
- Unemo, M.; Golparian, D.; Nicholas, R.; Ohnishi, M.; Gallay, A.; Sednaoui, P. High-Level Cefixime- and Ceftriaxone-Resistant Neisseria gonorrhoeae in France: Novel penA Mosaic Allele in a Successful International Clone Causes Treatment Failure. Antimicrob. Agents Chemother. 2012, 56, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Balduck, M.; Laumen, J.G.E.; Abdellati, S.; De Baetselier, I.; de Block, T.; Manoharan-Basil, S.S.; Kenyon, C. Tolerance to Ceftriaxone in Neisseria gonorrhoeae: Rapid Induction in WHO P Reference Strain and Detection in Clinical Isolates. Antibiotics 2022, 11, 1480. [Google Scholar] [CrossRef]
- Ma, K.C.; Mortimer, T.D.; Hicks, A.L.; Wheeler, N.E.; Sánchez-Busó, L.; Golparian, D.; Taiaroa, G.; Rubin, D.H.F.; Wang, Y.; Williamson, D.A.; et al. Adaptation to the cervical environment is associated with increased antibiotic susceptibility in Neisseria gonorrhoeae. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- A Morse, S.; Lysko, P.G.; McFarland, L.; Knapp, J.S.; Sandstrom, E.; Critchlow, C.; Holmes, K.K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect. Immun. 1982, 37, 432–438. [Google Scholar] [CrossRef]
- Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother 2016, 71, 3096–3108.
- Mcdermott PF, White DG, Zhao S, Simjee S, Walker RD. Antimicrobial Susceptibility Testing. Preharvest Postharvest Food Saf Contemp Issues Futur Dir 2008, 25.
- BioMérieux. ETEST - Trusted Leader in MIC Gradient Strip Technology. https://www.biomerieux-usa.com/sites/subsidiary_us/files/prn_056750_rev_03.a_etest_brochure_final_art_2.pdf.
- Liu, J.; Gefen, O.; Ronin, I.; Bar-Meir, M.; Balaban, N.Q. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 2020, 367, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Burns, J.L.; Lory, S.; Lewis, K. Emergence of Pseudomonas aeruginosa Strains Producing High Levels of Persister Cells in Patients with Cystic Fibrosis. J. Bacteriol. 2010, 192, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Honsa, E.S.; Cooper, V.S.; Mhaissen, M.N.; Frank, M.; Shaker, J.; Iverson, A.; Rubnitz, J.; Hayden, R.T.; Lee, R.E.; Rock, C.O.; et al. RelA Mutant Enterococcus faecium with Multiantibiotic Tolerance Arising in an Immunocompromised Host. mBio 2017, 8, e02124–16. [Google Scholar] [CrossRef]
- Lazarovits, G.; Gefen, O.; Cahanian, N.; Adler, K.; Fluss, R.; Levin-Reisman, I.; Ronin, I.; Motro, Y.; Moran-Gilad, J.; Balaban, N.Q.; et al. Prevalence of Antibiotic Tolerance and Risk for Reinfection Among Escherichia coli Bloodstream Isolates: A Prospective Cohort Study. Clin. Infect. Dis. 2022, 75, 1706–1713. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Camilleri, S.; Ward, C.; Huffam, S.; Chen, M.Y.; Bradshaw, C.S.; Fairley, C.K. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex. Heal. 2016, 13, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- Galiwango, R.M.; Park, D.E.; Huibner, S.; Onos, A.; Aziz, M.; Roach, K.; Anok, A.; Nnamutete, J.; Isabirye, Y.; Wasswa, J.B.; et al. Immune milieu and microbiome of the distal urethra in Ugandan men: impact of penile circumcision and implications for HIV susceptibility. Microbiome 2022, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akomoneh, E.A.; Laumen, J.G.E.; Abdellati, S.; Van Dijck, C.; Vanbaelen, T.; Britto, X.B.; Manoharan-Basil, S.S.; Kenyon, C. The Discovery of Oropharyngeal Microbiota with Inhibitory Activity against Pathogenic Neisseria gonorrhoeae and Neisseria meningitidis: An In Vitro Study of Clinical Isolates. Microorganisms 2022, 10, 2497. [Google Scholar] [CrossRef] [PubMed]
- Baquero F, Moreno F. The microcins. FEMS Microbiol Lett 1984, 23, 117–124.
- Simpson, D.M.; Davis, C.P. Properties of a Gonococcal Inhibitor Produced by Escherichia coli. J. Gen. Microbiol. 1979, 115, 471–477. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.E.; Duncan, W.C.; Knox, J.M. Bacterial interference of Neisseria gonorrhoeae by alpha-haemolytic streptococci. Sex. Transm. Infect. 1980, 56, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.-H.; Wang, J.-T.; Wei, S.-C.; Ni, Y.-H. Host-microbial interactions and regulation of intestinal epithelial barrier function: From physiology to pathology. World J. Gastrointest. Pathophysiol. 2012, 3, 27–43. [Google Scholar] [CrossRef]
- Tedijanto, C.; Olesen, S.W.; Grad, Y.H.; Lipsitch, M. Estimating the proportion of bystander selection for antibiotic resistance among potentially pathogenic bacterial flora. Proc. Natl. Acad. Sci. 2018, 115, 201810840–E11995. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.S.; Hatzis, C.L.; Lau, A.; A Williamson, D.; Chow, E.P.F.; Fairley, C.K.; Hocking, J.S. Treatment efficacy for pharyngeal Neisseria gonorrhoeae: a systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2020, 75, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Levin-Reisman, I.; Brauner, A.; Ronin, I.; Balaban, N.Q. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. 2019, 116, 14734–14739. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ma, K.; Wan, I.; Willcox, M.D. Ciprofloxacin resistance and tolerance of Pseudomonas aeruginosa ocular isolates. Contact Lens Anterior Eye 2023, 46, 101819. [Google Scholar] [CrossRef]
- Manoharan-Basil S, Balduck M, Laumen J, Kenyon C. Transcriptomic profiling of ceftriaxone-tolerant phenotypes of Neisseria gonorrhoeae WHO P reference strain. ECCMID 2023, Copenhagen; 2023.
| Antibiotics | Tolerance | Heterotolerance | ||
| Number | WHO isolates | Number | WHO isolates | |
| Azithromycin | 1 | Z | 0 | |
| Ciprofloxacin | 2 | P, U | 1 | U |
| Ceftriaxone | 8 | K, M, N, O, P, U, V, W | 0 | |
| Antibiotics |
Tolerance | Heterotolerance | ||||
| Yes | No | Proportion | Yes | No | Proportion | |
| Azithromycin | ||||||
| Anorectal | 10 | 20 | 33% | 6 | 24 | 20% |
| Urogenital | 1 | 31 | 3% | 1 | 31 | 3% |
| p value | 0.002 | p value | 0.050 | |||
| Ciprofloxacin | ||||||
| Anorectal | 5 | 25 | 17% | 3 | 27 | 10% |
| Urogenital | 9 | 23 | 28% | 7 | 25 | 22% |
| p value | 0.367 | p value | 0.304 | |||
| Ceftriaxone | ||||||
| Anorectal | 10 | 20 | 33% | 4 | 26 | 13% |
| Urogenital | 3 | 29 | 9% | 1 | 31 | 3% |
| p value | 0.029 | p value | 0.189 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





