1. Introduction
Due to economic growth, increasing population, urbanisation, industrialisation, and agricultural production, some new toxic substances are detected in the water environment. A significant group of new chemical compounds is included in the group termed emerging contaminants (ECs). These substances are characterised by relatively low concentrations in water, typically in concentrations ranging from ng·L
−1 to μg·L
−1, and in many cases, their presence in the water environment is generally not monitored. However, they are recognised as significant and dangerous water pollutants due to their long term stability in water and tendency to accumulate in the environment [
1].
One of the types of EC are pharmacological substances belonging to a family of compounds called, in short, PPCPs (pharmaceuticals and personal care products). Most of these substances are toxic to organisms and increase the phenomenon of microorganisms' drug resistance. Although pharmaceuticals are in low concentrations, their bioaccumulating capacity makes them unsafe for organisms [
2,
3]. Moreover, the studies show that the transformation products' metabolites and derivatives are more toxic than the primary forms of pharmaceuticals [
4].
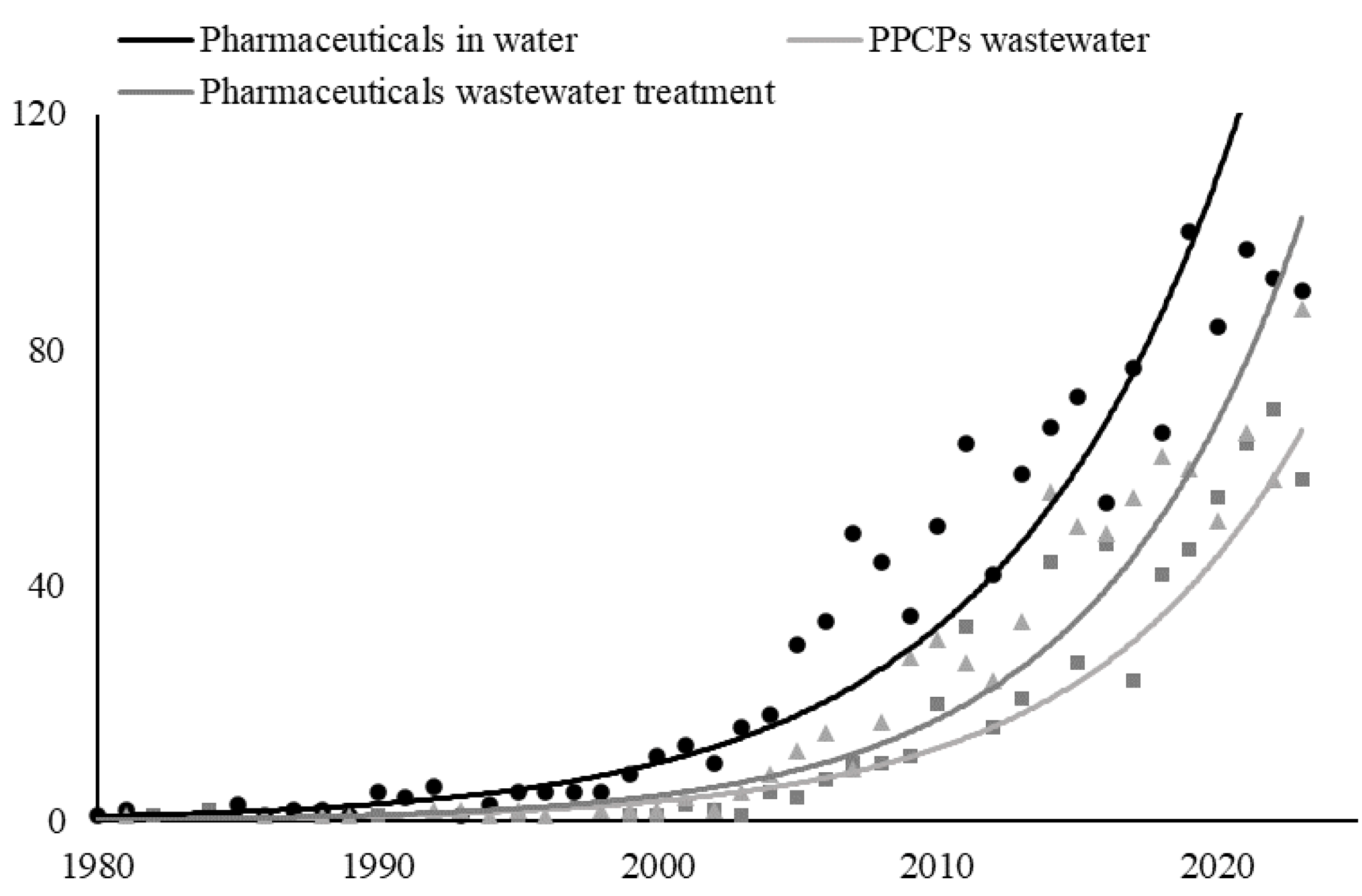
The increasing interest in contamination of water by pharmaceutical and PPCP components might be confirmed by an increase in the number of scientific papers in recent years related to the subject of pharmaceutical ingredients in water, pharmaceutical wastewater treatment and PPCP wastewater (
Figure 1).
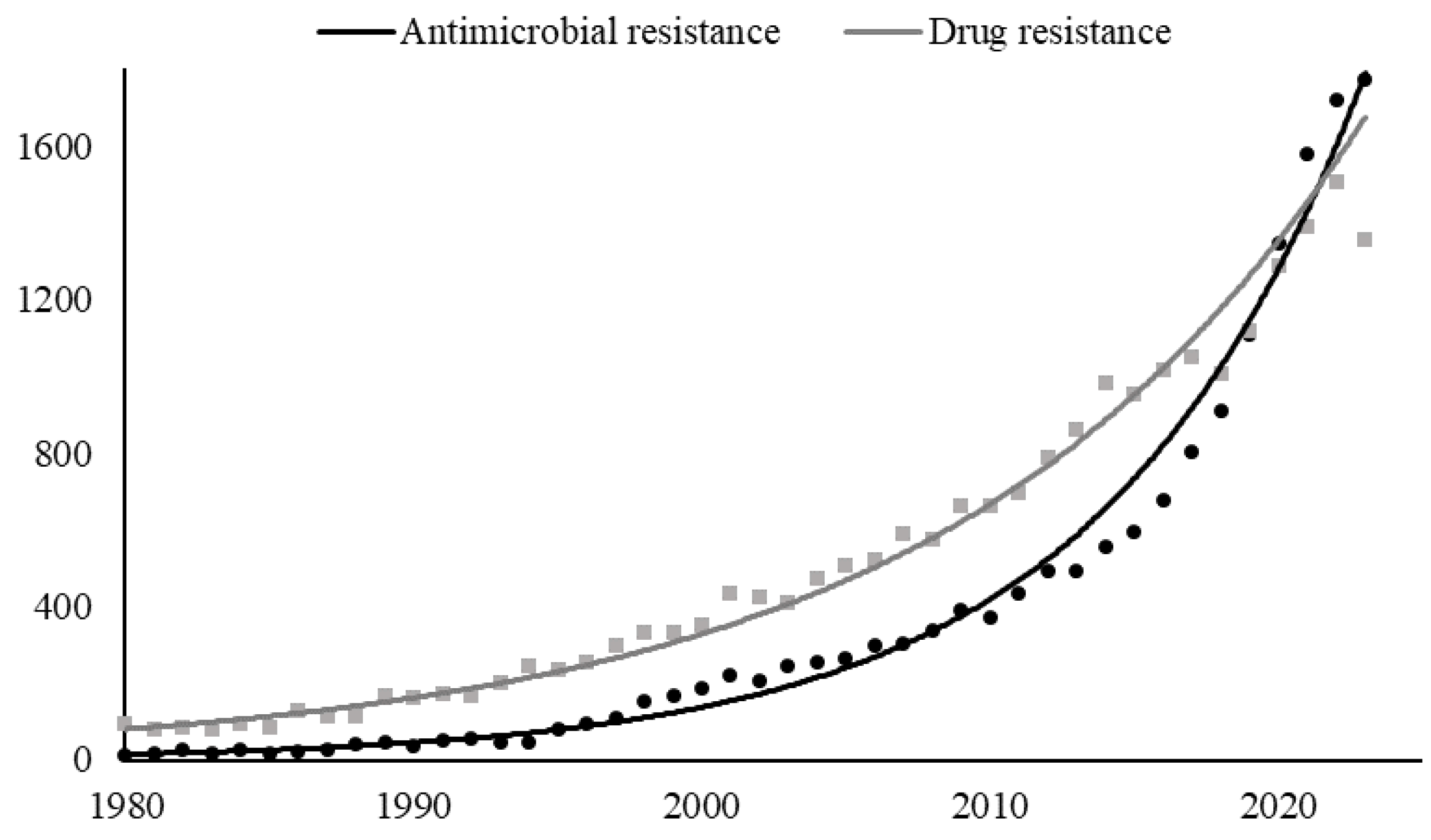
Another aspect related to the presence of pharmaceuticals in the water environment is the phenomenon of antimicrobial resistance. It occurs when bacteria, fungi or other microorganisms no longer respond to medicines. Due to that, the medicines become ineffective and treating infections are harder and could lead to death at times [
5,
6,
7]. One of the top 10 global public health threats facing humanity by WHO is antimicrobial resistance. To underline how awareness of the phenomenon increased during years number of science articles with different keywords are shown in the
Figure 2.
The source of pharmaceuticals in water might be domestic, urban, hospital, and industrial wastewater, as well as effluents from sewage treatment plants (STPs), aquaculture, and intensive livestock farming. However, it should also be noted that except for a few different sources of pharmaceuticals, the problem also refers to many different substances with other chemical properties detected in various types of water bodies [
8,
9,
10,
11]. For these reasons, using a single, efficient method to remove pharmaceutical substances from water is complicated, and it is necessary to develop new treatment methods or improve the efficiency of existing technologies.
Several treatment processes for removing PPCPs from water are known, such as membrane techniques, advanced oxidation processes, and biological methods [
12,
13,
14]. Adsorption is one of well-known and usually cheap methods for removing pollutants from water. During the process, substances bind to the solid’s surface from water or gas. The most significant advantages of this type of purification are the lack of harmful products and the independence of toxic substances contained in the cleaned stream [
15,
16,
17,
18]. Many materials are used as adsorbents, such as activated carbon, carbon nanotubes, and graphite. However, despite their high efficiency, the relatively high cost of sorbent and operation (incl. need for regeneration or bed replacement after a number of cycles) is a serious disadvantage [
12,
19,
20,
21,
22,
23]. Due to that, natural materials have been evaluated into non-conventional adsorbents such as biochar, zeolites, chitosan, coconut shells, metal-organic frameworks (MOFs), and others [
23,
24]. They are developing not only because they are cheaper but also more environmentally friendly and available in large amounts from industries or agriculture.
One of the non-conventional adsorbents is the calcium carbonate (CaCO
3). This substance is a natural mineral which is known for its good thermal stability and low cost [
25,
26]. In the literature, many studies consider the molecular level of calcium carbonate precipitation, aggregation, and formation [
27,
28,
29]. It is related to the increasing use of CaCO
3 nanoparticle aggregates in industry, e.g. as an adsorbent for cationic dye molecules [
30], for pharmaceutical substances [
31], or in biomedical applications [
26,
32,
33].
This work aimed to determine the adsorption potential of CaCO3 for PPCPs, which was produced using our own method. Additionally, the adsorption tests using the CaCO3 as a sorbent were carried out in different systems, where CaCO3 particles were suspended in pharmaceutical solutions or where CaCO3 particles were an inorganic filler in the produced non-homogeneous polymeric membranes. Additionally, the antibacterial properties of CaCO3 particles and the membranes modified by CaCO3 were determined.
2. Materials and Methods
2.1. Materials
To prepare pharmaceuticals solutions for experiments, sulfadiazine (Sigma Aldrich, Darmstadt, Germany), tetracycline (Pol-Aura, Olsztyn, Poland) and ultrapure water (own reverse osmosis laboratory installation) were used. The solution concentration was 40 mg/dm3. Additionally, NaCl (Pol-Aura, Olsztyn, Poland) and SiO2 (Sigma Aldrich, St. Louis, USA) were used to prepare test solutions.
Calcium carbonate, CaCO3, was produced by the original method, which is presented in the next section. Calcium hydroxide (Chempur, Piekary Slaskie, Poland), carbon dioxide (99,99%) (Multax, Stare Babice, Poland) and ultrapure water were used to produce calcium carbonate.
Polysulfone (PS) polymer (Sigma Aldrich, Finland Oy) was used to produce the membranes. N-methyl pyrrolidone (NMP) (Sigma Aldrich, Poznan, Poland) (ACS reagent, ≥99.0%) was used as the solvent. The membranes were produced by the wet-phase inversion process [
34]. All important parameters related to the membrane process production were determined during our own research.
Additionally, commercial flat sheet ultrafiltration polyvinylidene fluoride (PVDF) membranes (Microdyne Nadir, Wiesbaden, Germany) were used during experiments.
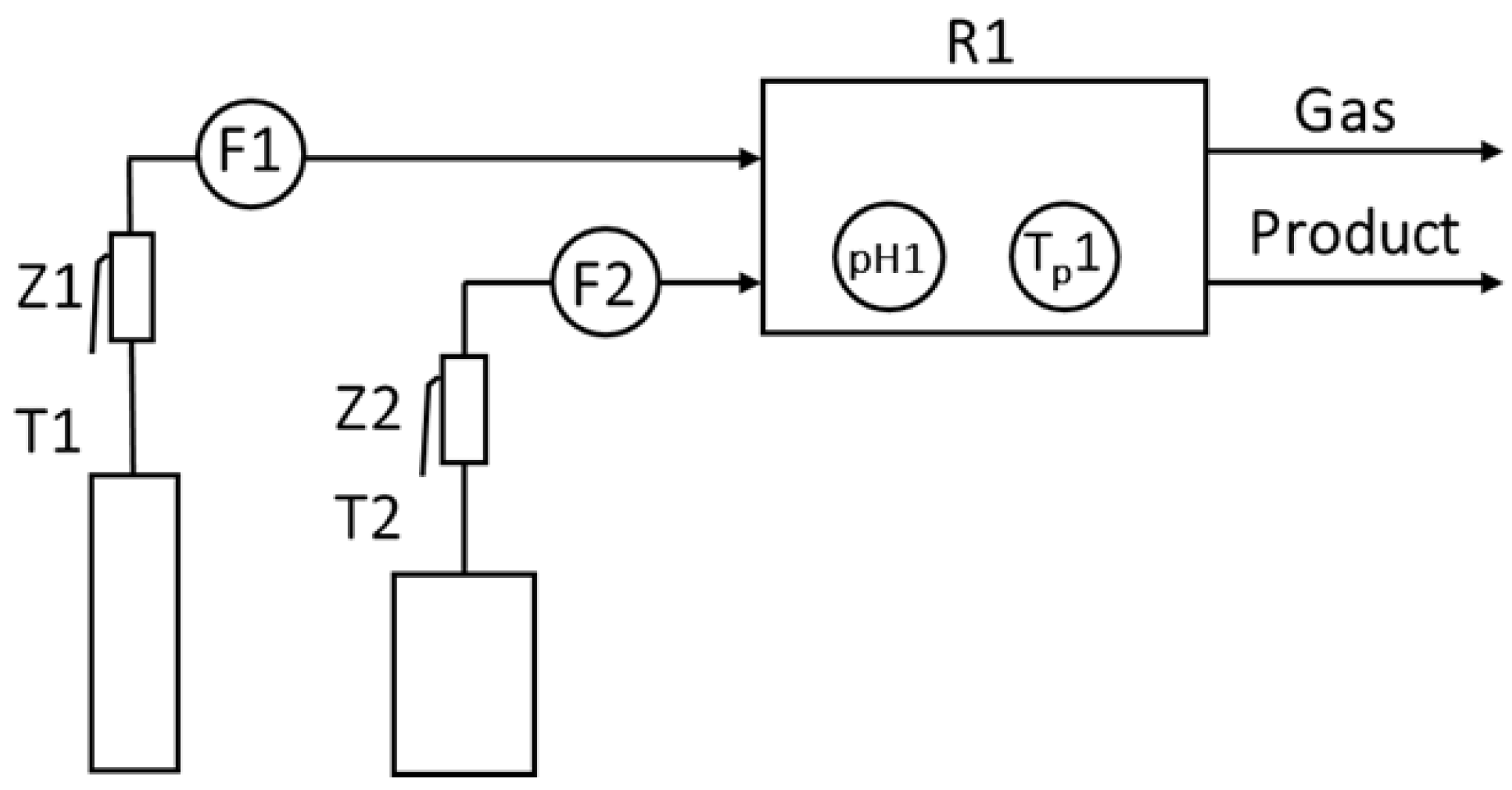
2.2. CaCO3 Production Method
Calcium carbonate has been produced in the laboratory using the method previously developed by our team’s members [
27,
35]. The installation with a rotated disc is shown in
Figure 3. In the first step, a calcium hydroxide solution, which was oversaturated and mixed for 48 hours, was prepared. Then, the solution was filtered using a 0.1 μm filter. Both processes were performed under atmospheric pressure and at room temperature (25°C). The temperature and pH of the calcium hydroxide solution were controlled during the production process. The initial pH of the solution was between 10.80 – 11.00. The production starts when the gas CO
2 flows and contacts with the solution. Gas flow was 2 dm
3/min all the time. The process was stopped when the pH decreased to 7.00. It means that the solution became neutralised.
The solution of calcium carbonate from the reactor was also filtered on a vacuum set. The crystals were poured with ultrapure water, dried in an oven at 80°C until the weight was stable, and finally stored in a desiccator.
2.3. Method of Membrane Preparation
The membranes were prepared by the wet-phase inversion method. The membrane-forming solution was prepared by dissolving PS in NMP with a concentration of 11% (w/w). Our own earlier research determined the concentration of the polymer. Using a commercially available casting knife, a film of the appropriate thickness was formed. In turn, for the preparation of membranes with calcium carbonate particles as a filler, the CaCO3 was added to the polymer solution in 10% or 20% concentration relative to the weight of the polymer. The suspension was stirred and placed in an ultrasonic bath before forming the layer with the casting knife. The temperature of the membrane-forming solution or suspension was 25°C.
2.4. Testing of CaCO3 Adsorption Properties
Testing of CaCO3 adsorption properties was conducted in three different systems. In the first system - the adsorption stationary system, CaCO3 particles acted as adsorbent. In this study, three distinct masses of calcium carbonate were prepared in samples and analysed in a stationary system. The pharmaceutical solutions were added to the adsorbents. Three concentrations of suspensions were considered - 0.05%, 0.1% and 0.5% (weight of calcium carbonate/weight of suspension). After the adsorption process, the suspension had to be clarified before quantitative pharmaceutical analysis. In this system, centrifugation was used to separate CaCO3 particles from the suspensions. The centrifugation time was included in the adsorption time because the particles remained in contact with the removed substance.
In the second system – the adsorption flow system the CaCO3 particles played the role of adsorption bed in the flow system. In this system, a suspension of CaCO3 particles and pharmaceutical solution circulated during the test. The PVDF membrane was used to separate CaCO3 particles from the solutions.
In the third system, two processes were combined into one integrated process. Therefore, the filtration-adsorption process was analysed. In this system, CaCO
3 particles were used as fillers in the produced membranes. Additionally, in this system, the impact of the presence of salt (NaCl) and solid particles (SiO
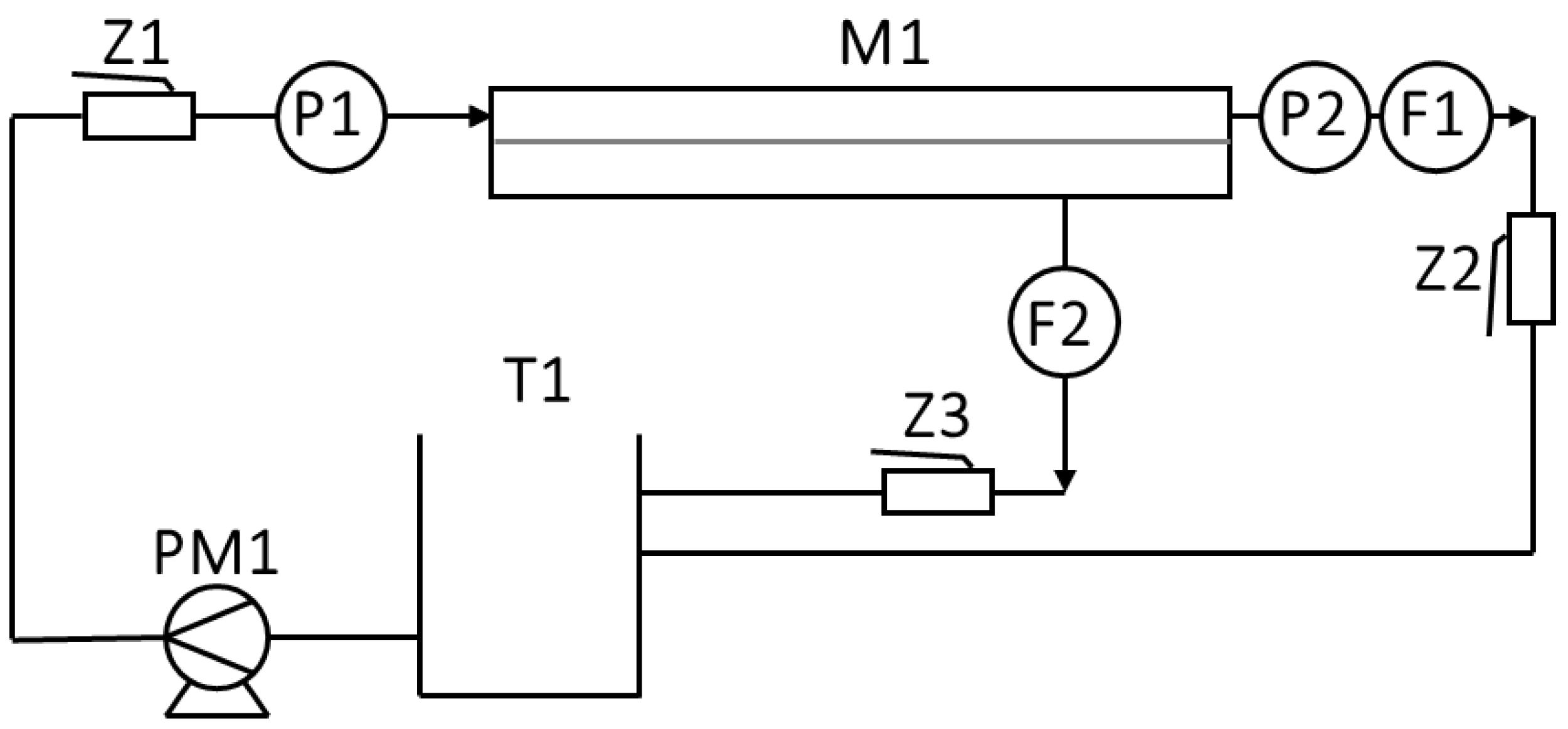
2 particles) on the filtration-adsorption process was tested. In both flow systems, the standard membrane filtration process was performed. A laboratory filtration plant (
Figure 4) was used to carry out the membrane filtration process.
The effectiveness of pharmaceutical removal in all used systems was determined based on changes in the concentration of tested substance in water during the processes. In the case test with suspension, the mass of removed pharmaceutical was given per unit mass of CaCO3 mCaCO3 [g]. In turn, in the case of an adsorption-filtration system, the mass was given per unit surface of membranes Amem [m2]. However, knowing the volume of the membrane and the filling particle content, it is possible to estimate the mass of removed pharmaceuticals per unit mass of CaCO3. The tested substance concentration was determined by using UV-vis spectroscopy. Additionally, turbidity measurement was carried out to determine the efficiency of retaining calcium carbonate by the membrane in the case of a flow system and to determine the efficiency of suspension separation in a stationary system. An MI 415 turbidimeter (Milwaukee, Rocky Mount, USA) was used.
2.5. Morphology of Membranes Testing
The morphology of the cross-section of the membranes was examined by PhenomPro scanning electron microscopy (SEM) (PhenomWorld, Eindhoven, the Netherlands). Before tests, all the membrane samples were frozen in liquid nitrogen and broken.
2.6. Testing of CaCO3 Antibacterial Properties
The evaluation of antibacterial properties of CaCO3 particles and prepared membranes were carried out for two strains of bacteria commonly encountered in the aquatic environment: Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive). The procedure based on the ASTM E2149-13a "Standard Test Method for Determining the Antimicrobial Agents Under Dynamic Contact Conditions" was used in the tests. Each test was repeated three times, and the results were averaged.
The percentage reduction in the number of bacteria
RP was determined based on the equation 1
where
A is concentration of living cells after 1h of contact with the sample [CFU/mL] and
B is the concentration after 1h in the control sample [CFU/mL].
The initial concentration of bacteria was 4.42·104 CFU/ml for E.coli and 4.55·105 CFU/ml for S.aureus.
2.7. Mechanisms of Adsorption
Considering the mechanism of combining an organic substance with some solid particles, adsorption can be divided into physical adsorption and chemical adsorption. Physical adsorption means that the substance at the interface is bound by van der Waals forces or hydrogen bonds [
36,
37]. These interactions are not strong. A physically adsorbed molecule may deform as a result of interaction with the surface, but it does not change as in the case of chemical adsorption. In case of physically adsorption the primary adsorption mechanisms strongly depend on the experimental adsorption conditions like pH or thermodynamics, which is connected with such adsorbate characteristics - solubility, pKa and surface functional groups of adsorbent [
39]. The electrostatic interactions, hydrogen-bonding, π-π electron donor-acceptor interactions between pharmaceuticals and solid depend on pH value. Tetracycline and sulfadiazine are amphoteric molecules with two dissociation constants pKa = 3,30; 7,68 [
39] and pKa = 2,20; 6,5 [
40], respectively. The effect of pH on the intensity of the adsorption process of pharmaceutical substances on solid materials is widely described in the literature [
41,
42,
43,
44]. It should also be noted that the literature underlines the effect of ionic strength, physicochemical properties of adsorbent particles, and process parameters on the intensity of the adsorption process.
3. Results and Discussion
3.1. Adsorption Stationary System
The first research step focused on the determination of CaCO
3 sorption properties in the stationary system. Experiments lasted 90 minutes, and samples were analysed after 90 minutes from the beginning of the experiments. The final results calculated as mass of pharmaceuticals removed per unit mass of calcium carbonate
qI [mg/g] (Equation 2) and process efficiency
η [%] (Equation 3) are presented in
Table 1.
where
c0 [mg/dm
3] is initial concentration of pharmaceutical substance,
ct [mg/dm
3] is concentration of pharmaceutical substance during the process,
mCaCO3 is mass of CaCO
3 [g], in turn
VI [dm
3] is volume of tested solution.
The clear liquid is necessary for UV-Vis spectroscopy analysis, where particles could interfere with light and change the result. Due to that, centrifugation was used to separate adsorbent particles from suspension before analyses. The centrifugation parameters should allow for obtaining the solution with the lowest possible turbidity in a short time, indicating a high level of CaCO
3 separation from the pharmaceutical solution. The chosen centrifugation method has three stages, each lasting 3 minutes with 4000 rpm spin speed. The obtained values of solution turbidity are presented in
Table 1.
The amount of CaCO3 particles corresponding to the obtained solution does not negatively affect the measurements by UV-vis spectroscopy.
Based on the results (
Table 2), it can be noticed that the adsorbed mass of sulfadiazine increases with a more significant amount of sorbent. In the case of tetracycline, removal efficiency at the end of the experiment for 0.05%, 0.1% and 0.5% are, respectively, 1.07%, 4.03% and 10.01%. In the case of sulfadiazine, this parameter reached 19.87%, 17.66%, and 21.91%, respectively. However, the process efficiency for sulfadiazine do not increase significantly. It can result from the particle agglomeration phenomenon. The particle agglomeration decreases mass transfer surfaces, and as a result, the intensity of the adsorption process decreases. The adsorbed mass of tetracycline varies without any trend. The results obtained for measurements for the suspension with the smallest amount of calcium carbonate are questionable. However, no reason for this result was found during testing.
Additionally, based on the results (
Table 2), the difference in the adsorbed mass of tetracycline and sulfadiazine might be the result of the differences in the physicochemical properties of the tested substances. In the case of solid particles without chemical groups on their surface, such as CaCO
3, physical interactions are responsible for the adsorption of substances on their surface. The differences in the amount of adsorbed mass of tetracycline and sulfadiazine can be explained by electrostatic interactions between the tested substances and calcium carbonate. In neutral pH (used ultrapure water was 6.5), the zeta potential on the surface of calcium carbonate is positive [
45]. In turn, tetracycline [
46] and sulfadiazine [
40] are characterized by more than one dissociation constant, which depends on the value of pH. However, for pH=6.5 the percentage of anionic species to neutral or cationic species is higher for the sulfadiazine than tetracycline. As a result, the mass of adsorbed sulfadiazine is higher than tetracycline in the system used.
3.2. Adsorption Flow System
The second research step focused on the determination of CaCO3 sorption properties in the adsorption flow system. During this process, the effectiveness of pharmaceuticals results from filtration on an ultrafiltration membrane and sorption onto calcium carbonate. Additionally, the ultrafiltration process is responsible for removing CaCO3 from the feed solution.
In the stationary system, the highest adsorption concerning the mass of the material was achieved for 0.10% concentration of CaCO
3 in the suspension with tetracycline and for the lowest concentration of CaCO
3, in the case of sulfadiazine. A concentration of 0.05% was chosen to study the integrated filtration and adsorption process, due to the results of sulfadiazine described above and a problem of membrane blocking at higher calcium carbonate concentrations in the suspension. The experiments lasted 90 minutes, and samples were analysed 90 minutes after the beginning of the experiments. The final results calculated as mass of pharmaceuticals removed per unit area of membrane
qII [mg/m
2 ] (Equation 4) are presented in
Table 3.
where
c0 [mg/dm
3] is the initial concentration of a pharmaceutical substance,
ct [mg/dm
3] is the concentration of a pharmaceutical substance during the process,
Amem is the membrane area [m
2], in turn
VI [dm
3] is the volume of tested solution. For the comparison, in
Table 3 also, the results for the tests carried out without CaCO
3 are presented, as a blind probe for membrane.
Based on the result, it can be concluded that the presence of CaCO
3 increases the adsorbed mass in the flow system compared to the pure membrane. However, there is also a significant difference in the results between the adsorbed mass of the tested substances. The adsorbed mass of the substance indicates a lower affinity of sulfadiazine for membranes made of PVDF. This is probably due to the structure and properties of the material, which is hydrophobic [
47,
48]. Moreover, sulfadiazine is characterized by a good affinity for hydrophilic materials, which plays an essential role in its adsorption.
Based on the results presented in
Table 3, the adsorbed mass of pharmaceuticals removed per unit mass of calcium carbonate can be calculated according to Equation 2. The mass of tetracycline removed by calcium carbonate is 232 mg/g, and the mass of sulfadiazine is 524 mg/g.
Additionally, it should also be noted that the PVDF ultrafiltration membrane makes it possible to remove CaCO3 particles from the feed stream, which is confirmed by the results of permeate turbidity. The initial turbidity of each suspension was higher than 1000 NTU. The average value of permeate turbidity for different tests is about 1 NTU. However, the presence of CaCO3 particles in the feed solution is responsible for the fouling phenomena on the membrane surface. As a result, the permeate volume stream insignificantly decreases during the process.
3.3. Filtration-Adsorption System
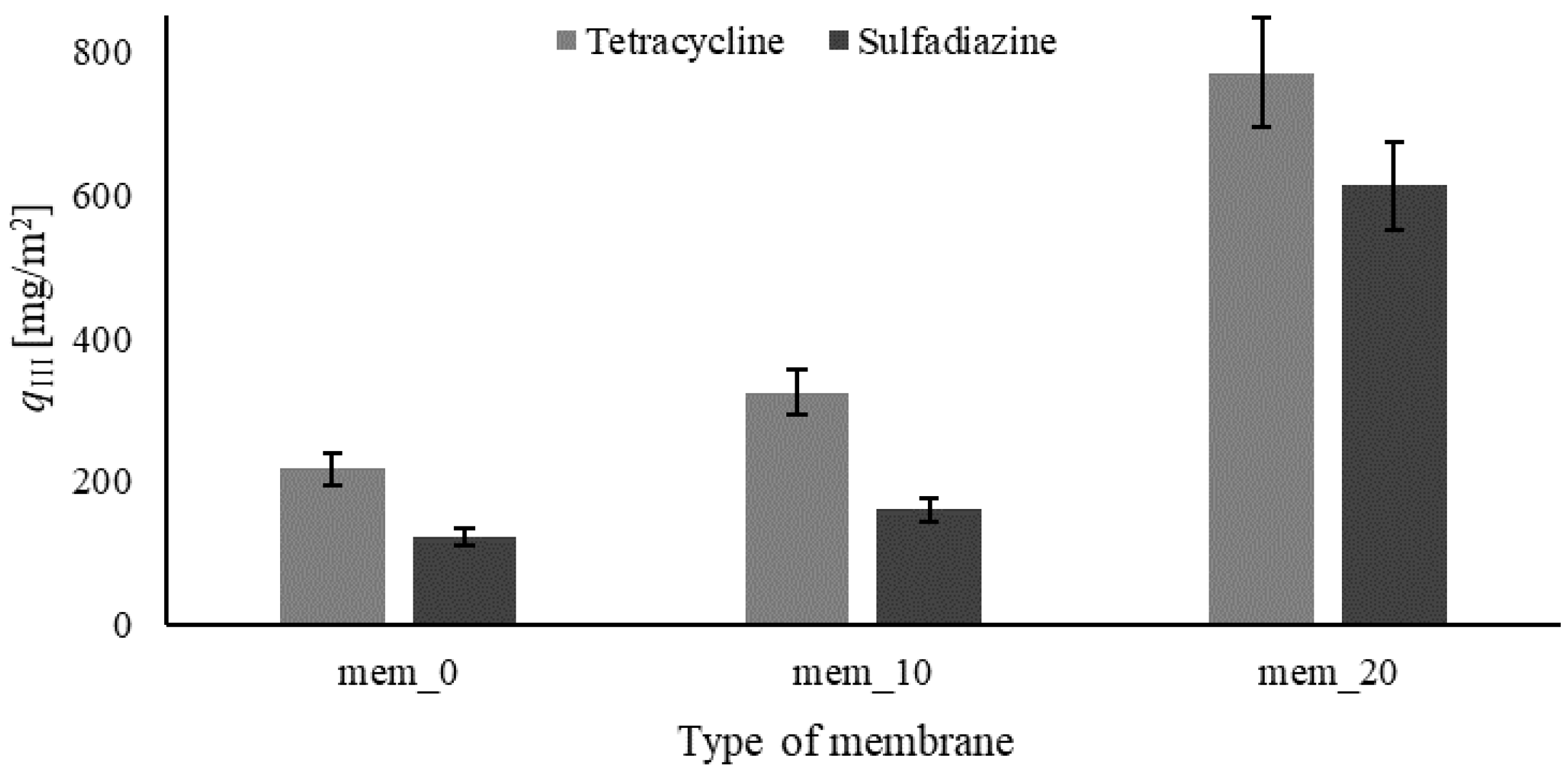
The last part of the research focused on the use of calcium carbonate for membrane modification to improve the adsorption properties of membranes. In order to determine the effect of CaCO
3 particles on membrane adsorption properties, membranes with 0% (labelled as mem_0), 10% (mem_10) and 20% (mem_20) concentrations of CaCO
3 were prepared. The content of particles was calculated in relation to the weight of the polymer. Experiments lasted 90 minutes, and samples were analysed after 90 minutes from the beginning of the experiments. The final results calculated as mass of pharmaceuticals removed per unit area of membrane,
qIII [mg/m
2], are presented in
Figure 5. The mass of pharmaceutical removed was calculated according to Equation 5.
where
c0 [mg/dm
3] means the initial concentration of pharmaceutical substance,
ct [mg/dm
3] means concentration of pharmaceutical substance during the process,
Amem [m
2] means the area of the membrane, in turn,
VI [dm
3] means the volume of tested solution. The concentrations refer to the amount of pharmaceuticals in the feed stream.
Based on the results (
Figure 5), the presence of CaCO
3 improved membrane adsorption properties. The higher CaCO
3 concentration, the higher the amount of pharmaceutical substance removed mass from the feed solution. The removed mass of pharmaceutical is about four times higher for the process where the membrane with 20% concentration of CaCO
3 was used compared to the unmodified membrane. However, over 20% CaCO
3 concentration, the membrane's mechanical properties dramatically decrease. Therefore, the results for membranes with higher CaCO
3 are not presented. Additionally, a higher CaCO
3 concentration increases the intensity of agglomeration phenomena which makes it impossible to prepare a homogenous membrane casting solution, and as a result, the membrane structure is not uniform.
According to the results shown in
Figure 5, the mass of removed pharmaceuticals per unit mass of calcium carbonate can be calculated according to Equation 6:
where
x [%] means the concentration of calcium carbonate in the membrane,
d [g/m
3] means the density of the membrane, it is 0.258 g/cm
3,
Amem [m
2] means the area of the membrane, is 0.0160 m
2, in turn, δ [m] means the thickness of the membrane, it is 98±6 µm. The mass of tetracycline and sulfadiazine removed by unit mass of calcium carbonate in the membranes with 10% of particles are 43 mg/g and 15 mg/g, respectively. For the membranes with 20% calcium carbonate, the mass of pharmaceuticals removed per unit mass of the filling is 109 mg/g in the case of tetracycline, and 97 mg/g in the case of sulfadiazine. It shows that the removed mass does not increase proportionally with calcium carbonate content into the membrane.
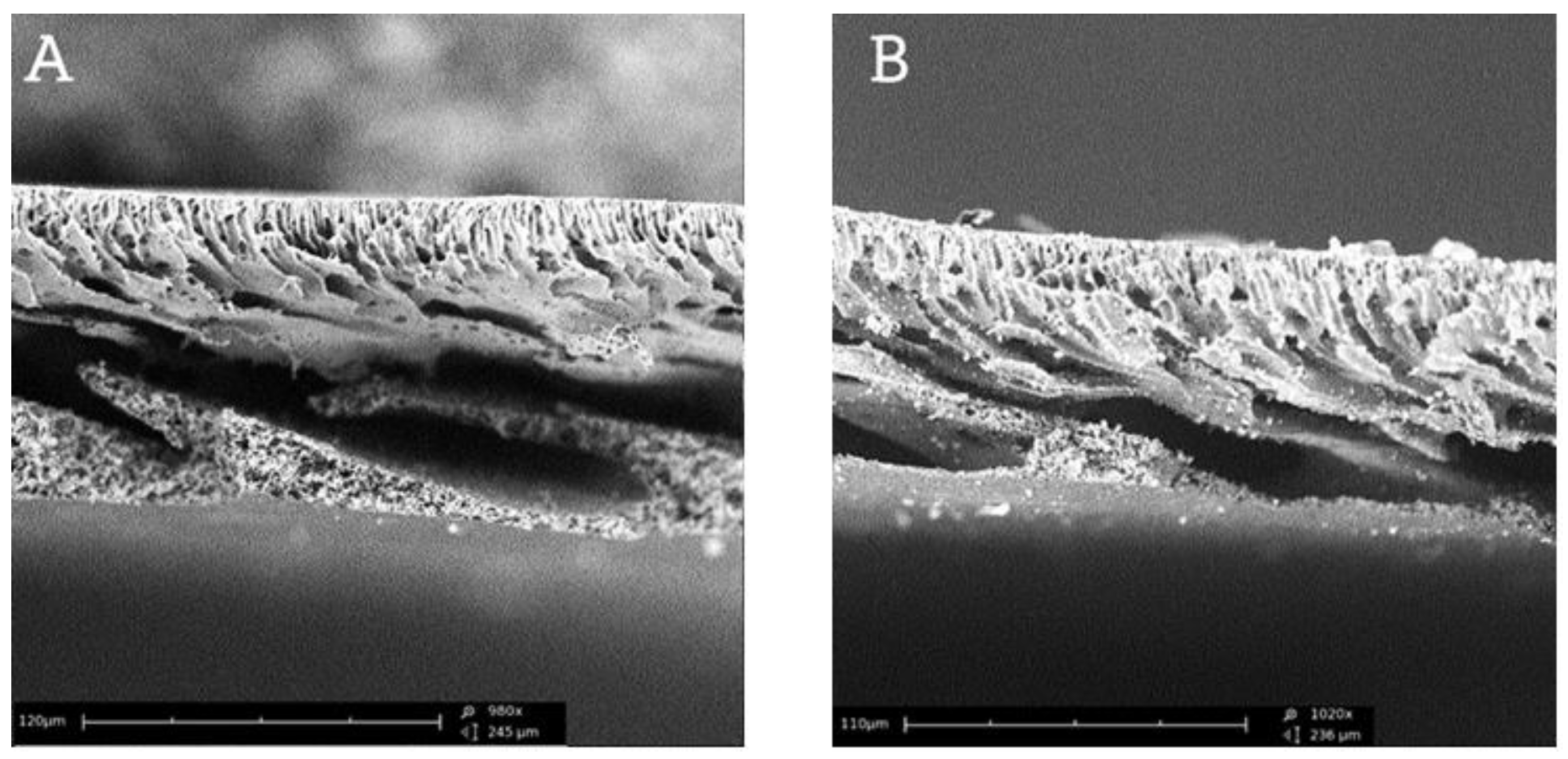
Figure 6 shows the cross-sections through the membrane without and with the 20% filling addition. The calcium carbonate particles can be observed both on the outside surface of membrane and into the pores. Moreover, it should be emphasized that adding the filling does not change the membrane structure visibly.
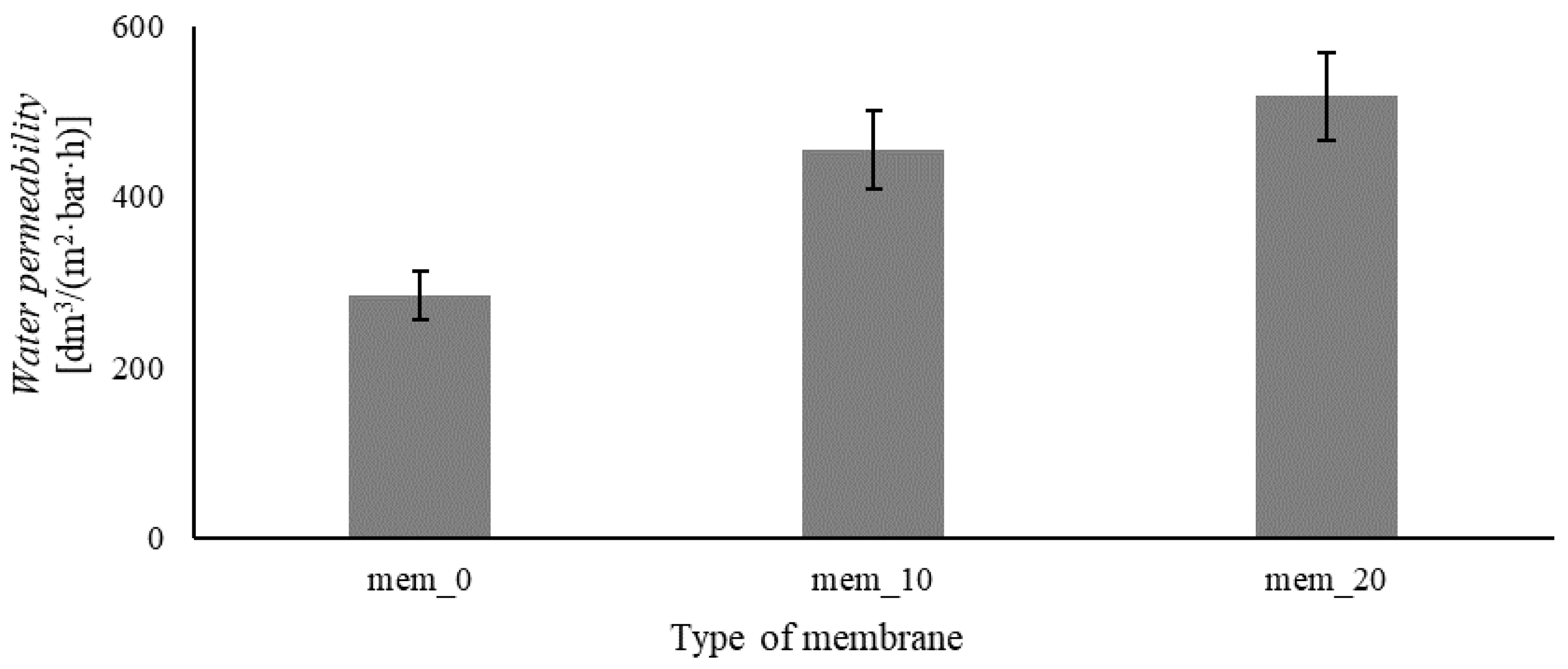
It should also be noted that the modified membranes have higher permeability than the unmodified membrane, which is confirmed by the results of the water permeability [dm
3/(m
2·bar·h)] (
Figure 7). The increase in the permeability of the modified membranes might be a result of an increase in membrane wettability, pore size, and porosity. The increase in the membrane wettability is related to the presence of hydrophilic CaCO
3 particles in the membrane structure [
49]. Additionally, the presence of hydrophilic particles in the membrane casting solution affects the process of membrane formation by the wet-phase method. The presence of hydrophilic particles facilitates the penetration of non-solvent (water) into the formed membrane-forming film. In addition, the presence of hydrophilic particles reduces the affinity of the solvent (NMP) to the polymer chains. Both the facilitated penetration of water and faster diffusion of NMP into the non-solvent volume accelerates the extraction process, resulting in increased porosity and pore diameter [
50,
51].
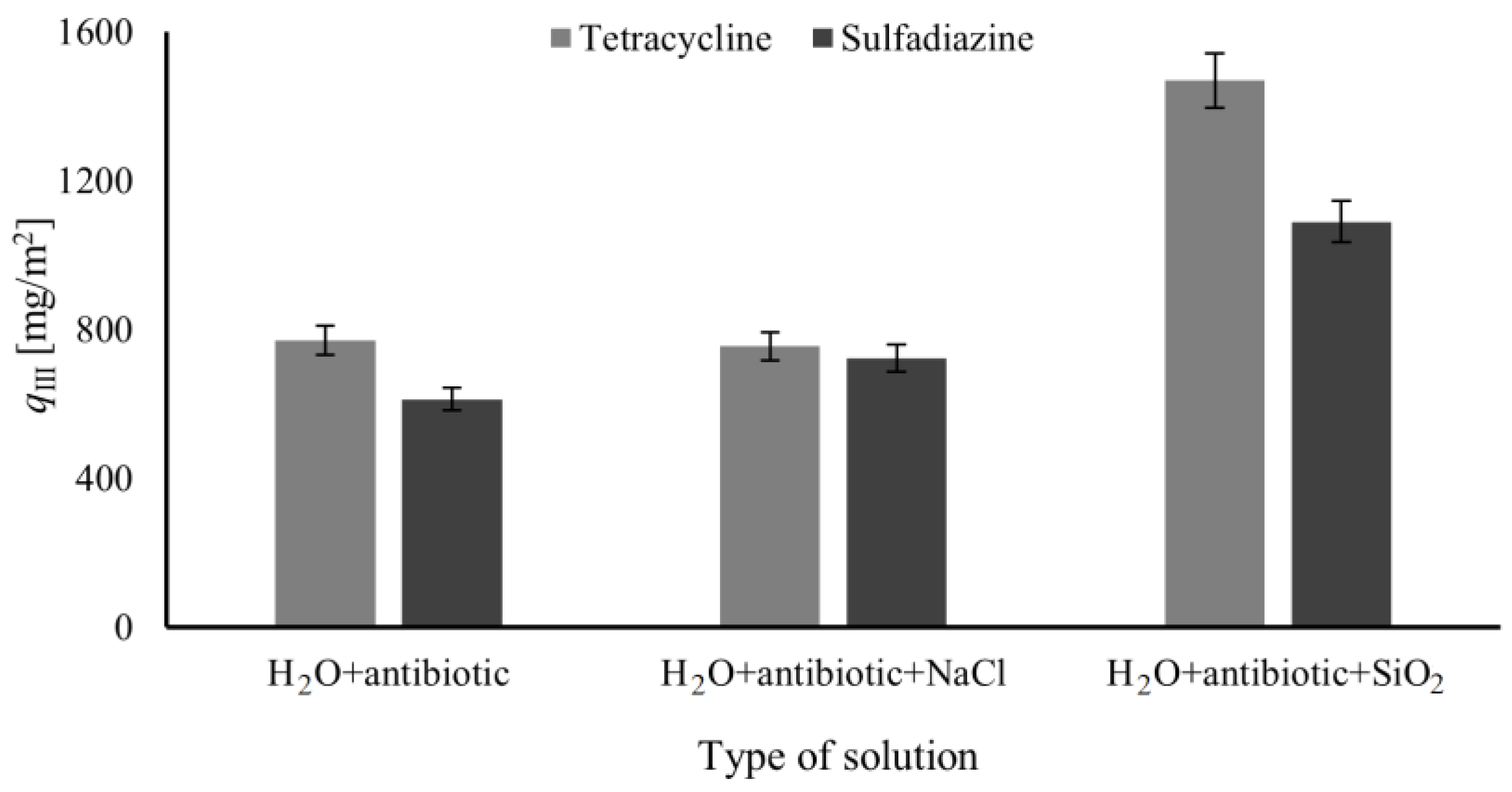
In the last part of the research, the influence of present ions (NaCl) or solid particles (SiO
2) on the efficiency of removing pharmaceutical substances from water was investigated. The obtained results for different compositions of the feed solutions are presented in
Figure 8.
Based on the results (
Figure 8), the presence of NaCl in pharmaceutical solutions has a statistically insignificant effect on tetracycline or sulfadiazine adsorption. However, the impact of salt on pharmaceutical adsorption might be completely different than in the presented results because it depends on the type of salt, its concentration, and the physicochemical properties of pharmaceutical substances [
40,
42,
53,
54,
55].
In the case of SiO
2 presence in the pharmaceutical solutions it can be noticed an increase in the removed mass of antibiotics compared to the solution without SiO
2 (
Figure 8). The increase in removed mass of antibiotics is results of adsorption on SiO
2 surface [
56,
57] but also it might be effect of fouling phenomena. A newly created layer on the membrane surface or blocked pore with SiO
2 particles can improve the filtration effect of pharmaceutical substances from water. However, due to the fouling phenomena, the permeate volume stream increases during the process. Additionally, the insignificant differences in percentage change in the adsorbed mass of tetracycline and sulfadiazine, an increase of about 40% in ratio to the solution without SiO
2, indicated that the presence of SiO
2 similarly affects the adsorption of both substances.
3.4. Antibacterial Properties
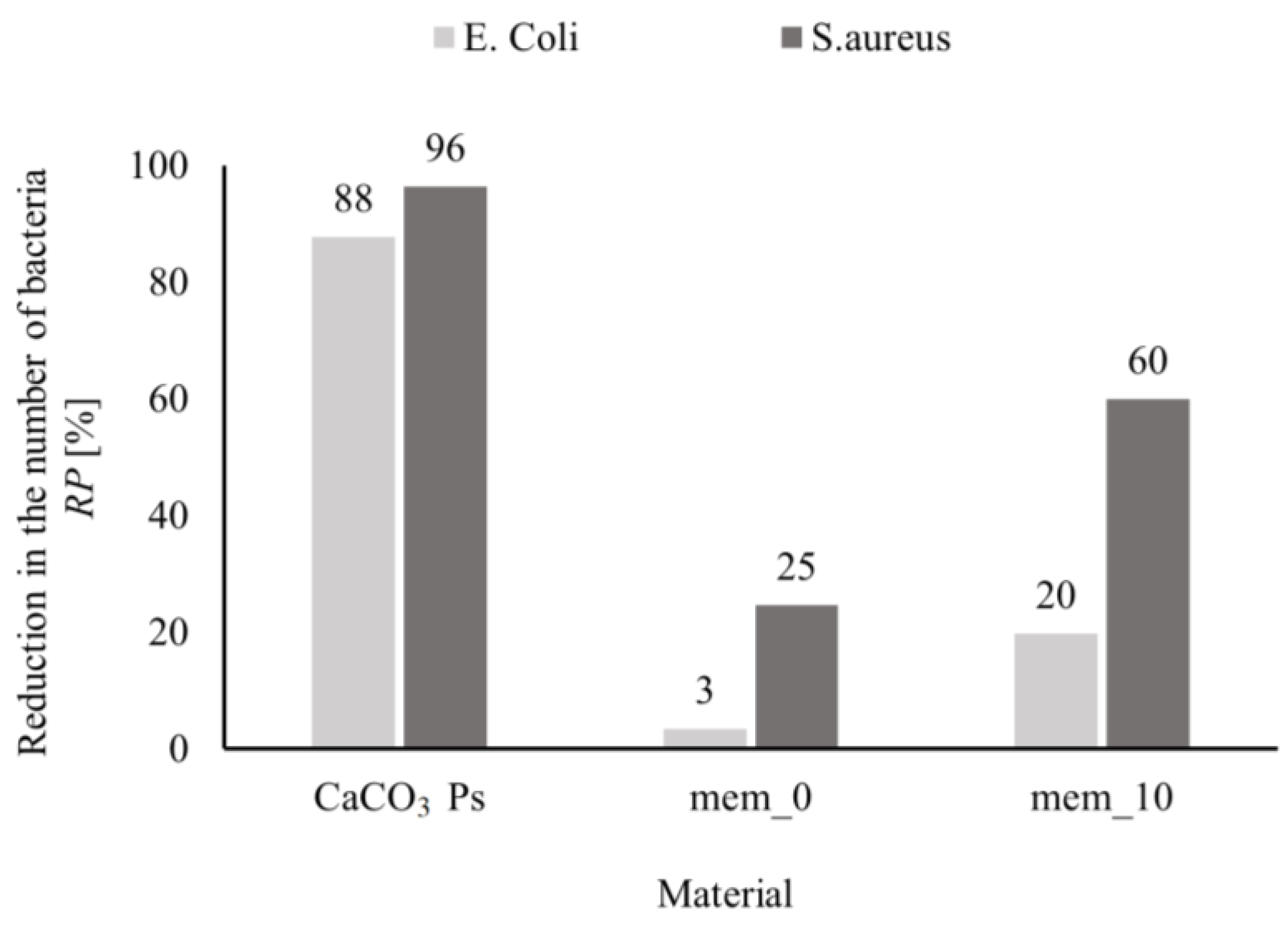
The results of bacteriostatic tests are presented in
Figure 9. It can be concluded that CaCO
3 particles (CaCO
3-Ps) exhibit a pronounced antibacterial properties against both Gram-negative and Gram-positive bacteria, for which reduction of bacteria population clearly exceeds 80% and 90%, respectively. Accordingly, the presence of CaCO
3 particles on the membrane surface translates to their antibacterial properties. For both types of bacteria, the modified membranes (mem_10) have a clearly better antibacterial properties than the unmodified membrane (mem_0).
It should be noted that CaCO
3 particles have the best antibacterial properties compared to analysed materials. The antimicrobial effects of calcium carbonate against gram-negative bacteria are also confirmed in the literature [
58]. In the case of the membrane with CaCO
3 particles, the polymer plays an important role in the growth of bacteria [
59,
60].
4. Conclusions
To sum up, it can be noticed that the CaCO3 particles, which were produced by our method, have the ability to adsorb tetracycline and sulfadiazine from water solutions, which was confirmed by the test carried out in three different systems. Additionally, the produced CaCO3 can be used to modify the developed membranes to improve their adsorption properties. However, the amount of adsorbed mass of pharmaceuticals depends on their physicochemical properties as well as the properties of membrane materials. It is related to the possible mechanism of adsorption of tested substances, which might be electrostatic interactions between pharmaceutical molecules and materials. This phenomenon is confirmed by the obtained results. According to them, the best adsorption properties are observed for the filtration process with calcium carbonate suspension. The mass of tetracycline or sulfadiazine removed by unit mass of particles is 232 mg/g and 524 mg/g, respectively. The integrated adsorption-filtration process is less effective per unit mass of calcium carbonate relative to the non-integrated process. However, this process can be conducted longer because the problem of membrane blocking does not occur. It should be also noticed that the produced CaCO3 particles have antibacterial properties against both Gram-negative and Gram-positive bacteria.
Author Contributions
Conceptualization, I.Z., M.S. and D.P.; methodology, all authors; writing—original draft preparation, I.Z. and D.P.; writing—review and editing, M.S.; supervision, M.S.; funding acquisition, P.G. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was partially supported by the Warsaw University of Technology Grant (IDUB TM-3: Advanced 504/04496/1070/45.010508). The research was partially supported by National Centre for Research and Development (AMROCE financed under the ERA-NET Aquatic Pollutants Joint Transnational Call GA No. 869178).
Acknowledgments
The authors would like to thank the company VTT for helping to acquire the plastic material, in particular we would like to thank Dr. Mika Paajanen, Dr. Jani Pelto and Dr. Milad Mosallaei for the fruitful discussion.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rodriguez-Narvaez, O. M., Peralta-Hernandez, J. M., Goonetilleke, A., Bandala, E. R., Treatment technologies for emerging contaminants in water: A review. Chemical Engineering Journal, 2017, 323, 361-380. [CrossRef]
- O'Flynn, D., Lawler, J., Yusuf, A., Parle-McDermott, A., Harold, D., Mc Cloughlin, T., Holland, L., Regan, F., White, B., A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Analitical Methods, 2021, 13, 575-594. [CrossRef]
- Xu, L., Zhang, H., Xiong, P., Zhu, Q., Liao, C., Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Science of The Total Environment, 2021, 753. 141975. [CrossRef]
- Angeles L.F., Mullen, R.A., Huang, I.J., Wilson, C., Khunjar, W., Sirotkin, H.I., McElroy, A. E., Aga, D.S. Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems. Environmental Science: Water Research & Technology, 2020, 6. 62-77. [CrossRef]
- Dadgostar, P., Antimicrobial resistance: implications and costs. Infection and drug resistance, 2019, 3903-3910. [CrossRef]
- Holmes, A. H., Moore, L. S., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A., Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet, 2015, 387(10014), 176-187. [CrossRef]
- Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., Alqumber, M. A., Antimicrobial resistance: a growing serious threat for global public health. In Healthcare, 2023, Vol. 11, No. 13, p. 1946. [CrossRef]
- Mompelat, S., Le Bot, B.,Thomas, O., Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environment international, 2019, 35(5), 803-814. [CrossRef]
- Nikolaou, A., Meric, S., Fatta, D., Occurrence patterns of pharmaceuticals in water and wastewater environments. Analytical and bioanalytical chemistry, 2017, 387, 1225-1234. [CrossRef]
- Bottoni, P., Caroli, S., Caracciolo, A. B., Pharmaceuticals as priority water contaminants. Toxicological & Environmental Chemistry, 2010, 92(3), 549-565. [CrossRef]
- Ranjan, N., Singh, P. K., Maurya, N. S., Pharmaceuticals in water as emerging pollutants for river health: A critical review under Indian conditions. Ecotoxicology and Environmental Safety, 2022, 247, 114220. [CrossRef]
- Grassi, M., Kaykioglu, G., Belgiorno, V., Lofrano, G. 2012. Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process. Emerging Compounds Removal from Wastewater. SpringerBriefs in Molecular Science, 2012, 15-37. [CrossRef]
- Gadipelly, C., Pérez-González, A., Yadav, G.D., Ortiz, I., Ibáñez, R., Rathod, V.K., Marathe, K.V., Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Industrial & Engineering Chemistry Research, 2014, 53(29). 11571-11592.
- Homem, V., Santos, L. Degradation and removal methods of antibiotics from aqueous matrices – A review. Journal of Environmental Management, 2011, 92. 10. 2304-2347. [CrossRef]
- Grassi, M., Kaykioglu, G., Belgiorno, V., Lofrano, G., Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process. Emerging Compounds Removal from Wastewater, 2012, 15-37. [CrossRef]
- Gadipelly, C., Pérez-González, A., Yadav, G.D., Ortiz, I., Ibáñez, R., Rathod, V.K., Marathe, K.V., Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse, Industrial & Engineering Chemistry Research, 2014, 53(29). 11571-11592.
- Homem, V., Santos, L., Degradation and removal methods of antibiotics from aqueous matrices – A review. Journal of Environmental Management, 2011, 92. 10. 2304-2347. [CrossRef]
- N’diaye, A.D., Kankou, M.S., Modeling of adsorption isotherms of pharmaceutical products onto various adsorbents: A Short Review. Journal of Materials and Environmental Science, 2020, 11(8). 1264-1276.
- Al-Khateeb, L. A., Almotiry, S., Salam, M. A., Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chemical Engineering Journal, 2014, 248, 191-199. [CrossRef]
- Zielinska, I., Polak, D., Szwast, M., Analysis of the adsorption of selected pharmaceuticals on a composite material PEBAX/GO, Journal of Water Process Engineering, 2021, 44, 1-7. [CrossRef]
- Kim, H., Hwang, Y. S., & Sharma, V. K., Adsorption of antibiotics and iopromide onto single-walled and multi-walled carbon nanotubes, Chemical engineering journal, 2014, 255, 23-27. [CrossRef]
- Zhu, X., He, M., Sun, Y., Xu, Z., Wan, Z., Hou, D., Tsang, D. C., Insights into the adsorption of pharmaceuticals and personal care products (PPCPs) on biochar and activated carbon with the aid of machine learning. Journal of Hazardous Materials, 2022, 423, 127060. [CrossRef]
- Crini, G., Non-conventional low-cost adsorbents for dye removal: A review. Bioresource Technology, 2006, 97. 9. 1061-1085. [CrossRef]
- de Andrade, J. R., Oliveira, M. F., da Silva, M. G. C. , Vieira, M. G. A., Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Industrial & Engineering Chemistry Research, 2018, 57. 9. 3103–3127. [CrossRef]
- Song X., Cao, Y., Bu, X., Luo, X. Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process. Applied Surface Science. 2021, 536. 147958. [CrossRef]
- Shamsuri, A. A., & Sumadin, Z. A., Influence of hydrophobic and hydrophilic mineral fillers on processing, tensile and impact properties of LDPE/KCF biocomposites. Composites Communications, 2018, 9, 65-69. [CrossRef]
- Wszelaka-Rylik, M., Piotrowska, K., Gierycz, P., Simulation, aggregation and thermal analysis of nanostructured calcite obtained in a controlled multiphase process. Journal of Thermal Analysis and Calorimetry, 2015, 119, 1323–1338. [CrossRef]
- Schlomach, J., Quarch, K., Kind, M. 2006. Investigation of precipitation of calcium carbonate at high supersaturations. Chemical Engineering&Technology, 2006, 29. 2. 215–220. DOI : 10.1002/ceat.200500390.
- Rehage, H., Orthey, J., Kind, M., On the complete similitude of technical precipitation. Part II: Stirred-tank reactors. Chemical Engineering Journal, 2021, 426. 131788. DOI : 10.1016/j.cej.2021.131788.
- Jahani, D., Nazari, A., Ghourbanpour, J. Ameli, A., Polyvinyl alcohol/calcium carbonate nanocomposites as efficient and cost-effective cationic dye adsorbents. Polymers, 2020, 12(10), 2179. [CrossRef]
- Zhou, L., Peng, T., Sun, H., Guo, X., Fu, D., The characterization and amoxicillin adsorption activity of mesopore CaCO3 microparticles prepared using rape flower pollen. Minerals, 2019, 9(4), 254. [CrossRef]
- Hui, T. S., Ibrahim, N. A., Zhi, L. L., Zaini, M. A. A., Endocrine-disrupting chemical removal by carbon nanocomposites. Handbook of Advanced Approaches Towards Pollution Prevention and Control, 2021, 45-74. [CrossRef]
- Wang, C., He, C., Tong, Z., Liu, X., Ren, B., Zeng, F., Combination of adsorption by porous CaCO3 microparticles and encapsulation by polyelectrolyte multilayer films for sustained drug delivery. International journal of pharmaceutics, 2006, 308(1-2), 160-167. [CrossRef]
- Blanco, J. F., Sublet, J., Nguyen, Q. T., Schaetzel, P., Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process. Journal of membrane science, 2006, 283(1-2), 27-37.
- Kędra-Królik, K., Gierycz P., Obtaining calcium carbonate in a multiphase system by the use of new rotating disc precipitation reactor. Journal of Thermal Analysis and Calorimetry, 2006, 83. 579-582. [CrossRef]
- Ruthven, D. M., Adsorption (Chemical Engineering). 2003. Encyclopedia of Physical Science and Technology (Third Edition). Academic Press. 251 – 271. [CrossRef]
- Chang, Q., Chapter 10 - Surface of Solids. Colloid and Interface Chemistry for Water Quality Control. Academic Press, 2016, 175 – 225. [CrossRef]
- Oba, S. N., Ighalo, J. O., Aniagor, C. O., & Igwegbe, C. A. Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Science of The Total Environment, 2021, 780, 146608. doi:10.1016/j.scitotenv.2021.1466.
- Zhou, Y., Liu, X., Xiang, Y., Wang, P., Zhang, J., Zhang, F., Wei, J., Luo, L., Lei, M., Tang, L., Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: adsorption mechanism and modelling, Bioresource Technology, 2017, 245, 266 - 273. doi:10.1016/j.biortech.2017.08.178.
- Hu, S., Zhang, Y., Shen, G., Zhang, H., Yuan, Z., Zhang, W., Adsorption/desorption behaviour and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil and Tillage Research, 2019, 186, 233-241. [CrossRef]
- Zhang, L., Wang, Y., Jin, S., Lu, Q., Ji, J., Adsorption isotherm, kinetic and mechanism of expanded graphite for sulfadiazine antibiotics removal from aqueous solutions. Environmental technology, 2017, 38(20), 2629-2638. [CrossRef]
- Boxall, A. B., Blackwell, P., Cavallo, R., Kay, P., & Tolls, J., The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicology letters, 2002, 131(1-2), 19-28. [CrossRef]
- Ersan, G., Apul, O. G., Perreault, F., & Karanfil, T., Adsorption of organic contaminants by graphene nanosheets: A review. Water Research, 2017, 126, 385-398. [CrossRef]
- Gao, Y., Li, Y., Zhang, L., Huang, H., Hu, J., Shah, S. M., Su, X., Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. Journal of colloid and interface science, 2012, 368(1), 540-546. [CrossRef]
- M. Xiaoming et al, Ma, X., Li, L., Yang, L., Su, C., Wang, K., Yuan, S., Zhou, J., Adsorption of heavy metal ions using hierarchical CaCO3–maltose meso/macroporous hybrid materials: Adsorption isotherms and kinetic studies. Journal of hazardous materials, 2012, 209, 467-477. [CrossRef]
- Conde-Cid, M., Núñez-Delgado, A., Fernández-Sanjurjo, M. J., Álvarez-Rodríguez, E., Fernández-Calviño, D., & Arias-Estévez, M., Tetracycline and sulfonamide antibiotics in soils: presence, fate and environmental risks. Processes, 2020, 8(11), 1479. [CrossRef]
- Kuo, C. Y., Lin, H. N., Tsai, H. A., Wang, D. M., Lai, J. Y., Fabrication of a high hydrophobic PVDF membrane via nonsolvent induced phase separation. Desalination, 2008, 233(1-3), 40-47. DOI 10.1016/j.desal.2007.09.025.
- Li, Q., Xu, Z. L., & Liu, M., Preparation and characterization of PVDF microporous membrane with highly hydrophobic surface. Polymers for Advanced Technologies, 2011, 22(5), 520-531. [CrossRef]
- Wang, C., Piao, C., Zhai, X., Hickman, F. N., Li, J., Synthesis and characterization of hydrophobic calcium carbonate particles via a dodecanoic acid inducing process. Powder Technology, 2010, 198(1), 131-134. [CrossRef]
- Abedini, R., Mousavi, S. M., & Aminzadeh, R., A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination, 2011, 277(1-3), 40-45. [CrossRef]
- Blanco, J. F., Sublet, J., Nguyen, Q. T., Schaetzel, P., Formation and morphology studies of different polysulfones-based membranes made by wet phase inversion process. Journal of membrane science, 2006, 283(1-2), 27-37. [CrossRef]
- Hu, S., Zhang, Y., Shen, G., Zhang, H., Yuan, Z., & Zhang, W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil and Tillage Research, 2019, 186, 233-241.
- Wang, S., Wang, H., Adsorption behavior of antibiotic in soil environment: a critical review. Frontiers of Environmental Science & Engineering, 2015, 9, 565-574. [CrossRef]
- Liu, S., Xu, W. H., Liu, Y. G., Tan, X. F., Zeng, G. M., Li, X., Cai, X. X., Facile synthesis of Cu (II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Science of the Total Environment, 2017, 592, 546-553. [CrossRef]
- Schmitt, M. O., & Schneider, S., Spectroscopic investigation of complexation between various tetracyclines and Mg 2+ or Ca 2+. PhysChemComm, 2000, 3(9), 42-55. [CrossRef]
- Lotfi, R., Hayati, B., Rahimi, S., Shekarchi, A. A., Mahmoodi, N. M., Bagheri, A., Synthesis and characterization of PAMAM/SiO2 nanohybrid as a new promising adsorbent for pharmaceuticals. Microchemical Journal, 2019, 146, 1150-1159. [CrossRef]
- Balarak, D., Baniasadi, M., Removal of Ciprofloxacin from of pharmaceutical wastewater by adsorption on SiO2 nanoparticle. Journal of Pharmaceutical Research International, 2018, 25(6): 1-9.
- Ataee, R.A, Derakhshanpour, J., Mehrabi Tavana, A., Eydi, A., Antibacterial effect of calcium carbonate nanoparticles on Agrobacterium tumefaciens, Iranian Journal of Military Medicin, 2011, 13, 2, 65-70.
- Sabri, S., Najjar, A., Manawi, Y., Eltai, N.M., Al.-Thani, A., Atieh, M.A., Kochkodan, V., Antibacterial Properties of Polysulfone Membranes Blended with Arabic Gum, Membranes, 2019, 9(2), 29. [CrossRef]
- Prihandana, G.S., Sriani, T., Muthi’ah, A.D., Machmudah, A., Mahardika, M., Miki, N., Nanomaterials, 2022, 12(3), 388. [CrossRef]
Figure 1.
Number of articles connected with pharmaceutical in water, pharmaceutical wastewater treatment and PPCPs wastewater (own study based on the Scopus database).
Figure 1.
Number of articles connected with pharmaceutical in water, pharmaceutical wastewater treatment and PPCPs wastewater (own study based on the Scopus database).
Figure 2.
Number of articles connected with drug or antimicrobial resistance during years (own study based on the Scopus database).
Figure 2.
Number of articles connected with drug or antimicrobial resistance during years (own study based on the Scopus database).
Figure 3.
Scheme of rotation disc reactor for calcium carbonate production (based on [
27]), where R1 – disc reactor, T1 – CO2 tank, T2 - Ca(OH)
2 tank, pH1 – phmeter, F1, F2 – flowmeters, T
p1 – thermometer, Z1, Z2 - regulation valves.
Figure 3.
Scheme of rotation disc reactor for calcium carbonate production (based on [
27]), where R1 – disc reactor, T1 – CO2 tank, T2 - Ca(OH)
2 tank, pH1 – phmeter, F1, F2 – flowmeters, T
p1 – thermometer, Z1, Z2 - regulation valves.
Figure 4.
Scheme of filtration installation,, where: M1 – membrane module, PM1 – feed pump, T1 – feed tank, P1, P2 – manometers, F1, F2 – flowmeters, Z1, Z2, Z3 – regulation valves.
Figure 4.
Scheme of filtration installation,, where: M1 – membrane module, PM1 – feed pump, T1 – feed tank, P1, P2 – manometers, F1, F2 – flowmeters, Z1, Z2, Z3 – regulation valves.
Figure 5.
Mass of removed antibiotic in the adsorption-filtration system for different types of membrane.
Figure 5.
Mass of removed antibiotic in the adsorption-filtration system for different types of membrane.
Figure 6.
The cross-sections of membrane: A – without CaCO3, B – with 20% concentration of CaCO3.
Figure 6.
The cross-sections of membrane: A – without CaCO3, B – with 20% concentration of CaCO3.
Figure 7.
Water permeability for different types of membrane.
Figure 7.
Water permeability for different types of membrane.
Figure 8.
Mass of removed antibiotic in the adsorption-filtration system for different types of solution.
Figure 8.
Mass of removed antibiotic in the adsorption-filtration system for different types of solution.
Figure 9.
Reduction of the number of bacteria in contact with the tested material (CaCO3 Ps –particles, mem_0 – membrane without nanoparticles, mem_10 – membrane with 10% of CaCO3 Ps).
Figure 9.
Reduction of the number of bacteria in contact with the tested material (CaCO3 Ps –particles, mem_0 – membrane without nanoparticles, mem_10 – membrane with 10% of CaCO3 Ps).
Table 1.
Turbidity of suspension after the centrifugation process.
Table 1.
Turbidity of suspension after the centrifugation process.
| The concentration of calcium carbonate in suspension [%] |
0.05 |
0.1 |
0.5 |
| Turbidity [NTU] |
Initial |
>1000 |
>1000 |
>1000 |
| After all stages |
2.97 |
3.31 |
4.16 |
Table 2.
The process efficiency and the adsorbed mass of pharmaceuticals by using calcium carbonate for different suspension concentrations.
Table 2.
The process efficiency and the adsorbed mass of pharmaceuticals by using calcium carbonate for different suspension concentrations.
| Substance |
Suspension concentration
[%] |
Efficiency, η
[%] |
Adsorbed mass, qI
[mg/g] |
| Tetracycline |
0.05
0.10
0.50 |
1.07 ± 0.07
4.03 ± 0.03
10.01 ± 0.07 |
0.81 ± 0.01
1.52 ± 0.04
0.75 ± 0.04 |
| Sulfadiazine |
0.05
0.10
0.50 |
19.87 ± 0.21
17.66 ± 0.17
21.92 ± 0.19 |
15.40 ± 4.27
6.85 ± 1.96
1.69 ± 0.44 |
Table 3.
The adsorbed mass of pharmaceuticals in an adsorption flow system with CaCO3 and without CaCO3.
Table 3.
The adsorbed mass of pharmaceuticals in an adsorption flow system with CaCO3 and without CaCO3.
| Substance |
Suspension concentration [%] |
Adsorbed mass, |
| Tetracycline |
0.00 |
3477 ± 56 |
| 0.05 |
3742 ± 76 |
| Sulfadiazine |
0.00 |
494 ± 61 |
| 0.05 |
954 ± 85 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).