1. Introduction
The microbiome-gut-brain axis has emerged as a contributing factor to the aetiology and pathophysiology of depression[
1,
2]. Preclinical evidence suggests that many physiological pathways influenced by the gut microbiota are also implicated in depression[
3]. In humans, differences in gut microbiota composition have been associated with depression[
4,
5], and interventions that modulate the gut microbiota, such as diet[
6,
7,
8] and probiotic supplementation[
9], can alleviate depressive symptoms. Manipulation of the gut microbiota may also further our understanding of how the gut microbiota influences, and can be targeted to treat, depressive disorders. Reciprocally, understanding how common interventions affect the gut microbiota may have important clinical and pathophysiological implications.
Mechanical bowel preparations are osmotic laxatives consumed prior to colonoscopy. Bowel preparation is essential for visualisation of the colon and the detection of abnormalities during colonoscopy[
10,
11], and previous research has shown an impact of bowel preparation on the gut microbiome. Indeed, osmotic laxatives have strong associations with gut microbiota composition and function, with large effect sizes[
12]. Previous intervention studies that have examined the impact of bowel preparation and colonoscopy (hereafter referred to as the ‘procedure’) on the gut microbiota have reported mixed results. Whilst decreases in the number and distribution (i.e., alpha diversity) and relative abundances of bacterial taxa have been observed immediately after bowel preparation (before colonoscopy)[
13,
14], very minimal or no changes have also been described[
15,
16]. Differences in gut microbiota composition have been reported one week[
14,
15,
17,
18,
19] to one month[
13,
17,
20,
21] post-procedure compared to baseline, however in other studies there were no statistically significant differences at these follow up time points[
17,
19,
20]. Few studies have measured changes in gut microbiota composition beyond one month after bowel preparation and colonoscopy, and those that have provided limited evidence of ongoing changes six weeks and six months later[
18,
22].
Whilst studies have aimed to characterise changes in gut microbiota composition, no studies, to our knowledge, have investigated changes in depressive symptoms after bowel preparation and colonoscopy, nor explored the potential associations between changes in gut microbiota composition and changes in depressive symptoms. Considering the millions of colonoscopies conducted annually[
23,
24], there is a need to better understand the potential impact of this procedure on the microbiome-gut-brain axis.
Therefore, the aim of this study was to investigate changes in self-reported depressive symptoms one week before versus one month after bowel preparation and colonoscopy. We also aimed to explore changes in gut microbiota composition and functional potential post-procedure and identify any associations with changes in depressive symptoms.
2. Materials and Methods
2.1. Study Design
The data used for this manuscript were derived from the Micro-Scope study, which used a pre–post intervention study design to investigate changes in depressive symptoms and gut microbiota composition after bowel preparation and colonoscopy. Data are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement[
25], and the Strengthening the Organising and Reporting of Microbiome Studies (STORMS) checklist[
26]. This study had ethical approval from the Barwon Health (#15-129), Epworth Healthcare (#EH2016-146), and Deakin University (#2016-391) Human Research Ethics Committees. Microbial data were not uploaded to an online public repository as consent for the sharing of data was not obtained. This study was pre-registered on the Open Science Framework (OSF):
https://osf.io/fv7xd/.
2.2. Participants and Setting
Participants were community-dwelling adults referred for colonoscopy between May 2017 and November 2018 at University Hospital Geelong, Australia. Colonoscopies were performed at University Hospital or Epworth Hospital, Geelong, Australia. Recruitment of participants occurred at the time of their initial outpatient consultation with the General Surgery or Gastroenterology services. Any adults referred for colonoscopy during the study time frame were considered for recruitment. Exclusion criteria included those: highly dependent on medical care; unable to give informed consent (e.g., language barriers, significant intellectual or cognitive disability); or children under 18 years of age. There were no exclusions for antibiotic use. Those deemed eligible to participate by their clinician were directed to a member of the research team to discuss the study further and provide informed consent. Those with a diagnosis of cancer post-colonoscopy were withdrawn and did not provide follow up data.
2.3. Data Collection
Baseline data were collected one week pre-procedure. Participants completed paper-based questionnaires and collected a fresh faecal sample into a sterile collection jar with a scoop lid at home. The sample was stored in their freezer (−20℃) for approximately one week until transported on ice to the research team on the day of colonoscopy, when it was transferred to a −80℃ freezer for storage until DNA extraction. Luminal aspirates were collected during colonoscopy to demonstrate the potential immediate changes in alpha diversity associated with the procedure. Faecal residue from within the colon was aspirated into a sterile collection jar (which had been flushed with saline for collection if necessary) and placed on ice, then transferred to a −80℃. One month post-procedure, participants completed another set of questionnaires, and collected a final faecal sample at home as previously.
2.4. Intervention
Bowel preparation was prescribed and carried out as per normal advice and practice for the colonoscopy service. Participants were instructed to commence a low-fibre (“white”) diet two days before their colonoscopy, and then fast for 12–24 hours prior to their procedure and consume a sodium picosulfate–based bowel preparation product in three separate doses. Adequacy of bowel preparation was reported during colonoscopy by the endoscopist using a modified overall Boston Bowel Preparation Scale score[
27].
2.5. Outcomes
Depressive symptoms (primary outcome) were measured pre- and post-procedure using the depression sub-score of the Hospital Anxiety and Depression Scale (HADS)[
28]. All other outcomes were considered exploratory. The severity of depressive symptoms was measured using the Patient Health Questionnaire-9 (PHQ-9)[
29]; anxiety symptoms were measured using the anxiety sub-score of the HADS[
28]; total, psychosocial, and physical quality of life were measured using the Assessment of Quality of Life-8 (AQOL-8D)[
30]; and stress was measured using the Perceived Stress Scale (PSS)[
31]. Gut microbiota alpha diversity was measured using the Shannon index — a within-sample index of both richness and evenness — and the number of observed amplicon sequencing variants (ASVs) at the genus level. Beta-diversity was measured using the Aitchison distance and differential abundances of ASVs were determined using centred-log ratio transformed count abundance data.
2.6. Covariates
Age at the time of recruitment and sex (male/female/other) were obtained from medical records. A triage nurse collected participant height and weight at their initial outpatient consultation, which were used to calculate body mass index (BMI; kilograms/metre
2). Participants self-reported their residential postcode and suburb, which were used to calculate socioeconomic status using an area-based measure called the Index of Relative Socio-economic Advantage and Disadvantage (IRSAD)[
32]. Each suburb has an IRSAD classification ranging from 1-10, where a lower IRSAD score suggests greater disadvantage. Smoking status, lifetime history of medical conditions (including depression), and current medication use were self-reported. Diet quality was measured using the Simple Dietary Questionnaire[
33], where the total score (out of 100) rated dietary adherence to the Australian Dietary Guidelines, higher scores representing greater compliance[
34]. The ROME III Diagnostic Questionnaire for Adult Functional Gastrointestinal Disorders was used to determine if participants met irritable bowel syndrome (IBS) criteria[
35]. Colonoscopy indication and outcomes were obtained from medical records.
2.7. DNA Extraction
Microbial DNA extraction from stool was performed using the commercial QIAamp Fast DNA Stool Mini Kit (QIAGEN, Germany) per manufacturer instructions, with an additional mechanical lysis step using PowerBead tubes (QIAGEN, Germany). Extracted DNA was stored at −80℃ until couriered on dry ice to the Australian Genomic Research Facility (AGRF) for sequencing.
2.8. Sequencing and Annotation
Sequencing of the 16S rRNA gene sequence was performed using the Illumina MiSeq platform. The V1–V3 hypervariable region of the 16S rRNA gene was amplified by polymerase chain reaction using 27F (AGAGTTTGATCMTGGCTCAG) and 519R (GWATTACCGCGGCKGCTG) primers with a read length of 300 base pairs. Diversity profiling analysis was performed with QIIME 2 2019.7[
36]. The demultiplexed raw reads were primer trimmed and quality filtered using the cutadapt plugin followed by denoising with DADA2 (via q2-dada2)[
37]. Taxonomy was assigned to amplicon sequence variants (ASVs) using the q2-feature-classifier[
38] classify-sklearn naïve Bayes taxonomy classifier. Taxonomy was assigned using the SILVA (v.132) database.
2.9. Pre-Processing
Pre-processing and filtering was performed per Callahan et al[
39]. Zero count bacterial features and non-bacterial taxa were removed prior to calculating the alpha diversity metrics Shannon index and observed ASVs. Additional filtering removed low prevalence taxa (those present in less than 5% of samples), and data were centred-log ratio transformed to calculate Aitchison distances (i.e., beta-diversity) and for differential abundance testing. Functional potential was predicted using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSTt2)[
40], which maps to MetaCyc pathways.
2.10. Power Calculation
A sample size of 59 has more than 80% power to detect mean differences on paired before-after comparison in HADS score of 1.5 or greater, which has been considered clinically important[
41]. We assumed a standard deviation of 3.7 for the differences[
42], and significance level of 0.05 using a two-sided paired t-test was used in the power calculation.
2.11. Statistical Analyses
Statistical analyses were performed within the RStudio[
43] 4.3.1 environment using complete case analysis. Packages used for analysis are listed within the Supplementary Material. All univariate and multivariate models included age, sex, BMI, diet quality, and IBS at baseline as covariates, as these have previously been associated with depression. Multiple imputation with predictive mean matching was performed for missing covariate data, including age, sex, and BMI as auxiliary variables for precision.
The primary outcome was the change in average HADS depressive symptom scores one week pre- versus one-month post-procedure using complete case analysis. This was analysed using generalised estimating equations (GEE) assuming a Gaussian distribution with an AR(1) correlation structure to account for within-participant autocorrelation. Changes in average depressive symptom severity, anxiety symptoms, quality of life, perceived stress scores, Shannon index, observed ASVs, and the Shannon index of MetaCyc pathways were considered exploratory outcomes and measured with GEEs as above. Robust standard error estimates were reported for all GEE models. Beta-diversity pre- and post-intervention was plotted using Principal Component Analysis (PCA) of Aitchison distances. Changes in beta-diversity metrics across time points were calculated using pairwise permutational ANOVA with 999 permutations, stratified by participant ID to consider the paired nature of the data. Differential abundance analyses at the genus level and of functional MetaCyc pathways were calculated using linear mixed models in the Maaslin2 package, with minimum abundance and prevalence set to zero, time point and covariates as fixed effects, participant as the random effect, and a centred–log ratio transformation.
Exploratory linear regression models were used to examine potential associations between the changes in average HADS depressive symptom scores with change in bacterial genera and alpha diversity. A post-hoc sensitivity analysis was also performed, whereby all participants with a baseline sample were included in a modified intention-to-treat analysis with missing follow up data imputed using predictive mean matching, with age, sex, BMI, marital status, employment status, socioeconomic decile, diet quality, IBS, and bowel preparation adequacy used as auxiliary variables.
For differential abundance and exploratory analyses, the Benjamini-Hochberg procedure was applied to control the false discovery rate (FDR), with taxa below an FDR of 0.05 reported in results.
3. Results
3.1. Recruitment
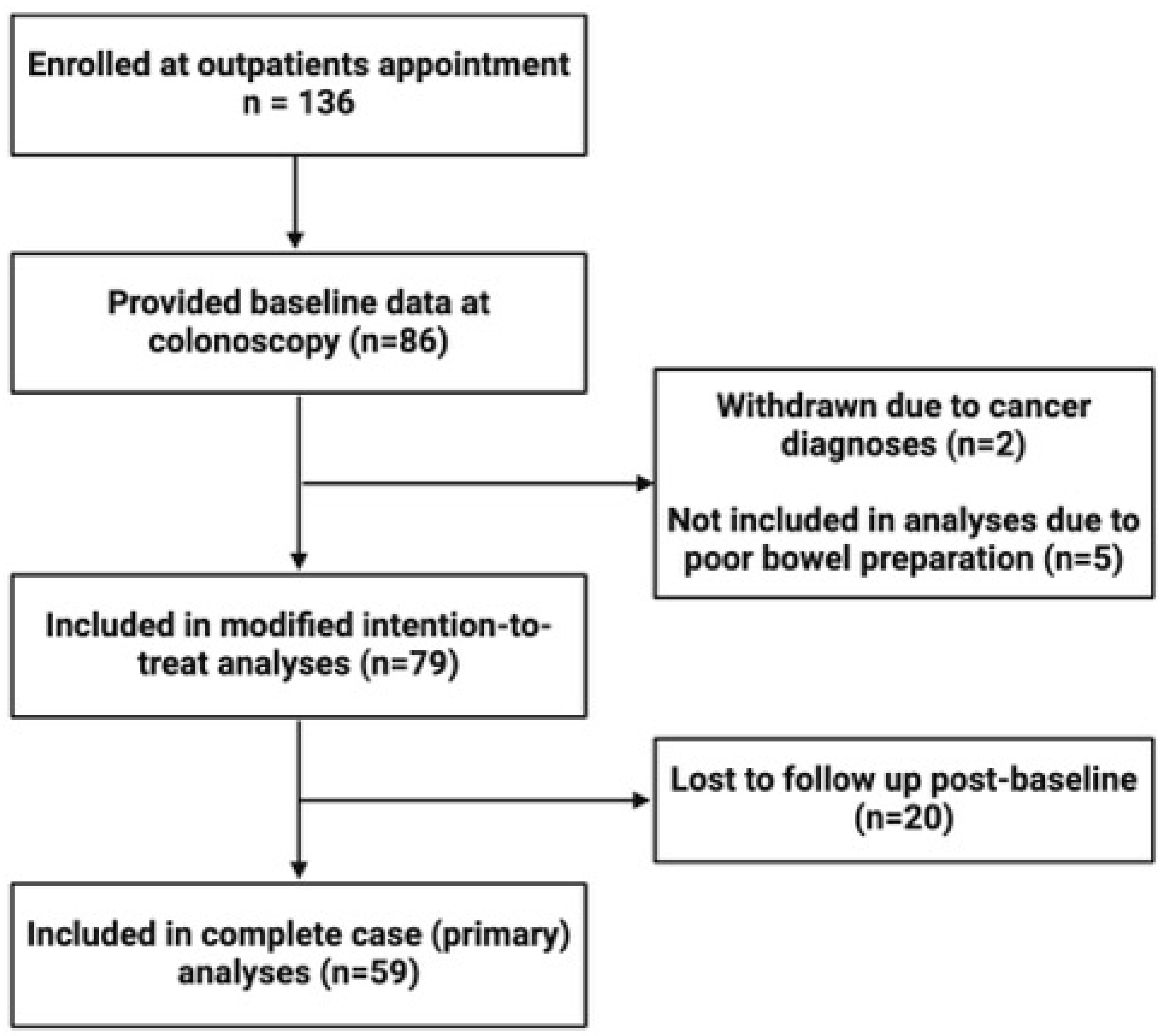
Our study enrolled a total of 136 participants who provided informed consent at the time of their outpatient appointment. Eighty-six participants were successfully contacted, consented, and provided baseline data. Of these, five participants were excluded from analyses due to inadequate bowel preparation (rated as ‘poor’ by their endoscopist) and two participants were excluded because of a cancer diagnosis post-procedure. A further 20 participants were lost to follow-up between baseline and their one-month follow up. Therefore, 59 participants were included in complete case analyses, and 79 participants were included in modified intention-to-treat sensitivity analyses (
Figure 1). In addition, two participants did not have intra-colonoscopy luminal samples collected, and three intra-colonoscopy samples were of too low biomass to yield sufficient DNA, leaving 56 luminal samples for analysis.
3.2. Participant Characteristics
Characteristics of this sample are presented in
Table 1. Participants had a mean age of 58.5 years, almost equal distributions of sex (54% female) and socioeconomic advantage (56% IRSAD >5), and an average BMI of 29.7. Most of the study participants were taking at least one medication (92%), had poor average diet quality (46.8/100 SDQ points), with 12% current smokers, 22% meeting diagnostic criteria for IBS, and 22% self-reporting a lifetime history of depression. No participants self-reported antibiotic use within one month prior to colonoscopy. Procedural characteristics are presented in
Table S1. Faecal samples were collected (on average) seven days pre-procedure, and 33 days post-procedure. The bowel preparation adequacy of most participants was rated as ‘good’ (but not excellent) by the endoscopists (63%), and polyps were the most common finding during colonoscopy (63%).
3.3. Change in Mental Health Symptoms
There were decreases in average HADS depressive symptom scores (adjusted β=−0.64; 95%CI: −1.18, −0.11) and PHQ-9 depression severity scores (−0.68; −1.33, −0.02) one-month post-procedure versus baseline (
Table 2). There were also increases in the average total (0.02; 0.01, 0.04), psychosocial (0.04; 0.02, 0.06), and physical (0.02; 0.00, 0.04) quality of life scores, but no statistical evidence of changed average HADS anxiety symptom scores or perceived stress scores (
Table 2). Results of modified intention-to-treat sensitivity analyses (n=79) were similar; however, after imputation of missing data there was a statistically significant reduction in perceived stress scores (−1.86; −3.21, −0.51) (
Table 2).
3.4. Changes in Gut Microbiota Composition
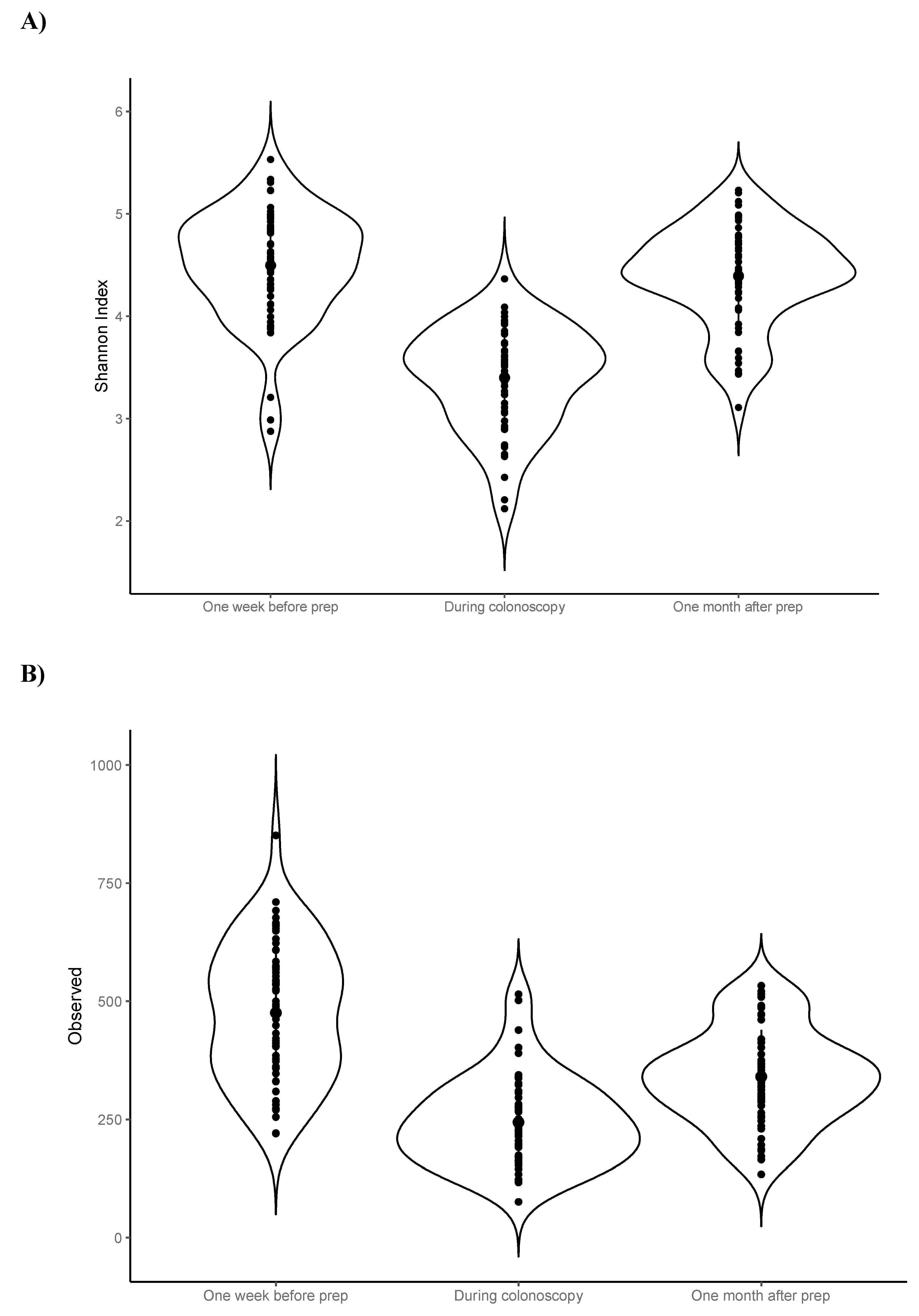
Visual inspection of the data suggested that luminal samples (n=56) collected from colonic aspirates during colonoscopy – as expected – had lower average alpha diversity (i.e., observed ASVs and Shannon index) compared to faecal samples collected pre- and post-procedure (
Figure 2).
As luminal samples are not directly comparable to faecal samples, statistical testing of alpha diversity metrics was performed using only the faecal samples collected one week pre- and one-month post-procedure. The average number of observed ASVs was decreased one-month post-procedure compared to baseline (−135; −168, −102), however there was no statistically significant change in the average Shannon index (
Table 2). There was a modest change in overall community composition (i.e., beta diversity) one-month post-procedure (p<0.001), however time point only explained 0.4% of the variance after adjusting for baseline covariates (
Table S2).
There were 22 differentially abundant genera one-month post-procedure compared to baseline after adjusting for baseline covariates and multiple comparisons (q<0.05) (
Table S3). Of these, only an unidentified Lachnospiraceae genus was decreased one-month post-procedure compared to baseline, whereas the other 21 genera were increased post-procedure, and included the following annotated/identified taxa: Cutibacterium, Prevotella 9, Megamonas, Ruminococcaceae UCG-009, Oxalobacter, Lactonifactor, Ruminococcaceae CAG352, Lachnospiraceae GCA900066575, Eubacterium, Gordonibacter, Solobacterium, Rikenellaceae RC9 group, Lachnospiraceae UCG-010, and Megasphaera (
Table S3).
There were no statistically significant changes in the average alpha diversity (i.e., Shannon index) of functional MetaCyc pathways (−0.01; −0.04, 0.02) (
Table 2). Differential abundance analyses identified increases in ten MetaCyc pathways one-month post-procedure compared to baseline after adjustment for baseline covariates and multiple comparisons (q<0.05), which included lipopolysaccharide lipid A biosynthesis and denitrification as the only annotated pathways (
Table S4).
3.5. Exploratory Analyses
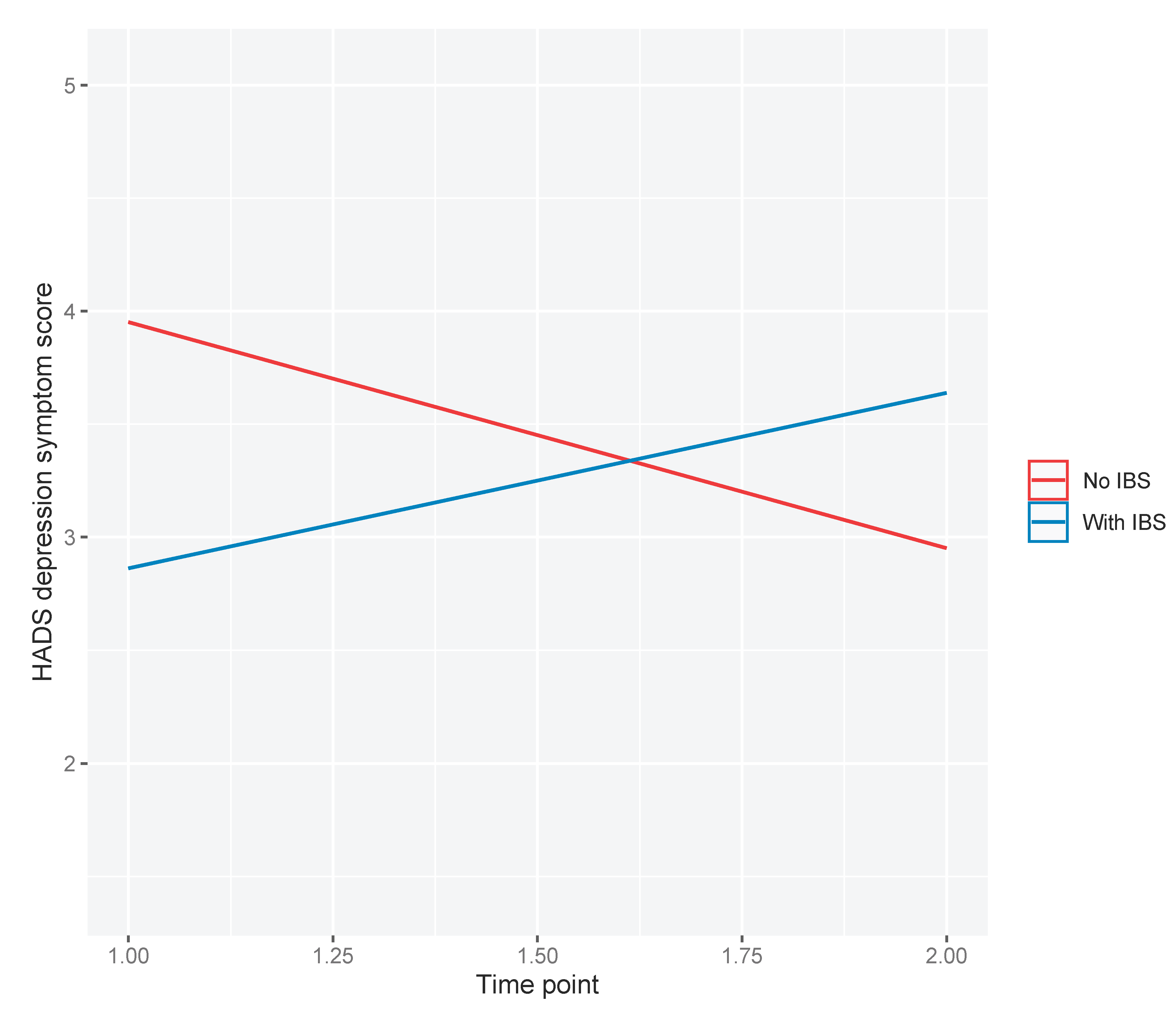
As 22% of the cohort met diagnostic criteria for IBS, exploratory analyses were conducted to examine whether IBS at baseline moderated the observed decrease in average depressive symptom scores post-procedure. We observed a statistically significant two-way interaction between IBS and time point (β=1.78; 95%CI: 0.292, 3.26; p=0.019). Those without IBS appeared to have reductions in depression symptom scores, whereas those with IBS appeared to have increased depression symptom scores, one-month post-procedure compared to baseline (
Figure 3).
We also examined whether colonoscopy outcome moderated the observed decrease in average depressive symptom scores post-procedure. Due to our small sample size and some participants receiving more than one colonoscopy outcome (i.e., both polyps and diverticular disease), we dichotomized participants into two groups – those with (80%) and without abnormalities identified. We did not observe evidence of a statistically significant two-way interaction between colonoscopy outcome and time point (β=−0.69; 95%CI: −2.11, 0.72; p=0.338).
3.6. Post-Hoc Analyses
We also explored whether there were associations between the changes in gut microbiota composition and changes in average HADS depressive symptoms. There were 8 ASVs whose change was associated with changes in depressive symptoms after adjusting for covariates (p<0.05) (
Table S5). Of those identified at the genus level, only the inverse association between
Ruminococcaceae UCG-009 and the change in average HADS depression scores remained significant after adjusting for multiple comparisons (q=0.028) (
Table S5).
4. Discussion
This is the first study, to our knowledge, to investigate the potential impact of colonoscopy and bowel preparation on depressive symptoms, and to associate changes in depressive symptoms with changes to gut microbiota composition. We observed a decrease in average depressive symptom scores, and increases in average quality of life scores, one-month post-procedure compared to baseline, but there was little evidence to support decreased average perceived stress or anxiety symptom scores. The changes in average depression symptom scores appeared to be moderated by IBS, whereby those with IBS experienced worsening of their depressive symptoms, and those without IBS experienced improvements. Compared to baseline, we also observed changes in gut microbiota composition, including reduced alpha-diversity, modest changes in beta-diversity, and genera and functional pathways that were differentially abundant one-month post-procedure. Finally, we observed that increases in the genus Ruminococcaceae UCG-009 were associated with decreases (i.e., improvements) in depressive symptom scores.
The finding that average depressive symptom scores decreased one-month post-procedure is novel and has not been previously explored. This finding is somewhat concordant with studies of other microbiome-modulating interventions that have reported improvements in depressive symptoms, such as probiotics[
9], faecal microbiota transplant[
44]
,[
45], and diet[
6]
,[
8]. However, it is plausible that our participants experienced improvements in their depressive symptoms due to no major adverse findings (i.e., cancer) during colonoscopy, although we did not observe any changes in stress and anxiety measures, which might be expected if this were the explanation. Moreover, levels of depressive symptoms at baseline were low, and the average reduction observed, although statistically significant, was very small.
We observed a two-way interaction between time point and IBS status that suggested that those with IBS at baseline had worsening of their depressive symptoms post-procedure whereas those without IBS appeared to experience improvements. Interpretation of this finding is limited by the small number of participants with IBS within the study, however it is possible that the increase in depression symptoms in those with IBS could be due to an aggravation of their symptoms post-procedure. Worsening of gastrointestinal symptoms occurs in ~20% of patients after colonoscopy and bowel prep[
46], and those with IBS are more likely to experience prolonged post-procedural abdominal pain[
47]. Worsening of depressive symptoms could also be due to a lack of findings to adequately explain their gastrointestinal symptoms. A previous study of IBS patients showed no improvement in reassurance or health-related quality of life in those that received a negative colonoscopy result[
48]. Additionally, a study observed that those with IBS were more likely to experience lower satisfaction, and higher burden and embarrassment, immediately after colonoscopy[
49], and that these perceptions worsened six weeks compared to immediately post-colonoscopy[
49]. Whether IBS is an at-risk group that require additional disease management after colonoscopy may be an important focus of further research. Our findings suggest that the gut microbiota may still be re-establishing one month after bowel preparation and colonoscopy, however the health implications are unclear.
Interestingly, many bacterial genera that were higher in abundance one-month post-procedure compared to baseline have potential functionality that may be advantageous for health. We observed an increase in the relative abundance of bacteria with the ability to metabolise dietary polyphenols into metabolites that confer health benefits, including
Gordonibacter[
50]
,[
51] and
Lactonifactor[
52]. A recent Mendelian Randomisation study identified low hippurate, which is produced by bacterial metabolism of polyphenols, as causally related to depression[
53]. There were also increases in bacteria with butyrate-producing capacity, including
Eubacterium,
Solobacterium, and
Megasphaera; health benefits of short-chain fatty acids such as butyrate have been reviewed extensively[
54]. Thus, it could be postulated that the flushing out of gut bacteria by bowel preparation and colonoscopy may ‘reset’ the gut microbiota, resulting in a composition better able to metabolise foods and harness benefits from their diet.
We also observed that as
Ruminococcaceae UCG009 increased, depressive symptoms decreased (i.e., improved). Our previous systematic review found that
Ruminococcaceae was consistently lower in mental disorders compared to healthy controls[
4]. More recently, a large association study found
Ruminococcaceae UCG002,
UCG003, and
UCG005 all to be negatively associated with depressive symptoms[
55]. The
Ruminococcaceae family is largely considered beneficial to health, particularly due to the many butyrate-producing genera within this family including
Ruminococcus,
Faecalibacterium,
Caproiciproducens,
Agathobaculum,
Butyricicoccus, and
Gemmiger. Future studies employing whole genome metagenomic sequencing with greater resolution at the species and strain level, or that specifically target changes in the abundance of
Ruminococcaceae taxa, may afford further insight into how these bacteria change after intervention, and mechanistic studies such as reverse translation preclinical models may assist to elucidate how these bacteria influence host health and behaviours, including depressive symptoms.
Our study has notable limitations. We used 16S rRNA gene sequencing, which is subject to variability in sequencing depth and has only genus-level resolution. Our study did not collect any additional biological samples such as blood or urine to investigate changes to bacterial metabolites. Our study had a small sample size, and colonoscopy indications and outcomes were heterogenous, which limited our statistical power and prevented subgroup investigations. Some of the changes observed may be associated with colonoscopy itself rather than bowel preparation. For example, the use of propofol, a sedative used for colonoscopy in the present study, has been previously found to have antidepressant potential[
56]. All participants in this study specifically used a sodium picosulfate-based bowel preparation product, and different results may be observed with alternative bowel preparation products. Those lost to follow-up had poorer mental health compared to those that completed the study, and the impact of their missing data, particularly for perceived stress, is unknown. Finally, we only collected comparable faecal samples at two time points, and greater sampling frequency within and beyond one month post-procedure may provide additional information regarding how the gut microbiota re-establishes.
Our study provides preliminary evidence of a potential impact of bowel preparation and colonoscopy on depressive symptoms that may relate to changes in the gut microbiota. Future research leveraging bowel preparation as a method of gut microbiota modulation may further our understanding of the microbiota-gut-brain axis. To better elucidate any potential mental health impact of bowel preparation itself, clinical trials of this intervention in a population experiencing heightened psychological distress and without colonoscopy are needed. Finally, clinical implications of the potential differential impact of bowel preparation and colonoscopy in those with and without IBS deserves further exploration.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Table S1. Bowel preparation adequacy and colonoscopy indications and findings. Supplementary Table S2. Unadjusted and adjusted models of the change in beta-diversity of faecal samples collected one week before and one month after bowel preparation and colonoscopy using the Aitchison distance metric. Supplementary Table S3. Adjusted analyses of the change in differential abundance of taxa at the genus level. Taxa were adjusted for age, sex, body mass index, diet quality, and irritable bowel syndrome at baseline, and for multiple comparisons using Benjamini-Hochberg correction (q<0.05). Supplementary Table S4. Adjusted analyses of the change in differential abundance of MetaCyc pathways. Analyses were further adjusted for age, sex, body mass index, diet quality, and irritable bowel syndrome at baseline, and for multiple comparisons using Benjamini-Hochberg correction (q<0.05). Supplementary Table S5. Associations between the change in depressive symptom scores and change in the relative abundance of taxa at the genus level one month after bowel preparation and colonoscopy compared to baseline. Linear regression analyses were adjusted for age, sex, body mass index, diet quality, irritable bowel syndrome at baseline, and for multiple comparisons using Benjamini-Hochberg correction (q<0.05).
Author Contributions
Amelia McGuinness: Conceptualization (Supporting); Data curation (Lead); Formal analysis (Lead); Investigation (Equal); Methodology (Equal); Project administration (Lead); Writing – original draft (Lead); Writing – review & editing (Lead). Martin O’Hely: Data curation (Supporting); Formal analysis (Supporting); Methodology (Supporting); Writing – review & editing (Equal). Douglas Stupart: Conceptualization (Equal); Funding acquisition (Supporting); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Equal). David Watters: Conceptualization (Equal); Funding acquisition (Supporting); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Samantha Dawson: Formal analysis (Supporting); Methodology (Supporting); Writing – review & editing (Equal). Chris Hair: Conceptualization (Equal); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Michael Berk: Conceptualization (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Mohammadreza Mohebbi: Formal analysis (Supporting); Methodology (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Amy Loughman: Formal analysis (Supporting); Methodology (Supporting); Supervision (Supporting); Writing – review & editing (Equal). Glenn Guest: Conceptualization (Equal); Funding acquisition (Supporting); Investigation (Equal); Methodology (Equal); Project administration (Supporting); Supervision (Supporting); Writing – review & editing (Supporting). Felice Jacka: Conceptualization (Lead); Data curation (Supporting); Formal analysis (Supporting); Funding acquisition (Lead); Investigation (Supporting); Methodology (Equal); Project administration (Supporting); Supervision (Lead); Writing – review & editing (Equal).
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Financial Support
AJM is funded through the NHMRC supported Centre for Research Excellence for the Development of Innovative Therapies (CREDIT CRE). MB is supported by a NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. AL is supported by a Deakin Dean’s Postdoctoral Research Fellowship. AL has received grant, research or travel support from Deakin University, The University of Melbourne, RMIT University, National Health and Medical Research Council, Australian Academy of Science, The Jack Brockhoff Foundation, Epilepsy Foundation of Australia, American Epilepsy Society and has received speakers’ honoraria from European Space Agency, Swisse Australia – all unrelated to this work. MM has received Grant/research support from the NHMRC, Deakin University School of Medicine, Deakin Biostatistics Unit, Institute for Mental and Physical Health and Clinical Translation, Stroke Foundation and Medibank Health Research Fund. FNJ has received: competitive Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, The University of Melbourne; industry support for research from Meat and Livestock Australia, Woolworths Limited, the A2 Milk Company, Be Fit Foods; philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, the Waterloo Foundation and; travel support and speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Network Nutrition, Angelini Farmaceutica, Eli Lilly and Metagenics. She is on the Scientific Advisory Board of the Dauten Family Centre for Bipolar Treatment Innovation and Zoe Limited. FNJ has written two books for commercial publication. She is currently supported by an NHMRC Investigator Grant L1 (#1194982). The Food & Mood Centre has received Grant/Research support from the a2 Milk Company, Be Fit Foods, Meat and Livestock Australia, and Woolworths Limited, and philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, and the Waterloo Foundation.
Conflicts of interest
MOH has a financial interest in Prevatex Pty Ltd, a company developing probiotic-based biotherapeutics. AL is a named inventor on a patent relating to Prevotella. FNJ has written two books for commercial publication. The remaining authors declare no conflicts of interest.
References
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber HLt Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol 2021, 19, 241–55. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C.S.M. , et al. The Microbiota-Gut-Brain Axis. Physiol Rev 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Molecular Psychiatry. [CrossRef] [PubMed]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry 2021, 78, 1343–54. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. The effect of a Mediterranean diet on the symptoms of depression in young males (the "AMMEND: A Mediterranean Diet in MEN with Depression" study): a randomized controlled trial. Am J Clin Nutr 2022, 116, 572–80. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O'Neil, A.; Opie, R. , et al. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Med 2017, 15, 23. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S. , et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom Med 2019, 81, 265–80. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Faghfouri, A.H. , et al. Probiotics as an effective therapeutic approach in alleviating depression symptoms: an umbrella meta-analysis. Crit Rev Food Sci Nutr 2022, 1–9. [Google Scholar]
- Sharma, P.; Burke, C.A.; Johnson, D.A.; Cash, B.D. The importance of colonoscopy bowel preparation for the detection of colorectal lesions and colorectal cancer prevention. Endosc Int Open 2020, 8, E673–E83. [Google Scholar] [CrossRef]
- Sulz, M.C.; Kroger, A.; Prakash, M.; Manser, C.N.; Heinrich, H.; Misselwitz, B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One 2016, 11, e0154149. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Nishijima, S.; Miyoshi-Akiyama, T. , et al. Population-level Metagenomics Uncovers Distinct Effects of Multiple Medications on the Human Gut Microbiome. Gastroenterology 2022, 163, 1038–52. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; De Grandi, R.; Casini, V.; Pace, F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur J Gastroenterol Hepatol 2016, 28, 532–7. [Google Scholar] [CrossRef] [PubMed]
- Gorkiewicz, G.; Thallinger, G.G.; Trajanoski, S. , et al. Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS One 2013, 8, e55817. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Tian, H.; Yang, R. , et al. Oral Probiotics Alleviate Intestinal Dysbacteriosis for People Receiving Bowel Preparation. Front Med (Lausanne) 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Tohya, M.; Fukuda, S. , et al. Effects of bowel preparation on the human gut microbiome and metabolome. Sci Rep 2019, 9, 4042. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Chen, C.C.; Chen, C.C.; et al. Gut microbiome changes in overweight male adults following bowel preparation. BMC Genomics 2018, 19 Suppl 10, 904. [Google Scholar] [CrossRef]
- Powles, S.T.R.; Gallagher, K.I.; Chong, L.W.L. , et al. Effects of bowel preparation on intestinal bacterial associated urine and faecal metabolites and the associated faecal microbiome. BMC Gastroenterol 2022, 22, 240. [Google Scholar] [CrossRef]
- Li, M.; Qian, W.; Yu, L. , et al. Multi-Time-Point Fecal Sampling in Human and Mouse Reveals the Formation of New Homeostasis in Gut Microbiota after Bowel Cleansing. Microorganisms 2022, 10. [Google Scholar] [CrossRef]
- Batista, L.; Robles, V.; Manichanh, C. , et al. Colonic bacterial diversity and dysbiosis in active microscopic colitis as compared to chronic diarrhoea and healthy controls: effect of polyethylene glycol after bowel lavage for colonoscopy. BMC Gastroenterol 2022, 22, 320. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.J.; Kwon, H.J. , et al. Effect of gut microbiome on minor complications after a colonoscopy. Intest Res 2021, 19, 341–8. [Google Scholar] [CrossRef] [PubMed]
- Nalluri-Butz, H.; Bobel, M.C.; Nugent, J. , et al. A pilot study demonstrating the impact of surgical bowel preparation on intestinal microbiota composition following colon and rectal surgery. Sci Rep 2022, 12, 10559. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.A.; Meester, R.G.; Zauber, A.G. , et al. Colorectal cancer screening: Estimated future colonoscopy need and current volume and capacity. Cancer 2016, 122, 2479–86. [Google Scholar] [CrossRef] [PubMed]
- Shine, R.; Bui, A.; Burgess, A. Quality indicators in colonoscopy: an evolving paradigm. ANZ J Surg 2020, 90, 215–21. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M. , et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014, 12, 1495–9. [Google Scholar] [CrossRef] [PubMed]
- Mirzayi, C.; Renson, A.; Genomic Standards, C. , et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med 2021, 27, 1885–92. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.J.; Calderwood, A.H.; Doros, G.; Fix, O.K.; Jacobson, B.C. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009, 69 Pt 2, 620–625. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983, 67, 361–70. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001, 16, 606–13. [Google Scholar] [CrossRef]
- Richardson, J.; Iezzi, A.; Khan, M.A.; Maxwell, A. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient 2014, 7, 85–96. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J Health Soc Behav 1983, 24, 385–96. [Google Scholar] [CrossRef] [PubMed]
- Statistics ABo. 2033.0.55.001 - Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016; Internet: Canberra, Australia, 2018. [Google Scholar]
- Parletta, N.; Zarnowiecki, D.; Cho, J. , et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutritional neuroscience 2019, 22, 474–87. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.L.; Mohebbi, M.; Craig, J.M. , et al. Targeting the perinatal diet to modulate the gut microbiota increases dietary variety and prebiotic and probiotic food intakes: results from a randomised controlled trial. Public Health Nutr 2021, 24, 1129–41. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R. Rome III: The functional gastrointestinal disorders, third edition, 2006. World J Gastroenterol 2008, 14, 2124–5. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R. , et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019, 37, 1091. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13, 581–3. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y. , et al. q2-longitudinal: Longitudinal and Paired-Sample Analyses of Microbiome Data. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: from raw reads to community analyses. F1000Res 2016, 5, 1492. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R. , et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 2020, 38, 685–8. [Google Scholar] [CrossRef]
- Puhan, M.A.; Frey, M.; Buchi, S.; Schunemann, H.J. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008, 6, 46. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K. , et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–59.e8. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, 2022. [Google Scholar]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Green, J.E.; Berk, M.; Mohebbi, M. , et al. Feasibility, Acceptability, and Safety of Faecal Microbiota Transplantation in the Treatment of Major Depressive Disorder: A Pilot Randomized Controlled Trial. Can J Psychiatry 2023, 68, 315–26. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.W.; Riffle, S.; Shapiro, J.A. , et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc 2007, 65, 648–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Wang, H.P.; Chiu, H.M. , et al. Factors determining post-colonoscopy abdominal pain: prospective study of screening colonoscopy in 1000 subjects. J Gastroenterol Hepatol 2006, 21, 1575–80. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, B.M.; Gralnek, I.M.; Bolus, R. , et al. Is a negative colonoscopy associated with reassurance or improved health-related quality of life in irritable bowel syndrome? Gastrointest Endosc 2005, 62, 892–9. [Google Scholar] [CrossRef] [PubMed]
- Denters, M.J.; Schreuder, M.; Depla, A.C. , et al. Patients' perception of colonoscopy: patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol 2013, 25, 964–72. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Beltran, D.; Frutos, M.D.; Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens. Food Funct 2020, 11, 7012–22. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Antony, P.J.; Ceasar, S.A. , et al. Health functions and related molecular mechanisms of ellagitannin-derived urolithins. Crit Rev Food Sci Nutr 2022, 1–31. [Google Scholar]
- Correa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front Nutr 2019, 6, 188. [Google Scholar] [CrossRef]
- van der Spek, A.; Stewart, I.D.; Kuhnel, B. , et al. Circulating metabolites modulated by diet are associated with depression. Mol Psychiatry 2023, 28, 3874–87. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int 2016, 99, 110–32. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G. , et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun 2022, 13, 7128. [Google Scholar] [CrossRef]
- Mickey, B.J.; White, A.T.; Arp, A.M. , et al. Propofol for Treatment-Resistant Depression: A Pilot Study. Int J Neuropsychopharmacol 2018, 21, 1079–89. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).