1. Introduction
Psoriasis was initially characterized as a dermatologic disorder primarily affecting epidermal keratinocytes. However, recent studies have shed light on its immune-mediated pathogenesis, making it one of the most prevalent immune-mediated conditions (Kadam et al., 2010). Psoriasis is a chronic autoimmune disease characterized by systemic manifestations and inflammatory changes that predominantly affect the skin and joints. The disease can significantly compromise the overall quality of life of those affected. Autoimmunity is believed to be the underlying cause of psoriasis, an autoimmune disorder that can be triggered by environmental factors. Psoriasis is a multifaceted disease, and its pathogenesis is not yet fully understood. Despite the availability of several treatment options, psoriasis remains a challenging disease to manage. Therefore, further research is necessary to understand the disease's pathogenesis and develop more effective treatments to improve patients' quality of life (Rendon and Schäkel, 2019). Up to 2.5% of the world's population is affected by a chronic skin disorder known as Psoriasis Vulgaris (Elder, 2018). Several clinical phenotypes of psoriasis are recognised, with chronic plaque (Psoriasis Vulgaris) accounting for 90% of cases (Griffiths and Barker, 2007). It is believed to be triggered in genetically susceptible individuals by environmental factors, which in turn initiates the inflammatory cells in the adaptive and innate immune pathways. Lesions are frequently highly visible, leading to a considerable amount of physical, psychological, social and emotional disability in the patients (Nestle et al., 1994).
The cause of psoriasis is still unknown. However, one study found that patients with psoriasis had increased levels of serum malondialdehyde and nitric oxide end products, as well as decreased erythrocyte-superoxide dismutase activity.(Kadam et al., 2010). The pathogenesis of psoriasis is not fully elucidated; three factors contribute to the pathogenesis of the disease: tumour necrosis factor-alpha (TNFα), dendritic cells, and T-cells (Matsuzaki and Umemura, 2018). Although psoriasis's major clinical findings are present on the skin and histologically on the epidermal layer, inflammation is not limited just to the epidermal layer. Still, it is the interaction of keratinocytes with many other different cell types (innate and adaptive immune cells and vasculature) found in the dermal layer of the skin (Rendon and Schäkel, 2019). IL-17, along with T-helper (Th) 17, play a central part in the pathogenesis of psoriasis. IL-17A, usually called IL-17, is a fundamental component of the IL-17 group. There are six components of IL 17 (A-F). Therefore, the human T cell has several subsets of cells, and one of these cells is Th17, which produces the cytokine IL-17. The research findings from animal knockout models suggest that Th17 and IL-17 have a major part in the pathogenesis of this disease. Defined T cells are responsible for activating the adaptive immune response, which drives the maintenance phase of psoriatic inflammation. This phase includes Th17 cytokines, namely IL-17, IL-21, and IL-22, which activate keratinocyte proliferation in the epidermis. IL-17A has been found to have an important efficacy when it is used to treat moderate and severe psoriasis plaque. IL-17A inhibition works rapidly and produces sustained responses with a considerable safety profile.
The treatment of psoriasis is determined by the clinical severity of the disease, which is assessed based on various factors such as the extent of the affected area, the PSAI score, and the Dermatology Life Quality Index. Mild conditions can be addressed with topical therapies, whereas moderate to severe cases may necessitate phototherapy or systemic therapy, comprising biological or non-biological interventions or a combination of both. Secukinumab, also known as AIN457, is a biological medication that functions as an anti- IL17A monoclonal antibody (mAb). It is employed to treat moderate and severe psoriasis, as well as psoriatic arthritis (Wagener et al., 2013). Secukinumab is an IgG1/K-class property and it has been produced in the Chinese Hamster Ovary (CHO) cells (Molden et al., 2021). Secukinumab has been shown to have a superiority over other biological treatments in terms of achieving severity index PASI 75 (Bagel et al., 2021). Also, it has the ability to sustain efficacy over time compared to other biological treatments (Bissonnette et al., 2018). Secukinumab works in the treatment of psoriasis by neutralising and binding aspects of the cytokine interleukin-17A (IL-17A). Secukinumab is a fully human monoclonal antibody that selectively binds and neutralises the pro-inflammatory cytokine interleukin- 17A (IL-17A31009130) (Reich et al., 2019) without affecting IL-17F and Th17 cells. Additionally, it has no direct influence on the Th1 pathway. Recent studies have shown that increased oxidative stress (OS) and T-cell abnormalities are central to the pathogenesis of this disease (psoriasis). The resulting reactive oxygen species (ROS) induces the proliferation and differentiation of Th17/Th1/Th22 cells and inhibits the anti-inflammatory activities of regulatory T lymphocytes (Treg). Subsequent secretions of inflammatory cytokines, such as interleukin (IL)-17, IL-22, tumour necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ), and vascular endothelial growth factor (VEGF), stimulate keratinocyte proliferation and angiogenesis (Lai et al., 2018, Heidenreich et al., 2009, Ciazynska et al., 2021)
Liposomes are vesicles that consist of several lamellae or shells composed of a phospholipid bilayer covering a small amount of aqueous liquid, used for biotechnological and biomedical roles. They vary in size ranging from several nanometres to several hundred micrometres based on preparation and use (Lombardo and Kiselev, 2022). Biologically active compounds of liposomes, such as chelating agents, enzymes, vaccines, genetic materials, proteins, peptides, antimicrobial agents, and hormones, have been evaluated for patient use. The fundamental reason behind the popularity of liposomes in the technological world can be attributed to the considerable increase in proportion between surface area and volume at the nanoscale, which brings quantum mechanics into the display, providing an evolution of novel and improved mechanical, optical and electronic properties to nanomaterials (Sercombe et al., 2015, Nsairat et al., 2022). Lispomes have also been found to reduce the systemic side effects of treatments by prolonging the localisation of the drug.
This study aimed to determine the effects of Secukinumab bulk form compared to liposome on the DNA damage in the peripheral blood lymphocytes from psoriasis patients and healthy individuals using the Comet assay and Micronucleus (CBMN) assay. Additionally, the study aimed to determine the best concentration of secukinumab liposome to prepare topically for treating psoriasis in the future. The cellular response to the bulk and liposome forms was investigated and the optimal dose for bulk and liposome forms was obtained. In the presented study, the genotoxicity assays with different endpoints were used to assess the effect of secukinumab bulk and liposome forms on lymphocytes from psoriasis patients compared to healthy controls.
Figure 1.
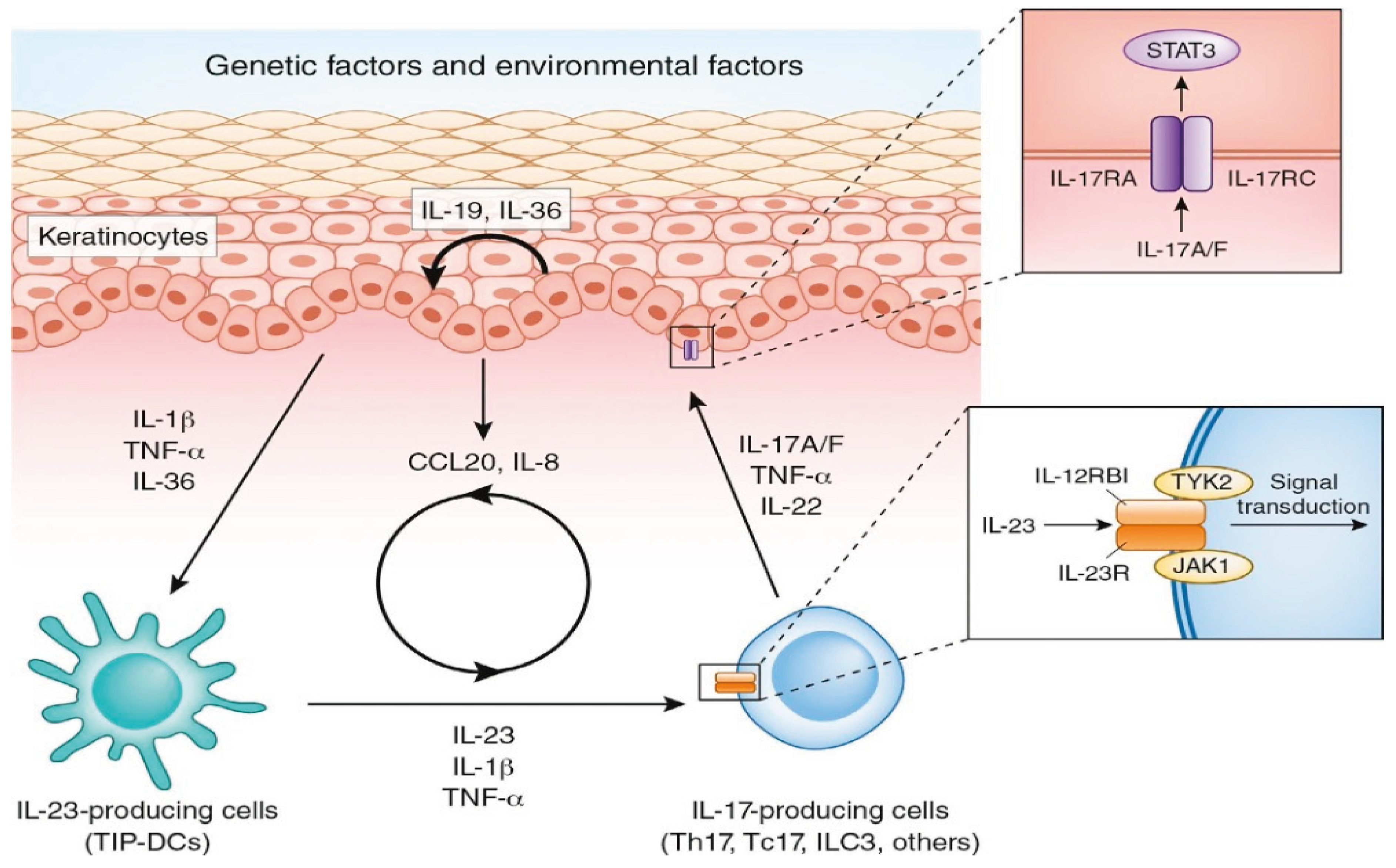
pathogenesis of psoriasis, The cytokines were activated in psoriasis and their further cascades in the skin. Interleukin (IL)-23 produced by various IL-23- producing cells induces IL-17 production from various IL-17-producing cells such as T-helper (Th)17, IL-17 producing CD8+ T cells (Tc17), and innate lymphoid cells type 3 (ILC3). IL-17 stimulates keratinocytes to produce inflammatory cytokines/chemokines, which further activate IL-23-producing cells and recruit IL-17-producing cells and neutrophils (Yamanaka, 2021).
Figure 1.
pathogenesis of psoriasis, The cytokines were activated in psoriasis and their further cascades in the skin. Interleukin (IL)-23 produced by various IL-23- producing cells induces IL-17 production from various IL-17-producing cells such as T-helper (Th)17, IL-17 producing CD8+ T cells (Tc17), and innate lymphoid cells type 3 (ILC3). IL-17 stimulates keratinocytes to produce inflammatory cytokines/chemokines, which further activate IL-23-producing cells and recruit IL-17-producing cells and neutrophils (Yamanaka, 2021).
2. Materials and Methods
2.1. Materials
Secukinumab was obtained from Novartis Pharmaceuticals (Cosentyx). 1,2-Dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt (DPPG), 1,2-dioleoyl-sn-glycero-3-phsphoethanolamine (DOPE), purity >99%, Cholesterol (Chol), Methanol, Dichloromethane (DCM) were obtained from Sigma-Aldrich Company Ltd. (UK). All other reagents used were derived from Sigma Aldrich U.K. unless otherwise stated. Secukinumab solutions were prepared by dissolving the drug in distilled water (150mg/ml). A 75μg/ml stock solution was prepared. The prefilled secukinumab syringes (150 mg) were used in this study to produce the liposome format of this biological agent and investigate its comparison with bulk form. The compound was diluted to a suitable concentration for treating the cells in vitro.
2.2. Preparation and Characterisation of Peptide-Loaded Liposome
Liposomes were prepared using the thin film rehydration method. In Dichloromethane (DCM) and methanol (3:1 v/v), 1,2-dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt (DPPG) (2 mg), 1,2-dioleoyl-sn-glycero-3-phsphoethanolamine (DOPE) (4 mg), and cholesterol (2:2:1 molar ratio) DCM and methanol were dissolved (3:1 v/v). The solution was transferred to a rotary evaporator flask and the organic solvent mixture was evaporated at 40 oC under a vacuum for 6 hours to ensure no traces of the organic solvents were left. The thin film was then hydrated by 10 ml of distilled water inserted into 1 ml of Secukinumab for 1 h at 60 0C using a bath sonicator (150 W) to help shear particles and decrease their size and, therefore, increase encapsulation efficiency. The sample was then subjected to 4 repeated freeze-thaw cycles (freezing at -20 °C and thawing at 60 °C in the bath sonicator). The final solution was diluted with 75 μg/ml using distilled water. The sample was then centrifuged at 22,000 x g for 30 minutes at 4 °C to remove the non-encapsulated medication and purify it.
The average size and polydispersity index (PDI) of the liposome preparations were determined by dynamic light scattering (DLS) using Zetasizer ZS-90, Model ZEN 3600 (Malvern Instruments, UK). All measurements were performed in triplicate. Measures were found to be < 150 nanometres.
2.3. Ethical Approval
Ethical approval has been granted for Genetic and environmental effects in lymphocytes from different cancerous, precancerous and inflammatory conditions using various genetic endpoints reviewed by Leeds east research ethics committee (rec) (rec reference number: 12/YH/0464). Ethical approval was granted by the University of Bradford's Sub-Committee for Ethics in Research involving Human Subjects (Reference no.: 0405/8). The Research Support and Governance Office Bradford Teaching Hospital NHS Foundation granted the Re DA number: 1202.
2.4. Blood Collection
Whole blood was collected from 20 healthy individuals and 20 patients by venepuncture after informed consent. Basic information about health and lifestyle factors was gathered using a questionnaire. Blood was collected from a patient with moderate Psoriasis under systemic treatment; non were taking any biological treatment.
2.5. Cell Viability Determination
Cell viability was determined using the cell counting kit 8 (CCK8) from VWR, UK. Cell cultures were prepared by adding blood samples with RPMI-1640 medium and the chemicals in Eppendorf tubes as described earlier for the Comet assay treatment step. The tubes were centrifuged at 3000 rpm (705 g) and the supernatant was discarded, and the cell pellet was re-suspended. The contents of the kit were directly added to the cell suspension and the procedure was followed according to the manufacturer's protocol. Cell viability of ≥80% was considered for use in all experiments (
Figure 2).
Table 1.
the list of patients' information confounding.
Table 1.
the list of patients' information confounding.
| No |
Sample Code |
Age |
Ethnicity |
Gender |
Smoking History |
| 1 |
P3 26719 |
31 |
Caucasian |
M |
Ex S |
| 2 |
P1 26719 |
41 |
Asian |
M |
N |
| 3 |
P3 26719 |
65 |
Caucasian |
F |
Ex S |
| 4 |
P4 25719 |
64 |
Asian |
F |
N |
| 5 |
P2 21519 |
23 |
Caucasian |
F |
N |
| 6 |
P3 21519 |
47 |
Caucasian |
M |
Ex S |
| 7 |
P1 21519 |
50 |
Caucasian |
M |
N |
| 8 |
P128519 |
35 |
Caucasian |
F |
N |
| 9 |
P228519 |
38 |
Caucasian |
M |
Y |
| 10 |
P125619 |
80 |
Caucasian |
F |
N |
| 11 |
P2 25619 |
64 |
Caucasian |
M |
N |
| 12 |
P2 18619 |
62 |
Caucasian |
F |
N |
| 13 |
P1 18619 |
44 |
Caucasian |
M |
N |
| 14 |
P1 2719 |
80 |
Caucasian |
F |
Ex S |
| 15 |
P2 2719 |
53 |
Caucasian |
F |
N |
| 16 |
P3 2719 |
63 |
Caucasian |
F |
Ex S |
| 17 |
P1 9719 |
35 |
Caucasian |
M |
Y |
| 18 |
P2 9719 |
55 |
Caucasian |
F |
N |
| 19 |
P3 9719 |
42 |
Caucasian |
F |
N |
| 20 |
P4 9719 |
35 |
Asian |
M |
N |
Table 2.
the list of healthy controls confounding factors.
Table 2.
the list of healthy controls confounding factors.
| No |
Age |
Ethnicity |
Gender |
Smoking History |
| 1 |
33 |
Caucasian |
F |
Non-smoker |
| 2 |
34 |
Asian |
M |
Past smoker |
| 3 |
20 |
Asian |
F |
Non-smoker |
| 4 |
42 |
Asian |
F |
Non-smoker |
| 5 |
21 |
Caucasian |
F |
Non-smoker |
| 6 |
20 |
Asian |
M |
Non-smoker |
| 7 |
21 |
Asian |
M |
Non-smoker |
| 8 |
22 |
Asian |
F |
Non-smoker |
| 9 |
21 |
Caucasian |
F |
Non-smoker |
| 10 |
22 |
Asian |
F |
Non-smoker |
| 11 |
24 |
Asian |
F |
Non-smoker |
| 12 |
35 |
Caucasian |
F |
Non-smoker |
| 13 |
37 |
Caucasian |
M |
Non-smoker |
| 14 |
40 |
Asian |
M |
Non-smoker |
| 15 |
39 |
African |
F |
Non-smoker |
| 16 |
33 |
Asian |
M |
Non-smoker |
| 17 |
39 |
Caucasian |
M |
Non-smoker |
| 18 |
28 |
Caucasian |
M |
Non-smoker |
| 19 |
35 |
Arab |
Male |
Non-smoker |
| 20 |
48 |
Arab |
Male |
Non-smoker |
2.6. Cell Treatment and the Comet Assay
Whole blood samples from 20 psoriasis patients and 20 healthy individuals were collected and supplemented with RPMI medium and 10% DMSO and immediately stored at -80 ℃. Five treatment groups were used to conduct the experiment, including an untreated group (NC) containing the blood sample and RPMI 1640 medium. A positive control containing media, blood, and a total of 10μl, 75μM/ml hydrogen peroxide H2O2, while the others were two treatment groups containing different concentrations of Secukinumab (2.8µg /ml, 2.1µg/ml) with or without H2O2. The total treatment volume used was 1000μl for the experiment. Samples were incubated at 37°C for 30 minutes, followed by centrifugation at 3000 rpm (705 g). The method used hereafter was followed as described in Tice et al. and Najafzadeh et al. (Tice et al., 2000, Najafzadeh, 2016).
A total of 100 cells were scored from each treatment using the fluorescence microscope (20X magnification) equipped with CCD camera, connected to a computer terminal using Komet 6 software and Kinetic Imaging (Andor Technology Ltd, Belfast). Two parameters of the Comet assay were used, Olive tail moment (OTM) and % tail DNA.
2.7. Cytokinesis Block Micronucleus Assay (CBMN)
Fresh blood sample (350 μl) and 130μl of phytohaemagglutinin (PHA) were added to 25cm3 vented cap corning flasks containing 4.5 ml RPMI-1640 medium supplemented with 1% penicillin-streptomycin and 15% foetal bovine serum (FBS). Followed by incubation for 24h at 37⁰C in the presence of 5% CO2 and then the test chemicals (Secukinumab bulk and liposome, H2O2) were added. The plain basic medium was used for the untreated negative control, and mitomycin C (MMC) (0.4μM) and H2O2 (75μM) were used as the positive controls. Thirty μl of cytochalasin B (cyt B) (1mg/ml) was added to each flask at 44h and cultured under the same conditions for another 20 h. Hereafter the CBMN procedures were performed as described in Fenech et al.,2016 (Fenech et al., 2016).
The frequency of MNi was determined by scoring 1000 cells per treatment group under 40X magnification using a light microscope according to the criteria adapted from Fenech (2007) and (Fenech, 2007). The MNi frequency in binucleated and mononucleated cells, nuclear division index (NDI), percentage of binucleated cells per 1000 cells scored and other parameters of the micronucleus assay were also determined. The nuclear division index (NDI) was calculated using the equation of NDI = M1 + 2(M2) + 3(M3) / N, where M1 = mononucleated cells, M2= binucleated cells, M3 = multinucleated cells and N = total number of viable cells scored (Fenech et al., 2016).
2.8. Statistical Analysis
The data were analysed by one-way ANOVA and t-tests to determine the significant values. A value <0.05 was considered statistically significant. Analysis was performed using Graph Pad Prism 8.
3. Results
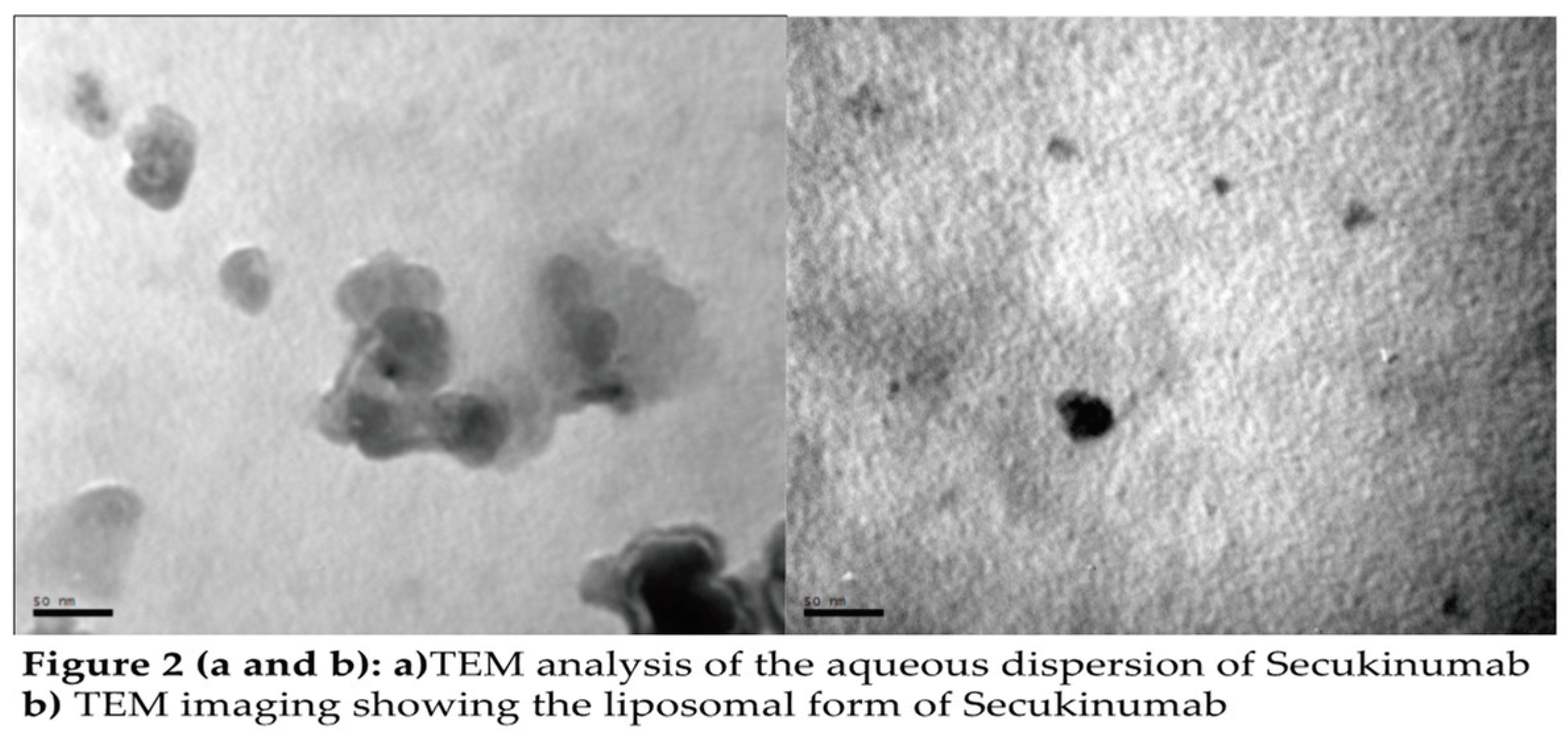
The surface morphology of secukinumab liposome was evaluated by Transmission Electron Microscopy (TEM), with liposome showing a uniform morphology, spherical bioparticles, with diameters ranging from 100 to 200 nm.
A cell counting kit was used to measure the cellular viability and integrity of lymphocytes. Cell viability is defined as the number of live or viable cells as a percentage of the total number counted (
Figure 3).
3.1. The Effects of Secukinumab and H2O2 on the Lymphocytes from Healthy
Figure 4 displays the responses of bulk and liposome forms of Secukinumab on lymphocyte DNA from healthy and psoriatic patients treated with H
2O
2 using Olive Tail Moment (OTM), standard errors and significance. Using the one-way ANOVA, it is clear that H
2O
2 treatments showed a significant increase in DNA damage compared to untreated cells by an increase in OTM, **p ≤ 0.01in psoriatic and ***p≤0.001 in healthy individuals. However, Secukinumab treatments in the two different forms with or without H
2O
2 were not significant (ns). On the other hand, comparing the treatments with the positive control, a significant decrease in the DNA damage was observed after the addition of Secukinumab ***p≤0.001 for the bulk in patients without the H
2O
2 and **p ≤ 0.01 for all the other treatments.
Results presented in
Figure 4 illustrate the responses of bulk and liposome forms of Secukinumab on lymphocyte DNA from healthy and psoriatic individuals treated with H
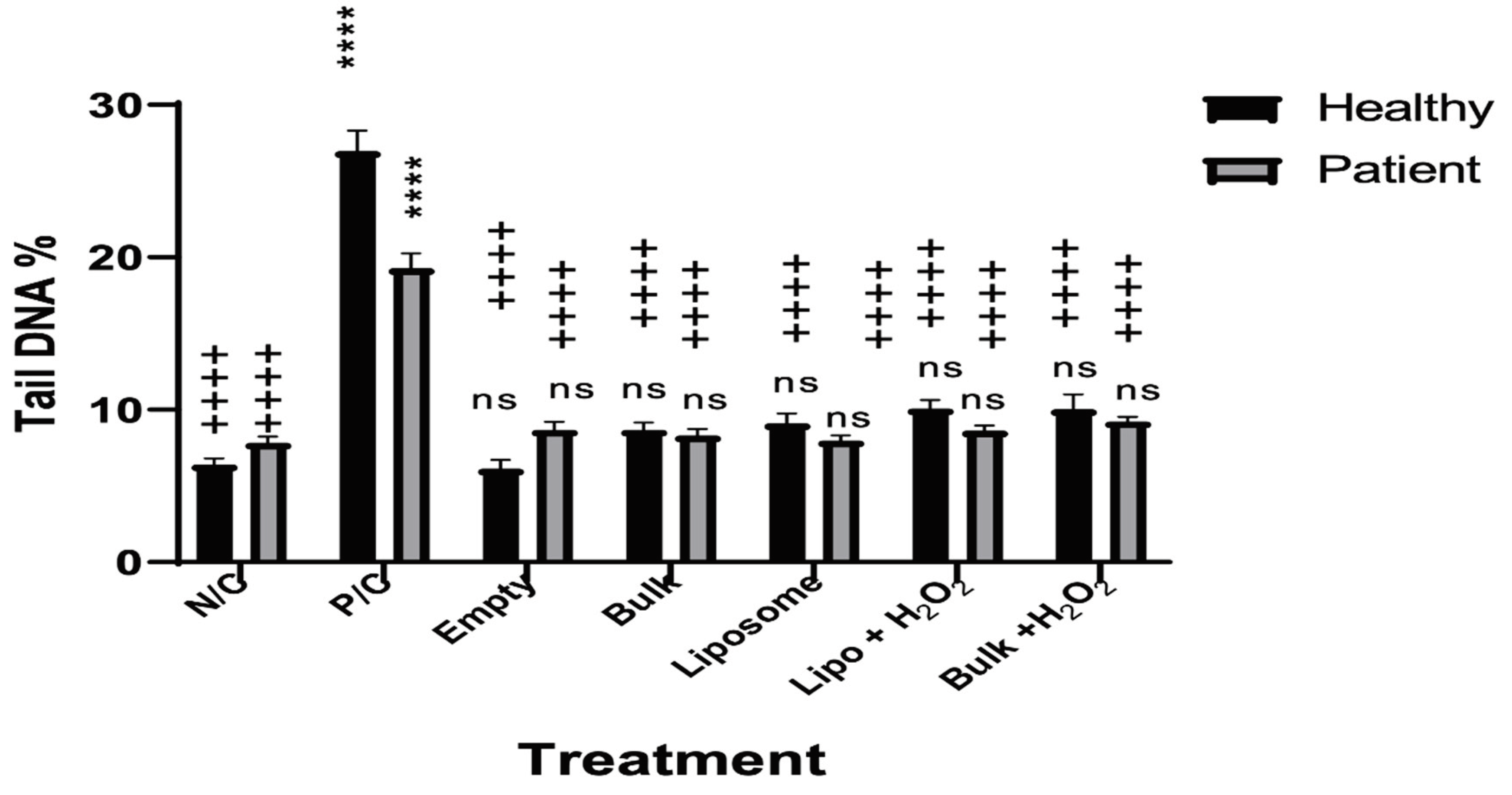
2O
2 using Tail DNA %, standard errors SE and significance. DNA damage observed in the patient group was higher compared to the healthy as expected. The PC showed a significant increase in the Tail DNA control cells, ****p ≤ 0.0001. However, Secukinumab treatments in both bulk and liposome form, with or without H
2O
2 were not significant when compared to the NC. On the other hand, comparing the treatments with the PC showed a significant decrease in the damage after the addition of Secukinumab ++++p ≤ 0.0001.
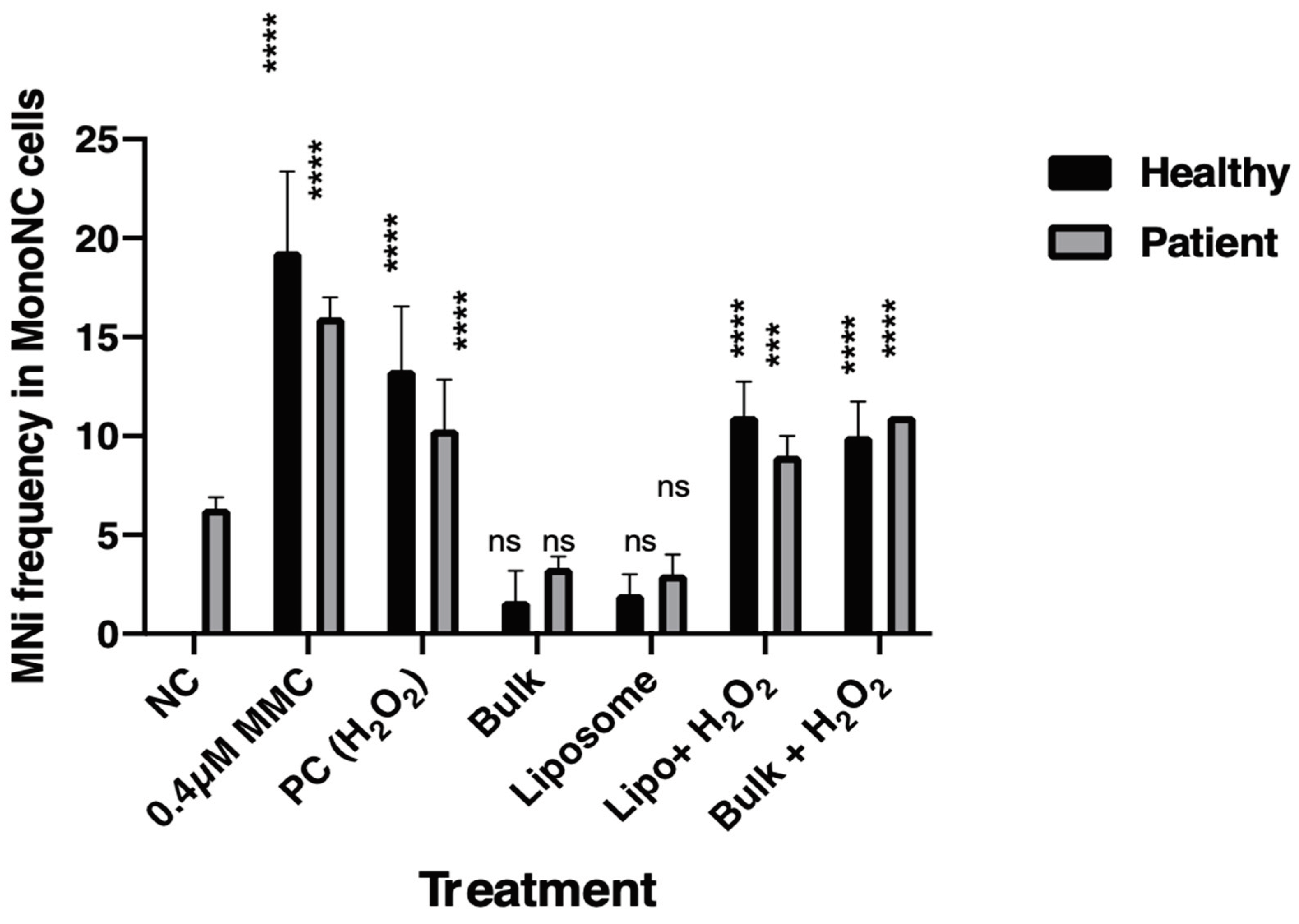
3.2. Cytokinesis Block Micronucleus Assay (CBMN)
The micronucleus assay is highly reliable, simple and an appropriate procedure to investigate DNA damage at chromosomal levels, particularly to determine genotoxicity as the MNi frequency represents cell damage. A significantly higher level of MNis in MonoNC was calculated in the patient group without treatment (NC) through the
in vitro treatment. Secukinumab 2.1µg/ml in bulk and liposome forms has shown some attenuation of the effects caused by H
2O
2 (
Figure 4).
4. Discussion
Secukinumab currently is one of the best choices with proper efficacy on psoriasis conditions, even on the increased level of IL-23 and JAK inhibitors. However, in the case of secukinumab failure, combined therapy would be ideal (Damiani et al., 2022). Also, secukinumab is a well-known biological therapy considered the most cost-effective biological agent (Schweikert et al., 2020). Moreover, a range of side effects linked with these biological compounds, such as paradoxical reactions (PRs), which are the result of worsening of immune-mediated inflammatory disease (Miyagawa, 2022) and increased eosinophilic disorders (Bridgewood et al., 2022). It is therefore desirable to reduce the concentration of the antibody in the blood and increase its residency in the psoriatic dermal layer by encapsulation in liposomes. This
in vitro study examined the effects of Secukinumab bulk and liposome forms on human peripheral lymphocytes from twenty psoriasis patients and twenty healthy individuals using the Comet assay and on whole blood from five volunteers in each group using the CBMN assay. In addition, the potential protective effects of Secukinumab in both forms on H
2O
2-induced DNA damage were determined in both study groups. Although the DNA damage induced by Secukinumab alone was comparable to the untreated group, in the presence of H
2O
2, inducing DNA damage on lymphocytes and both forms of Secukinumab anti-oxidant effect on the extent of DNA damage were demonstrated. Our results showed that Secukinumab significantly decreased the percentage of DNA damage in lymphocytes in the Comet assay (
+++ p < 0.
001). Therefor, data from the Comet assay showed that H
2O
2 had induced significant levels of DNA damage in both groups, although, Secukinumab administered in both forms (liposomal and bulk) suppressed the effect of H
2O
2 and significantly attenuated the adverse effects caused by this oxidising agent. Again, there was not much difference in DNA damage between healthy and patient groups after treatment with both forms of Secukinumab when supplemented with or without H
2O
2. Empty liposomes had no significant damage on the cell, which shows that it in itself is safe on cells.These results suggest the ability of Secukinumab to arrest and reduce the DNA damage caused by H
2O
2 in healthy individuals and psoriasis patients (
Figure 4 and
Figure 5). This could be due to the fact that oxidative stress results in the production of many pro-inflammatory cytokines. Since Secukinumab is an anti-IL-17, it can reverse the action of the oxidation (Zou and Meng, 2021). Oxidative stress has been linked to a number of pathologies through the elevation of intracellular levels of reactive oxygen species (ROS) that cause damage to lipids, proteins and DNA. However, elevated ROS are also signalling molecules, i.e. redox biology that maintains physiological functions. Oxidative stress has been shown to have a significant role in different inflammatory conditions, including psoriasis (Wagener et al., 2013).
Our results show that Secukinumab did not induce any significant DNA damage in lymphocytes from healthy individuals or patient groups when all treatments were compared to the negative control. The results were almost similar to the negative control (
Figure 4 and
Figure 5). This suggests that the concentration of 2.1μg/ml of Secukinumab is non-genotoxic to the cells in both groups. Hydrogen peroxide is an oxidative stress inducer compound which causes significant amounts of oxidative stress-related DNA damage in peripheral lymphocyte cells (Najafzadeh et al., 2009, Anderson et al., 2014, Stanić et al., 2016). Our results are in accordance with these studies as H
2O
2 significantly induced DNA damage in both groups; healthy individuals and psoriasis patients. However, when these groups were treated with two different forms of Secukinumab, bulk form and liposome, the DNA damage induced by H
2O
2 was significantly decreased compared to the positive control alone. In addition, the damage decreased to a level similar to the untreated control. The results were consistent for both the OTM and % tail DNA. There could be several molecular properties of Secukinumab involved in the reduction of this damage such as antioxidant defence. It has been found that,
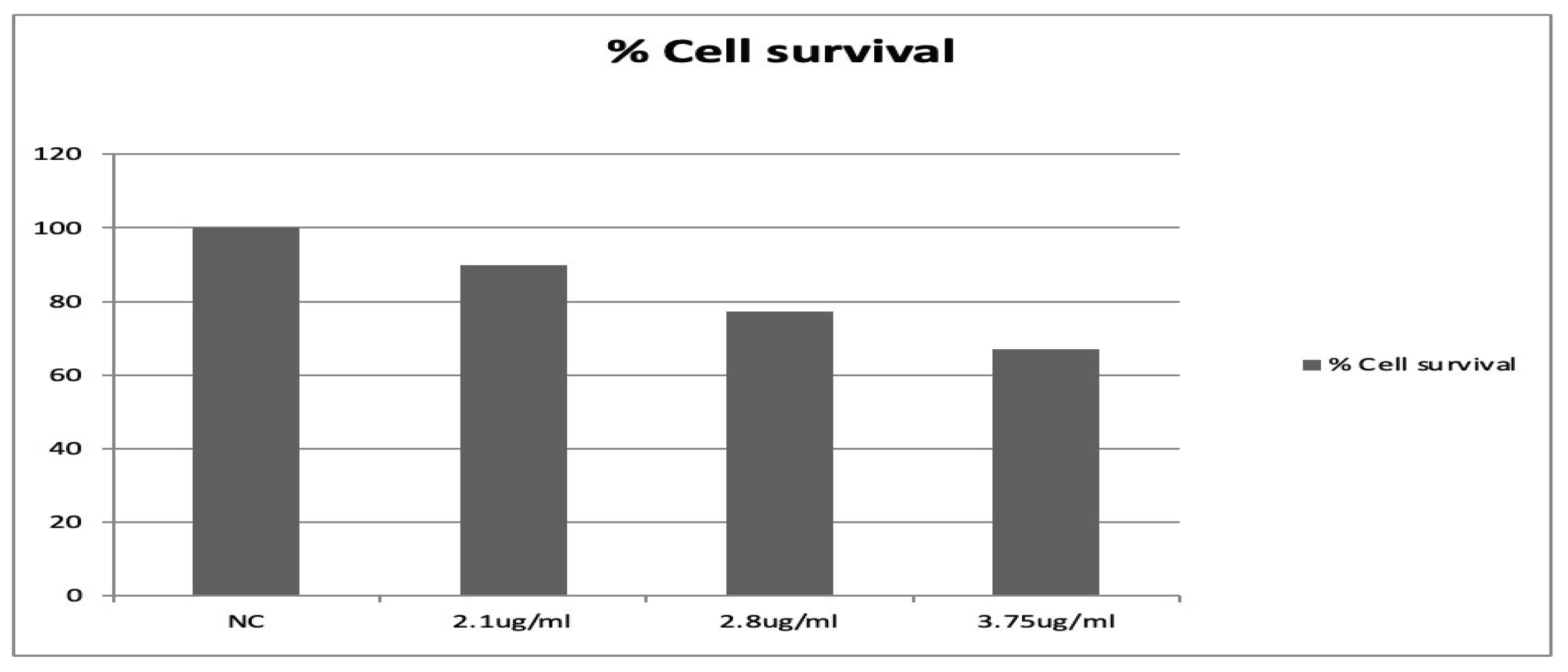
a positive correlation between oxidative stress markers and psoriasis area and severity index (PASI) values and a negative correlation between antioxidant markers and PASI values in patients with psoriasis (Lin and Huang, 2016). Different concentrations of Secukinumab were screened for their cytotoxicity in peripheral lymphocytes from healthy individuals using the CCK8. The results disclosed concentration-dependent cytotoxicity of Secukinumab in lymphocytes. A higher concentration of Secukinumab (3.75µg/ml) was found to be cytotoxic, although the two lower concentrations of the drug tested, 2.1µg/ml and 2.8µg/ml, had cell survival rates of over 75% (
Figure 3).
The CBMN assay is an effective test to study the ability of genotoxic agents to cause various clastogenic (chromosome breakage) and aneugenic effects (causing daughter cells to have an abnormal number of chromosomes during cell division) (Fenech, 2009). It was utilised in the current study to evaluate the effects of Secukinumab on healthy individuals versus psoriasis patients. Also, to determine the protective potential of the drug against H
2O
2-induced genotoxicity. This assay assesses several parameters, including micronuclei (MNi), which form during anaphase and indicate chromosomal remains or lost chromosomes generated during nuclear division. To enhance the sensitivity and reliability of the assay, cytokinesis is blocked using cytochalasin B, which facilitates the accumulation of BiNC. The presence of MNi in BiNC only reflects damage induced after treatment, reducing the possibility of scoring pre-existing damage. This approach effectively determines the effects of the test chemicals. By implementing this methodology, the sensitivity and reliability of the assay are increased, and the effects of the test chemicals are accurately determined (Magdolenova et al., 2012, No, 2014, économiques, 2016). The lymphocytes from healthy individuals treated with Secukinumab in both forms had no effect on MNi frequency when compared with untreated cells. The MMC and 75 μM H
2O
2 both induced a significant increase in the MNi frequency in lymphocytes (***p<0.001), yet Secukinumab (bulk or liposome co-supplemented with H
2O
2) showed significant decreases in the number of MNi in lymphocytes (*p<0.05). It was evident that liposomal Secukinumab caused a significant reduction in the number of MNis in patients (***p<0.001 as compared to untreated cells). Furthermore, the addition of MMC and 75 μM H
2O
2 showed a significant increase (***p<0.001) in the MNi number in lymphocytes as compared to the untreated cells (
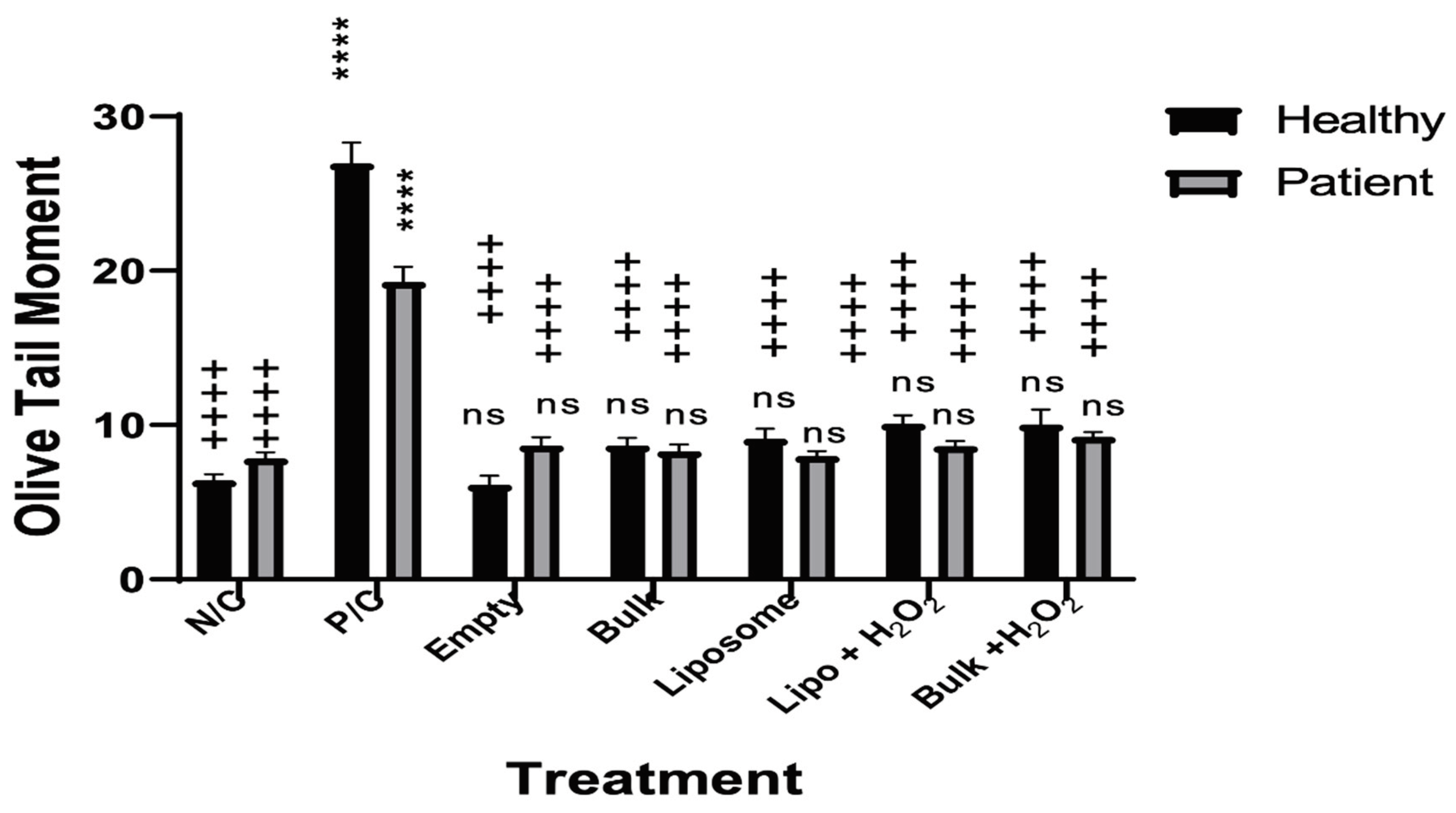
Figure 6). This all confirms the safety of secukinumab in both forms, bulk and liposome, on lymphocytes. This could be the first step in using the liposome form as a topical treatment.
Therefore, the results demonstrate that all the treatment sets from both investigative groups have shown the BiNC % and the NDI within the normal ranges: a typical NDI value represents a successful division. Evaluation of the assays shows that treatment of healthy and patient groups with Secukinumab in both forms did not induce a significant number of MNi in BiNC at basal levels. However, when 2.1μg/ml of the drug was co-treated with H
2O
2, Secukinumab provided substantial protection against H
2O
2-induced damage and reduced the frequency of MNi in healthy BiNC (
Figure 6). A significantly raised number of MNi were observed in MonoNC of the patient group compared to healthy individuals, which indicates pre-existing DNA and chromosomal damage due to the disease state, medications or other confounding factors. Secukinumab 2.1μg/ml reduced the frequency of MNi in MonoNC of the patient group at a basal level. However, the results were not statistically significant. The consistency of the data suggests that confounding factors were not an issue.
5. Conclusion
The aim of this study was to investigate the effect of Secukinumab, in both liposomal and non-liposomal forms, on DNA damage in healthy individuals and psoriasis patients. Negative control experiments revealed no significant effect on DNA damage. However, in the presence of H2O2, Secukinumab significantly reduced H2O2-induced damage and effectively attenuated its adverse effects. Throughout the in vitro study, the patient group exhibited higher levels of DNA damage compared to the healthy group. The results were consistent across the Comet and micronucleus assays, with Secukinumab 2.1μg/ml demonstrating enhanced efficacy against H2O2-mediated DNA damage. These findings suggest that both forms of Secukinumab exhibit protective effects against H2O2-induced oxidative stress, potentially facilitating repair, without inducing significant genotoxicity. Furthermore, the liposomal form of the drug was found to be safe for localized treatment via subcutaneous injection or transdermal drug delivery preparation. However, additional investigations are required to elucidate other possible molecular mechanisms involved.
In conclusion, this study demonstrated the ability to effectively deliver Secukinumab via liposomes, which may have clinical applications. These results highlight the potential of Secukinumab to serve as a protective agent against oxidative stress-induced DNA damage. Overall, this study provides valuable insight into the effects of Secukinumab on DNA damage and its potential clinical applications.
Abbreviations
| CHO |
Chinese Hamster Ovary cells |
| Chol |
Cholesterol |
| CBMN |
cytokinesis-block micronucleus |
| DCM |
methanol dichloromethane |
| DOPE |
Dioleoyl phsphoethanolamine |
| DPPG |
1,2-Dipalmitoyl-sn-glycerol sodium salt |
| IL-17 |
Interleukin 17 |
| mAb |
Monoclonal antibody |
| PSAI |
Psoriasis Area and Severity Index |
| Th |
T-Helper cells |
| TNFa |
Tumor necrosis factor-alpha |
| RPMI |
Mammalian cell culture media/ Roswell Park Memorial Institute |
References
- ANDERSON, D., NAJAFZADEH, M., GOPALAN, R., GHADERI, N., SCALLY, A. J., BRITLAND, S. T., JACOBS, B. K., REYNOLDS, P. D., DAVIES, J. & WRIGHT, A. L. 2014. Sensitivity and specificity of the empirical lymphocyte genome sensitivity (LGS) assay: implications for improving cancer diagnostics. The FASEB Journal, 28, 4563-4570.
- BAGEL, J.; BLAUVELT, A.; NIA, J.; HASHIM, P.; PATEKAR, M.; DE VERA, A.; AHMAD, K.; PAGUET, B.; XIA, S.; MUSCIANISI, E.; et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). J Eur Acad Dermatol Venereol 2021, 35, 135–142. [Google Scholar] [CrossRef]
- BISSONNETTE, R.; LUGER, T.; THACI, D.; TOTH, D.; LACOMBE, A.; XIA, S.; MAZUR, R.; PATEKAR, M.; CHAREF, P.; MILUTINOVIC, M.; LEONARDI, C.; MROWIETZ, U. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol 2018, 32, 1507–1514. [Google Scholar] [CrossRef]
- BRIDGEWOOD, C.; WITTMANN, M.; MACLEOD, T.; WATAD, A.; NEWTON, D.; BHAN, K.; AMITAL, H.; DAMIANI, G.; GIRYES, S.; BRAGAZZI, N. L.; MCGONAGLE, D. T Helper 2 IL-4/IL-13 Dual Blockade with Dupilumab Is Linked to Some Emergent T Helper 17Type Diseases, Including Seronegative Arthritis and Enthesitis/Enthesopathy, but Not to Humoral Autoimmune Diseases. J Invest Dermatol 2022, 142, 2660–2667. [Google Scholar] [CrossRef]
- CIAZYNSKA, M.; OLEJNICZAK-STARUCH, I.; SOBOLEWSKA-SZTYCHNY, D.; NARBUTT, J.; SKIBINSKA, M.; LESIAK, A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life (Basel) 2021, 11. [Google Scholar]
- DAMIANI, G.; ODORICI, G.; PACIFICO, A.; MORRONE, A.; CONIC, R.R.Z.; DAVIDSON, T.; WATAD, A.; PIGATTO, P.D.M.; COLOMBO, D.; MALAGOLI, P.; FIORE, M. Secukinumab Loss of Efficacy Is Perfectly Counteracted by the Introduction of Combination Therapy (Rescue Therapy): Data from a Multicenter Real-Life Study in a Cohort of Italian Psoriatic Patients That Avoided Secukinumab Switching. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- ÉCONOMIQUES, O. D. C. E. D. D. 2016. Test No. 487: In vitro mammalian cell micronucleus test, OECD Publishing.
- ELDER, J. T. Expanded genome-wide association study meta-analysis of psoriasis expands the catalog of common psoriasis-associated variants. Journal of Investigative Dermatology Symposium Proceedings, 2018. Elsevier, S77-S78.
- FENECH, M. 2007. Cytokinesis-block micronucleus cytome assay. Nat Protoc 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- FENECH, M. 2009. A lifetime passion for micronucleus cytome assays—reflections from Down Under. Mutation Research/Reviews in Mutation Research 2009, 681, 111–117. [Google Scholar] [CrossRef]
- FENECH, M.; KNASMUELLER, S.; BOLOGNESI, C.; BONASSI, S.; HOLLAND, N.; MIGLIORE, L.; PALITTI, F.; NATARAJAN, A. T.; KIRSCH-VOLDERS, M. Molecular mechanisms by which in vivo exposure to exogenous chemical genotoxic agents can lead to micronucleus formation in lymphocytes in vivo and ex vivo in humans. Mutat Res 2016, 770, 12–25. [Google Scholar] [CrossRef]
- GRIFFITHS, C.E.; BARKER, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef]
- HEIDENREICH, R.; ROCKEN, M.; GHORESCHI, K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol 2009, 90, 232–248. [Google Scholar] [CrossRef]
- KADAM, D.P.; SURYAKAR, A.N.; ANKUSH, R.D.; KADAM, C.Y.; DESHPANDE, K.H. Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem 2010, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- LAI, R.; XIAN, D.; XIONG, X.; YANG, L.; SONG, J.; ZHONG, J. Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cel. Redox Report 2018, 23, 130–135. [Google Scholar] [CrossRef] [PubMed]
- LIN, X.; HUANG, T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free radical research 2016, 50, 585–595. [Google Scholar] [CrossRef] [PubMed]
- LOMBARDO, D.; KISELEV, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- MAGDOLENOVA, Z.; LORENZO, Y.; COLLINS, A.; DUSINSKA, M. Can standard genotoxicity tests be applied to nanoparticles? Journal of Toxicology and Environmental Health, Part A 2012, 75, 800–806. [Google Scholar] [CrossRef] [PubMed]
- MATSUZAKI, G.; UMEMURA, M. Interleukin-17 family cytokines in protective immunity against infections: role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. Microbiol Immunol 2018, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- MIYAGAWA, F. Pathogenesis of Paradoxical Reactions Associated with Targeted Biologic Agents for Inflammatory Skin Diseases. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- MOLDEN, R.; HU, M.; YEN, E.S.; SAGGESE, D.; REILLY, J.; MATTILA, J.; QIU, H.; CHEN, G.; BAK, H.; LI, N. Host cell protein profiling of commercial therapeutic protein drugs as a benchmark for monoclonal antibody-based therapeutic protein development. MAbs 2021, 13, 1955811. [Google Scholar] [CrossRef]
- NAJAFZADEH, M.; GUEIDAN, C.; BADALI, H.; VAN DEN ENDE, A.G.; XI, L.; DE HOOG, G. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Medical mycology 2009, 47, 17–25. [Google Scholar] [CrossRef]
- NAJAFZADEH, M.A.D. The use of isolated peripheral lymphocytes and human whole blood in the comet assay. Nature Protocolexchange 2016. [Google Scholar] [CrossRef]
- NESTLE, F.O.; TURKA, L.A.; NICKOLOFF, B.J. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest 1994, 94, 202–209. [Google Scholar] [CrossRef]
- NO, O.T. 489: In vivo Mammalian alkaline Comet assay. OECD Guidelines for the Testing of Chemicals. OECD Publ 2014, 4, 1–21. [Google Scholar]
- NSAIRAT, H.; KHATER, D.; SAYED, U.; ODEH, F.; AL BAWAB, A.; ALSHAER, W. Liposomes: structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- REICH, K.; BLAUVELT, A.; ARMSTRONG, A.; LANGLEY, R.G.; DE VERA, A.; KOLBINGER, F.; SPINDELDREHER, S.; REN, M.; BRUIN, G. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits low immunogenicity in psoriasis patients treated up to 5 years. J Eur Acad Dermatol Venereol 2019, 33, 1733–1741. [Google Scholar] [CrossRef]
- RENDON, A.; SCHÄKEL, K. Psoriasis Pathogenesis and Treatment. International journal of molecular sciences 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- SCHWEIKERT, B.; MALMBERG, C.; AKERBORG, O.; KUMAR, G.; NOTT, D.; KIRI, S.; SAPIN, C.; HARTZ, S. Cost-Effectiveness Analysis of Sequential Biologic Therapy with Ixekizumab Versus Secukinumab in the Treatment of Active Psoriatic Arthritis with Concomitant Moderate-to-Severe Psoriasis in the UK. Pharmacoecon Open 2020, 4, 635–648. [Google Scholar] [CrossRef] [PubMed]
- SERCOMBE, L.; VEERATI, T.; MOHEIMANI, F.; WU, S.Y.; SOOD, A.K.; HUA, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- STANIĆ, D.; PLEĆAŠ-SOLAROVIĆ, B.; PETROVIĆ, J.; BOGAVAC-STANOJEVIĆ, N.; SOPIĆ, M.; KOTUR-STEVULJEVIĆ, J.; IGNJATOVIĆ, S.; PEŠIĆ, V. Hydrogen peroxide-induced oxidative damage in peripheral blood lymphocytes from rats chronically treated with corticosterone: The protective effect of oxytocin treatment. Chemico-biological interactions 2016, 256, 134–141. [Google Scholar] [CrossRef]
- TICE, R.R.; AGURELL, E.; ANDERSON, D.; BURLINSON, B.; HARTMANN, A.; KOBAYASHI, H.; MIYAMAE, Y.; ROJAS, E.; RYU, J.C.; SASAKI, Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 2000, 35, 206–221. [Google Scholar] [CrossRef]
- WAGENER, F.A.; CARELS, C.E.; LUNDVIG, D. Targeting the redox balance in inflammatory skin conditions. International journal of molecular sciences 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- YAMANAKA, K. Special Issue: "Skin Disease and Comorbidities. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- ZOU, Y.; MENG, Z. Literature Overview of the IL-17 Inhibition from Psoriasis to COVID-19. J Inflamm Res 2021, 14, 5611–5618. [Google Scholar] [CrossRef] [PubMed]
Figure 3.
Cytotoxicity of Secukinumab on human peripheral lymphocytes after 24 hrs exposure to different concentrations (2.1, 2.8, and 3.751 μg/mL) by using CCK8.
Figure 3.
Cytotoxicity of Secukinumab on human peripheral lymphocytes after 24 hrs exposure to different concentrations (2.1, 2.8, and 3.751 μg/mL) by using CCK8.
Figure 4.
The effects of Secukinumab and H2O2 on the lymphocytes from healthy and patient groups measuring OTM (* comparing the treatments to the without treatments or negative control or NC, ns (non-significant) comparing the groups with different treatments to the NC group, + comparing the groups with different treatments to the positive control or PC) ( N=20 in the healthy control group and 20 in the patient group), ), N/C stands for the negative control, P/C; positive control, Bulk; secukinumab bulk, liposome (liposome form of secukinumab), empty; liposomes with no drug, hydrogen peroxide (H2O2).
Figure 4.
The effects of Secukinumab and H2O2 on the lymphocytes from healthy and patient groups measuring OTM (* comparing the treatments to the without treatments or negative control or NC, ns (non-significant) comparing the groups with different treatments to the NC group, + comparing the groups with different treatments to the positive control or PC) ( N=20 in the healthy control group and 20 in the patient group), ), N/C stands for the negative control, P/C; positive control, Bulk; secukinumab bulk, liposome (liposome form of secukinumab), empty; liposomes with no drug, hydrogen peroxide (H2O2).
Figure 5.
The effects of Secukinumab and H2O2 on the lymphocytes from 20 healthy and 20 patient groups measuring Tail DNA% (* comparing the treatments to the NC, ns (non-significant) comparing the treatments to the NC, + comparing the treatments to the PC) ( N=20 in the healthy control group and 20 in the patient group), ), N/C stands for the negative control, P/C; positive control, Bulk; secukinumab bulk, liposome (liposome form of secukinumab), empty; liposomes with no drug, hydrogen peroxide (H2O2).
Figure 5.
The effects of Secukinumab and H2O2 on the lymphocytes from 20 healthy and 20 patient groups measuring Tail DNA% (* comparing the treatments to the NC, ns (non-significant) comparing the treatments to the NC, + comparing the treatments to the PC) ( N=20 in the healthy control group and 20 in the patient group), ), N/C stands for the negative control, P/C; positive control, Bulk; secukinumab bulk, liposome (liposome form of secukinumab), empty; liposomes with no drug, hydrogen peroxide (H2O2).
Figure 6.
The average of MNi in BiNC scored per 1000 cells from 5 healthy individuals and 5 psoriasis patients, n=1000. Data are expressed as means ± standard error (SE).Seven treatment groups included the negative control, two positive control groups (0.4µM MMC) (75µМ of H2O2), 2.1µg/ml of Secukinumab, 2.1µg/ml of Secukinumab with H2O2 and the liposome form of both (*** represents P< 0.0001, **p<0.001, *p<0.02, *p<0.018, ns=not significant). ( N=5 in the healthy control group and 5in the patient group), ), N/C stands for the negative control, P/C; positive control, Bulk; secukinumab bulk, liposome (liposome form of secukinumab), empty; liposomes with no drug, hydrogen peroxide (H2O2).
Figure 6.
The average of MNi in BiNC scored per 1000 cells from 5 healthy individuals and 5 psoriasis patients, n=1000. Data are expressed as means ± standard error (SE).Seven treatment groups included the negative control, two positive control groups (0.4µM MMC) (75µМ of H2O2), 2.1µg/ml of Secukinumab, 2.1µg/ml of Secukinumab with H2O2 and the liposome form of both (*** represents P< 0.0001, **p<0.001, *p<0.02, *p<0.018, ns=not significant). ( N=5 in the healthy control group and 5in the patient group), ), N/C stands for the negative control, P/C; positive control, Bulk; secukinumab bulk, liposome (liposome form of secukinumab), empty; liposomes with no drug, hydrogen peroxide (H2O2).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).