Submitted:
07 March 2024
Posted:
07 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
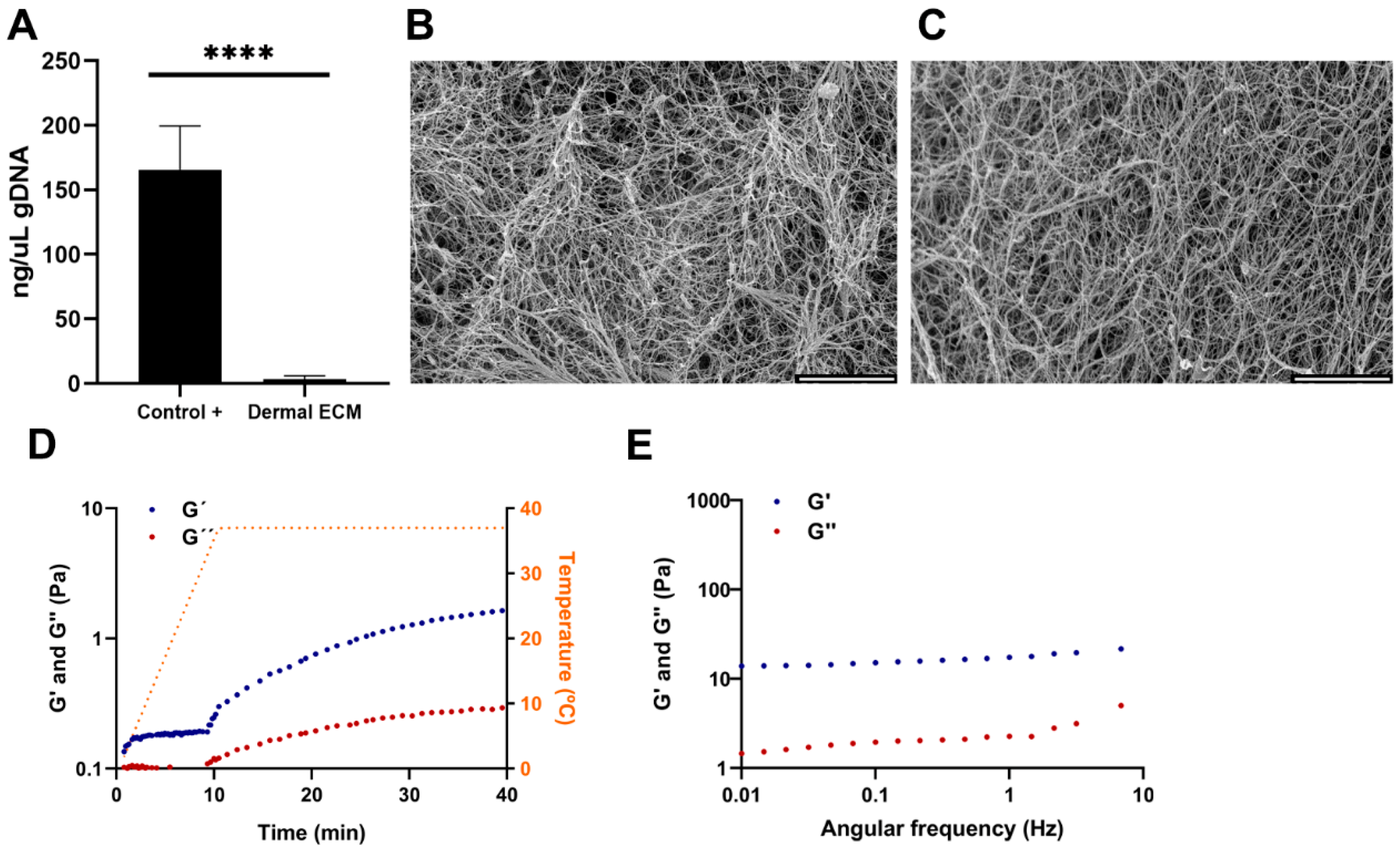
2.1. Sol-Gel Transition of Adult Human Dermal ECM at Physiologic Temperatures
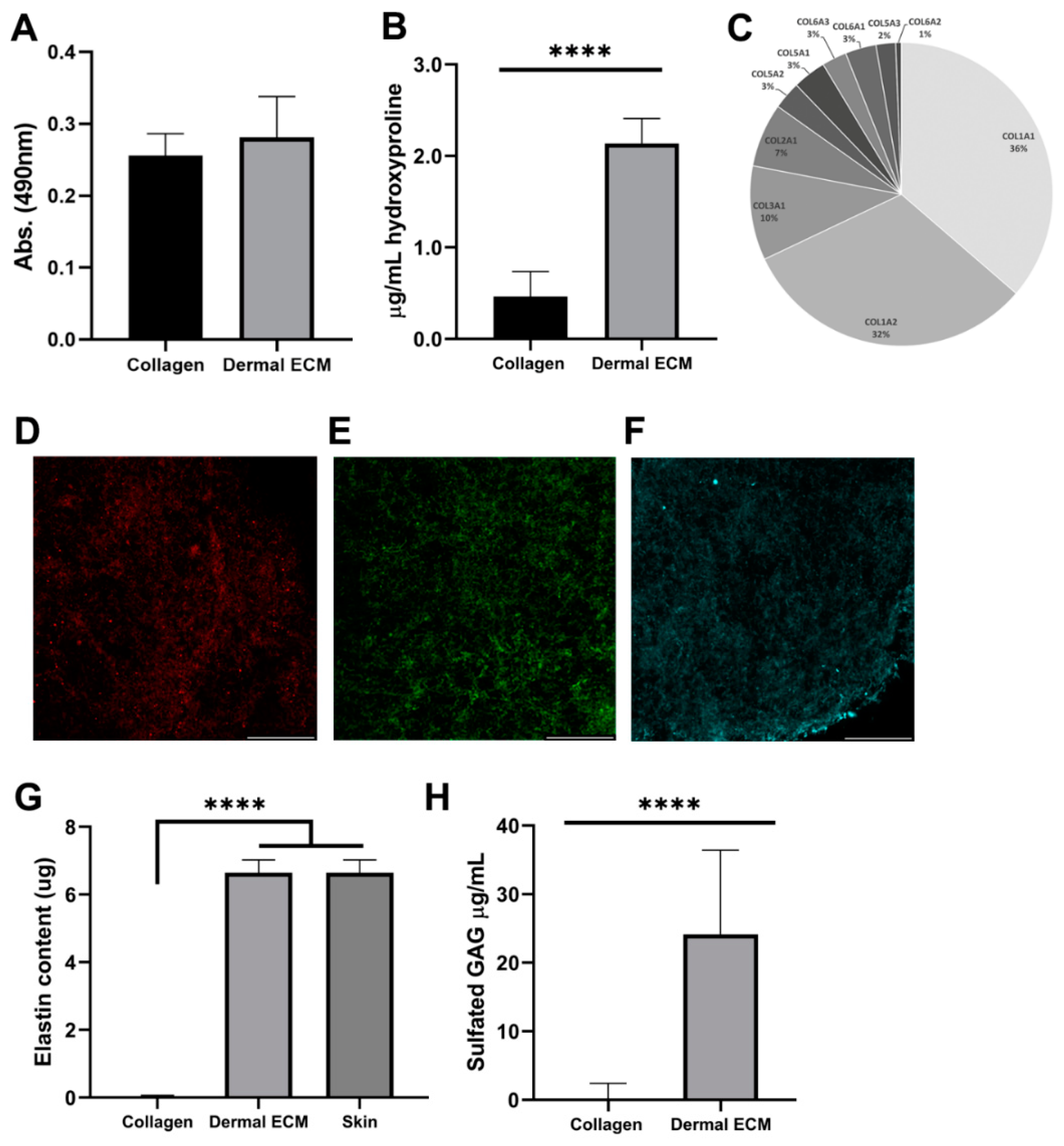
2.2. Human Dermal ECM Hydrogels Preserve Components Present in the Adult Native Tissue
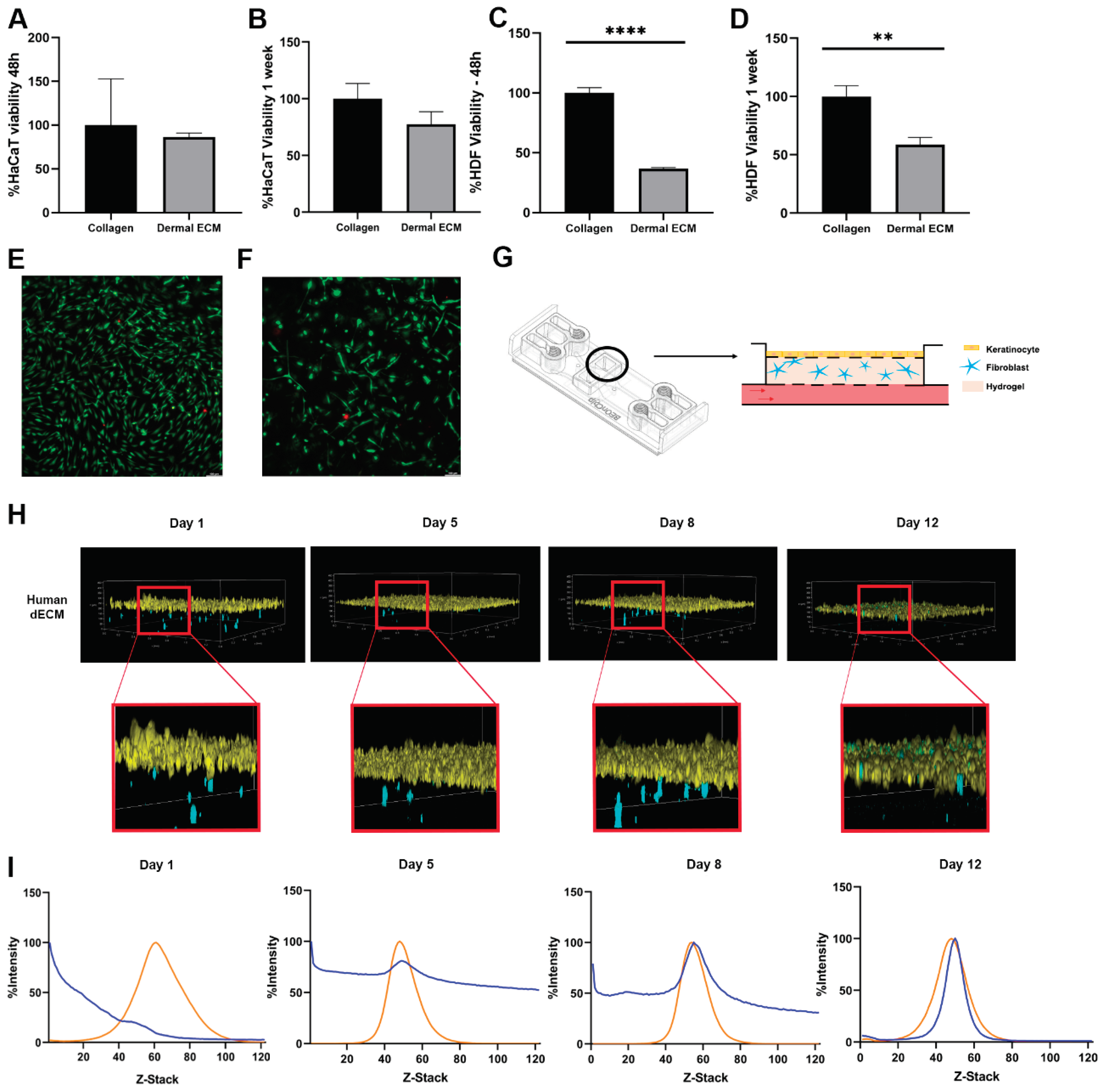
2.3. Human dECM Hydrogels Are Biocompatible and Suitable for Use as Scaffolds in a Skin Aging-on-Chip Model
3. Materials and Methods
3.1. Decellularized Human Dermal ECM Hydrogels
3.2. Human dECM Gelation
3.3. DNA Quantification
3.4. Rheological Characterization
3.5. Human dECM Protein Identification by Liquid Chromatography Mass Spectrometry (LC-MS)
3.6. Biomolecules Quantification within Human Dermal ECM
3.7. Immunofluorescence Microscopy
3.8. Scanning Electron Microscopy
3.9. Metabolic Activity and Viability Assays
3.10. Cell Tracking of Dermal Fibroblast Embedded in Human Dermal ECM Hydrogels within a Microfluidic Device
3.11. Statistical Analysis
4. Conclusion
Supplementary Materials
Acknowledgments
Disclosure of Potential Conflicts of Interest
Data availability statement
References
- Broek, L.J.v.D.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N. A. Structure and Function of Skin. Dermal Absorption Models in Toxicology and Pharmacology 200620-01-379, 1.
- Pissarenko, A.; Meyers, M.A. The materials science of skin: Analysis, characterization, and modeling. Prog. Mater. Sci. 2020, 110. [Google Scholar] [CrossRef]
- Fernandez-Carro, E.; Angenent, M.; Gracia-Cazaña, T.; Gilaberte, Y.; Alcaine, C.; Ciriza, J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022, 14, 1417. [Google Scholar] [CrossRef]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Risueño, I.; Valencia, L.; Jorcano, J. L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioengineering 2021, 5. [Google Scholar] [CrossRef]
- Beißner, N.; Albero, A.B.; Füller, J.; Kellner, T.; Lauterboeck, L.; Liang, J.; Böl, M.; Glasmacher, B.; Müller-Goymann, C.C.; Reichl, S. Improved in vitro models for preclinical drug and formulation screening focusing on 2D and 3D skin and cornea constructs. Eur. J. Pharm. Biopharm. 2018, 126, 57–66. [Google Scholar] [CrossRef]
- Lee, S.; Jin, S.-P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, S.H.; Seo, W.-Y.; Jeong, Y.; Shin, M.C.; Ryu, D.; Lee, S.B.; Choi, Y.J.; Kim, Y.J.C.&.K. Effects of human collagen α-1 type I-derived proteins on collagen synthesis and elastin production in human dermal fibroblasts. BMB Rep. 2021, 54, 329–334. [Google Scholar] [CrossRef]
- Woodley, J.P.; Lambert, D.W.; Asencio, I.O. Understanding Fibroblast Behavior in 3D Biomaterials. Tissue Eng. Part B: Rev. 2022, 28, 569–578. [Google Scholar] [CrossRef]
- Mithieux, S. M.; Weiss, A. S. ELASTIN.
- Adamo, C.S.; Zuk, A.V.; Sengle, G. The fibrillin microfibril/elastic fibre network: A critical extracellular supramolecular scaffold to balance skin homoeostasis. Exp. Dermatol. 2020, 30, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.T.; Holbrook, K.A.; Madri, J.A. Collagen types I, III, and V in human embryonic and fetal skin. Am. J. Anat. 1986, 175, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Heng, S.; Zhang, W.; Liu, Y.; Xia, T.; Ji, C.; Zhang, L.-J. Dermal extracellular matrix molecules in skin development, homeostasis, wound regeneration and diseases. Semin. Cell Dev. Biol. 2022, 128, 137–144. [Google Scholar] [CrossRef]
- Uitto, J.; Olsen, D.R.; Fazio, M.J. Extracellular Matrix of the Skin: 50 Years of Progress. J. Investig. Dermatol. 1989, 92, 61S–77S. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J. K.; Ou, G.; Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. Nature reviews. Molecular cell biology 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Penc, S.F.; Pomahac, B.; Winkler, T.; Dorschner, R.A.; Eriksson, E.; Herndon, M.; Gallo, R.L. Dermatan Sulfate Released after Injury Is a Potent Promoter of Fibroblast Growth Factor-2 Function. J. Biol. Chem. 1998, 273, 28116–28121. [Google Scholar] [CrossRef]
- Zhou, H.-M.; Wang, J.; Elliott, C.; Wen, W.; Hamilton, D.W.; Conway, S.J. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J. Cell Commun. Signal. 2010, 4, 99–107. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Prince, S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Takeda, U.; Utani, A.; Wu, J.; Shinkai, H.; Adachi, E.; Koseki, H.; Taniguchi, M.; Matsumoto, T.; Ohashi, T.; Sato, M. Targeted Disruption of Dermatopontin Causes Abnormal Collagen Fibrillogenesis. J. Investig. Dermatol. 2002, 119, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef]
- Sarmin, A.M.; El Moussaid, N.; Suntornnond, R.; Tyler, E.J.; Kim, Y.-H.; Di Cio, S.; Megone, W.V.; Pearce, O.; Gautrot, J.E.; Dawson, J.; et al. Multi-Scale Analysis of the Composition, Structure, and Function of Decellularized Extracellular Matrix for Human Skin and Wound Healing Models. Biomolecules 2022, 12, 837. [Google Scholar] [CrossRef]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and functional comparisons of four anatomical regions of porcine skin with human abdominal skin. Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Casarrubios, L.; Feito, M.J.; Madarieta, I.; Garcia-Urkia, N.; Murua, O.; Olalde, B.; Briz, N.; Diez-Orejas, R.; Portolés, M.T. Effects of Human and Porcine Adipose Extracellular Matrices Decellularized by Enzymatic or Chemical Methods on Macrophage Polarization and Immunocompetence. Int. J. Mol. Sci. 2021, 22, 3847. [Google Scholar] [CrossRef]
- Liao, P.; Wang, Z. Thiel-embalming technique: investigation of possible modification in embalming tissue as evaluation model for radiofrequency ablation. J. Biomed. Res. 2019, 33, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chaudhary, P.P.; D’souza, B.N.; Spathies, J.; Myles, I.A. Impact of Skin Tissue Collection Method on Downstream MALDI-Imaging. Metabolites 2022, 12, 497. [Google Scholar] [CrossRef]
- Crosado, B.; Löffler, S.; Ondruschka, B.; Zhang, M.; Zwirner, J.; Hammer, N. Phenoxyethanol-Based Embalming for Anatomy Teaching: An 18 Years’ Experience with Crosado Embalming at the University of Otago in New Zealand. Anat. Sci. Educ. 2019, 13, 778–793. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory Effects of Plant Phenols on the Activity of Selected Enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Heal. Mater. 2020, 9, 2000734. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- White, L. J.; Taylor, A. J.; Faulk, D. M.; Keane, T. J.; Saldin, L. T.; Reing, J. E.; Swinehart, I. T.; Turner, N. J.; Ratner, B. D.; Badylak, S. F. The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomaterialia 2016, 50, 207. [Google Scholar] [CrossRef] [PubMed]
- Lamers, E.; Van Kempen, T. H. S.; Baaijens, F. P. T.; Peters, G. W. M.; Oomens, C. W. J. Large amplitude oscillatory shear properties of human skin. Journal of the Mechanical Behavior of Biomedical Materials 2013, 28. [Google Scholar]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Ski. Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Siperko, L.M.; Seehra, G.P. Mechanobiology of force transduction in dermal tissue. Ski. Res. Technol. 2003, 9, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Pailler-Mattei, C.; Debret, R.; Vargiolu, R.; Sommer, P.; Zahouani, H. In vivo skin biophysical behaviour and surface topography as a function of ageing. J. Mech. Behav. Biomed. Mater. 2013, 28, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.; Laquièze, L.; Le Bot, A.; Laquièze, S.; Zahouani, H. Dynamic indentation on human skinin vivo: ageing effects. 2009, 15, 55.
- Jachowicz, J.; McMullen, R.; Prettypaul, D. Indentometric analysis of in vivo skin and comparison with artificial skin models. Ski. Res. Technol. 2007, 13, 299–309. [Google Scholar] [CrossRef]
- Castro-Abril, H.; Heras, J.; del Barrio, J.; Paz, L.; Alcaine, C.; Aliácar, M.P.; Garzón-Alvarado, D.; Doblaré, M.; Ochoa, I. The Role of Mechanical Properties and Structure of Type I Collagen Hydrogels on Colorectal Cancer Cell Migration. Macromol. Biosci. 2023, 23, e2300108. [Google Scholar] [CrossRef] [PubMed]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef]
- Uitto, J.; Perejda, A.J.; Abergel, R.P.; Chu, M.L.; Ramirez, F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc. Natl. Acad. Sci. 1985, 82, 5935–5939. [Google Scholar] [CrossRef]
- Uitto, J.; Olsen, D.R.; Fazio, M.J. Extracellular Matrix of the Skin: 50 Years of Progress. J. Investig. Dermatol. 1989, 92, 61S–77S. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, O.; Fujiwara, S. Dermatopontin, a Novel Player in the Biology of the Extracellular Matrix. Connect. Tissue Res. 2006, 47, 177–189. [Google Scholar] [CrossRef]
- Lewandowska, K.; Choi, H.U.; Rosenberg, L.C.; Sasse, J.; Ame, P.J.N.; Culp, L.A. Extracellular matrix adhesion-promoting activities of a dermatan sulfate proteoglycan-associated protein (22K) from bovine fetal skin. J. Cell Sci. 1991, 99, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.M.; Watson, R.E.B. Review Article: A new wrinkle on old skin: the role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Actin cytoskeleton assembly regulates collagen production via TGF-β type II receptor in human skin fibroblasts. J. Cell. Mol. Med. 2018, 22, 4085–4096. [Google Scholar] [CrossRef] [PubMed]
- Theocharidis, G.; Connelly, J.T. Minor collagens of the skin with not so minor functions. J. Anat. 2017, 235, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kwon, Y.W.; Kong, J.-S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.-B.; Lee, H.; Kim, J.H.; Cho, D.-W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Mouw, J. K.; Ou, G.; Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. 2014, 15, 771-785.
- Ingber, D.E. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.; Kim, H.; Sung, G.Y. Effect of α-Lipoic Acid on the Development of Human Skin Equivalents Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2021, 22, 2160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Sung, G.Y. Coenzyme Q10 Efficacy Test for Human Skin Equivalents Using a Pumpless Skin-On-A-Chip System. Int. J. Mol. Sci. 2020, 21, 8475. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef]
- Ozpinar, E.W.; Frey, A.L.; Arthur, G.K.; Mora-Navarro, C.; Biehl, A.; Snider, D.B.; Cruse, G.; Freytes, D.O. Dermal Extracellular Matrix-Derived Hydrogels as an In Vitro Substrate to Study Mast Cell Maturation. Tissue Eng. Part A 2021, 27, 1008–1022. [Google Scholar] [CrossRef]
- Schneider, C. A.; Rasband, W. S.; Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis: for the past 25 years NIH image and ImageJ software have been pioneers as open tools for the analysis of scientific images. We discuss the origins, challenges and solutions of these two programs, and how their history can serve to advise and inform other software projects. 2012, 9, 671. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




