1. Introduction
As soon as we fall asleep, we suddenly stop to perceive sensory stimuli from the external world and instead get invaded by internal thoughts and hallucinations that are often unrelated to our previous immediate experiences (Mildner and Tamir, 2019). Indeed, even though disconnecting us from the outside world, sleep still hosts internal conscious sensory experiences, or dreams, triggered by the generation of an internal, virtual world (Mazzarello, 2000). Strikingly, these experiences usually give us the feeling of being awake, as similar features to our external sensorium (characters, objects, colors, places, or sounds) are incorporated in a realistic manner (Nir and Tononi, 2010). Moreover, similarly to waking experiences, dreams reflect our current concerns, interests and personality, and are highly rich in emotions (Nielsen and Stenstrom, 2005).
Despite their realism, dreams, especially from rapid-eye-movement (REM) sleep, are often bizarre and creative, mostly due to the fact that they do not simply replay previous experiences (Fosse et al., 2003; Schwartz, 2003; Wamsley, 2014). In a study examining dream reports and waking activities from participants over 14 days, Fosse et al. (2003) showed that while 65% of dream reports incorporate aspects of waking life experiences, the exact replay of waking events was found in only 1-2 %. Instead, dreams are made of various isolated episodic fragments, sometimes non-obviously related (Llewellyn, 2016b; Lewis et al., 2018; Zadra and Stickgold, 2021), for example representing an acquaintance outside of its usual context, which partly explains their creative aspect.
The observed novelty in our dreams raises the question of their potential function. How such a virtual, hallucinatory and fantastic experience would benefit our cognitive functions? A prominent theory of why dreams combine memories into a new, virtual scenario is that they enhance creativity (Cai et al., 2009; Lewis et al., 2018). Motivated by anecdotal evidence of scientific discoveries from dreams, e.g., benzene structure by Kekule (1865) or the chemical neurotransmission by Loewi (1936) (Mazzarello, 2000), the role of dreams in creativity has been taken into wider consideration. It has been proposed that the creative associations between unrelated memories during dreaming could lead to the discovery of unexpected solutions which lies at the essence of creative problem solving (Lewis et al., 2018). Through this process, the dreamer would make creative experimentations for potential future situations (Hobson, 2009; Llewellyn, 2016b), e.g., rehearsing threat perception and avoidance. However, studies report that dreams rarely contain practical solutions to real-life problems, in addition to the fact that most dreams are forgotten (Malcolm-Smith and Solms, 2004; Zadra et al., 2006; Zadra and Stickgold, 2021; Hoel, 2021).
In contrast, in a recent computational study, we (the authors of Deperrois et al., 2022) argued that the creative aspect of dreams serves a more basic function than creativity itself, that is, learning semantic concepts from the external world. In this study, we proposed a cortical architecture, where sensory inputs are perceived through feedforward pathways of sensory cortices, while dreams are generated through the feedback pathways. In particular, we show that the generation of dreams during REM sleep can be explained by an adversarial learning mechanism inspired by Generative Adversarial Networks (GANs, Goodfellow et al., 2014) where feedback pathways trick feedforward pathways into believing that the dream comes from outside. Crucially, this mechanism leads to the acquisition of structured, semantic cortical representations, essential to perform downstream tasks such as object recognition.
In this article, we provide an accessible overview of this computational approach, thereby explaining how creative dreams could facilitate learning. We discuss the conclusions of the model in light of previous theories of dream origin and function. We finally propose extensions of this framework to already established functions of dreams, such as enhancing creativity and insight.
2. A Computational Model for Creative Dreams
Even though dreaming is a universal phenomenon, characterizing its role is still, up to this day, a challenging task. It is, for instance, difficult to assess whether the improvement of skills after a night of sleep is due to the occurrence of a certain dream, or to other physiological features of sleep such as hippocampal replay during NREM sleep (Nadasdy et al., 1999). Nonetheless, there have been some attempts to investigate these effects, notably through the use of pharmacological interventions to suppress REM sleep in participants (see, e.g., Oudiette et al., 2012). Despite these efforts, disentangling the effects of dreams within sleep remains complex and largely unclear.
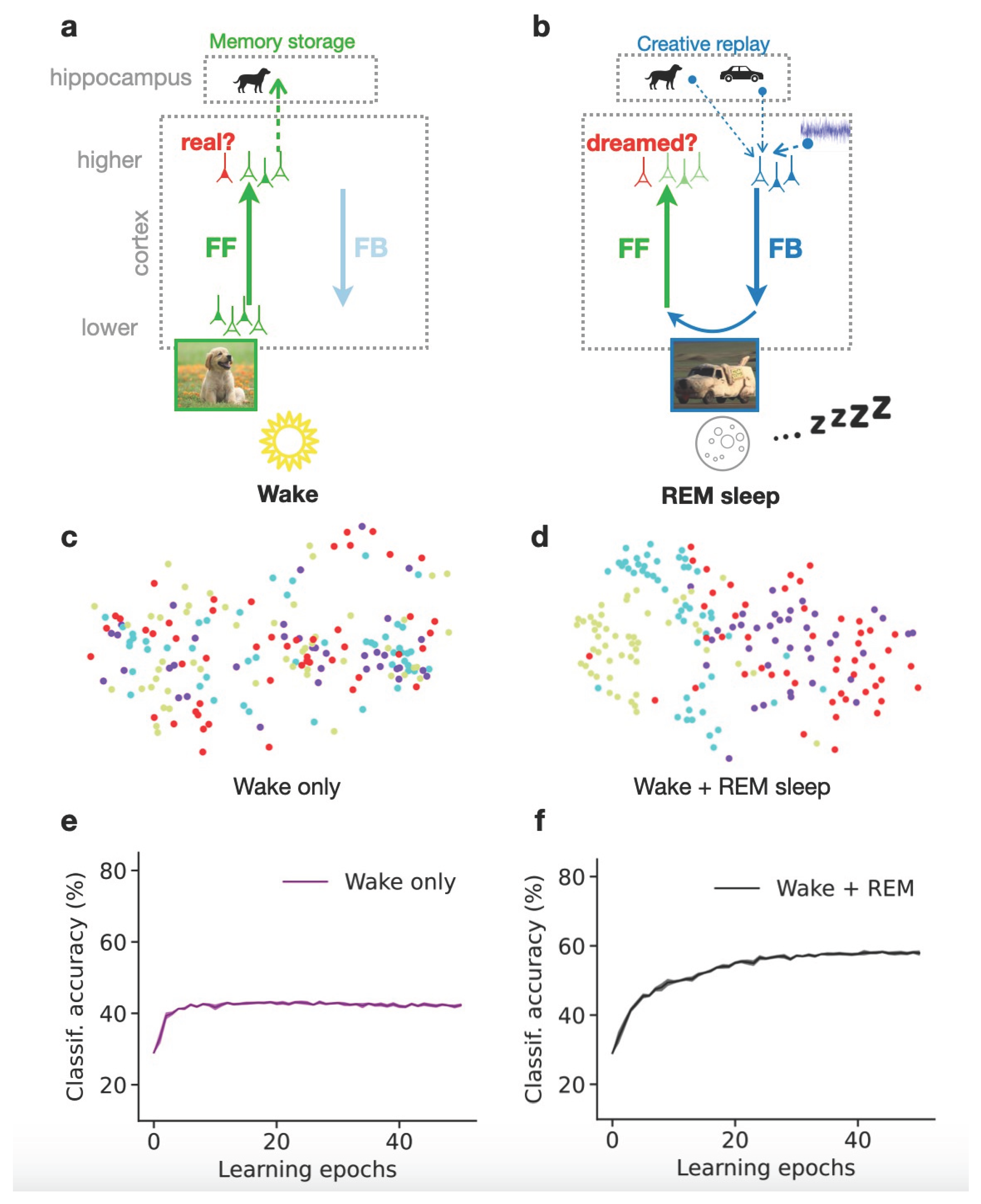
Here, computational models can help to quantify physiological features such as dreams and replay through simulations, and thereby decipher their contributions within a defined task. This was the challenge of our study Deperrois et al. (2022) that, through the construction of a “perturbed and adversarial dreaming” (PAD) model, suggests a role for dreams in learning. The PAD model is composed of cortical feedforward (FF) and feedback (FB) pathways within which synapses are modified through accumulated sensory experience, along with a hippocampus model storing and retrieving memories from the day. The system can either be in a wake state, where external stimuli are perceived through FF pathways, or in a dream state, where sensory activity is driven by memory replay and FB pathways.
During wakefulness (
Figure 1a), the cortex is exposed to diverse natural images that are processed along the cortical hierarchy through FF pathways until forming high-level neuronal representations, for example in the Inferior-Temporal (IT) cortex (Grill-Spector et al., 2001). These representations are temporarily stored in the hippocampus. Simultaneously, FF pathways learn to recognize that the observed images are
real, as they come from the outside world.
During the REM sleep phase (
Figure 1b), the representation stored from the previous day is replayed (“dog” memory) along with past, sometimes unrelated memories (“car” memory) and some additional cortical background activity (which is modeled as noise). The resulting activity is processed along FB pathways down to low-level sensory areas where details to the dream are added. Due to the combination of diverse memories, the dream might contain elements from each of them, such as a car having the texture or shape of a dog. However, combining contents does not tell how the FB pathway can generate a realistic experience of the novel combination. This is where the principle of GANs, i.e., adversarial learning, comes into play. The dream is processed though FF pathways that learn to distinguish it from real sensory inputs observed during wakefulness. Simultaneously, the FB pathway adversarially learns to generate a dream that could be recognized as “real” by the FF pathway, referred to as “adversarial dreaming”. By trying to fool the FF pathway, the FB pathway learns to generate a creative dream that combines aspects from both replayed memories, while still keeping some overall resemblance with the external inputs observed during wakefulness.
Simulations of this model, obtained by repeating many wake-sleep cycles, reveal the quality of the learned high-level representations at the end of training (
Figure 1c-d). Note that these representations are not learned with explicit teaching signals, indicating the category of the observed object, but tend to discover them (unsupervised learning). Higher-level neuronal representation of visual objects be- came clustered, as illustrated by projecting them to two dimensions via Principal Component Analysis (PCA, Pearson, 1901).
If only the wake phase is present, the obtained PCA projection shows that representations from dif- ferent object categories are entangled, showing that wakefulness is not sufficient to construct semantic representations (
Figure 1c). When both Wake and REM sleep phases are simulated (
Figure 1d), the PCA projection shows relatively distinct clusters of latent representations according to the semantic category (“class identity”) of their corresponding images. The model thus tends to organize latent representations such that high-level, semantic clusters are discernable, potentially helping humans and other animals to discern different object categories from their sensorium. This is in particular impor- tant for animals, that do not receive explicit teaching signals in the way humans and their children do throughout development.
These results can be quantified by evaluating the performance of a linear decoder (classifier) trained on high-level cortical representations obtained throughout the model simulation. If the REM phase is included in training, the accuracy of the classifier tends to be much higher than if only the Wake phase is simulated (
Figure 1e-f). The results show that the generation of dreams during REM sleep is essential to organize high-level representations according to the semantics of the sensorium, suggesting that dreaming is an essential component of learning.
3. Semantization Requires More than Memory Replay
Previous cognitive theories of sleep, such as “semantization” theories (Nadel and Moscovitch, 1997; Winocur et al., 2010), suggest that the commonalities between multiple experienced episodes is ex- tracted during NREM sleep to form a cortical semantic representation. A cognitive model (Lewis and Durrant, 2011) proposed that semantic formation is based on the invariant overlapping and statistical regularities between replayed episodic memories, where areas of overlap are strengthened via Hebbian learning, allowing the abstraction of shared elements among these memories, or the semantic “gist”. For example, the reactivation of various memories of “cat experiences” facilitates the extraction and consolidation the “cat” concept from repeating features with episodic memories (four legs, pointed ears, tail, etc.) in cortical representations.
In contrast, Deperrois et al. (2022) suggested that dreams during REM sleep, rather than the replay during NREM sleep, are more likely to trigger the semantization of cortical representations. In fact, we also model a NREM sleep phase where stored memories are replayed without combining with other memories. We find that NREM has little or no impact on learning semantic representations, even when adversarial learning is enabled in FF and FB pathways. This shows that in order to extract semantic concepts from the sensorium, the brain must go beyond merely replaying previous experiences. Instead, novel but realistic contents can be created out of stored memories. The postulate of creative REM dreams may refine cognitive theories about sleep function and delineate the role of NREM and REM sleep in memory semantization through future experimental investigations.
4. Adversarial Dreams on the Edge between Fantasy and Reality
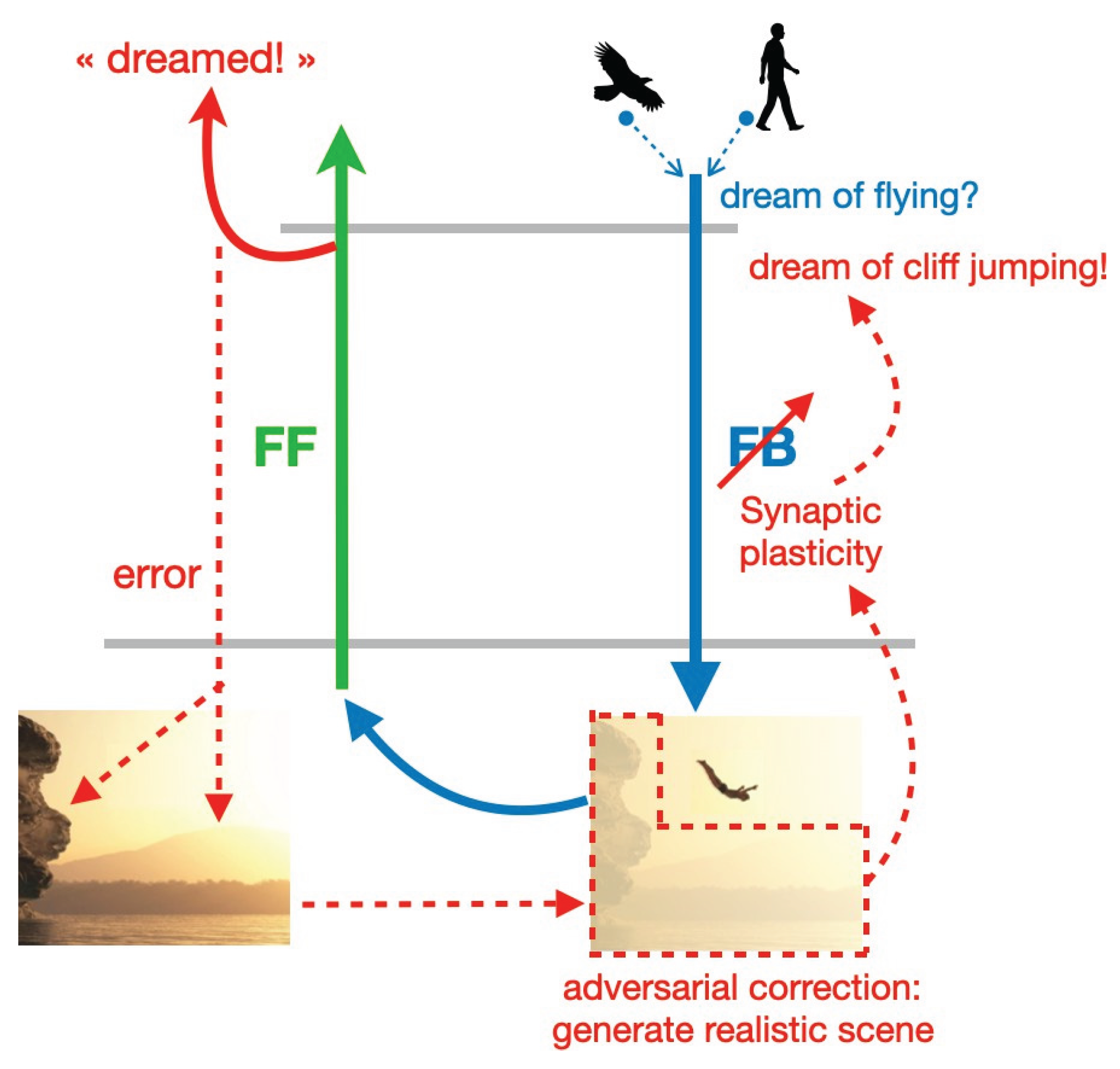
Aside from proposing a role for dream function, the adversarial dreaming principle of the PAD model suggests a mechanism for how dreams are generated in the brain, and how they maintain an equilibrium between fantasy and realism. Following the PAD framework, one could assume that two different memories, e.g., of an eagle and a human (me), are concurrently replayed from hippocampus and combined in high-level areas (
Figure 2). The combined activity is sent through FB pathways, leading to the generation of a dream representing a flying human, that while being novel and creative, is not realistic. This dream is passed through FF pathways that, from the cumulative observation of real sensory inputs during wakefulness, can easily detect that this experience is dreamed through their discriminative function.
However, a key point of adversarial dreaming is that FB pathways attempt to make FF pathways believe that the dream indeed comes from the outside. This is performed by computing an error between the discriminator prediction “dreamed”) and what FB pathways would like it to be (“real”). This error is then backpropagated through FF pathways, down to low-level sensory areas. At this level, the error contains the visual elements that the FB pathway should generate in order to make the FF pathway classify the dream as “real”.
For instance, we may dream of flying by combining the hippocampal memory of an eagle and us watching it. The visual elements making our dream of flying more realistic are the cliff and the sea. The missing of these elements are representing the errors that should be corrected to make the dream of flying more plausible. In a next REM phase we are therefore dreaming of jumping from the cliff and feel like flying across the sea. The synaptic connections of the FB pathway are then modified in order to generate a more realistic, plausible dream. The novel dreams created during the REM phase will also change the early cortical activity produced during wakefulness when mind wandering through the latent representation. This in turn may influence our future actions. For instance, we may go cliff jumping the next day after the REM dream has generated the corresponding scene.
Adversarial learning could thus explain how dreams, initiated by a creative combination of mem- ories, can be constrained to look realistic and make them compatible with our waking experiences and actions. This is in line with the pioneer activation-synthesis theory from Hobson and McCarley (1977) that claims that REM dreams result from the brain “making the best of a bad job in producing even partially coherent dream imagery from the relatively noisy signals sent up to it from the brain stem”. In the spirit of this citation, the REM model starts with noise signals added up to random combinations of memories, and out of this tries to produce a coherent and realistic cortical activity through adversarial learning. We will next see how this balance between fantasy and realism can be useful to trigger creative insights.
5. Is Adversarial Dreaming at the Heart of Creativity?
As a consequence of adversarial dreaming, FB projections can lead to the generation of sensory activity patterns that have unlikely been evoked by previously experienced stimuli, but that nevertheless may be part of the external world. This can have functional benefits in terms of creative thinking and gaining insights in general.
New insights may result from a period of “incubation” where non-obvious, remote associations amongst memory (or knowledge) elements are made (Dijksterhuis and Meurs, 2006; Baird et al., 2012). These associations can sometimes be compatible with reality, in which case they can provide a solution to a complex problem through a creative insight (Kounios and Beeman, 2009, “Aha moments”,) that awake reasoning alone may not provide. Dreaming seems to be an ideal stage to promote novel associations and eventually enhance creative insights, as previously suggested (Llewellyn, 2016a; Lewis et al., 2018).
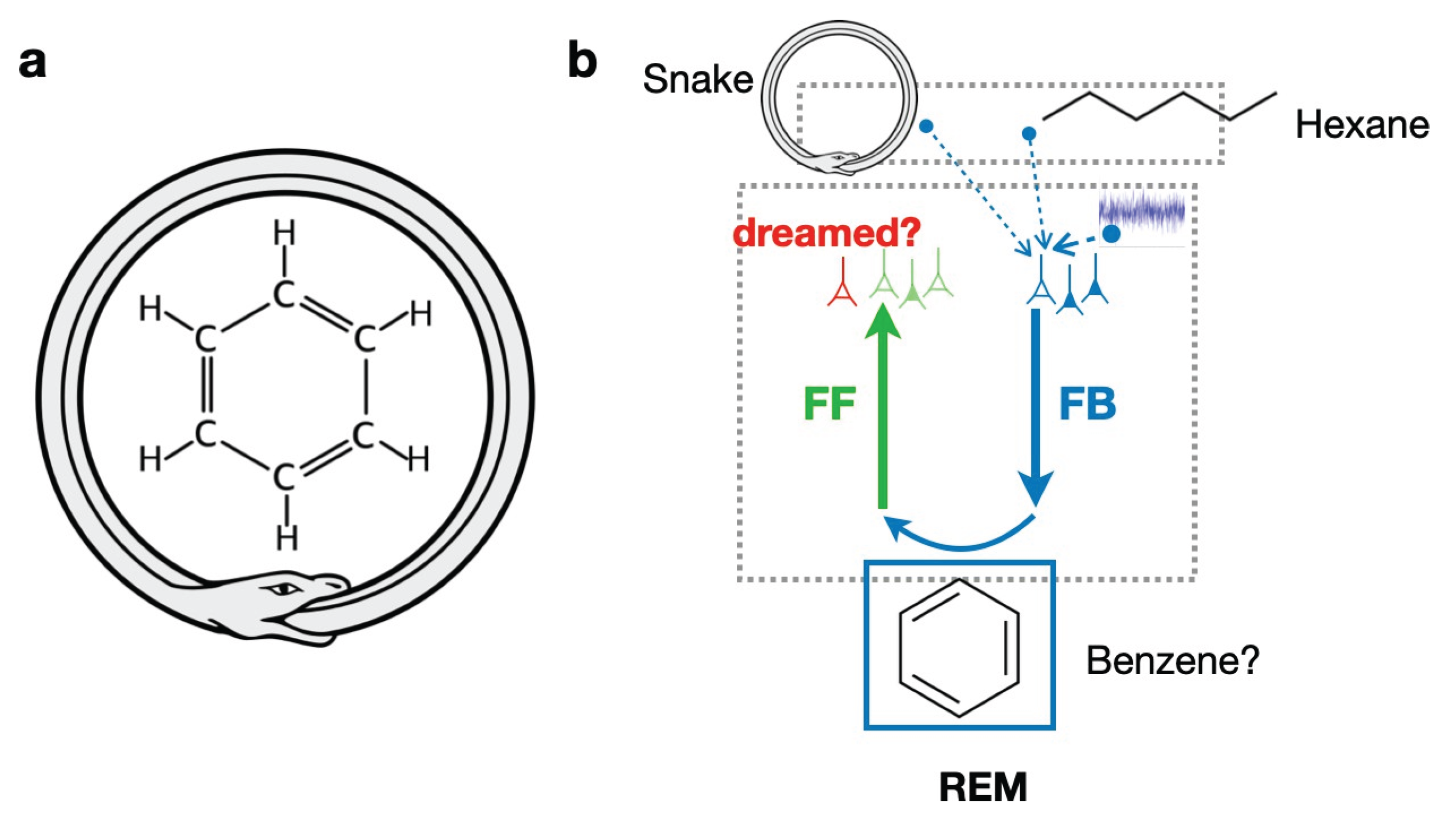
As an anecdotical evidence, the Benzene structure was for instance discovered through a dream by Kekulé (Mazzarello, 2000), by combining two non-related concepts, such as a snake biting its own tail, and the carbohydrate molecular chain. In this example, the adversarial dreaming framework could explain the occurrence of such insights (
Figure 3). The memories of a snake and the hexane molecule could be randomly replayed from the hippocampus and combined in high-level areas during REM sleep. By sending down this activity through FB pathways, the adversarial learning mechanism could allow the generation of a dreamed molecule that contains aspects of the snake, such as its cyclic shape. By forcing this dream to be realistic against the FF judgement, this generated dream could respect known properties of chemistry and exist in the external world. Naturally, not all creative combinations experienced during dreaming are useful, and their usefulness is ultimately determined by how compatible they are with the actual external world.
This suggests that additional steps may be involved, such as verifying experimentally the potential existence of the suggested insights, reflecting with Kekulé ’s words: “Let us learn to dream, gentlemen, and then perhaps we shall learn the truth. but let us beware of publishing our dreams before they have been put to the proof by the waking understanding.” (Account of his famous dream of the benzene structure, as quoted in Olah (2002), p. 54)
6. NREM Dreams: A Role for Replay
For the past decades, NREM sleep has been classically associated with memory consolidation (Mc- Clelland et al., 1995; Diekelmann and Born, 2010; Klinzing et al., 2019) and memory semantization (Nadel and Moscovitch, 1997; Lewis and Durrant, 2011). The main mechanism hypothesized to drive these consolidation processes is the observed reactivation of hippocampal representations during the deepest stage of NREM sleep, slow-wave sleep (Girardeau and Zugaro, 2011). According to these theories, replaying hippocampal memories allows a transfer to cortical networks for long-term reten- tion via Hebbian learning (Diekelmann and Born, 2010), possibly allowing an abstraction of semantic concepts by losing spatiatemporal details and keeping the commonalities between replayed memories (Nadel and Moscovitch, 1997; Winocur et al., 2010; Lewis and Durrant, 2011).
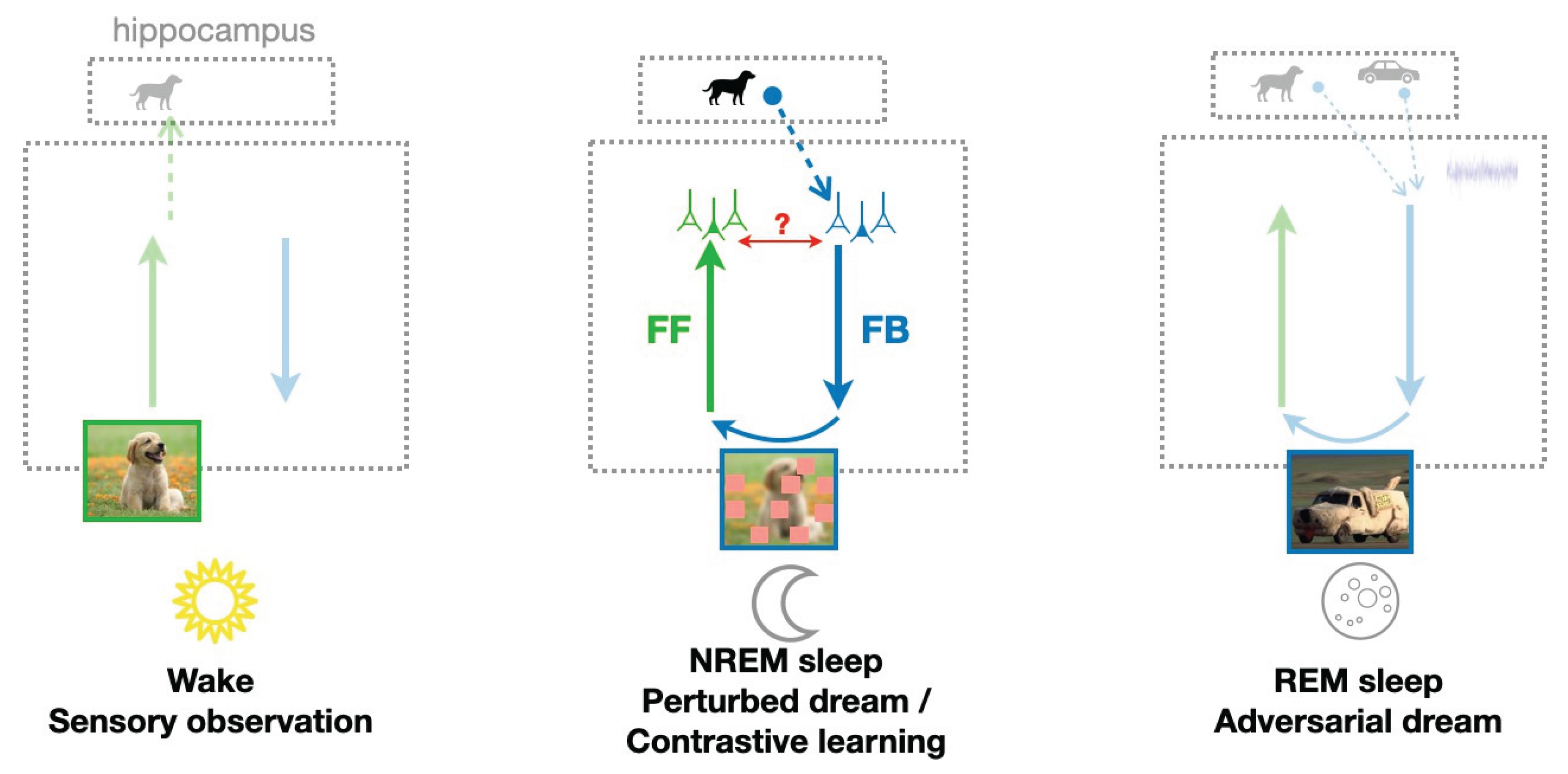
Considering these hypotheses, the PAD model also characterizes hippocampal replay during NREM sleep and thereby suggests a complementary role to REM sleep in memory consolidation and semanti- zation. While memory replay has been extensively associated with memory consolidation, as such, it is not obvious how the reactivation of previous memories alone could improve cortical representations. However, non-creative dreams can still be beneficial if they are additionally altered with some pertur- bations, making the recognition of an object more challenging. In this line, during the NREM phase of the model, a single episodic memory is retrieved from the hippocampus (instead of multiple memories in the case of REM) (
Figure 4, middle), leading to the dream of a sensory input previously experi- enced, such as the image of a dog observed the previous day. This dream is additionally perturbed by some occlusions and the FF pathway is trained to map this perturbed dream to the initially replayed representation. Such learning paradigm is remniscient of the sleep phase of the Wake-Sleep algorithm (Hinton et al., 1995), necessary to train the FF pathway to invert the FB generative pathway learned during the Wake phase.
By replaying and perturbing previously experienced inputs, the model shows that learned cortical representations are more robust to perturbations that could potentially occur in the visual field when an object is partially hidden by obstacles. While REM dreams tend to semantize cortical representa- tions through their creative process, NREM dreams make them more robust to environmental noise. Together, NREM and REM dreams act in a complementary fashion to construct semantic, robust representations.
While in the PAD model, REM sleep is the main driver of semantization, future work could further investigate whether NREM sleep, through the replay of a single memory only, could also drive semantization. Recent models of artificial intelligence use contrastive learning (Le-Khac et al., 2020; Chen et al., 2019; Ericsson et al., 2022) as a way to efficiently learn semantic representations. The main idea is to construct representations such as similar inputs are mapped to similar representations, while dissimilar inputs are pushed apart within the representational space. Similar inputs are simply obtained by creating two different versions of the same input, for instance by adding different sets of transformations on top of them.
As recently suggested (Deperrois 2023), NREM sleep could host a contrastive learning process to further drive semantization. The clustering of similar inputs could be implemented by the proposed NREM phase of the PAD model. Subsequently, other memories stored in the hippocampus could be replayed, from which the encoded representation through the FF pathway would be pushed away. This would force cortical network to separate sensory inputs from different categories.
7. Discussion
In this perspective article, we have reviewed the PAD (Perturbed & Adversarial Dream) model — a novel proposal for both the formation and function of NREM and REM dreams (Deperrois et al., 2022). The model is built upon the idea of GANs (Generative Adversarial Networks, Goodfellow et al. (2016)) that have been proposed to also be implemented in the brain (Gershman, 2019). GANs come with a discriminator network that tells the internally generated sensory activity apart from the externally triggered sensory activity. Such a discriminator network may be realized in the brain by a reality monitoring region located in the anterior prefrontal cortex (Simons et al., 2017).
The PAD model suggests that during REM sleep, new sensory contents are created out of previous memories, which are shaped by an adversarial game between FB and FF pathways improving the realism of the novel sensory activity. While the proposed adversarial mechanism has shown benefits for learning semantic representations in silico, we have also discussed its potential implications in higher- level cognitive functions, such as enhancing creative insight. Finally, we suggested a complementary role of non-creative dreams, mostly occurring during NREM sleep, in improving the robustness of cortical representations. These insights implicate some promising directions for empirical studies in human subjects.
8. Experimental Verifications
8.1. Semantization
A first line of experimental investigation arises from the model’s prediction that REM dreams facilitate the emergence of semantic representations. In order to test these predictions, experimental studies could record the stimulus-evoked activity in high-cortical areas in human subjects (Grill-Spector et al., 2001; Hung et al., 2005). Doing so over a long period of time would allow to evaluate how well subjects separate object categories. To reveal a potential effect of REM dreams on this semantization process, pharmacological agents such as anti-depressants could be administered to repeatedly impair REM sleep (Boyce et al., 2017) over the course of the experiment.
8.2. Assessing Dreams
Evaluating the effects of REM dreaming on representation learning in hu- mans encompasses several significant problems. First, sleep deprivation is not only experimentally challenging but also potentially ethically questionable. Second, it is difficult to directly attest whether a subject is dreaming or not, or determine what the subject is dreaming about (although a real-time dialogue between experimenter and dreamer is possible Konkoly et al. (2021) and images can be re- constructed from fMRI activity (Takagi and Nishimoto, 2022)). Third, given that sleep and dreams naturally co-occur, it becomes challenging to disentangle the specific effects of dreams from those of sleep. When only considering the FF and FB pathways, the PAD model does not allow for distin- guishing whether a subject becomes aware of cortical activity (dream) or not (dreamless sleep). Yet, learning of the real/dream-discriminator assumes a meta-instance (’conductor’) that encodes whether activity in the sensory areas generated from inside or outside (Deperrois et al., 2022). Considering these difficulties, we suggest testing the experimental predictions of the PAD model by investigat- ing the effects of mental imagery—another process that internally generates visual experience—on learning representations.
8.3. Mental Imagery
The cognitive process of mental imagery is assumed to cause perception-like experience of visual stimuli in the absence of corresponding external stimulation (e.g., Currie and Ravenscroft, 2002; Pearson and Kosslyn, 2013). In contrast to dreaming, mental imagery is volun- tarily triggered and its content is relatively controllable (Pearson, 2019). These characteristics render it comparably more suitable for testing the effects of internally generated experiences on learning. Considering that mental imagery shares the same neuronal substrates as dreaming (Nir and Tononi, 2010; Pearson, 2019), we suggest that mental imagery is a valid proxy process to test the predictions of the PAD model on representation learning. We propose to employ a category learning task in which subjects must acquire representations for novel objects. During this task, some subjects are asked to perform mental imagery training sessions, whereby the objects to be learned (for instance a ‘doggy car’) need to be imagined. In parallel, a control group of subjects would perform the category learning task without engaging in any mental imagery. The PAD model predicts that subjects who internally generate additional visual input by mental imagery, learn representations that live in a space that features a better linear separability (as compared to the control group).
8.4. Creativity
A second line of experimental investigation arises from the central claim that cre- ativity is nurtured by REM dreams, as has previously been shown (Cai et al., 2009; Lewis et al., 2018). Further evidence that adversarial learning could be involved comes from the observation that prefrontal networks implicated in reality monitoring (Siegel, 2009) are generally deactivated during REM sleep (Muzur et al., 2002), while leaving the question open whether specific REM activation cites may also be related to daydreaming and creative visual imagery during wakefulness (Uitermarkt et al., 2020).
We also postulate a difference between NREM dreams (non-creative; only replay of memories) and REM dreams (creative; recombination of memories). In this line, semantic analysis of dream protocols showed that REM dreams are likely composed of more minimal-story-units than NREM dreams (Nielsen et al., 2001), consistent with the model assumption that REM dreams are composed of a mixture of episodic memories. Moreover, the analysis of dream protocols by non-semantic word graphs has shown that REM dreams are more complex and have a larger connectedness (although they are graph-theoretically less random-like) than NREM dreams (Marti et al., 2020).
8.5. REM Dreams Becoming More Realistic
The PAD model posits that REM dreams become more realistic during the course of a learning process, and during the refinement of cortical represen- tations in general. While anecdotal evidence suggests that exotic REM dreams decrease with age, scientific evidence exists for a reduced nightmare frequency with aging (Scarpelli et al., 2019). The hypothesis that REM dreams become more realistic with age, or also across a period of reporting sim- ilar dreams, has yet to be tested. Several questionnaires could be employed to quantitatively assess the reduction of bizarreness of dreams across time, like the bizarreness score (Yu and Shen, 2020), in combination with the dream frequency scale (Schredl and Erlacher, 2004) or the Creative Achievement Questionnaire (Carson et al., 2005).
9. Conclusion
Inspired from modern artificial intelligence, the PAD model connects cortical structures and dream phenomenology to a functional model of sleep. The model complements the memory consolidation theory during sleep with a creational process that combines old memories to form new contents. The adversarial dreams during REM sleep allow for exploring, testing and structuring the newly formed cortical representations while keeping these representations compatible with wake experiences. Ad- versarial dreams may improve our creative abilities by reenacting the past to generate novel virtual experiences. The suggested experimental approaches may help to validate these concepts, and hope- fully help to elucidate the mystery of sleep.
References
- Baird, B., Smallwood, J., Mrazek, M. D., Kam, J. W., Franklin, M. S., and Schooler, J. W. (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological science, 23(10):1117–1122.
- Boyce, R., Williams, S., and Adamantidis, A. (2017). REM sleep and memory. Current Opinion in Neurobiology, 44:167–177.
- Cai, D. J., Mednick, S. A., Harrison, E. M., Kanady, J. C., and Mednick, S. C. (2009). REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences, 106(25):10130–10134.
- Carson, S. H., Peterson, J. B., and Higgins, D. M. (2005). Reliability, validity, and factor structure of the creative achievement questionnaire. Creativity research journal, 17(1):37–50.
- Chen, T., Zhai, X., Ritter, M., Lucic, M., and Houlsby, N. (2019). Self-supervised GANs via auxiliary rotation loss. In 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), pages 12146–12155, Long Beach, CA, USA. IEEE.
- Currie, G. and Ravenscroft, I. (2002). Recreative minds: Imagination in philosophy and psychology. Oxford University Press on Demand.
- Deperrois, N., Petrovici, M. A., Senn, W., and Jordan, J. (2022). Learning cortical representations through perturbed and adversarial dreaming. eLife, 11:e76384.
- Deperrois, N.,Petrovici,M.A., Senn, W., and Jordan, J. (2023). Learning beyond sensa- tions: how dreams organize neuronal representations. Neuroscience and Biobehavioral Reviews, 157. [CrossRef]
- Diekelmann, S. and Born, J. (2010). The memory function of sleep. Nature Reviews Neuroscience, 11(2):114–126.
- Dijksterhuis, A. and Meurs, T. (2006). Where creativity resides: The generative power of unconscious thought. Consciousness and cognition, 15(1):135–146.
- Ericsson, L., Gouk, H., Loy, C. C., and Hospedales, T. M. (2022). Self-Supervised Representation Learning: Introduction, advances, and challenges. IEEE Signal Processing Magazine, 39(3):42–62.
- Fosse, M. J., Fosse, R., Hobson, J. A., and Stickgold, R. J. (2003). Dreaming and episodic memory: A functional dissociation? Journal of Cognitive Neuroscience, 15(1):1–9.
- Gershman, S. J. (2019). The Generative Adversarial Brain. Frontiers in Artificial Intelligence, 2.
- Girardeau, G. and Zugaro, M. (2011). Hippocampal ripples and memory consolidation. Current opinion in neurobiology, 21(3):452–459.
- Goodfellow, I., Bengio, Y., and Courville, A. (2016). Deep Learning. MIT Press. http://www. deeplearningbook.org.
- Goodfellow, I. J., Pouget-Abadie, J., Mirza, M., Xu, B., Warde-Farley, D., Ozair, S., Courville, A., and Bengio, Y. (2014). Generative adversarial networks.
- Grill-Spector, K., Kourtzi, Z., and Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41(10):1409 – 1422.
- Hinton, G., Dayan, P., Frey, B., and Neal, R. (1995). The ”wake-sleep” algorithm for unsupervised neural networks. Science, 268(5214):1158–1161.
- Hobson, J. A. (2009). REM sleep and dreaming: towards a theory of protoconsciousness. Nature Reviews Neuroscience, 10(11):803–813.
- Hobson, J. A. and McCarley, R. W. (1977). The brain as a dream state generator: an activation- synthesis hypothesis of the dream process. The American journal of psychiatry.
- Hoel, E. (2021). The overfitted brain: Dreams evolved to assist generalization. Patterns, 2(5):100244.
- Hung, C. P., Kreiman, G., Poggio, T., and DiCarlo, J. J. (2005). Fast readout of object identity from macaque inferior temporal cortex. Science, 310(5749):863–866.
- Klinzing, J. G., Niethard, N., and Born, J. (2019). Mechanisms of systems memory consolidation during sleep. Nature Neuroscience, 22(10):1598–1610.
- Konkoly, K. R., Appel, K., Chabani, E., Mangiaruga, A., Gott, J., Mallett, R., Caughran, B., Witkowski, S., Whitmore, N. W., Mazurek, C. Y., Berent, J. B., Weber, F. D., Türker, B., Leu- Semenescu, S., Maranci, J. B., Pipa, G., Arnulf, I., Oudiette, D., Dresler, M., and Paller, K. A. (2021). Real-time dialogue between experimenters and dreamers during REM sleep. Current Biology, 31(7):1417–1427.e6.
- Kounios, J. and Beeman, M. (2009). The aha! moment: The cognitive neuroscience of insight. Current directions in psychological science, 18(4):210–216.
- Le-Khac, P. H., Healy, G., and Smeaton, A. F. (2020). Contrastive Representation Learning: A Framework and Review. IEEE Access, 8:193907–193934.
- Lewis, P. A. and Durrant, S. J. (2011). Overlapping memory replay during sleep builds cognitive schemata. Trends in Cognitive Sciences, 15(8):343–351.
- Lewis, P. A., Knoblich, G., and Poe, G. (2018). How Memory Replay in Sleep Boosts Creative Problem-Solving. Trends in Cognitive Sciences, 22(6):491–503.
- Llewellyn, S. (2016a). Crossing the invisible line: De-differentiation of wake, sleep and dreaming may engender both creative insight and psychopathology. Consciousness and Cognition, 46:127–147.
- Llewellyn, S. (2016b). Dream to Predict? REM Dreaming as Prospective Coding. Frontiers in Psychology, 6.
- Malcolm-Smith, S. and Solms, M. (2004). Incidence of threat in dreams: A response to revonsuo’s threat simulation theory. Dreaming, 14(4):220.
- Marti, J. M., Andriano, D. W., Mota, N. B., Mota-Rolim, S. A., Araujo, J. F., Solms, M., and Ribeiro,S. (2020). Structural differences between REM and non- REM dream reports assessed by graph analysis. PLoS ONE, 15(7):1–20.
- Mazzarello, P. (2000). What dreams may come? Nature, 408(6812):523–523.
- McClelland, J. L., McNaughton, B. L., and O’Reilly, R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3):419–457.
- Mildner, J. N. and Tamir, D. I. (2019). Spontaneous thought as an unconstrained memory process. Trends in neurosciences, 42(11):763–777.
- Muzur, A., Pace-schott, E. F., and Hobson, J. A. (2002). The prefrontal cortex in sleep. Trends in Cognitive Sciences, 6(11):475–481.
- Nadasdy, Z., Hirase, H., Czurkó, A., Csicsvari, J., and Buzsáki, G. (1999). Replay and time com- pression of recurring spike sequences in the hippocampus. The Journal of Neuroscience, 19:9497 – 9507.
- Nadel, L. and Moscovitch, M. (1997). Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology, 7:217–227.
- Nielsen, T., Kuiken, D., Hoffmann, R., and Moffitt, A. (2001). REM and NREM Sleep Mentation Differences: A Question of Story Structure? Tore. Sleep and Hypnosis, 3(1):9–17.
- Nielsen, T. A. and Stenstrom, P. (2005). What are the memory sources of dreaming? Nature, 437(7063):1286–1289.
- Nir, Y. and Tononi, G. (2010). Dreaming and the brain: from phenomenology to neurophysiology. Trends in Cognitive Sciences, 14(2):88–100.
- Olah, G. A. (2002). A life of magic chemistry: Autobiographical reflections of a nobel prize winner. John Wiley & Sons.
- Oudiette, D., Dealberto, M.-J., Uguccioni, G., Golmard, J.-L., Merino-Andreu, M., Tafti, M., Garma, L., Schwartz, S., and Arnulf, I. (2012). Dreaming without REM sleep. Consciousness and Cognition, 21(3):1129–1140.
- Pearson, J. (2019). The human imagination: the cognitive neuroscience of visual mental imagery. Nature Reviews Neuroscience, 20(10):624–634.
- Pearson, J. and Kosslyn, S. M. (2013). Mental imagery. Frontiers in Psychology, 4(198.10):3389. Pearson, K. (1901). Liii. on lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 2(11):559–572.
- Scarpelli, S., Bartolacci, C., D’Atri, A., Gorgoni, M., and De Gennaro, L. (2019). Mental sleep activity and disturbing dreams in the lifespan. International Journal of Environmental Research and Public Health, 16(19).
- Schredl, M. and Erlacher, D. (2004). Lucid dreaming frequency and personality. Personality and Individual Differences, 37(7):1463–1473.
- Schwartz, S. (2003). Are life episodes replayed during dreaming? Trends in Cognitive Sciences, 7(8):325–327.
- Siegel, J. M. (2009). Sleep viewed as a state of adaptive inactivity. Nature Reviews Neuroscience, 10(10):747–753.
- Simons, J. S., Garrison, J. R., and Johnson, M. K. (2017). Brain mechanisms of reality monitoring. Trends in Cognitive Sciences, 21(6):462–473.
- Takagi, Y. and Nishimoto, S. (2022). High-resolution image reconstruction with latent diffusion models from human brain activity. bioRxiv, page 2022.11.18.517004.
- Uitermarkt, B. D., Bruss, J., Hwang, K., and Boes, A. D. (2020). Rapid eye movement sleep patterns of brain activation and deactivation occur within unique functional networks. Human Brain Mapping, 41(14):3984–3992.
- Wamsley, E. J. (2014). Dreaming and Offline Memory Consolidation. Current Neurology and Neuro- science Reports, 14(3).
- Winocur, G., Moscovitch, M., and Bontempi, B. (2010). Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal–neocortical interactions. Neuropsychologia, 48(8):2339–2356.
- Yu, C. and Shen, H. (2020). Bizarreness of lucid and non-lucid dream: effects of metacognition. Frontiers in psychology, 10:2946.
- Zadra, A., Desjardins, S., and Marcotte, E. (2006). Evolutionary function of dreams: A test of the threat simulation theory in recurrent dreams. Consciousness and Cognition, 15(2):450–463.
- Zadra, A. and Stickgold, R. (2021). When brains dream: Exploring the science and mystery of sleep. WW Norton.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).