1. Introduction
Polyhydroxyalkanoates (PHAs) are natural polyesters consisting of various chains of hydroxyalkanoates (HAs) produced by numerous microorganisms and have attracted attention due to the increase in demand for environmentally friendly compounds from renewable sources as a substitute to conventional petroleum-based plastics [
1,
2,
3].
PHAs in microorganism are principally synthesized as lipid inclusions in granular forms within the cellular structure and are used as an energy storage material mainly under nutrient-limited conditions in the presence of excess carbon source [
4,
5]. According to the Institute for Bioplastics and Biocomposites production of biopolymers reached in Europe 1.7 million tones and was expected to grow in 2022 to 2.44 million tones (European Bioplastics, 2017).
A wide variety of PHAs and their co-polymers are isolated from different bacterial species, and currently exists over 150 possible constituent monomers reported that are classified based on the number of carbons, in monomer via short chain length (scl) PHAs with less to 5 carbon atoms, i. e. PHB, PHV and PHB-co-V; medium chain length (mcl) PHAs from 6 to 14 carbon atoms i. e. polyhydroxyheptanoate, polyhydroxyoctanoate, polyhydroxynonanoate and long chain length (lcl) PHAs with more than 14 carbon atoms [
6,
7]. Among these, PHB and PHB-co-V are used most extensively for biomaterial investigations and are commercially available.
The principal biomedical applications include drug delivery, implants, biosensors, agriculture, adhesives, textile, paper coating, performance additives and energy materials [
8,
9]. To production of PHAs several sources of carbon have been used including whey [
10], lignocellulosic biomass [
11]; coffee waste [
12] for example. In the present work we evaluated the production of produced Polyhydroxyalkanoates by
H. gomseomensis using different carbon sources, polymers were characterized by Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), gel permeation chromatography (GPC) and elemental analysis by energy-dispersive X-ray spectroscopy (EDX).
2. Materials and Methods
2.1. Sampling, Isolation and Maintenance of Halophilic Bacteria
Saline water samples, were collected from salt ponds at Las Coloradas, Yucatan, Mexico. The water samples were diluted (10_4, 10_5 and 10_6), and 100 µL of each dilution was plated on sterile YGS solid media, which consist in 0.5% (w/v) yeast extract, 1% (w/v) glucose, sea water (25% v/v) and 4% bacteriological agar. The plates were incubated at 37°C for 72 h and analyzed to determine the total viable bacterial load. The colonies formed in the plates were picked and surface streaked several times until pure culture was obtained. The isolates were labelled as halophile series (JCCOL) and maintained on YGS solid medium at 4 °C.
2.2. Screening of H. gomseomemsis for PHA Production
The ability of the halophilic bacteria to accumulate PHA was tested under nitrogen limited conditions and using a modified YGS medium containing 0.25% (w/v) yeast extract and sea water 25% (v/v) and varying the 1% (w/v) of supplemented carbon source in addition to glucose, such as sodium acetate, starch, sodium citrate, fructose, soybean oil, glycerol and sucrose, the medium were incubated al 37 °C and 250 rpm by 48h.
2.3. Confocal Microscopy Analysis
Nile blue- A staining was applied as described by [
13] from 48-h culture grown. A 1% (w/v) aqueous solution of Nile blue A was prepared and filtered with a 0.45 µm Millex-HV PVDF membrane before use. Heat-fixed smears of bacterial cells were stained with the Nile blue A solution at 55 °C for 10 min in a Coplin staining jar. After being stained, the slides were washed with deionized water to remove excess staining and with 1M acetic acid for 1 min. The stained smear was washed and blotted dry with absorbent paper, remoistened with deionized water, and covered with a No. 1 glass cover slip. Images were acquired using an LSCM FV 1000 laser scanning confocal microscope (Olympus, Tokyo, Japan) and a Plan UPLFLN 40 oil immersion objective lens with a numerical aperture of 1.30.
2.4. Identification of Halophilic Isolates
Genomic DNA was isolated by the use of Wizard Genomic DNA Purification kit (Promega Biosciences, LLC., San Luis Obispo, CA) 16S rRNA gene fragment was amplified using fDl 5`-ccgaattcgtcgacaacAGAGTTTG ATCCTGGCTCAG-3´ and rDl 5`-cccgggatccaagcttAAGGAGGTGATCCAGCC-3´ or rP2 5`cccgggatccaagc ttACGGCTACCTTGTTACGACTT-3` primers under the following PCR conditions: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation (94 °C for 2 min), annealing (42 °C for 30 s) and extension (72 °C for 4 min) with a final extension (72 °C for 10 min). Amplified product was subjected to electrophoresis on a 15% agarose gel and was found to be approx. 13 kb in size. The purified product was sequenced unidirectionally using an automated DNA sequencer (Applied Biosystems, IBT, Cuernavaca, Mexico).
2.5. Polymer Extraction
Cells were harvested by centrifugation, suspended in commercial sodium hypochlorite solution and incubated at 37 °C for 1–2 h. Then, the mixture was centrifuged to collect the polymeric sediment and the supernatant was discarded. The sediment was washed with distilled water and centrifuged; this procedure was repeated using a mixture of acetone: methanol (1:1). The polymer granule was dissolved with chloroform and precipitated with methanol. The formed supernatant was evaporated to dryness in a DyNA-vap evaporator at 40 °C and 250 mbar for obtaining the PHA pellet.
2.6. Characterization of Obtained Polyhydroxyalkanoates
Infrared spectra of the polymer were obtained with a Nicolet FTIR Protégé 460, using attenuated total reflectance (ATR) mode, in the spectral range from 4000 to 650 cm-1, averaging 100 scans with a resolution of 4 cm-1. Elemental composition of the polymer was studied by means of energy-dispersive X-ray microanalysis (EDX) using an Oxford Inca Energy 200 energy dispersive spectrometry (EDS) system coupled to scanning electron microscope (SEM) JEOL JSM 6360 LV.
DSC analysis was performed to study the melting behavior of synthesized polymer. Samples were heated twice from 45 to 200 °C, at a heating rate of 10 °C min
-1. on a DSC-7 Perkin Elmer. The melting temperature (Tm) and the melting enthalpy (ΔHm) were determined from the endothermic peak on the DSC curve during the second scan. The crystallinity percentage (Xc) of polymeric sample was calculated using the following expression:
where ΔHm is the melting enthalpy of PHB sample and ΔH0 is that corresponding to the theoretical melting enthalpy of 100% crystalline PHB (146 J g
-1) [
14].
3. Results and Discussion
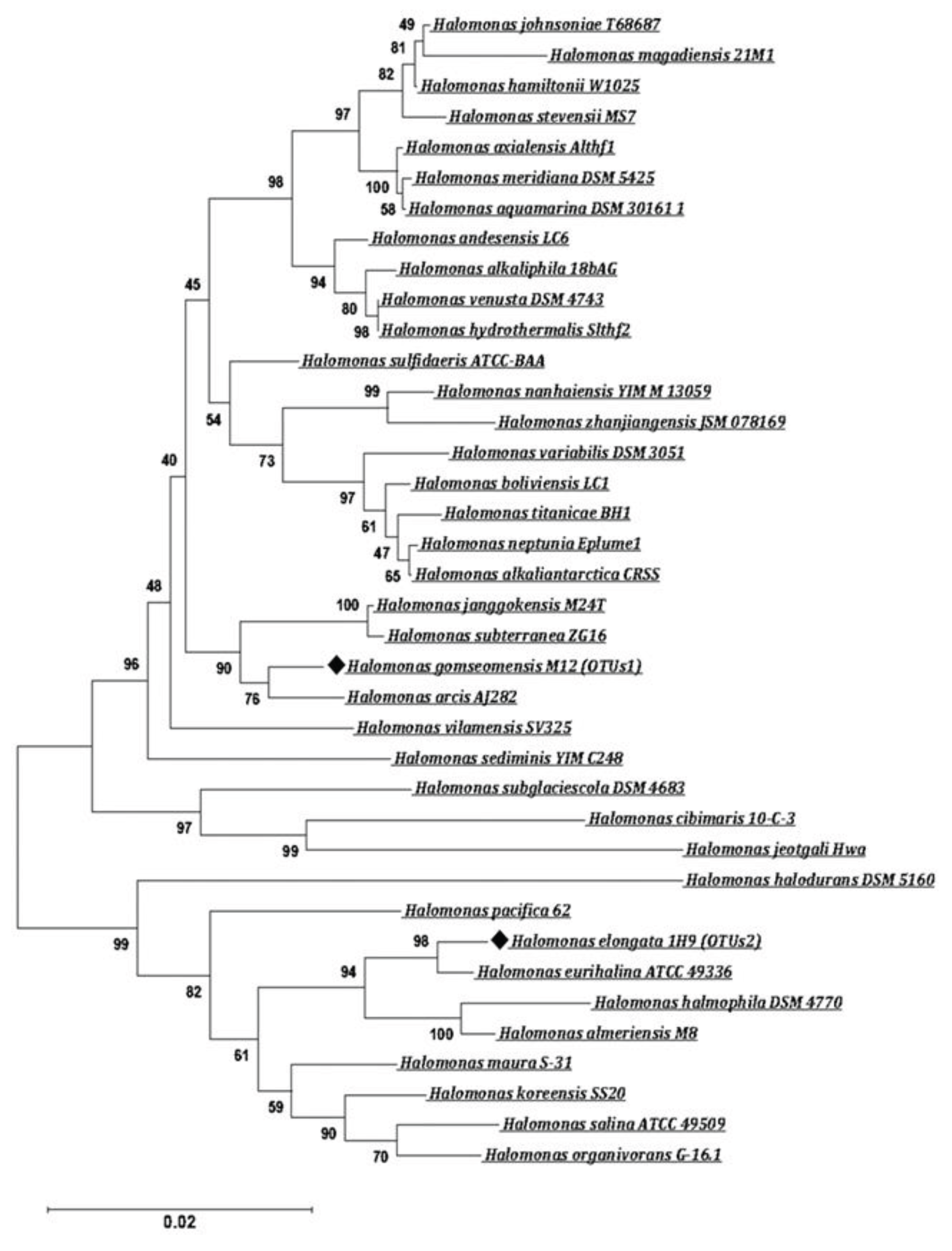
3.1. Identification of Halophile Microorganism through 16S rRNA
Previously, a strain characterized as JCCOL25.2 [
13] was isolated as a halophilic bacteria producer of PHB. The 16S rRNA sequence obtained for JCCOL25.2 strain was analyzed to relate taxa using BLAST search tool; multiple sequence alignment was performed using MUSCLE. MEGA 5.0 was used for the construction of the phylogenetic tree by neighbor-joining method with bootstrapping for 1000 replicates and tree displayed for 100 [
14]. Analysis revealed that the JCCOL25.2 strain was 99% similar to
H. gomseomensis (
Figure 1). The sequence obtained for this strain was deposited in EMBL Nucleotide Sequence Database with accession number LN835168.
3.2. Effect of Carbon Sources in Intracellular PHA Accumulation
To determine whether the use of different carbon sources could modify the PHAs detected in cells, 1% (w/v) of supplemented carbon source in addition to glucose, such as sodium acetate, starch, sodium citrate, fructose, soybean oil, glycerol and sucrose, were added to the culture. The tested carbon sources did not cause a significant effect on cell growth (data not shown). After evaluating the effect of glucose on the cell growth of
H. gomseomensis and given our previous results [
13] that support the ability of glucose an or other carbon sources to produce PHAs. We examined the effect of the application of each previously mentioned carbon source on PHA accumulation in a temporal course. To evaluate this effect, the isolated
H. gomseomensis culture was incubated for 72 hours in YGS media supplemented with the half of nitrogen source and 1% of each carbon source.
After 72 H of exposure to different carbon sources as mentioned before, the structure of
H. gomseomensis was observed by confocal microscopy to evaluate their structure and PHA accumulation. In
Figure 2 was observed the confocal microscopy analysis of
H. gomseomensis after 72 H of treatment in normal YGS medium, used as control from the evaluated carbon sources.
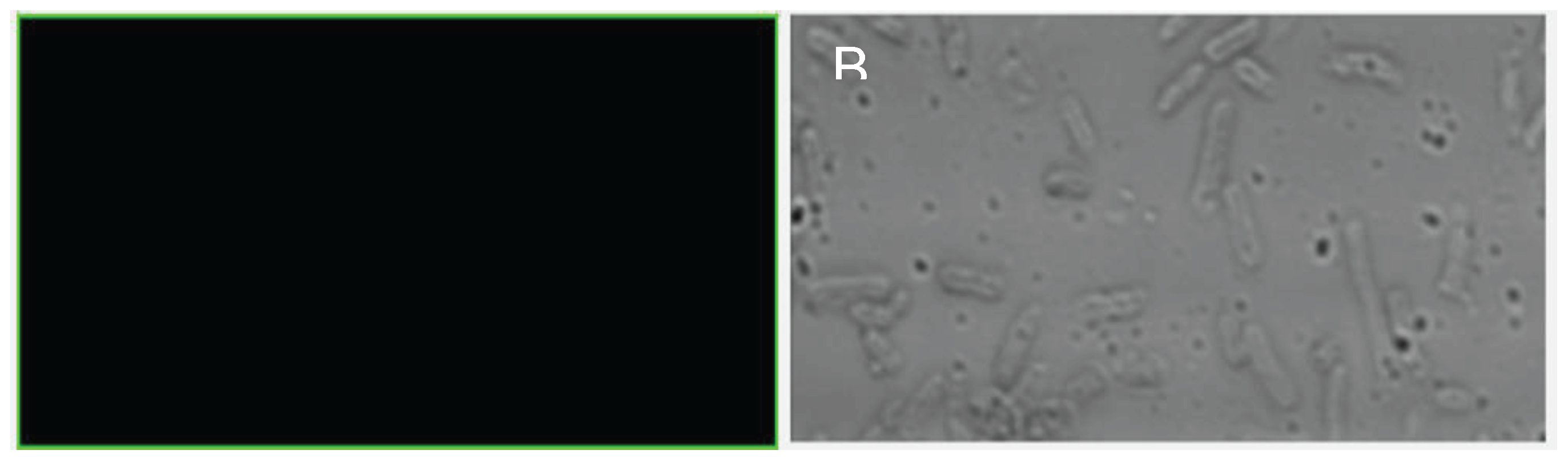
Figure 2A, negative control (growth with total nutrients) was stained with Nile blue A to evaluate accumulation of total intracellular PHA. Results showed no accumulation of PHAs. In
Figure 2B. Bright field to evaluate structure of cells, the untreated cells (control) showed an elongated shape with rounded ends and a bacillus-like structure. The cells treated with each different carbon source showed no alterations in structure and were similar in appearance to the control cells. In addition, a wide variety of cells showed more elongation compared with the control cells, this appearance of the cells could be due to fluid loss, causing them to lose firmness and plasticity. According to
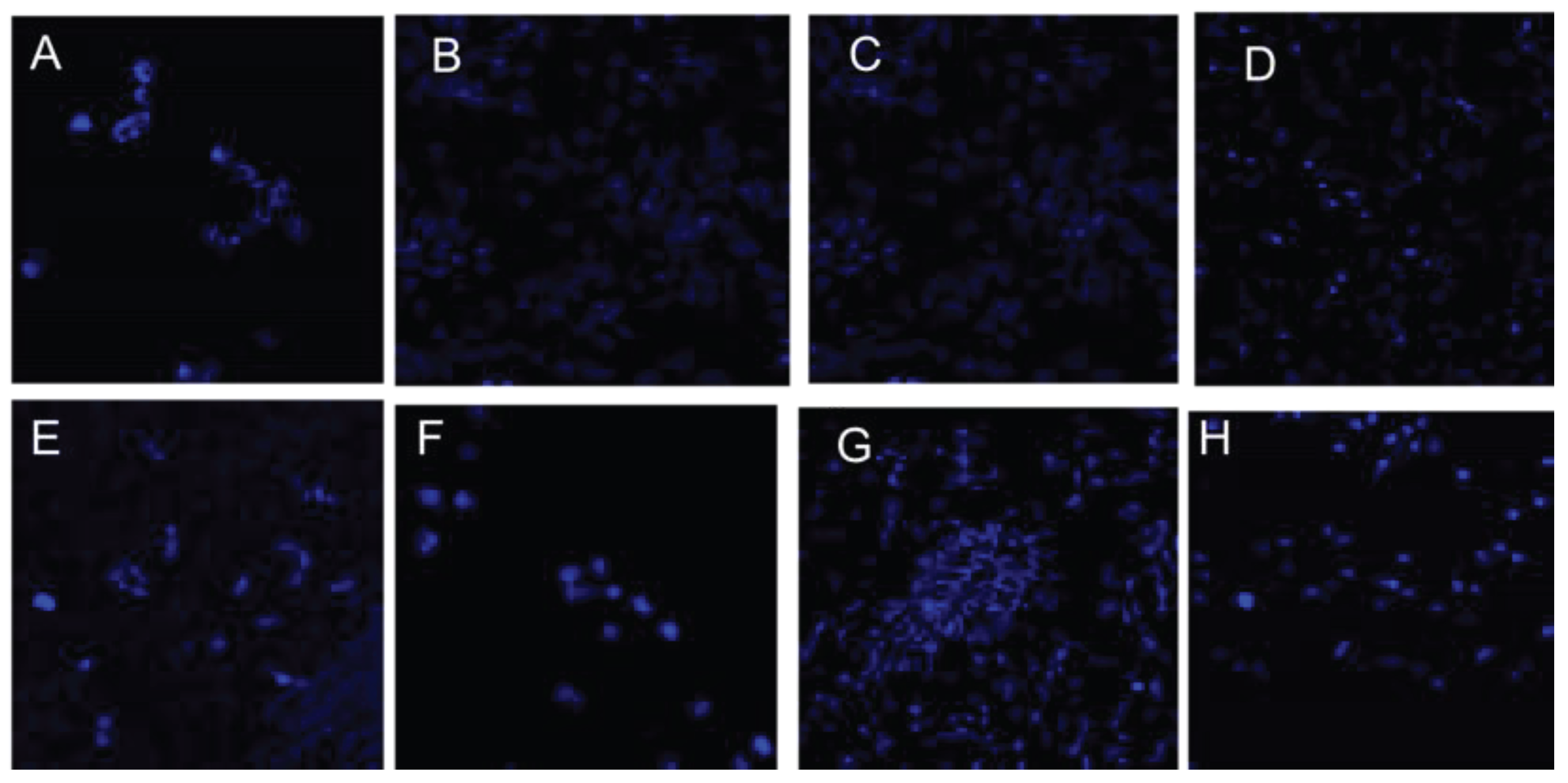
Figure 3 corresponds to the cells treated simultaneously exposed 72 H with N
2- limited YGS medium and supplemented with A. Sodium acetate, B. Starch, C. Sodium citrate, D. Glucose, E. Fructose, F. Soybean oil, G. Glycerol, H. Sucrose.
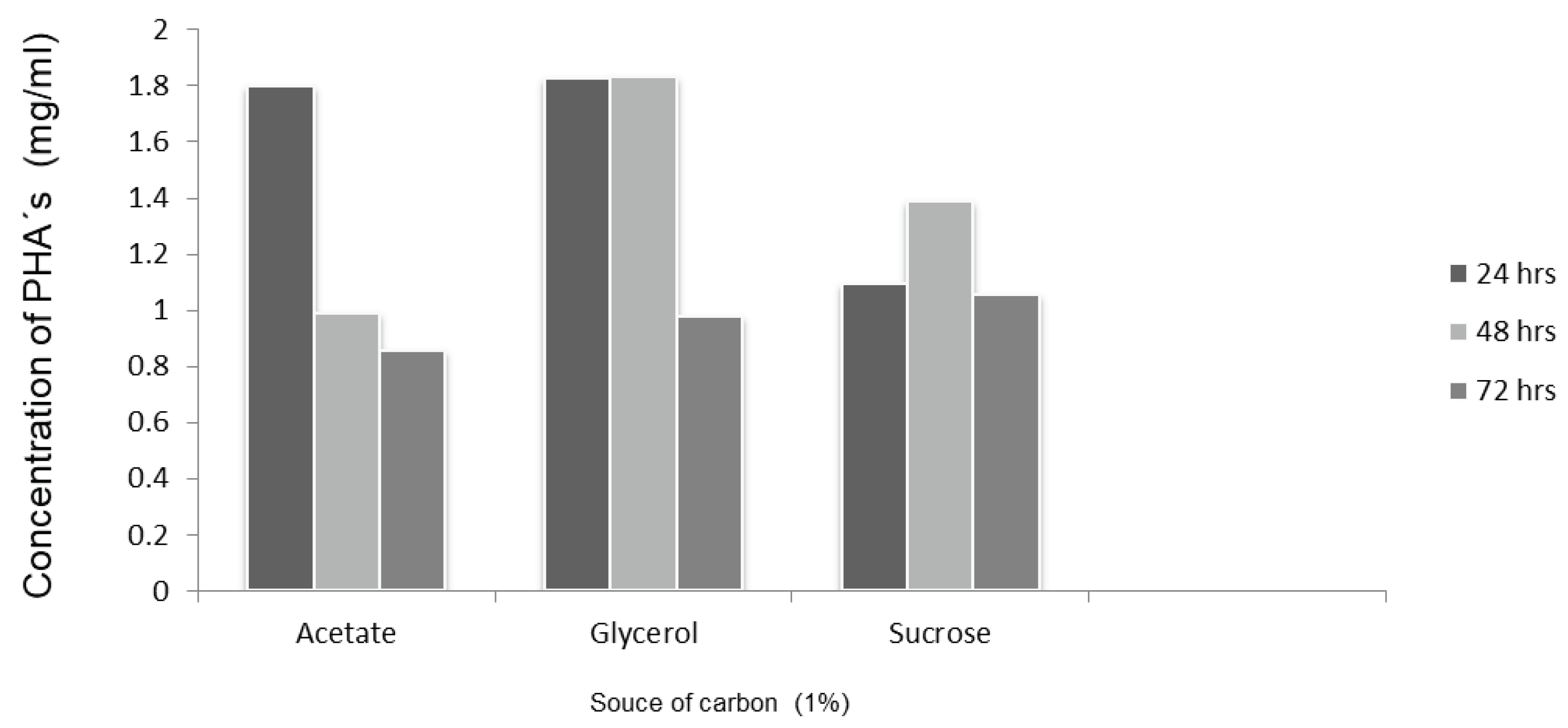
These results shows that during the exposition to each carbon source evaluated, the cells maintain the capacity to process and accumulate PHAs and also the cells subjected to the treatment with acetate, glucose and glycerol demonstrated a higher degree of accumulation of PHA than the other treatments (
Figure 4), obtaining the highest production of polymer to 24 h using acetate or glycerol (1.8 mg/mL), and 48h to sucrose (1.4 mg/mL). The results obtained show that the production pf PHAs H
. gomseomensis decreases after 72 hours of incubation this behavior coincides with the has been reported that microorganisms use PHAs as carbon and energy source reserves to continue their biological process [
15,
16]. Subsequently experiments were analyzed under N
2-limited YGS medium supplemented with glucose, acetate and glycerol independently.
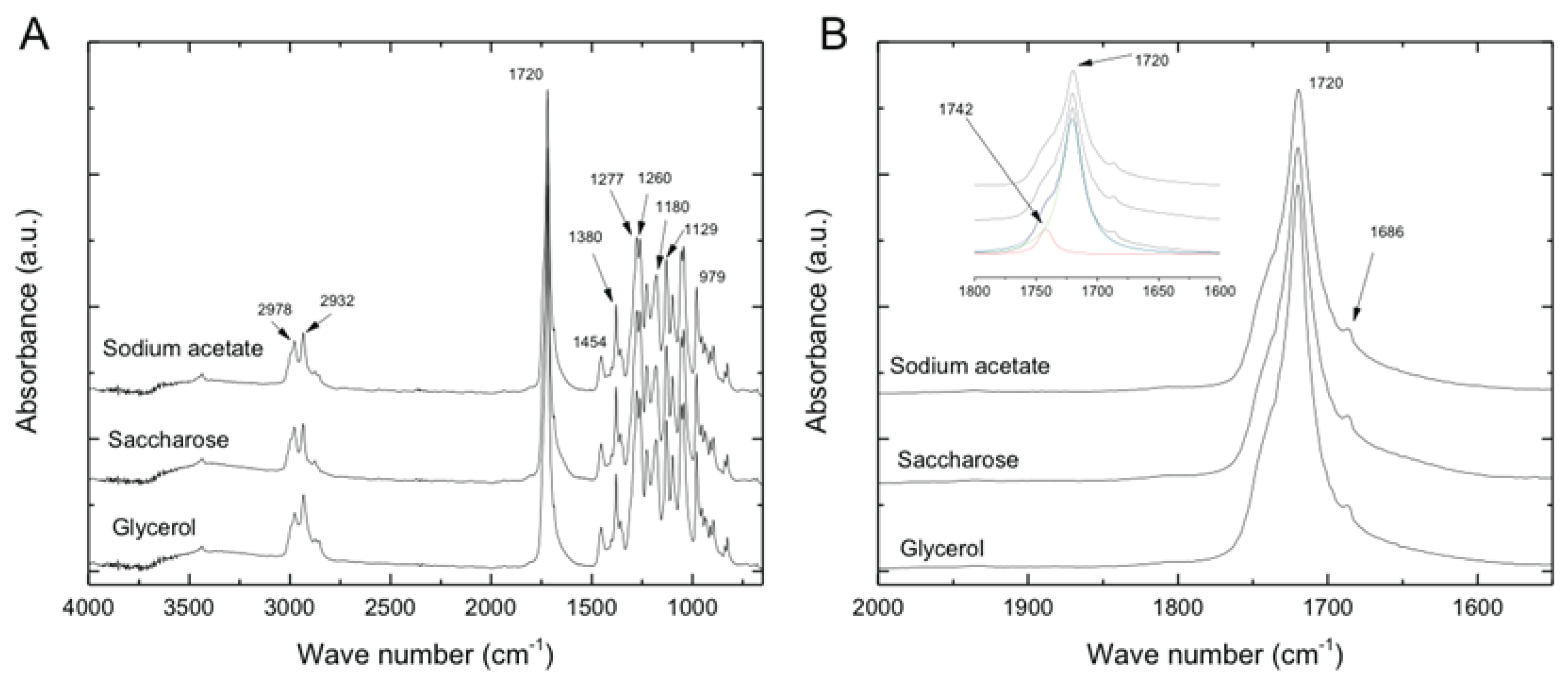
3.3. Fourier Transform Infrared Spectroscopy
Figure 5A shows the FTIR spectra of the polymers produced by
Halomonas gomseomensis cultured using different carbon sources (sodium acetate, saccharose and glycerol). As noted, spectra of the biopolymers were very similar among them which suggest that the carbon source has not a marked influence on the type of biopolymer produced. An intense band at 1720 cm-1, related to carbonyl stretching vibration belong to ester group is clearly observed [
13]. A close inspection of this band (
Figure 5B) revealed that the carbonyl band is composed by two overlapped peaks located at 1720 and 1742 cm-1; this fact has been reported previously and it has been associated to the presence of crystalline and amorphous phases in the polyhydroxyalkanoates, respectively [
17,
18,
19,
20]. A small peak was also observed at 1686 cm
-1, being typical of the crystalline state of PHA’s [
13,
20]. The spectra also showed bands in the range of 3000–2800 cm
-1, which are associated with C–H-stretching vibrations from methyl, methylene, and methyne groups [
21,
22]. Bands at 1454 and 1380 cm
-1, related to the asymmetric and symmetric deformation of the methyl groups, respectively, and peaks in the region of 1300–1000 cm
-1, associated to the stretching vibration of the C–O–C linkage were also observed.
It should be mentioned that spectra also showed a small broad peak centered at 3436 cm-1 which was attributed to the hydroxyl end group existing in the chemical structure of PHA’s, and/or due to the presence of moisture [
23].
3.4. Diferential Scanning Calorimetry
Figure 6 shows DSC curves of second heating scan for biopolymers obtained by
Halomonas gomseomensis using different carbon sources. As noted, thermograms are similar between them exhibiting three different thermal events: the first one is an endothermal base-line shift in the range of 2-4 °C which is typically associated to the glass transition (Tg); the second one is an exothermic peak around 51-55 °C related to crystallization phenomena (Tc) and; the third one, located between 159 and 168 °C, is an endothermic peak associated to polymer melting (Tm). Both Tg and Tm values fall within the temperature range reported for poly(3-hydroxybutyrate) (P3-HB) which is a highly crystalline thermoplastic polymer with a relatively high melting temperature (160–180 °C) and a glass transition temperature in the range of 0–5 °C. Therefore, it is possible to postulate that the polymer accumulated by
Halomonas gomseomensis using different carbon sources is P3-HB. In this regard, it should be mentioned that the precise Tm value for this polymer depends on stereoregularity of PHB [
24]. The composition of polyhydroxybutyrate obtained by microorganisms also plays a key role on the thermal transitions values as the presence of 3-hydroxybutyrate (3-HB)/4-hydroxybutyrate (4-HB) copolymers has been reported (an increase content of 4-HB yields a decrease melting point temperature [
25,
26]. In fact, [
26], reported that that composition of PHA’s accumulated by microorganism is function of carbon sources.
It is interesting to note that DSC curves exhibit a crystallization phenomenon which is not always displayed by P(3-HB); however, some authors have reported similar thermograms for PHB to those obtained in this work [
14,
27,
28]. A single melting peak was observed for P3-HB obtained from sodium acetate, although samples derived from other carbon sources showed a melting peak with a shoulder at lower temperatures. This behavior is not uncommon for PHB and/or its copolymers and depends on the crystallization conditions [
14,
29].
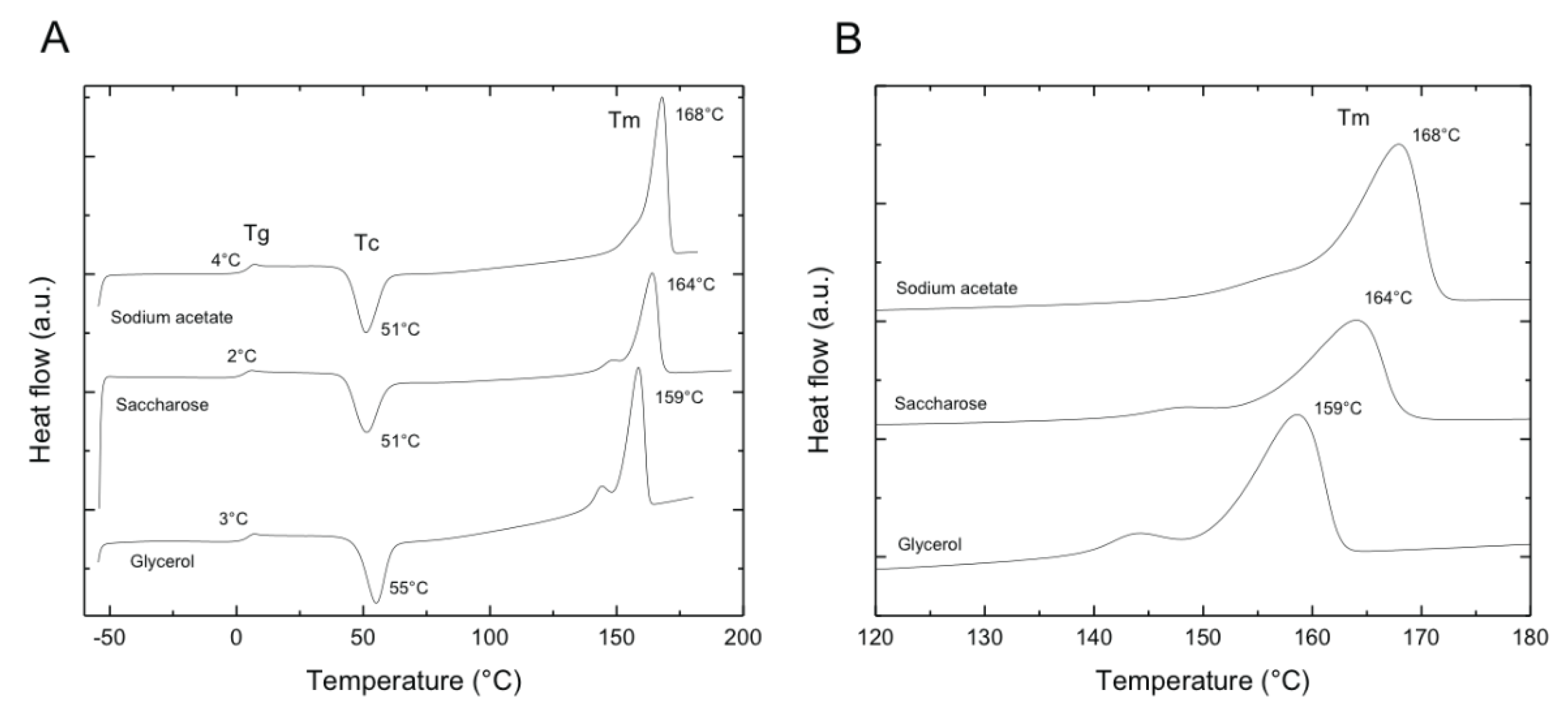
Crystallinity pf PHA samples was calculated from enthalpy of melting sample and that theroretical entalpy of meltong of pure cystal PHB 146 J/g. It was observed that values fall with the crystallinity range reported by other authors for PHB [
14,
30].
Table 1 summarizes the temperatures and crystallinity obtained for each PHB polymer sample.
3.5. Thermogravimetric Analysis
The thermal stability of PHA’s was investigated by thermogravimetric analysis and the TG and DTGA curves are shown in
Figure 7. As noted, all samples exhibit only one weight loss which started ca. 250°C and its maximum rate decomposition temperature is located in the range 290 -305 °C. Last values are similar to those reported by other authors for Td of P(3-HB) [
21,
29]. It is also interesting to note that PHB synthesized from glycerol as carbon source exhibited a wider transition in DTGA curve than those exhibited by PHB’s obtained from either sodium acetate or saccharose, which displayed sharp peaks. This could be related to the purity of the obtained product [
31,
32,
33].
4. Conclusions
The analyzes obtained from this work demonstrated that the JCCOL25.2 strain has a 99% similarity with Halomonas gomseomensi, and is capable of using different carbon sources to synthesize PHAs. The physicochemical analyzes allowed the identification of the biopolymer produced and classifying it as polyhydroxybutyrate (PHB), because the characteristics of this biopolymer have been reported for similar polymers. PHB has various applications in the pharmaceutical, medical, food and agronomy areas. Likewise, PHB has interesting properties for use in food packaging, due to its characteristics such as biodegradability, optical activity and good barrier properties. Finally, the importance and impact of our study, there is only one published study on the biosynthesis of PHAs by H. gomseomensis strains, although the characterization of the polymer obtained is not reported.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, R.T., and G.L. and E.O.; methodology, R.T., and G.L., and E. O.; software, E.R..; validation, E.O., M.F. and R.T.; formal analysis, E.O.; investigation, R.T.; resources, G.L. and E.O.; data curation, R.T.; writing—original draft preparation, E.O.; writing—review and editing, G.L. and E.O.; visualization, M.F.; supervision, E.R.; project administration, J.M.; funding acquisition, E.O. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
to all the laboratory technicians who helped develop the techniques to obtain results
Conflicts of Interest
“The authors declare no conflicts of interest.”
Abbreviations
DSC, Diferential Scanning Calorimetry; EDS, Energy Dispersive Spectrometry; EDX, Energy Dispersive X-ray microanalysis; GPC, Gel Permeation Chromatography; FTIR, Fourier Transform Infrared Spectroscopy; HAs, Hydroxyalkanoates; PHAs, Polyhydroxyalkanoates; PHB, Poly 3-hydroxybutyrate; PHV, Poly 3-hydroxyvalerate; PHB-co-V, Poly 3-hydroxybutyrate- co - hydroxyvalerate; YGS medium, Yeast extract, Glucose and Sea water medium.
References
- Hye-Young, S.; Hyeoncheol, F.; So, Y.; Sang, Y.; Up, L. Structural Insights into Polyhydroxyalkanoates Biosynthesis. Trends Biochem Sci. 2018, 43 (10), 790–805. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. GlobalChange Biol. 2018, 24, 1405–1405. [Google Scholar] [CrossRef]
- Zhang, X.; Rongcong, L.; Zhen, W., Yuan; Guo-Qiang, Ch. Application of (R)-3-hydroxyalkanoate methyl esters derived from microbial polyhydroxyalkanoates as novel biofuels. Biomacromolecules 2009, 10(4), 707-11. [Google Scholar] [CrossRef]
- Anbreen, A.; Mohammad, Z.; Khalid, M.; Aqdas, N.; Muhammad, N.; Shazia, T. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int J Biol Macromol 2016, 89, 161-74. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Muhammad, R.; Muhammad, I.; Ibrahim, M. Recent developments in bioreactor scale production of bacterial polyhydroxyalkanoates. Bioprocess and Biosystems Engineering 2019, 42, 901–919. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Shahina, R.; Ibrahim, M. Polyhydroxyalkanoates: Properties and chemical modification approaches for their functionalization. Biotechnol Prog. 2018, 34(1), 29-4. [Google Scholar] [CrossRef]
- Chethana, M.; Kona, M.; Urvashi, S.; Vimal, K. Production of Polyhydroxyalkanoates and Its Potential Applications. Advances in Sustainable Polymers 2019, 131–164. [Google Scholar] [CrossRef]
- Subhasree, R.; Vipin, Ch. Biomedical Applications of Polyhydroxyalkanoates. Indian J Microbiol. 2017, 57(3), 261–269. [Google Scholar] [CrossRef]
- Tiago, M.; Amaro, D.; Giuseppe, C.; Lucilla, I. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA). Production. Frontiers in Microbiology 2019, 10, 1.12. [Google Scholar] [CrossRef]
- Bhatia, S. K.; Gurav, R.; Choi, T.-R.; Jung, H.-R.; Yang, S.-Y.; Moon, Y.-M. et al.; et al. Bioconversion of plant biomass hydrolysare into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef]
- Bhatia, S. K.; Kim, J.-H.; Kim, M.-S.; Kim, J.; Wong, J. W.; Hong, Y. G. et al.; et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2018, 41, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Uc, J.M.; Catzin, J.; Vargas, I.; Herrera-Kao, W.; Miguel, F.; Rincón-Arriaga, S.; Lizama-Uc, G. Biosynthesis and characterization of polyhydroxyalkanoates produced by an extreme halophilic bacterium, Halomonas nitroreducens, isolated from hypersaline ponds. J Appl Microbiol 2014, 117, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, L.; Shanks, R.A.; Amarasinghe, G. Thermal history effects on crystallisation and melting of poly(3-hydroxybutyrate). Thermochimica Acta 2004, 423, 127–135. [Google Scholar] [CrossRef]
- Toh, P.; Jau, M.; Saw-Peng, Y.; Abed, R.; Sudesh, K. Comparison Of Polyhydroxyalkanoates Biosynthesis, Mobilization And The Effects On Cellular Morphology In Spirulina Platensis And Synechocystis Sp. Uniwg. 2008. Journal of Bioscience 19(2), 21–38. https://www.researchgate.net/publication/237628395_Comparison_of_polyhydroxyalkonates_biosynthesis_mobilization_and_the_effects_of_cellular_morphology_in_Spirulina_platensis_and_Synechocystis_sp_UNIWG_J_Biosci.
- Thomson, N.; Roy, I.; Summers, D.; Sivaniah, E. In vitro production of polyhydroxyalkanoates. 2009. Achievements and applications. [CrossRef]
- Ikejima, T.; Yagi, K.; Inoue, Y. Thermal properties and crystallization behavior of poly (3hydroxybutyric acid) in blends with chitin and chitosan. Macromol. Chem. Phys. 1999, 200, 413–421. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B-H.; Yang, R.; Wu, Q.; Chen, G-Q.; Zhang, Z-M. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Padermshoke, A.; Katsumoto, Y.; Sato, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Surface melting and crystallization behavior of polyhydroxyalkanoates studied by attenuated total reflection infrared spectroscopy. Polymer https://www.academia.edu/18874033/Surface_melting_and_crystallization_behavior_of_polyhydroxyalkanoates_studied_by_attenuated_total_reflection_infrared_spectroscopy. 2004, 45, 6547–6554. [Google Scholar] [CrossRef]
- Sato, H.; Dybal, J.; Murakami, R.; Noda, I.; Ozaki, Y. Infrared and Raman spectroscopy and quantum chemistry calculation studies of C–H/O hydrogen bondings and thermal behavior of biodegradable polyhydroxyalkanoate. Journal of Molecular Structure 2005, 744–747, 35–46. [Google Scholar] [CrossRef]
- Xiao, N.; Jiao, N. Formation of Polyhydroxyalkanoate in Aerobic Anoxygenic Phototrophic Bacteria and Its Relationship to Carbon Source and Light Availability. Applied and Environmental Microbiology 2011, 77, 7445–6450. [Google Scholar] [CrossRef]
- Shah, K.R. FTIR analysis of polyhydroxyalkanoates by novel Bacillus sp. AS 3-2 from soil of Kadi region, North Gujarat, India. J Biochem Tech. https://jbiochemtech.com/storage/models/article/jhGf8ERXqctMxostMWavZcj7BfBV9PEpcOVvw63gEXqsO8PefA1i3PPYJimo/ftir-analysis-of-polyhydroxyalkanoates-by-a-locally-isolated-novel-bacillus-sp-as-3-2-from-soil-of.pdf. 2012, 380–383. [Google Scholar]
- Bera, A.; Dubey, S.; Bhayani, K.; Mondal, D.; Mishra, S.; Ghosh, P.K. Microbial synthesis of polyhydroxyalkanoate using seaweed-derived crude levulinic Acid as co-nutrient. International Journal of Biological Macromolecules 2015, 72, 487–494. [Google Scholar] [CrossRef]
- Tanahashi, N.; Doi, Y. Thermal properties and stereoregularity of poly(3-hydroxybutyrate) prepared from optically active -butyrolactone with a zinc-based catalyst. Macromolecules 1991, 24, 5732–5733. [Google Scholar] [CrossRef]
- Kunioka, M.; Doi, Y. Thermal degradation of microbial copolyesters: poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate. Macromolecules 1990, 23, 1933–1936. [Google Scholar] [CrossRef]
- Anderson, A.J.; Dawes, E.A. Ocurrence, Metabolism, Metaboic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiological Reviews 1990, 54, 450–427. [Google Scholar] [CrossRef] [PubMed]
- Martino, L.; Cruz, M.V.; Scoma, A.; Freitas, F.; Bertin, L.; Scandola, M.; Reis, M. Recovery of amorphous polyhydroxybutyrate granules from Cupriavidus necator cells grown on used cooking oil. International Journal of Biological Macromolecules 2014, 71, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Bei, F-F.; Tian, H-L.; Chen, G-Q. Effects of crystallization of polyhydroxyalkanoate blend on Surface physicochemical properties and interactions with rabbit articular cartilage chondrocytes. Biomaterials 2005, 26, 3537–3548. [Google Scholar] [CrossRef] [PubMed]
- Corre, Y-M.; Bruzaud, S.; Audic, J-L.; Grohens, Y. Morphology and functional properties of comercial polyhydroxyalkanoates: A comprehensive and comparative study. Polymer Testing https://www.academia. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Porter, M.; Yu, J. Monitoring the in situ crystallization of native biopolyester granules in Ralstonia eutropha via infrared spectroscopy. Journal of Microbiological Methods 2011, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Padermshoke, A.; Katsumoto, Y.; Sato, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Surface melting and crystallization behavior of polyhydroxyalkanoates studied by attenuated total reflection infrared spectroscopy. Polymer 2004, 45, 6547–6554. [Google Scholar] [CrossRef]
- Teeka, J., Yuliani; Higuchi, T.; Yamamoto, K. Characterization of polyhydroxyalkanoates (PHAs) biosynthesis by isolated Novosphingobium sp. THA_AIK7 using crude glycerol. J Ind Microbiol Biotechnol 2012, 39, 749–758. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B-H.; Yang, R.; Wu, Q.; Chen, G-Q.; Zhang, Z-M. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).