INTRODUCTION
Background: New drugs are obtained from medicinal plants. it has been estimated that medicinal plants group comprises of more than 250,000 species of flowering plants, Khare et.al. (1997), Martinez, and Valencia, (2003). M. oleifera was introduced to Nigeria two decades ago, while A. hispidum is a native, there is ongoing research to ascertain their bioactive contents and the science behind their effects on certain african diseases. The knowledge of plant healing is made possible as a result of the study of medicinal plants, thereby preventing animals and humans from certain diseases, Duraipandiyan and Ignacimuthu, (2009).Although, the value of herbs has increased over time as a result of its aromatic, medicinal and flavoring potentials, but for sometimes, the modern age synthetic products are valued more than them.
Acanthospermum hispidum, (Bristly starbur, goat's head, hispid starburr, starbur) a member of Asteraceae family, an annual plant, which is originally native to South and Central America, Khare et.al. (1997), Martinez and Valencia, (2003). This plant was recognized as both a medical plant and a weed in cotton plantations in Brazil. It has grown wild in numerous scattered locations in Africa, North America, etc. The plant can flourish in a variety of soil and climatic situations. It can grow in both light and heavy textured soil but the most suitable is light textured soil. It is found usually amidst upland crops, roadsides, pastures, waste areas, around corals, and along railroads and cattle trails, Evani ., Araújo , Karina , Randau ., José ., Sena ., Rejane., Mendonça, Haroudo, (2008).
Moringa oleifera is a drought-resistant, fast-growing tree that belongs to Moringaceae family, native to subtropical and tropical regions of South Asia. It is commonly referred to as moringa, horseradish tree (from the root taste, that look like horseradish), drumstick tree (from the long, slender, triangular seed-pods), Khare et.al.(1997), Martinez and Valencia, (2003), Duraipandiyan and Ignacimuthu (2009). It is cultivated extensively due to its leaves and young seed pods, used as vegetables and for traditional herbal-medicine . The tree is small in size, height of about 5 to 10 m. As a result of its numerous utilities, it is planted all over the world. All its part is useful for specific nutritional or medicinal purposes. Along with being an excellent source of vitamins, oils, protein, fatty acids, essential minerals, and various phenolics. Anwar, Nadeem, Rashid, Kazi, Nadeem (2005). It has been established in the traditional medicinal system as a therapeutic remedy for several diseases due to its various pharmaceutical effects, Anwar and Bhanger, (2003).
M. oleifera has been utilized for the past 5000 years by Indians as a steady constituent of conventional eatables, Anwar, Nadeem, Rashid, Kazi, Nadeem (2005). In humid tropic or hot dry land, Moringa trees can thrive with an average height between 5 and 10 meters. It can endure hostile climates conditions as well as destitute soil with little drought effect It has a white corky, soft trunk and branches producing a gummy bark, Anwar, Nadeem, Rashid, Kazi, Nadeem (2005). On each trip pinnately compound leaves produce various small leaf legs. It has white flowers with three wings and seeds dispersed by wind. The tender leaves, pods, and flowers are edible vegetables, the leaf is extremely recommended for expectant mothers because of its high iron content.
Aim: The purpose of this study is to evaluate the bioactive compounds of Acanthospermum hispidum and Moringa oleifera, for antioxidant, anticancer, antifungal and antibacterial activities.
METHODS
Plant materials
Fresh leaves of Acanthospermum hispidum and Moringa oleifera were collected between August and September, 2021, from SPED International Secondary School, Akinmoorin and Awe town in Oyo State, Nigeria, where they were growing as ornamental plants, mandatory permission to collect these plants not applicable. They were kept and identified at the Herbarium of Biology Department, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria, by Professor of Plant Taxonomy, Professor A.T.J. Ogunkunle; a voucher of both plants were kept, the voucher number were Acanthospermum hispidum (LHO653) and Moringa oleifera (LHO654). The sample leaves were washed with tap water to remove adhering dust particles, rinsed with distilled water and air-dried in the laboratory at ambient temperature (30 ± 2°C) for five days. Thereafter, these leaves were cut into small pieces to increase their surface area. These dried leaves were ground separately in a mechanical grinder. The powdered leave materials were then passed through a sieve, weigh and stored in an airtight container until the time of use, voucher specimens were kept in this herbarium, Lee, Chou, Murugan , Shieh, Chen, (2007).
Figure 1.
Acanthospermum hispidum growing in the wild.
Figure 1.
Acanthospermum hispidum growing in the wild.
Figure 2.
Dried Leaves of Acanthospermum hispidum.
Figure 2.
Dried Leaves of Acanthospermum hispidum.
Figure 3.
Moringa oleifera cultivated along the school boundary.
Figure 3.
Moringa oleifera cultivated along the school boundary.
Figure 4.
Dried Leaves of Moringa oleifera.
Figure 4.
Dried Leaves of Moringa oleifera.
Ethics approval was not required, however, since we are working with plant materials, a voucher of the plant was identified and submitted to the Herbarium of Biology Department, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria, the voucher numbers were Acanthospermum hispidum (LHO653) and Moringa oleifera (LHO654).
Phytochemical Screening
Phytochemicals were identified through Qualitative Analysis and GC-MS. The results were compared. The Mass Spectrometer (MS) start time was 3mins and end time was about 35minutes and the solvent cut time was about 3minutes. While the identification of analogs and isomers was based on the molecular structure and calculated fragmentation patterns, the identification of standard compounds was based on mass spectral matching with the standard compounds in the National Institute of Standards and Technology (NIST) library. By comparing the mass spectra obtained with the reference spectra of compounds in the NIST mass spectra database, the primary chemical ingredients were determined. Each compound's peak area was used to calculate the quantitative GC-MS analysis, Martinez. and Valencia, (2003).
Detection of alkaloids:
With little changes, the general phytochemical screening procedures reported by Sofowora, (1986), Sofowora, (1993) and Trease and Evans, (1989), were used to identify the active components of these leaves in the extracts.
Extracts were soaked seperately in dilute HCl and filtered.
Mayer’s Test: Mayer's reagent, which is comprised of potassium mercuric iodide, was applied to the filtrates. Alkaloids can be seen in precipitates that are yellow in hue.
Wagner’s Test: Filtrates that have undergone Wagner's reagent with Iodine in Potassium Iodide treatment. Alkaloids can be seen in a brown or reddish precipitate.
Additionally, 0.2 g of the extract was heated for two minutes in 1% aqueous hydrochloric acid. After filtering the mixtures, Dragendorff's reagent was applied in little amounts. With the reagent, turbidity and leaves both took on a reddish-brown hue, which denotes the presence of alkaloids.
Detection of flavonoids:
Each extract was diluted in a small amount in 10% sodium hydroxide (NaOH) and hydrochloric acid (HCl). When HCl was added, the yellow solution became colorless, indicating the presence of flavonoids in both leaves.
NaOH test: Flavonoids are found in a little amount of extract that has been treated with aqueous NaOH and HCl, a yellow orange precipitate.
H2SO4 test: Flavonoids are detected in a portion of the extract that has been exposed to concentrated H2SO4 displaying an orange color.
Detection of anthraquinones
10 ml of benzene and 5g of each extract were mixed together. The solution was filtered, and the filtrate was then mixed with 5 ml of 10% NH4OH solution. When the ammoniacal phase is pink, red, or violet, anthraquinones are present in AH but not in MO. Rai, Obayemi, (1973).
Detecting steroids and terpenoids:
Detecting saponins:
Also, 0.1 g of the powdered extract were dissolved in 10 ml of distilled water and decanted while still hot. The filtrate was subjected to the tests listed below:
Frothing test: Shaking the mixture rapidly while adding 1 milliliter of filtrate to 4 milliliters of distilled water allowed for the detection of persistent foam that persisted for 15 minutes.
Emulsion test: This was accomplished by forcefully shaking the foaming solution while adding 2 drops of olive oil. Emulsion formation implies a favorable result for saponins.
Detectection of tannins:
Ferric chloride test: 20ml of water and 0.5g of dried powdered material were boiled in a test tube before being filtered. After adding a few drops of 0.1% FeCl3 observation of a brownish green-black or a blue-black coloration detected- tannins
Lead acetate test: 0.01g lead acetate was added after the mixture of 2ml of plant extract and 2ml of distilled water was thoroughly shaken. The emergence of white turbid precipitate is a sign of tannins.
Detection of glycosides:
Fehling’s test: Fehling's solutions A and B were diluted with distilled water and boiled for one minute to produce a translucent blue solution. This was then combined with 1 ml of Fehling's solution and eight drops of plant extract before boiling in a water bath for five minutes. A brick-red precipitate is a sign that glycosides are present.
Another technique known as Lieberman's test: The extracts were liquefied in 2 ml of acetic anhydride and cooled in ice in little amounts. Concentrated sulfuric acid was carefully added. A steroidal nucleus was identified in both leaves by a color shift from violet to blue to green.
Antimicrobial activity
The disc diffusion method was used for antibacterial and antifungal assay according to the method of Trease, and Evans, (1989). Nutrient Agar was prepared for bacterial isolates while Potato Dextrose Agar was prepared for the Fungi isolates following the manufacturer’s specifications. The selections include Salmonella typhi, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa for bacterial species, however Candida albicans, Fusarium oxysporum, Penicillium sclerotigenum and Aspergillus niger for fungi species. The Institute of Microbial Technology, Lautech Teaching Hospital, Osogbo, Nigeria, provided the bacteria cultures. All of the fungus cultures were collected from the Obafemi Awolowo Teaching Hospital's Department of Microbiology in Ile-Ife, Osun State, Nigeria. Cells were grown in Mueller-Hinton broth (Himedia) for 24 hours at body temperature to create the bacterial inoculum. On Potato dextrose agar slants, filamentous fungus were grown for 10 days at 28°C, and the spores were then harvested using sterile double-distilled water and homogenized. After the test cultures were swabbed on the media's surface and given 10 minutes to dry, a specific quantity of extracts was added to each disc. The loaded discs were then laid on the medium's surface and kept at room temperature for 30 minutes to allow compound diffusion. Equally, sterile water was used to prepare the negative control. The plates were incubated for bacteria for 24 hours at body temperature and for fungi for 48 to 72 hours at 280C. The experiment was repeated three times, with millimeter measurements of the zones of microbial inhibition.
Minimum Inhibitory Concentration
The purified ethanolic extracts were identified using a spectroscopic methods, mass spectroscopy. Studies on the isolated compound's minimum inhibitory concentration were carried out in accordance with the industry-reference standards for bacteria and filamentous fungi. Each medium in 10-well plates received the necessary amounts of the chemical (1.0, 2.0, 4.0, 6.0, 8.0, and 10.0 mg/mL). From each well, an inoculum was introduced. Ketoconazole, an antifungal drug, was used in the tests as a positive control for fungi, and streptomycin, a positive control for bacteria. The plates were incubated for bacteria for 24 hours at 370C and for fungi for 48 to 72 hours at 280C. The lowest extract concentration at which there is no discernible fungal growth after an incubation period is known as the MIC for fungi. The lowest concentration of the substance limiting the visible growth of the test cultures on the agar plate was identified as the minimum inhibitory concentration for bacteria.
Table 1.
Minimum Inhibitory Concentration for Bacteria (Mg/ML).
Table 1.
Minimum Inhibitory Concentration for Bacteria (Mg/ML).
| Microorganism |
Moringa oleifera |
Acanthospermum hispidum |
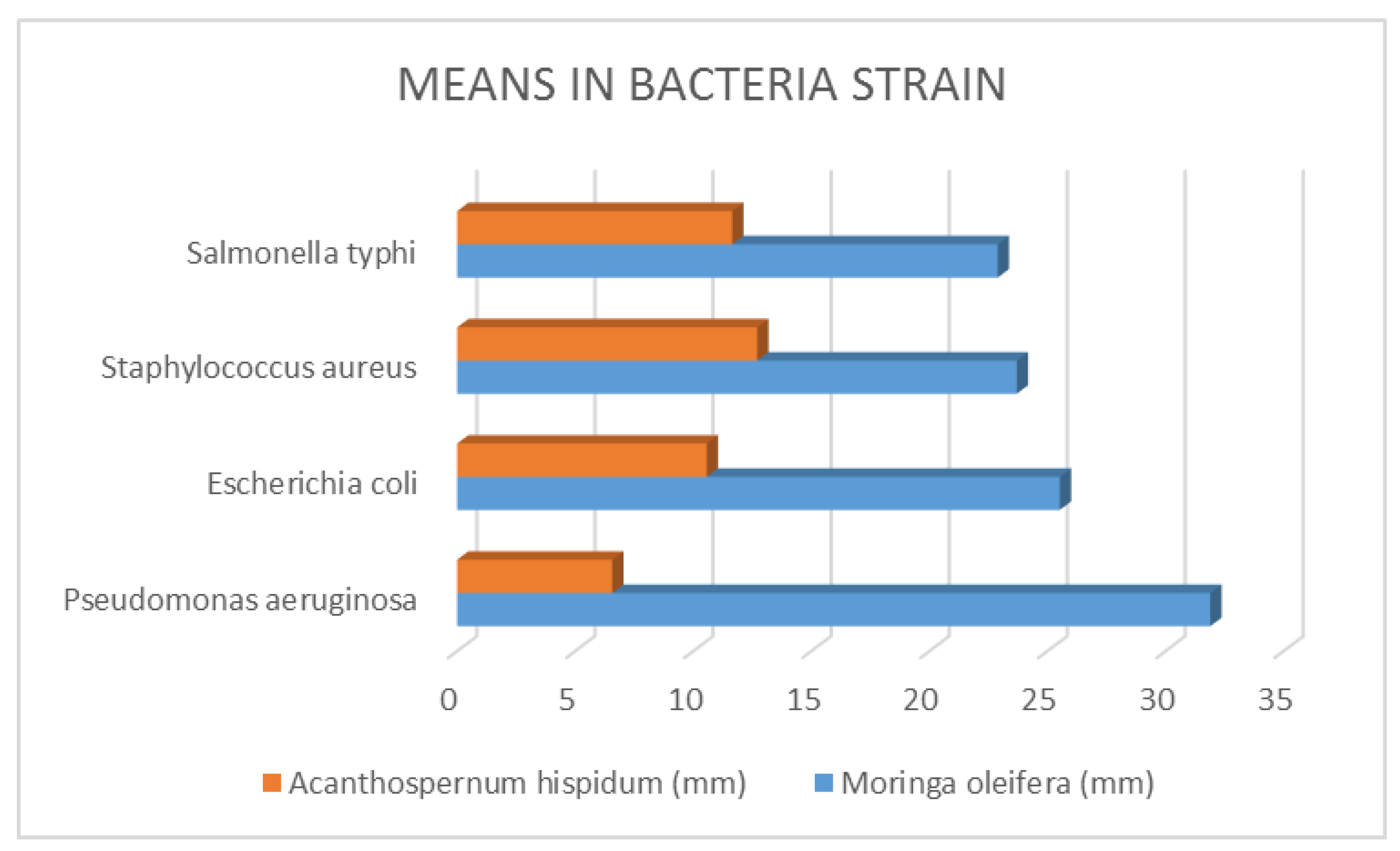
| Pseudomonas aeruginosa |
31±0.90 |
6±0.57 |
| Escherichia coli |
25±0.53 |
10±0.57 |
| Staphylococcus aureus |
23±0.71 |
12±0.71 |
| Salmonella typhi |
22±0.90 |
11±0.55 |
Figure 5.
Means in Bacteria Strain.
Figure 5.
Means in Bacteria Strain.
The minimum inhibitory concentration for bacteria was defined as the lowest concentration of the compound inhibiting the test cultures' ability to grow visibly on an agar plate, and the minimum inhibitory concentration for fungi was defined as the lowest extract concentration that resulted in no discernible fungal growth after an incubation period. From the above table, it was discovered that M. oleifera required the highest concentration compared with A. hispidum, this means that M. Oleifera have lower bioactive compounds than A. hispidum. Nevertheless, in areas where A. hispidum is not available, M. Oleifera could be used. Chuang, Lee, Chou, Murugan, Shieh, Chen, (2007).
Table 2.
Minimum Inhibitory Concentration for Fungi (Mg/ML).
Table 2.
Minimum Inhibitory Concentration for Fungi (Mg/ML).
| Microorganism |
Moringa oleifera |
Acanthospermum hispidum |
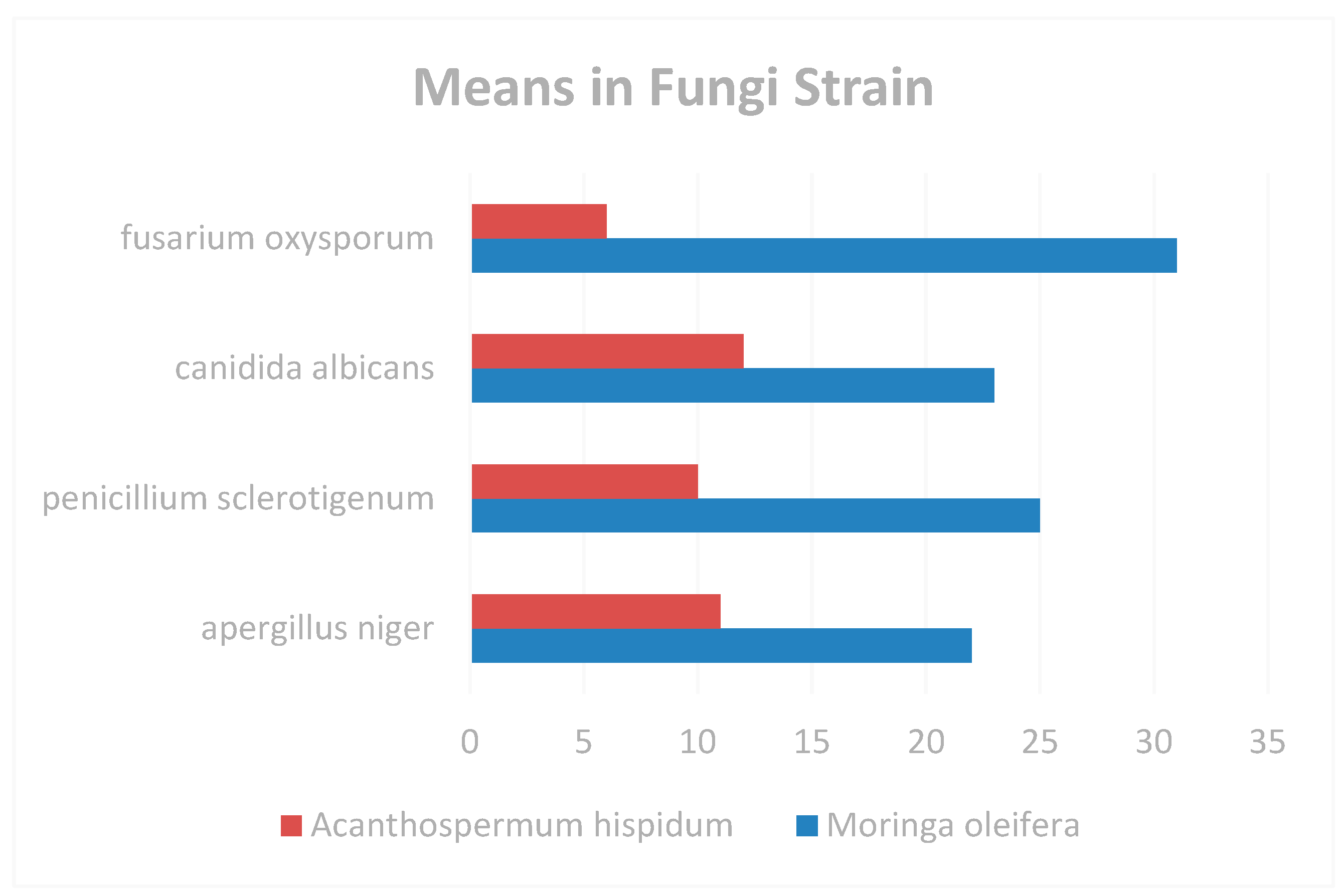
| Aspergillus niger |
26±0.61 |
12±0.55 |
| Candida albicans |
22±0.51 |
14±0.71 |
| Fusarium oxysporum |
16±0.71 |
12±0.71 |
| Penicillium sclerotigenum |
20±0.81 |
10±0.65 |
Figure 6.
Means in Fungi Strain.
Figure 6.
Means in Fungi Strain.
ANTIOXIDANT ASSAY DPPH RADICAL SCAVENGING ASSAY
The mixture of 35.23 mL of ethanolic solutions of M. oleifera and A. hispidum extracts (maximum dissolved content) and 2mL of freshly made 105M DPPH radical solution was incubated at 37°C for 30 minutes while the absorbance reduction of the mixture was observed at 520nm (As). Since DPPH radicals have a maximum absorption at 520 nm, the color of the sample changed from purple to pale yellow during this reduction process by the antioxidant. Every day at the same wavelength (Ab), blank samples made of 32.00 µL of ethanol extracts prepared in the aforementioned DPPH radical solution were monitored. Sterile water served as the positive control. The experiment was conducted three times. The comparatively stable DPPH radical was frequently employed to evaluate a compound's capacity as a hydrogen donor or free radical scavenger. Compounds with radical scavenger capacity can reduce DPPH radical using a donor hydrogen atom to DPPH free radical, based on an electron transfer or hydrogen atom to DPPH radical, antioxidant chemical and DPPH interact to form 1-1, diphenyl-2-picrylhydrazyl. The method was used to measure the DPPH radical scavenging activity of various plant leaves, Atawodi., Idakwo (
et.al.2010). The experiment was carried out three times, and the mean and standard deviation of the results were recorded.
CYTOTOXIC PROPERTIES OF ACANTHOSPERMUM HISPIDUM AND MORINGA OLEIFERA SELECTIVITY STUDIES
With certain adjustments, the cytotoxicity was assessed using the approach of Parvathy, and Umamaheshwari (2007). The cancer cell lines MDA-MB-231, which is a type of breast cancer, and HCT-15, which is a type of colon cancer, Duraipandiyan,; Al-Dhabi, Balachandran; Ignacimuthu; Sankar. and Balakrishna,(2015), were used in 20 well plates with media containing different doses of the plant extracts—1.0, 2.0, 4.0, 6.0, 8.0, and 10.0 mg/mL—cells were implanted at a rate of 1,000 cells per well. The cells were grown in 5% CO2, 95% air, and 100% relative humidity at body temperature. Following that, adherent cells were seeded and exposed to progressively stronger plant extracts over 48–72 hours. MTT assay was applied, and after 72 hours, the absorbance (560nm) was read. When provided with less than 50% survival following a 72-hour exposure, extracts were deemed active. An anticancer drug called cisplatin was utilized as a negative control. Triples of each therapy were carried out. The American National Cancer Institute (NCI) states that the threshold for cytotoxicity for crude extracts is an IC50 30 µg/mL during a 72-hour exposure period. Guevera, Vargas (.et.al.1999).
RESULTS AND DISCUSSIONS
Data were expressed as mean ± standard error of the mean (S.E.M.). All tests were performed with triplicate measurements, and data from in vitro experiments were collected in triplicates. Student's t-test for unpaired data was used for the statistical analysis. Levene's Test was used to compare experimental work after doing an ANOVA. P < 0.05 was used to determine whether differences between the data were significant.
Elemental Composition of the Plants
Particles Induced X-Ray Emission technique gives elemental concentrations of both
M. oleifera and
A. hispidum in
Table 1. It is well known that man, a heterotrophic species, needs both organic and inorganic nutrients for his metabolic processes. The majority of man's nutritional needs are met by his diet, Sofowora, (1993) and consuming enough of these nutrients is beneficial for human health to prevent deficiencies that are present in these two plants in appropriate amounts.
Table 3.
Particles Induced X-Ray Emission Results of M. oleifera and A. hispidum.
Table 3.
Particles Induced X-Ray Emission Results of M. oleifera and A. hispidum.
| Elements |
Moringa oleifera
(Concentration in ppm) |
Acanthospermum hispidum
(Concentration in ppm) |
| Mg |
3837.3±223.3 |
3538.8±206.0 |
| Si |
1475.0±42.5 |
1332.4±37.2 |
| P |
2661.2±72.4 |
1808.7±62.0 |
| S |
8772.6±60.5 |
5072.6±47.7 |
| Cl |
1412.9±39.7 |
1188.0±3O.7 |
| K |
17053.9±56.2 |
16908.4±50.7 |
| Ca |
14467.7±63.7 |
12567.7±61.6 |
| Mn |
71.3±5.4 |
32.6±5.1 |
| Fe |
113.5±4.3 |
329.7±6.6 |
| Cu |
3.7±1.7 |
5.0±1.7 |
| Zn |
15.3±14.3 |
11.4±2.2 |
| Rb |
29.2±14.3 |
25.1±10.8 |
| Sr |
ND* |
89.2±20.8 |
| Ba |
ND* |
662.0±162.9 |
GC-MS Results
The GC-MS results of both
M. oleifera and
A. hispidum are given in
Table 2 and
Table 3. These results show that both plants contain substances that are pharmacologically effective which includes the followings: Terpenoids, Flavonoids, Anthraquinones, Steroids, Glycosides, Alkaloids, and Tannins, Phenols and Saponins. Since these products are proven beneficial for human health, it is possible to use purified D’Souza., Kulkarni, (1993), form from these plants. Some of the detected products were not previously evaluated as efficacy. It was necessary to evaluate the effects of pure forms of these products on human health separately.
Table 4.
GC-MS Bioactive Data for Moringa oleifera Extracts.
Table 4.
GC-MS Bioactive Data for Moringa oleifera Extracts.
Peak
S/N |
Compound
Name |
Retention
Time(S) |
Composition
(%) |
Molecular
Formula |
Molecular
Weight
(g/mol) |
Pharmacological
Activity |
| 1 |
5-hydroxymethylfurfural |
18.217 |
6.46 |
C6H6O3
|
126.11 |
Anti-anemic activity. |
| 2 |
Benzeneacetonitrile,4-hydroxy- |
26.199 |
2.85 |
C8H7NO |
133.15 |
NAR |
| 3 |
Phenol-4-(3-hydroxy-1-propenyl)-2-methoxy |
33.211 |
1.34 |
C10H12O3
|
180.20 |
NAR |
| 4 |
n-hexadecanoic acid |
37.346 |
17.36 |
C16H32O2
|
256.43 |
CNS activity, Antioxidant Activity. |
| 5 |
Hexadecanoic acid, ethyl ester |
37.521 |
1.44 |
C18H36O2
|
284.48 |
Antioxidant activity |
| 6 |
Octadec-9-enoic acid |
38.547 |
40.27 |
C18H34O2
|
282.47 |
Cardiovascular activity, antimicrobial activity. |
| 7 |
Octadecanoic acid |
38.647 |
5.78 |
C18H36O2
|
284.48 |
Antimicrobial activity. |
| 8 |
Cis-11- Hexadecenal |
39.535 |
1.91 |
C16H30O |
238.42 |
Antimicrobial Activity
|
| 9 |
Eicosanoic acid |
39.635 |
1.68 |
C20H40O2
|
312.54 |
CNS activity
|
| 10 |
Sulphurous acid isohexyl pentyl ester |
40.304 |
9.27 |
C11H24O3S |
236.37 |
NAR
|
| 11 |
Glycerol-1- palmitate |
40.417 |
1.77 |
C38H19O4
|
330.51 |
Antimicrobial activity
|
| 12 |
Prop-2-one, 1-(4-iospropoxy-3-methoxyphenyl) |
40.767 |
2.27 |
C13H18O3
|
222.28 |
NAR
|
| 13 |
Benzeneaacetic acid, 4-hydroxy -3-methoxy-methyl ester |
40.917 |
1.59 |
C10H12O4
|
196.20 |
NAR |
| 14 |
n-propyl 11 octadecenoate |
41.737 |
6.02 |
C21H40 O2
|
324.55 |
NAR |
Table 5.
GC-MS Bioactive Data for Acanthospermum hispidum Extracts.
Table 5.
GC-MS Bioactive Data for Acanthospermum hispidum Extracts.
Peak
S/N |
Compound name |
Retention
Time(S) |
Composition
% |
Molecular
Formula |
Molecular
Weight
(g/mol) |
Pharmacological
Activity |
| 1 |
Thiophene, 2-methoxy-5- methyl- |
10.754 |
1.71 |
C6H8OS |
128.19 |
NAR |
| 2 |
4H- pyran-4-one, 2,3dihydro-3,5-dihydroxy-6-methyl |
14.808 |
2.06 |
C6H8O4
|
144.13 |
Antioxidant activity |
| 3 |
Benzeneacetonitrile,4-hydroxy- |
26.225 |
2.52 |
C8H7NO |
133.15 |
NAR |
| 4 |
E, Z-8, 10-dodecadine-1-ol |
35.894 |
1.56 |
C12H22O |
182.31 |
Antimicrobial Activity |
| 5 |
n-hexadecanoic acid |
37.337 |
24.98 |
C16H32O2
|
256.43 |
CNS activity, Antioxidant Activity. |
| 6 |
Hexadecanoic acid |
37.527 |
4.02 |
C18H36O2
|
284.48 |
Antioxidant Activity.
|
| 7 |
Phytol |
38.328 |
2.58 |
C2OH40O |
296.54 |
Hepatoprotective activity, Antioxidant activity, Antimicrobial Activity. |
| 8 |
9,12,15- octadecatrienoic acid, (z,z,z) |
38.565 |
47.33 |
C13H30O |
278.44 |
Antimicrobial activity, CNS activity, Anticancer activity, Hepatoprotectivity Anti-inflammatory activity. |
| 9, |
9, 12, 15- octadecatrienoic acid. (z,z,z), ethyl ester |
38.653 |
11.66 |
C20H34O2
|
306.49 |
NAR |
| 10 |
Hexadecanoic acid, 2 hydroxyl- 1- (hydroxymethyl) ester |
40.423 |
1.60 |
C19H38O4
|
330.51 |
Antioxidant activity, anti-inflammatory activity |
Both leaves show antibacterial, antifungal, antioxidants and anticancer properties against bacterial and fungal strains. When compared with A. hispidum, M. oleifera shows moderate antibacterial activity and pronounced antifungal activity. M. oleifera also shows a potent antioxidant activity, when DPPH radicals are reduced, the consequence is a discoloration from purple to pale yellow, which shows scavenging action, Bajpai, Pande, Tewari, Prakash, (2005). Cytotoxic activity was recorded against the cancer cell lines with IC50 recorded. The result revealed that the two plant extracts have in vitro anti-cancer activity against the selected cancer cell lines. M. oleifera and A. hispidum plant extracts have potential anti-cancer properties without any noticeable adverse effects, offering an unheard-of opportunity as a cancer preventive as well as healing beneficial to all cancer patients, regardless of the type of disease , R., Tabassum, J. and Azad,(2003). Without damaging the normal cells, extract treatment resulted in a considerable decrease in the number of malignant cells compared to the control. The presence of the identified phytochemicals makes these leaves pharmacologically active.
TOTAL ANTIOXIDANT CAPACITY
A number of chemical and biochemical methods have been employed to measure antioxidant activity Bharali.,Tabassum, and Azad, (2003), which include chemiluminescence (CL), oxygen radical absorbance capacity (ORAC), copper reduction assay (CUPRAC), total radical-trapping antioxidant potential (TRAP), ferric reducing antioxidant power (FRAP), total oxidant scavenging capacity (TOSC), 2,2′ azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay, low density lipoprotein (LDL) oxidation, croton bleaching, 2,2-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO), hydroxyl radical (OH) hydrogen peroxide (H2O2) and total phenolic assay among others. But, in this research work, 2,2-diphenyl-1-picrylhydrazyl (DPPH) was used to confirm the antioxidant properties of these two plant species as shown below:
Table 6.
Scavenging activity of Moringa oleifera and Acanthospermum hispidum ethanolic extract.
Table 6.
Scavenging activity of Moringa oleifera and Acanthospermum hispidum ethanolic extract.
| Concentration (µg/ml) |
(%)
MO |
(%)
AH |
(%)
Sterile Water |
| 0 |
0 |
0 |
0.00 |
| 20 |
63.17 |
46.30 |
0.00 |
| 40 |
65.20 |
51.35 |
0.00 |
| 60 |
72.16 |
55.92 |
0.00 |
| 80 |
78.14 |
70.02 |
0.00 |
| 100 |
80.02 |
76.00 |
0.00 |
From the above table, scavenging activity were shown in percentages, the result of reduction of DPPH radicals’ changes color from purple to pale yellow indicating the scavenging activity of both M. Oleifera and A. hispidum. Thus, from the experiment, it was concluded that M. Oleifera and A. hispidum have antioxidant capacity.
Independent Sample t-test of Elements in between Moringa oleifera and Acanthospermum hispidum
In
Table 7 above, the test confirmed the substantial flavonoid content in leaves; flavonoids are best recognized for their antioxidant properties, additionally, it might activate processes that prevent tumor invasion and possibly destroy cancer cells. Also, there was a significant amount of tannin in both leaf samples which are antioxidant agents.
CYTOTOXIC PROPERTIES OF ACANTHOSPERMUM HISPIDUM AND MORINGA OLEIFERA
Cancer is basically a disease of uncontrolled cell division. Tumor cells often form cultures that grow and divide indefinitely. Cell cycles controls are lost in cancer. Tumor suppression gene whose function is for cancer prevention known as P53; cancerous cells have no p53 gene (Molecular weight: 53000 Daltons) or already damaged. Four preventable cancers are: Breast, Prostate, Cervical and Colon cancer. The Following Lab – Cultivated Cancer Cell Lines were collected:
- -
Breast adenocarcinoma (MDAMB 231, MDAMB 468)
- -
Human colon cancer cell lines (HCT 15, HCT 116)
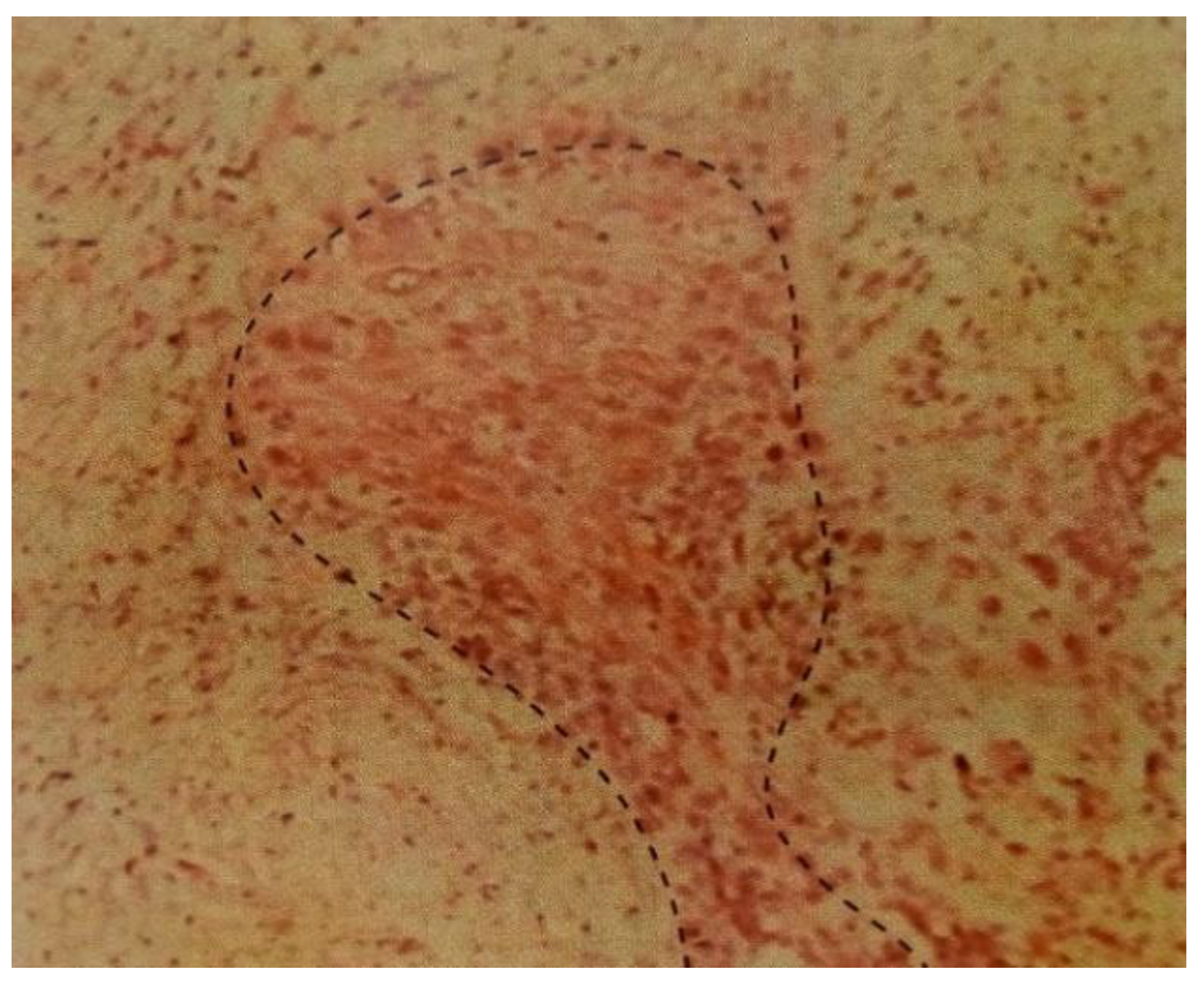
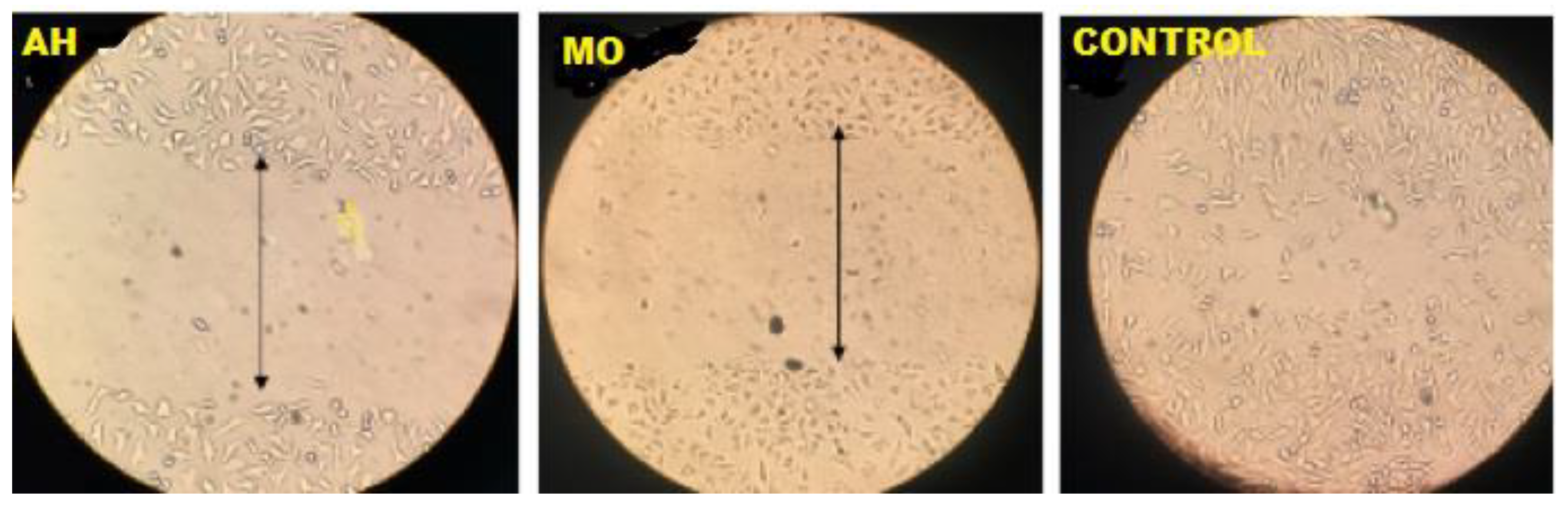
Figure 7.
Viable MDAMB 231 cell lines in the dotted line.
Figure 7.
Viable MDAMB 231 cell lines in the dotted line.
Table 8.
Moringa oleifera, Acanthospermum hispidum and Control on MDA-MB-231.
Table 8.
Moringa oleifera, Acanthospermum hispidum and Control on MDA-MB-231.
| DOSES (Mg/mL) |
MO (μg/mL) |
AH (μg/mL) |
CISPLATIN (μg/mL) |
| 1.0 |
18.0±1.61 |
5.0±1.05 |
9.0±0.11 |
| 2.0 |
22.0±2.54 |
10.0±1.84 |
15.0±1.32 |
| 4.0 |
24±2.34 |
16.0±1.46 |
18.0±1.51 |
| 8.0 |
28.0±2.52 |
23±2.54 |
22.0±1.61 |
| 10.0 |
30.0±2.53 |
26±2.61 |
24.0±1.81 |
From the above table, both M. Oleifera and A. hispidum shows lethal action against the Breast Cancer Cells in compliance with the IC50 recommended. Though, normal cells were not affected.
Table 9.
Moringa oleifera, Acanthospermum hispidum and Control on HTC-15.
Table 9.
Moringa oleifera, Acanthospermum hispidum and Control on HTC-15.
| DOSES (Mg/mL) |
MO (μg/mL) |
AH (μg/mL) |
CISPLATIN (μg/mL) |
| 1.0 |
16.0±1.61 |
3.0±1.05 |
8.0±0.11 |
| 2.0 |
20.0±2.54 |
9.0±1.84 |
13.0±1.32 |
| 4.0 |
22±2.34 |
14.0±1.46 |
16.0±1.51 |
| 8.0 |
25.0±2.52 |
20±2.54 |
19.0±1.61 |
| 10.0 |
28.0±2.53 |
24±2.61 |
23.0±1.81 |
Also, M. Oleifera and A. hispidum also show lethal action against the Human Colon Cancerous Cells in line with the IC50 recommended; equally, normal cell were not also affected.
The findings showed that the two plant extracts possessed an in vitro anticancer activity against the two selected cancer cell lines [
Figure 8]. The ethanolic extracts showed on the average a 50% reduction in cancer cell viability at 24.5μg mL−1 for
M. oleifera and 16.0μg mL−1 for
A. hispidum on MDA-MB-231 as compared to the standard reference point which have 17.65μg mL−1. Also, the ethanolic extracts shows on the average a 50% reduction in cancer cell viability at 22.2μg mL−1 for
M. Oleifera and 14.0μg mL−1 for
A. hispidum on HTC-15 as compared to control which have 15.80μg mL−1 Chumark, Khunawat, Sanvarinda, Phornchirasilp, Morales, Phivthong-Ngam, Ratanachamnong, Srisawat, Pongrapeeporn (2008). From the above,
M. Oleifera were more effective than
A. hispidum, this means that both can be used to complement each other as natural compounds as against the synthetic ones. This indicates that the extracts contained phenolic compounds and quercetin. This study demonstrates the potential for M. oleifera and A. hispidum leaves to limit the growth of cancer cells while also enhancing human health. Evani., Araújo., Karina., Randau., José., Sena., Rejane., Mendonça., Haroudo (2008).
Based on the in-vitro studies, the phytochemical components of
M. oleifera and A. hispidum leaves can be used as leading plants in treating cancer, Umamaheshwari (2007). When extract treatment was applied, a substantial number of dead cells were seen compared to the control, there was a substantial reduction in cell proliferation particularly,
M. Oleifera extracts while normal cells were not affected in the process. An aliquot of 100 mL of media containing 1 mg/mL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide was put into the plate following various cultivation times. The solution in the media was withdrawn after the cells had been cultivated for 4 hours. The plate received an aliquot of 100 lL of DMSO, which was added, and it was shaken until the dissolution of crystals. Each sample's cytotoxicity was quantified using its IC50 value, or half-maximal inhibitory concentration, Bharali., Tabassum and Azad, (2003). The average of three separate trials yielded the IC50 value, which is the concentration of the test sample that results in a 50% inhibition of cell growth.
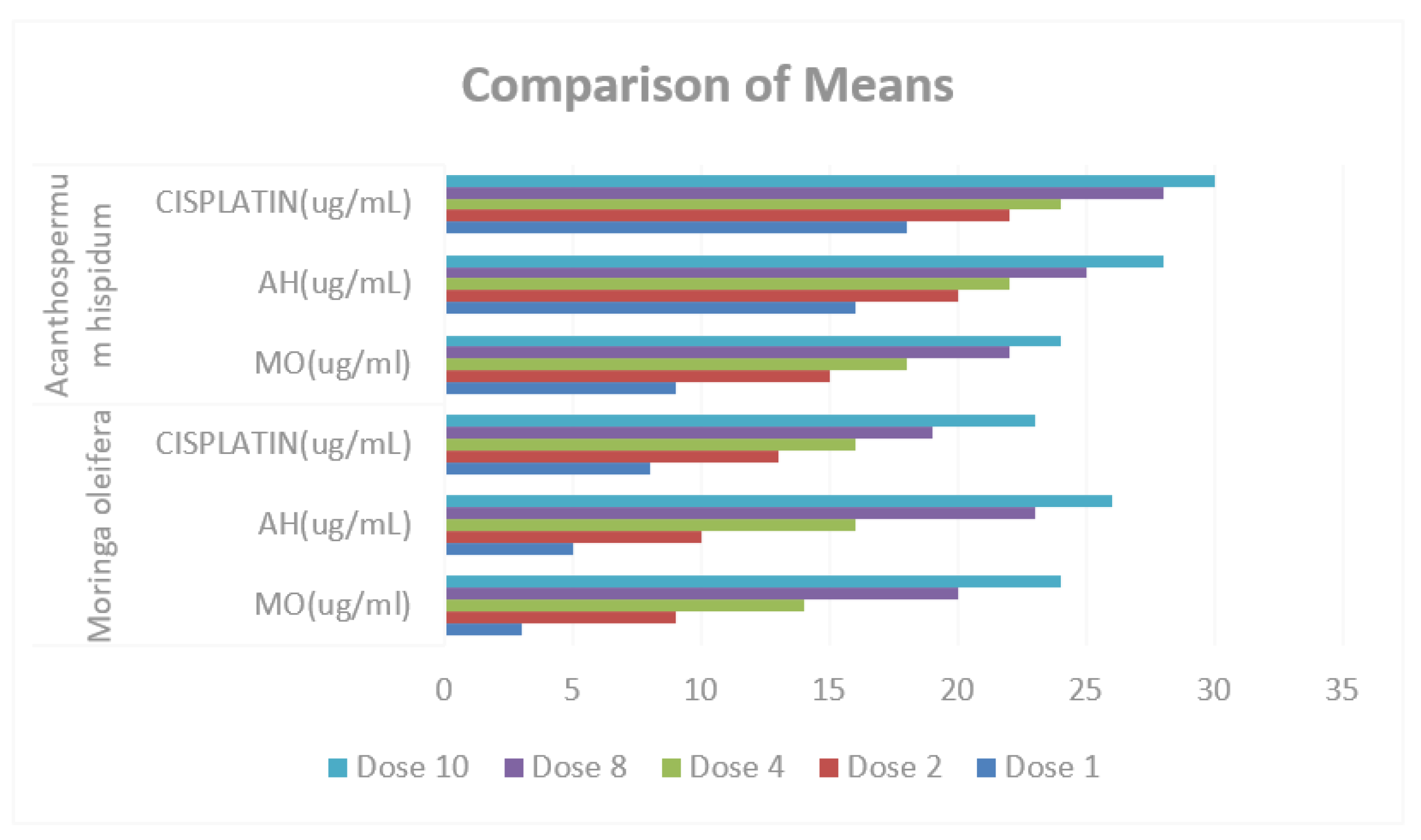
Table 7 and
Table 8 presents the comparative results of treatments in
M. oleifera and
A. hispidum as anti-cancer, the result shows that there is no significant difference in the means of MO (ug/ml), AH (ug/mL) and CISPLATIN (ug/mL), also in
M. oleifera and
A. hispidum since the p-values of (0.217, 0.505 and 0.380 respectively) greater than α-value (0.05).
Figure 9.
Comparison of Means of MO (ug/ml), AH (ug/ml) and CISPLATIN (ug/ml) in Moringa oleifera and Acanthospermum hispidum.
Figure 9.
Comparison of Means of MO (ug/ml), AH (ug/ml) and CISPLATIN (ug/ml) in Moringa oleifera and Acanthospermum hispidum.
Anticancer Properties of Moringa oleifera and Acanthospermum hispidum
Reports indicated M. oleifera having several bioactive compounds with anti-tumor activity. According to Guevera, Vargas (et.al 1999), niazimicin, a bioactive component present in M. oleifera leaves, has anti-cancer action. According to, Umamaheshwari (2007), M. oleifera leaf extract may have cytotoxic effects on human colon cancer cell lines. Additionally, it was noted that A. hispidum leaf extracts had an impact on the hepatic enzymes responsible for metabolizing carcinogens. Polyphenols, which have hypoglycemic effect, may be present in its leaves. Anti-tumor and bactericidal properties are all possessed by anthraquinones. Rai and Obayemi, (1973).
CONCLUSION
In conclusion, both leaves show antibacterial and antifungal properties against bacterial and fungal strains. When compared with A. hispidum, M. oleifera shows moderate antibacterial activity and pronounced antifungal activity. M. oleifera also shows a potent antioxidant activity, when DPPH radicals are reduced, the consequence is a discoloration from purple to pale yellow, which shows scavenging action, Wang and Li (2011). Cytotoxic activity was recorded against the cancer cell lines with IC50 recorded. The result revealed that the two plant extracts have in vitro anti-cancer activity against the selected cancer cell lines. M. oleifera and A. hispidum plant extracts have potential anti-cancer properties without any noticeable adverse effects, offering an unheard-of opportunity as a cancer prevention as well as healing beneficial to all cancer patients, regardless of the type of disease.
Without damaging the normal cells, extract treatment resulted in a considerable decrease in the number of malignant cells compared to the control. The presence of the identified phytochemicals makes these leaves pharmacologically active. It was discovered that M. oleifera has the highest zone of inhibition compared with A. hispidum, this means that M. oleifera have stronger bioactive compounds than A. hispidum. Nevertheless, in areas where M. oleifera is not available, A. hispidum could be used. Base on this research work, it is recommended that the active constituents be isolated and formulated as pharmaceutical raw materials.
Also, it is well known that man, a heterotrophic species, needs both organic and inorganic nutrients for his metabolic processes. The majority of a man's nutritional needs are met by his diet, and getting enough of these nutrients is beneficial for human health in order to prevent deficiencies.
References
- Anwar, F.; Bhanger, M.I. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J Agric food chem. 2003, 51, 6558–6563. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Anwar, F.; Nadeem, R.; Rashid, U.; Kazi, T.G.; Nadeem, M. Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab, Pakistan. Asian J. Plant Sci. 2005, 4, 417–421. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; et al. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Pande, A.; Tewari, S.K.; Prakash, D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutria. 2005, 56, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Duraipandiyan, V.; Al-Harbi, N.A.; Balakrishna, K.; Kalia, N.P.; Rajput, V.S.; Khan, I.A.; Ignacimuthu, S. Antimicrobial and antimycobacterial activities of methyl caffeate isolated from Solanum torvum swartz Fruit. Indian J Microbiol 2012, 52, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Duraipandiyan, V.; Ignacimuthu, S. Cytotoxic (A549) and antimicrobial effects of Methylobacterium sp. Isolate (ERI-135) from Nilgiris forest soil, India. Asian Pac J TropBiomed 2012, 2, 712–716. [Google Scholar]
- Bharali, R.; Tabassum, J.; Azad, M.R. Chemomodulatory Effect of Moringa oleifera, Lam, on Hepatic Carcinogen Metabolising Enzymes, Antioxidant Parameters and Skin Papillomagenesis in Mice. Asian Pacific Journal of Cancer Prevention 2003, 4, 131–139. [Google Scholar] [PubMed]
- Campbell, K.; et al. Collective Cell Migration and Metastases induced by an Epithelical to Mesenchymal transition in Drosophilia intestinal tumours. Nat. Commun 2019, 10, 2311. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.H.; Lee, C.W.; Chou, C.Y.; Murugan, M.; Shieh, B.J.; Chen, H.M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthong-Ngam, L.; Ratanachamnong, P.; Srisawat, P.; Pongrapeeporn, K.U. The invitro and in vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J. Ethnopharmacol. 2008, 116, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Costa-Lotufo, L.V.; Khan, M.T.; Ather, A.; Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; de Morae’s, M.E.; de Morae’s, M.O. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. 2005.
- D’Souza, J.; Kulkarni, A.R. Comparative Studies on nutritive values of tender foliage of seedlings and mature plants of Moringa oleiferaLam. J Econ Taxon Bot. 1993, 17, 479–485. [Google Scholar]
- Duraipandiyan, V.; Ignacimuthu, S. Antibacterial and antifungal activity of flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L. ) Lam. J Ethnopharmacol 2009, 123, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V; Al-Dhabi, N. A; Balachandran, C.; Ignacimuthu, S; Sankar, C.; Balakrishna, K. Antimicrobial, Antioxidant, and Cytotoxic Properties of Vasicine Acetate Synthesized from Vasicine Isolated from Adhatoda vasica L. Hindawi Publishing Corporation BioMed Research International 2015, 2015, 727304. [Google Scholar] [CrossRef]
- Elujoba, A.A.; Ajulo, O.O.; Iweibo, G.O. Chemical and Biological Analyses of Nigerian Cassia Species for Laxative Activity. Journal of Pharmaceutical and Biomedical Analysis 1989, 7, 1453–1457. [Google Scholar] [CrossRef]
- Evani, L.; Araújo, I.; Karina, P.; Randau, I.; José, G.; Sena, F.; Rejane, M.; Mendonça, P.; Haroudo, S. Acanthospermum hispidum DC (Asteraceae): Perspectives for a phytotherapeutic product. Rev. Bras. Farmacogn. 2008, 18. [Google Scholar]
- Guevera, A.P.; Vargas, C. ; et. al. An anti tumour promoter from MO Lam. Mutat Res 1999, 440, 181–188. [Google Scholar]
- Harbone, J.B. (1984). Phytochemical Methods: A guide to modern techniques of plant analysis. Chapman and Hall Ltd, London: Pp.279.
- Khare, et.al. (1997) Compendium of medicinal plants. National Institute of Industrial Research, Kamalanagar, New Delhi, India, pp 110–113.
- Martinez, A.; Valencia, G. (2003). Manual de practicas de Farmacognosia y Fitoquimia: 1999.1. Universidad de Antiquia, Medellin, pp: 59-65.
- Morton, F. The Horseradish tree, Moringaceae- A boon to Arid Lands, Economic Botany, 1991, 45, 318–333.
- Muangnoi, C.; Chingsuwanrote, P.; Praengamthanachoti, P.; Svasti, S.; Tuntipopipat, S. Moringa oleifera pod inhibits inflammatory mediator. 2011.
- Ndonga, A.; et al. Oesophageal cancer and experience with endoscopic stent incubation at St. Mary”s Hospital, Nairobi. Annuals of African Surgery 2008, 3.
- Parvathy, M.U.S.; Umamaheshwari, A. S. ; Umamaheshwari, A. Cytotoxic Effects of Moringa oleifera Extracts on Human Multiple Myeloma Cell Lines. Trends in Medical Research 2007, 2, 44–50. [Google Scholar]
- Rai, P. P, Obayemi, O. M. Anthraquinones from the leaves of cassiapodocerpa current science. 1973, 47, 457–460. [Google Scholar]
- Sofowora, A. (1986). The state of Medicinal Plants Research in Nigeria. University Press, Ibadan, Nigeria. P7.
- Sofowora, A. , 1993. Medicinal Plants and Traditional Medicine in Africa. 2nd Edn., Spectrum Books Ltd., Ibadan, Nigeria, ISBN-13: 9782462195, Pages: 289.
- Trease, G.E. and W.C. Evans, 1989. Trease and Evans' Pharmacognosy. 13th Edn., Bailliere Tindall, London, UK., ISBN-13: 9780702013577, Pages: 832Wallis, T. E (1967). Textbook of Pharmacognosy 5th Edition, London J and A. Church hill Ltd, P81-82, 135.
- Wang, X.; Li, X. Evaluation of antioxidant activity of isoferulic acid invitro. Nat Prod Commun 2011, 6, 1285–1288. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).