1. Introduction
Cancer death toll around the world has toppled all causes leading to loss of life. Annual new cases, due to this ailment, have surpassed ten million people, while death registry is estimated to be over six million. Yildiz et al [
1], found that more than 80% of the cancers, which the people suffer, are preventable and this can be achieved by good nutrition.
In United States in children under 15 years of age, malignant tumors are currently the main cause of death [
2].
In the management of cancer, chemotherapy has been a useful tool. However, it produces an increase in the accumulation of monoamine in the brain [
3]. Cisplatin (CDDP) is a chemotherapeutic agent frequently employed in cancer treatment [
4]; but, it has its demerits, since it is associated with the induction of neurotoxicity [
5]. This chemotherapeutic drug eliminates cancer cells at some points of development and growth [
6]. On the other hand, Temozolamide is now being employed as the most common chemotherapeutic agent for the treatment of glioblastomas; however, the results, as monotherapeutic agent, have been disappointing [
7]. Hence, the challenge in cancer treatment with these drugs is to reduce their neurotoxic and other side effects.
Chemotherapy in the young children, apart from inducing neurotoxicity, produces malnutrition complications [
8] among which protein malnutrition (PM), together with its concomitant compromise on antioxidant defense system of the body, can be highlighted. However, malnourished patients who received antioxidant supplementation as N-acetylcystein (NAC) were found to have considerable clinical benefits [
9]. Currently, this supplement is widely included in chemotherapy protocols, even when some studies have suggested the effective function of externally derived polyunsaturated fatty acids (PUFAs) in the modulation of cytotoxicity of anti-cancer drugs [
10].
Similarly, nitric oxide (NO) plays a crucial role as a neuromodulator. Nevertheless, it could be injurious to cells at a slightly higher concentration, due to its potential to increase the levels of reactive oxygen species (ROS) or its conversion into nitroso-glutathione (NOGSH) inside the cell [
11]. Free radicals (FR) produce detrimental effects on cell organelles [
12], especially the lipid constituent of the membranes of the plasma [
13]. Fortunately, the central nervous system (CNS) mediates not only the consumption of food but also the production of FR, but also plays an active role in food metabolism [
14]. Cell membrane is composed by different types of lipids, and modifications in this cell structure can affect many biological processes [
15]. In the brain, the phospholipids that compose the membrane of the plasma are contiguous with the protein architecture inside the double strand lipid layers of the membrane [
16]. The interchange of ions taking place in these double strand lipid layers is facilitated by Na
+, K
+ ATPase enzyme that stimulates the entrance and exit of Na
+ and K
+ in the cell [
17]. In the CNS, when the action of ATPase is inhibited, there is an induction of the release of excitatory amino acids [
18]. Therefore, it is of paramount importance to find a treatment, which can synergistically attenuate the toxic effects of most of the cancer drugs to ensure optimum benefit and tolerance of their use.
In view of the findings cited in the paragraphs above, this work aims at assessing the level of protection that NAC treatment offers to rats in which protein deficiency was artificially produced with CDDP treatment. This work performed a comparative analysis of the protection benefits of NAC in the maintenance of 5-HIAA monoamine levels as well as GSH, catalase, total ATPase and TBARS in the cortex, cerebellum, medulla oblongata and lung of animals treated with CDDP.
2. Results
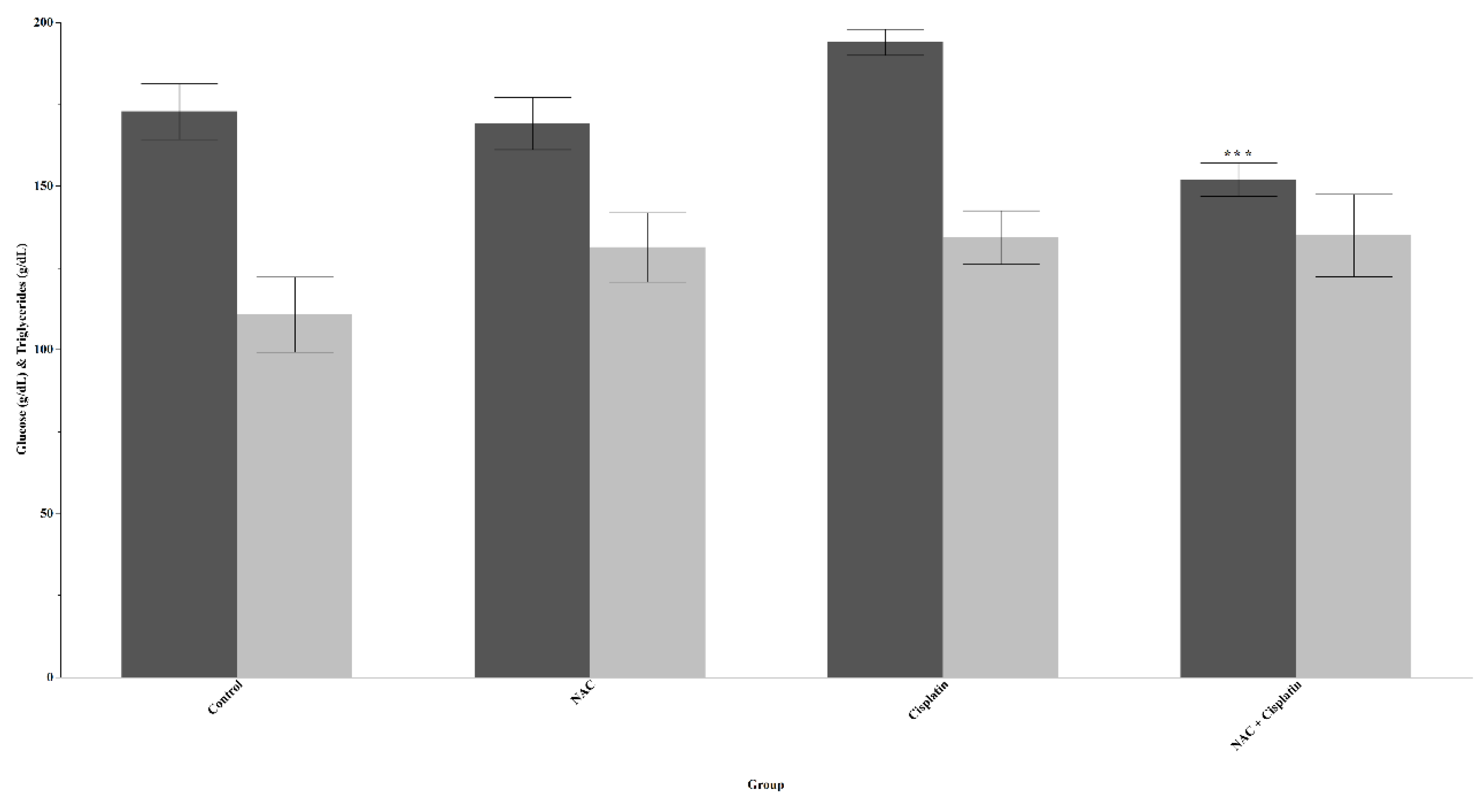
2.1. Glucose
The administration of Cisplatin increases glucose levels with respect to the control group; however, this increase was not statistically significant. In the group treated with NAC+Cisplatin, a reduction in the concentration of this element was observed in comparison with the rest of the experimental groups, but it only turned out to be significant when compared with the group treated with Cisplatin
p=0.0009. Triglycerides. There was an increase in triglyceride levels in the groups treated with NAC alone or in combination with Cisplatin; however, the statistical analysis did not reveal significant differences.
Figure 1.
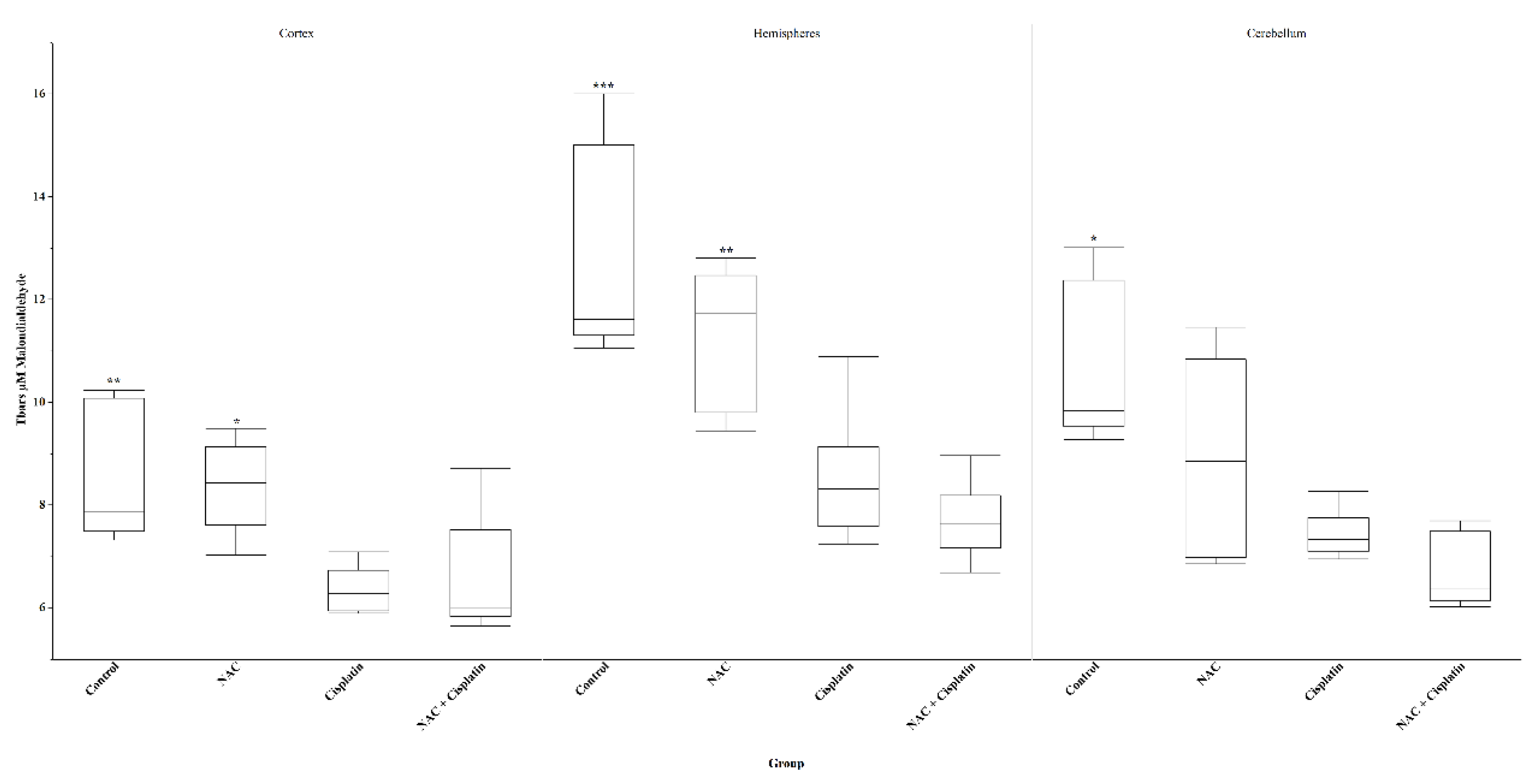
2.2. Lipid Peroxidation (TBARS)
In the groups of animals treated with Cisplatin and NAC+Cisplatin, a reduction in the concentration of Malondialdehyde was observed in the cortex (
p<0.02) and the hemispheres (
p<0.01) of the animals when compared both with the control group and with the group to which NAC was administered. In cerebellum, the reduction was significant only between the control group and the Cisplatin / NAC+Cisplatin groups (
p=0.04).
Figure 2.
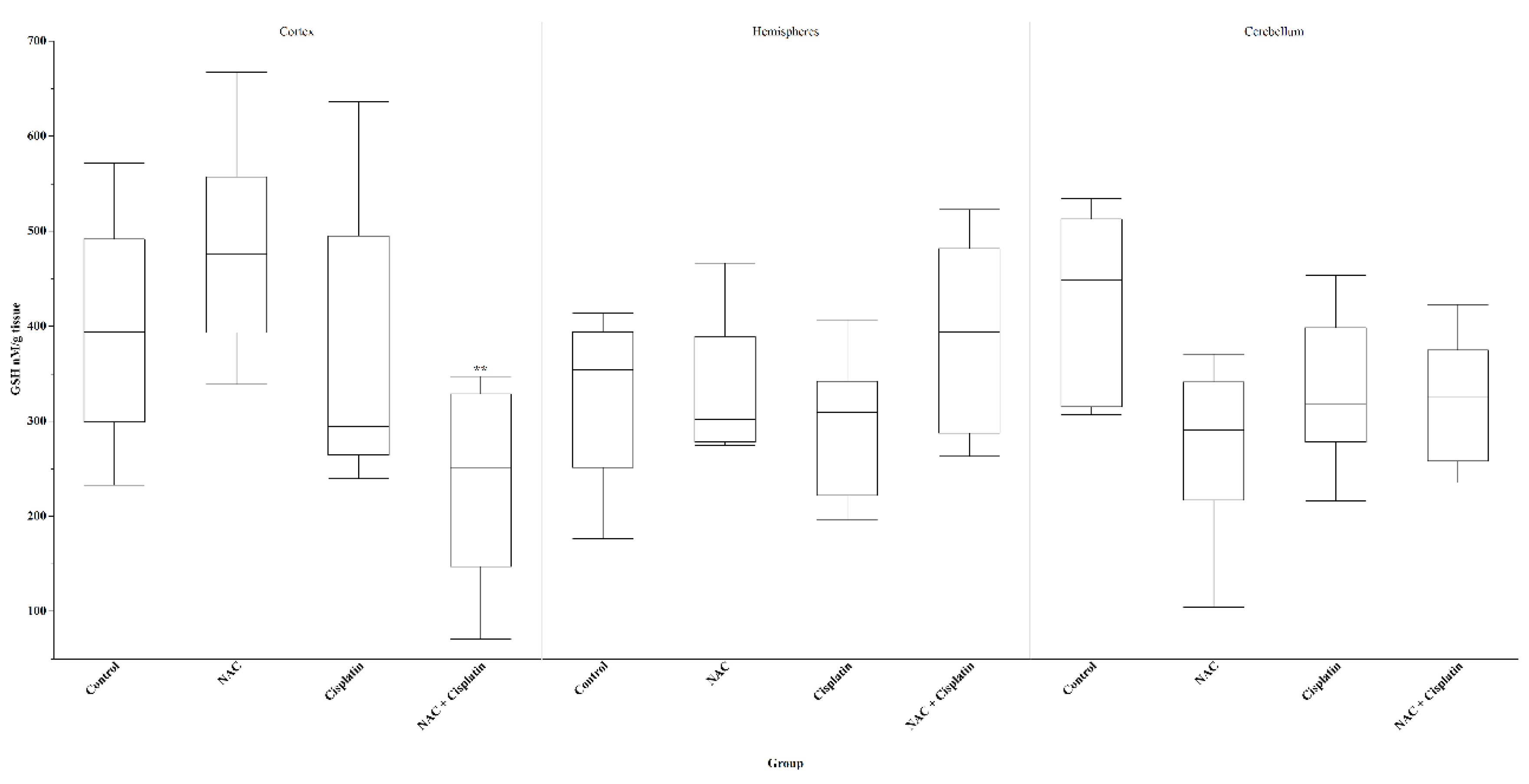
2.3. GSH
The GSH concentration showed a decrease in the cortex of the animals treated with NAC+Cisplatin with respect to the rest of the groups; however, the decrease in GSH was only statistically significant (
p=0.01) when compared with the group to which NAC was administered. In the hemispheres, there was an increase in the NAC+Cisplatin group, but the statistical analysis did not reveal significant differences. In the cerebellum, a decrease in GSH was observed in all the experimental groups, but again this difference did not reach statistical significance.
Figure 3.
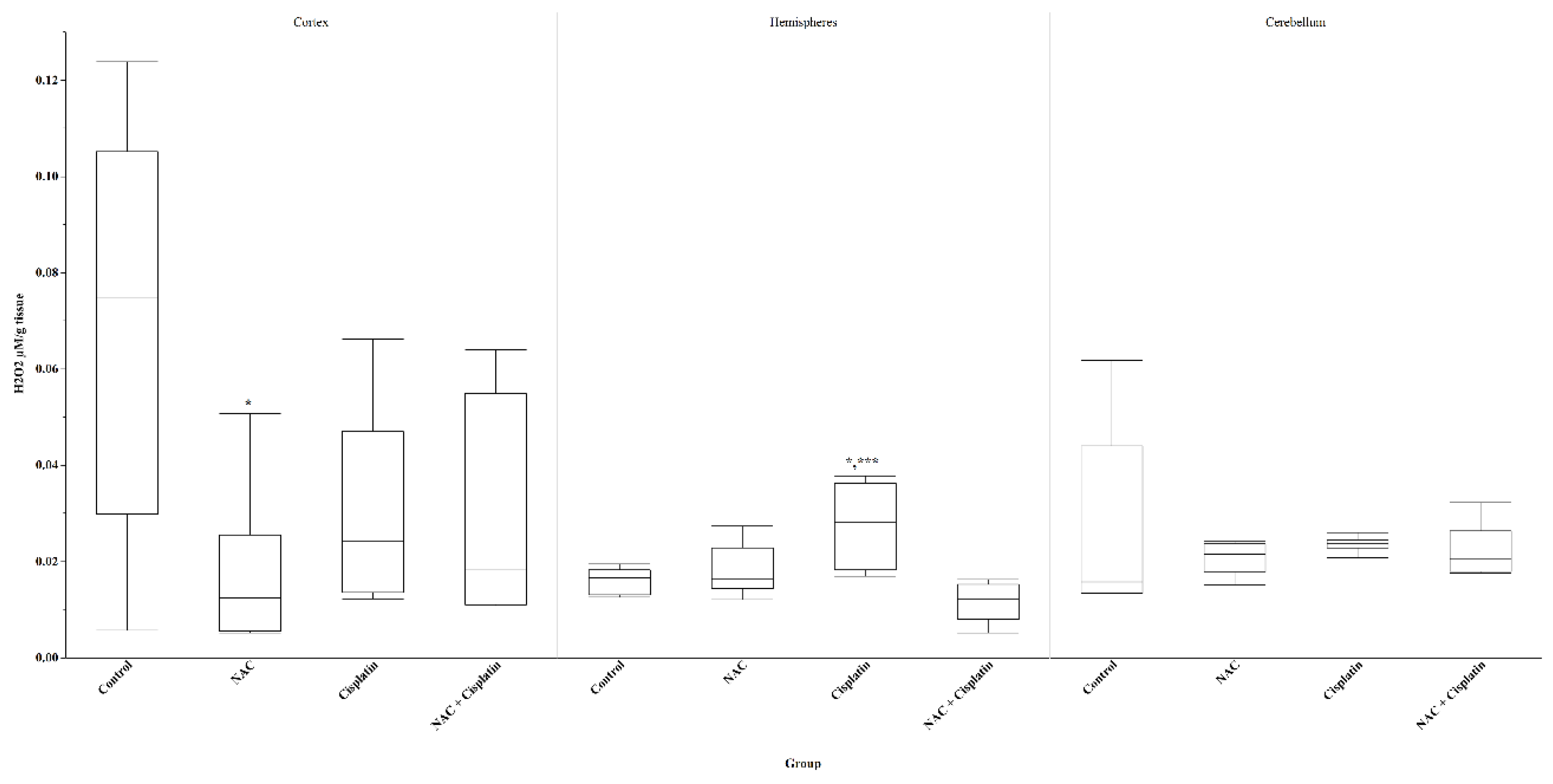
2.4. H2O2
In the cortex of the animals treated with NAC, Cisplatin or NAC+Cisplatin, a decrease in the H
2O
2 concentration was registered with respect to the animals of the control group, but the comparison between the groups shows a significant difference only between the control group and the treated groups, with NAC (
p=0.02). On the contrary, an insignificant increase in H
2O
2 was registered in the hemispheres of the group to which Cisplatin was administered when compared with the control group (
p=0.02) and the group treated with NAC+Cisplatin (
p=0.001). No significant differences were observed in the cerebellum.
Figure 4.
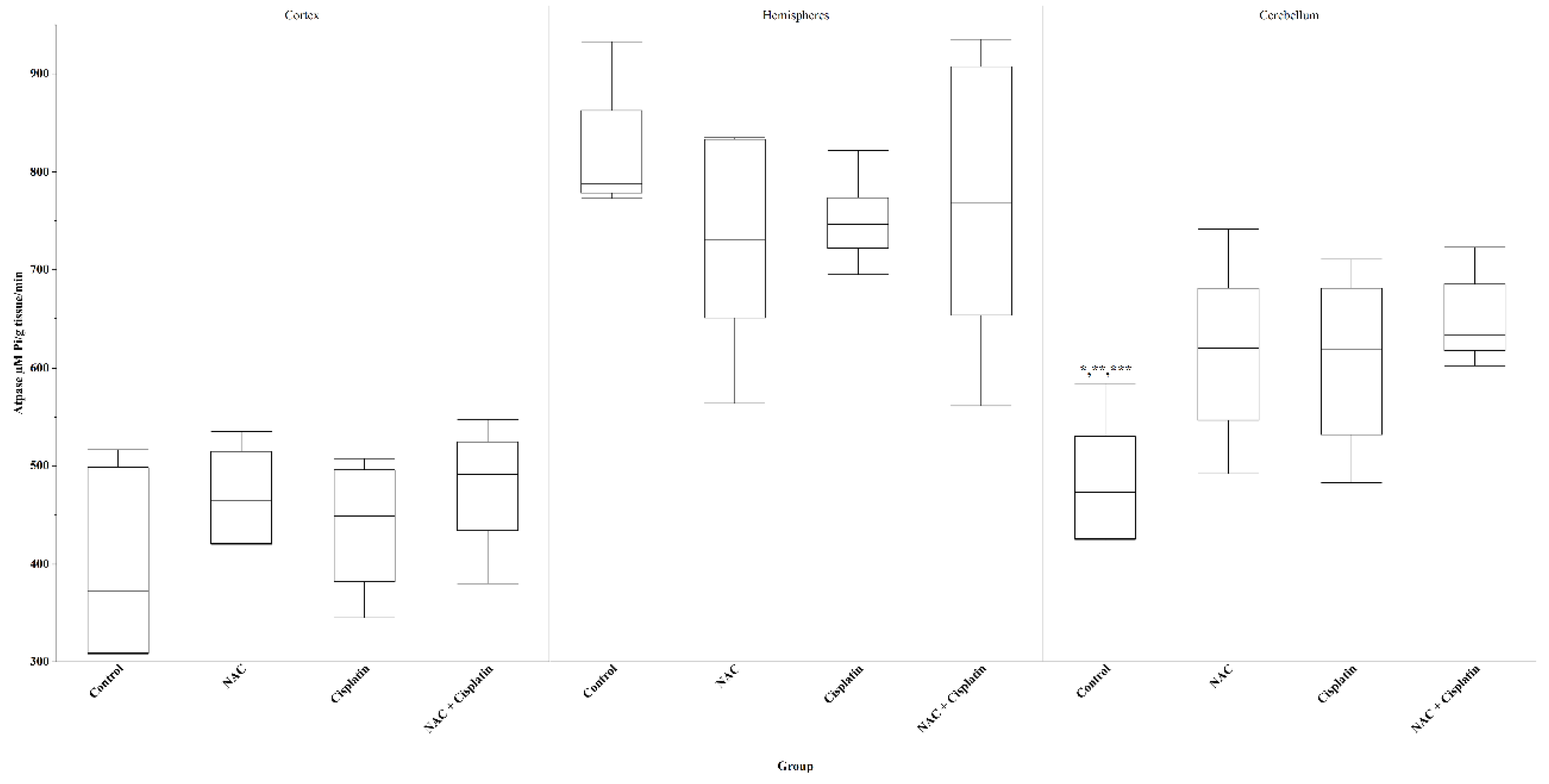
2.5. ATPase
The activity of ATPase enzyme registered a slight increase in the cortex of the experimental animals with respect to the control group, but this did not reach statistical significance. In the hemispheres, the highest ATPase activity was observed with a decrease in activity compared to the control group; however, the statistical difference was not significant. In the cerebellum, the administration of NAC, Cisplatin and NAC+Cisplatin combination produced a significant increase in ATPase activity on comparing them with the control group (
p=0.02) vs NAC, (
p=0.03) vs Cisplatin and (
p=0.004) vs NAC+Cisplatin.
Figure 5.
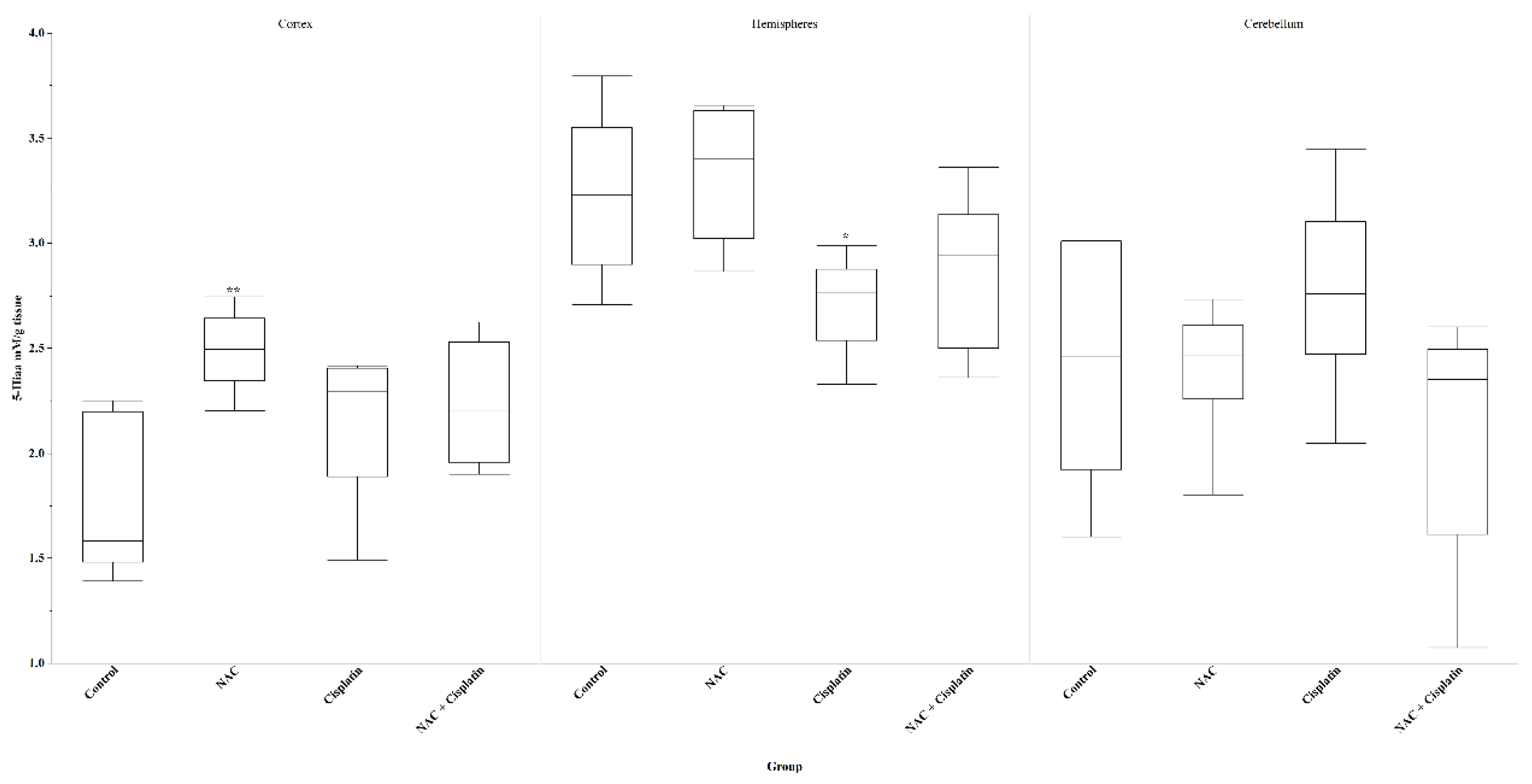
2.6. 5-HIAA
The levels of 5-HIAA in cortex showed an increase in animals that received NAC, Cisplatin and NAC+Cisplatin. Only the comparison between the control group and the group treated with NAC turned out to be statistically significant (p=0.007) while between Cisplatin / NAC+Cisplatin groups vs the control, no significant difference was observed.
In hemispheres, the Cisplatin and NAC+Cisplatin groups registered a decrease in 5HIAA levels, and only between the NAC and Cisplatin groups was this decrease statistically significant (
p=0.02). In the cerebellum, no effect of the treatments on 5HIAA levels was observed.
Figure 6.
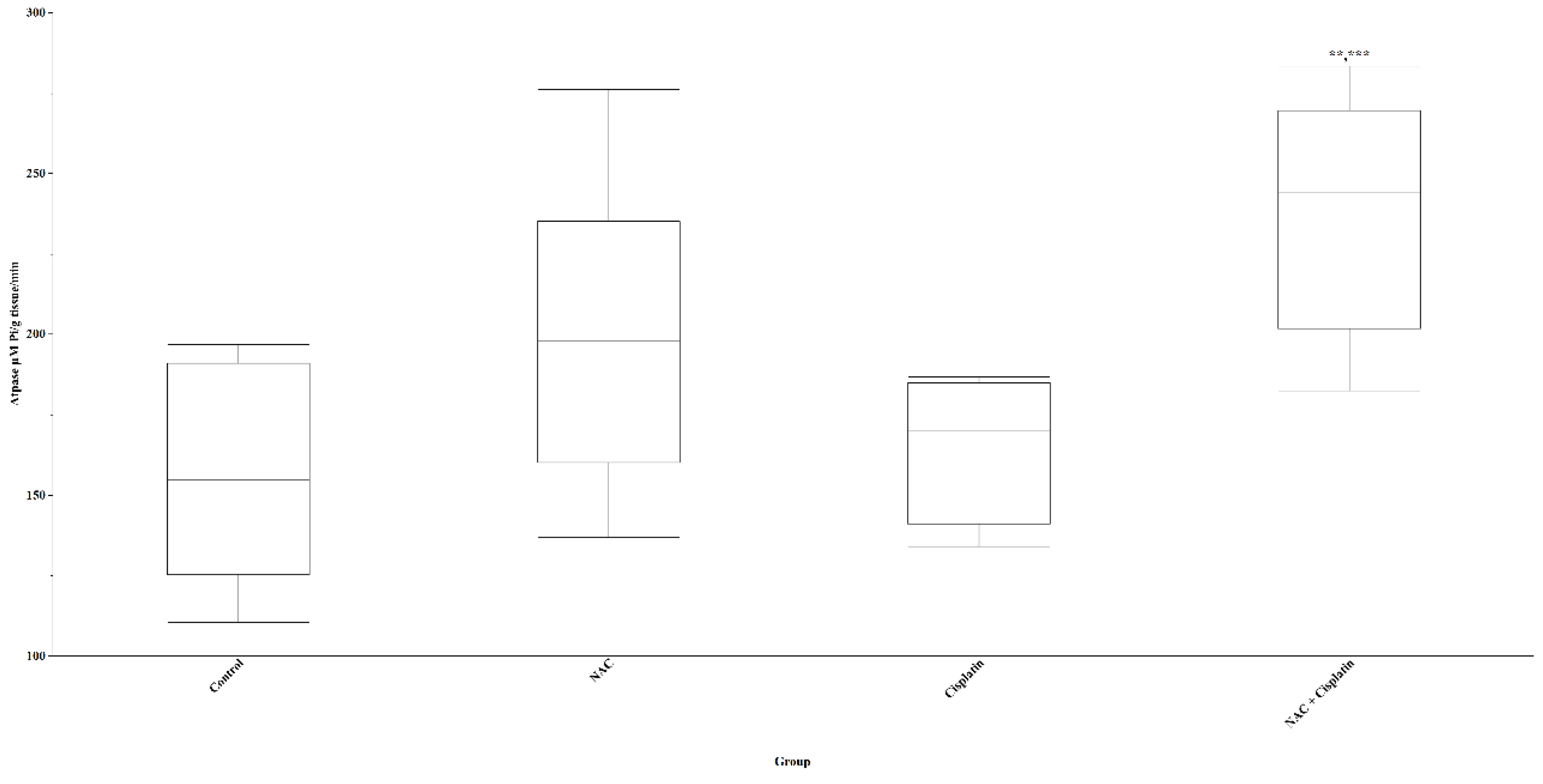
2.7. Lung
The results of the biochemical indicators evaluated in the lung showed that the levels of lipid peroxidation (TBARS) had a slight decrease in the groups treated with NAC and Cisplatin and a slight increase in the group treated with ACC+Cisplatin with respect to the control group without these variations being statistically significant (
p=0.07). The administration of Cisplatin and NAC+Cisplatin produced an increase in the concentration of GSH in comparison with the control and NAC groups; however, this increase did not reach statistical significance (
p=0.06). No effect of the treatments on the concentration of H
2O
2 in the lungs of the experimental animals was observed. Although there was an increase in the level of 5HIAA in the group treated with NAC+Cisplatin with respect to the level of this indoleamine in the control group, the difference did not reach statistical significance (
p=0.08). The activity of the ATPase enzyme in the lung increased significantly in the group of animals that were administered NAC+Cisplatin when this was compared with that of the control groups (
p=0.005) and those treated with Cisplatin (
p=0.01).
Figure 7.
3. Discussion
One of the common adverse effects of oncologic drugs is neurological dysfunction. Cisplatin is a member of a widely utilized class of chemotherapeutic agent that induce DNA damage response, cell cycle arrest, and p53-dependent apoptotic cell death in concert with DNA-platinum adduct formation, and normal programmed cell death (PCD) as oxidative damage [
27]. In this work, the use of CDDP in the treatment of the young animals, fed with protein deficient diet, decreased lipid peroxidation levels, glutathione (GSH) and hydrogen peroxide (H
2O
2) in cerebellum, cortex and medulla oblongata. In combination with NAC, the toxic effects of CDDP became mild [
28]. The increase of ATPase in lung and 5-HIAA in cerebellum, cortex and medulla oblongata indicates a reduction in oxidative stress due to the administration of NAC. Hence, these antioxidant molecules provide the scientific basis to design clinical trials aimed at reducing the oxidative stress, cognitive alterations and probably, the CNS changes elicited by chemotherapy in cancer patients.
Backway et al. suggest that chemotherapy agent-induced toxicity is accompanied by the loss of GSH, which leads to induction of oxidative stress and that the process seems to be a biphasic phenomenon where a state of high reactive oxygen generation proceeds permeability transition that occurs due to GSH depletion [
29]. In the study performed by Tchantchou et al. [
30], it was found that NAC supplement at 1 g/kg relieved oxidative damage, cognitive deterioration and reestablished glutathione synthase and GSH levels. NAC is a cell-permeant antioxidant and glutathione precursor. Indeed, NAC blocks neutral magnesium-dependent sphingomyelinase activation and inhibits ceramide formation. These properties make NAC to function as suppressor of caspase-3(-like) proteases activation and cisplatin generated apoptosis [
31].
Lung GSH increased in the group that received NAC while TBARS decreased in animals with protein deficient diet. These events suggest that cysteine prodrugs’ ability to restore GSH levels and redox status is tissue-specific [
32]. Hence, adjunctive therapy based on dietary supplement, which would facilitate the improvement of antioxidant status and reestablishment of tissue GSH, may be developed. This would replace the expensive high protein diet currently employed for this purpose in patients subjected to CDDP chemotherapy.
In this work, the animal model employed were subjected to diet restriction and according to literature, it is a suitable model to study how to maintain antioxidant status and tissue GSH reestablishment in severely compromised patients undergoing pharmacotherapy [
33].
Oxygen is an important element in keeping the viability of neuronal cells. Nevertheless, neuronal cells have relatively fragile protective antioxidant mechanisms. Therefore, regulation of the prooxidant-antioxidant balance could provide a therapeutic option, which can be used to improve neuroprotection in response to oxidative stress [
34].
The results of this work show that 5-HIAA and ATPase increased during NAC and CDDP treatment. This event may have given rise to neuroprotection and augment membrane fluidity. N-Acetylcysteine (NAC) portrays to have potential to mitigate neuronal and lung toxicity due to CDDP. Thus, the efficacy of combined NAC and CDDP drug therapy appears to be a promising strategy for future chemotherapy in malnourished patients. We suggest that more research work be carried out to thoroughly examine its neuroprotective mechanisms.
4. Methods
4.1. Experimental Animals
The twenty-four young male Wistar rats used in the study were purchased from certified laboratory animal breeding house of Instituto Politécnico Nacional, Mexico City. The animals were placed in four meshed plastic cages, each containing six rats and were exposed to both light and darkness, each with a duration of 12 hours in natural environmental conditions. The animals were fed with granulated laboratory rodent feed (Purine 5001®) to which they have no restriction, and water was freely allowed during the experiments. Before the study, the animals were allowed 1 – 2-week period of acclimatization to the animal house facility conditions with food and water.
4.2. Chemicals
Thiobarbituric acid (TBA), Glutathione, catalase, ATP and 5-HIAA were obtained from Sigma-Aldrich, St. Louis, MO, USA. Hydrochloric acid, Sulfuric acid, Nitric acid, Bisulfite, Trichloro acetic acid, Sodium phosphate and Magnesium chloride were purchased from Merck, Darmstad, Germany.
4.3. Experimental Drugs
Twenty-four young male Wistar rats, each weighing 200g, were employed in the study. The rats were indiscriminately assigned to a group. Each group consisted of six animals, making up a total of four groups. Every animal in the groups was fed with a protein diet at 7% for 15 days [
19]. Thereafter and based on the group, the animals were treated either with cisplatin® (CDDP) at 5mg per kg weight (unique dose) or Acetylcysteine® (NAC) at 5mg per kg weight (unique dose). The administration of the treatments was intraperitoneally and was performed in the following way: A vehicle of NaCl at 0.9% was administered to the control animals formed by first group. CDDP was given to the animals assigned to second group. The rats that formed the third group receive only NAC while those that formed the fourth group were administered NAC + CDDP, with 60min between each treatment.
The sacrifice of the rats was performed sixty minutes after drug administration. During the sacrifice, performed by decapitation without anesthesia, animal blood was obtained. This was employed in determining the levels of triglycerides and glucose using test strip devices. For the determination of lipid peroxidation (TBARS), serotonin metabolite (5-HIAA), glutathione (GSH), catalase as well as Ca+2, Mg+2 ATPase activities, a portion of the animal brain and lung, extracted immediately after the sacrifice, were used. The assays were based on previously validated methods. The dissection of the extracted brain was carried out and separated into hemispheres, cortex and medulla/oblongata. These brain structures were stored in a sodium chloride (NaCl) solution with a concentration of 0.9%. The homogenization of the hemispheres, cortex and medulla/oblongata as well as the lung was performed using 3ml of tris-HCl 0.05M, pH 7.2. The homogenates were utilized for the assay of the activity of Ca+2, Mg+2 ATPase, TBARS, 5-HIAA, GSH, and catalase determination. The assay of these bioamines were based on methods that were formerly validated. The preservation of the homogenized tissues was achieved by keeping them at –80°C until they were analyzed. The processes of the experiments were performed in accordance with the national and international standard procedures for the care and use of laboratory animals. The Ethics Committee of Instituto Nacional de Pediatria gave its approval to this research protocol with the reference number 049/2019. Besides, all experiments were performed in accordance with relevant guidelines and regulations.
4.4. 5-HIAA Assay and Assessment
The assessment of 5-hydroxyindol acetic acid was performed using the supernatant derived from the brain and lung tissue sections that were homogenized in perchloric acid (HCLO
4) and centrifuged at 9,000 rpm for 10 min. in a microcentrifuge (Hettich Zentrifugen, model Mikro 12-42, Germany), with a modified version of the technique reported by Beck et al, [
20] and modified by Peraza et al, [
21]. A portion of the supernatant was taken and put in a test tube containing 1.9 milliliters of a 0.1M acetate buffer at pH 5.5. A five-minute incubation of this mixture was performed at room temperature in total darkness. Thereafter, each sample was spectrofluorometrically read (Perkin Elmer LS 55, England) at emission and excitation lengths of 333 nm and 296 nm respectively. The software employed in this assessment was 4.00.02 version of FL Win Lab. Using a curve that was already standardized, the inference of 5-HIAA values was made and recorded in nM/g of wet tissue.
4.5. GSH assessment
Glutathione measurement was performed with the supernatant of the brain and lung tissue sections as described for 5-HIAA and in accordance with the technique of Hissin and Hilf [
22] and modified by Peraza et al [
23]. In a test tube, a mixture containing the supernatant (20 μL), ortho-phthaldehyde in methanol (100 mL at 1 mg/mL) and phosphate buffer (1.8 mL) with pH of 8.0 and EDTA 0.2% was prepared. A fifteen-minute incubation of this mixture was carried out at room temperature in absolute darkness. PERKIN ELMER LS 55 spectrophotometer, excitation 350 and emission 420 wavelengths, was used to assess the level of GSH. The software used in this assessment was 4.00.02 version of FL Win Lab. In an already standardized curve, the inference of GSH values was made and recorded as nM/g tissue.
4.6. Measurement of Catalase (CAT)
Catalase (CAT) activity in hemispheres, cortex and medulla/oblongata was assessed with the modified version of the technique of Sinha et al. [
23,
24] and reported in µmoles of H
2O
2 degraded/g tissue.
4.7. Total ATPase Assessment
The assay of ATPase was performed using Guzman et al technique [
25]. From the tris-HCl 0.05M pH 7.4 homogenized tissues of hemispheres, cortex and medulla/oblongata, 1 mg (10%) w/v was taken and added to a solution containing sodium chloride (100mM), potassium chloride (7mM) and magnesium chloride (13mM). Tris-ATP (4 mM) was added to the solution. In a shaking water bath (Dubnoff Labconco), the resultant solution was subjected to a 30-minute incubation at a temperature of 37
oC. The reaction was stopped using 100 µL of trichloroacetic acid w/v at a concentration of 10%. Thereafter, a 5-minute centrifugation of the solution was performed at 100 g under a temperature of 4 °C. With an aliquot of the supernatant and in accordance with Fiske and Subarrow`s method [
26], the measurement of inorganic phosphate (Pi) was made in duplicates. The supernatant absorbance was spectrophotometrically read at 660 nm using Helios-α, UNICAM. The activity of total ATPase was expressed as mM Pi/g wet tissue per minute.
4.8. Assessment of Lipid Peroxidation
TBARS was assessed with Gutteridge and Halliwell [
13] technique after modification and according to the works of Guzman et al [
25]. For this assessment, 1 ml of the tissues of hemispheres, cortex and medulla/oblongata that were subjected to homogenization in tris-HCl 0.05M pH 7.4, was mixed in a 2-ml TBA solution. This solution contains TBA (1.25g), a mixture of concentrated HCL (6.25 ml) and deionized water (250 ml), and trichloroacetic acid (TCA) (40g). The solution was put to a 30-minute heat until boiling point (Thermomix 1420). Thereafter, the solution was cooled by bathing it in ice during 5min. It was made to undergo a 15-minute centrifugation at 700g. (Sorvall RC-5B Dupont). To determine TBARS concentration, the absorbances of the brain tissue supernatants were spectrophotometrically interpreted in triplicate at 532nm using Heλios-α of UNICAM. The concentration of reactive substances to the Thiobarbaturic acid (TBARS) was expressed in µM of Malondialdehyde/g of wet tissue.
4.9. Statistical Analysis
The strategy for the inference analysis consisted in the comparison of the biochemical indicators between the control group and the different experimental groups, using tests to contrast hypotheses: Analysis of Variance (Anova) or Kruskal-Wallis, prior verification of variance homogeneity. Post hoc contrasts were performed with Tukey-Kramer or Steel-Dwass tests. Any associated probability value α <0.05 was considered statistically significant. Analysis was performed using SAS Systems JMP v12 software.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
All data generated or analyzed during this study are included in the published article.
Authors’ Contributions
DCGa,b,c,d,e, NOBb,c,d,e, MOHb,c,d,e, AVPb,c,d,e, NLRb,c,d,e, HJOb,c,d,e, DSAb,c,d,e, GBMb,c,d,e. (a) Contributed to the conception and design of the work. (b) Contributed to the collection, analysis or interpretation of data. (c) Critically revised the manuscript for important intellectual content. (d) Drafted the manuscript. (e) Gave final approval.
Funding
This work did not receive any kind of economic support.
Acknowledgement
The Ethics Committee of Instituto Nacional de Pediatria gave its approval to this research protocol with the reference number 049/2019. Besides, all experiments were performed in accordance with relevant guidelines and regulations.
Conflict of Interest
The authors declare that there are no conflict of interest.
Acknowledgement
Our thanks go to Dr. Cyril Ndidi Nwoye N., a native speaker and expert translator in medical research papers, as well as a physician and professor. The authors are very grateful to the National Institute of Pediatrics (NIP) for the support in the publication of this paper in the A022 program.
References
- Yildiz A, Kaya Y, Tanriverdi O. Effect of the interaction between selenium and zinc on DNA repair in association with cancer prevention. J. Cancer Prev. 2019, 24(3):146-154. [CrossRef]
- Quinn T Ostrom, Gino Cioffi, Kristin Waite, Carol Kruchko, Jill S Barnholtz-Sloan. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol 2021; 23(Suppl 3). [CrossRef]
- Matsutani T, Tamaru M, Hayakawa Y, Nagayoshi M, Nakahara T, Tsukada Y. A neurochemical study of developmental impairment of the brain caused by the administration of cytosine arabinoside during the fetal or neonatal period of rats. Neurochem Res 1983; 8(10):1295-306. [CrossRef]
- Noda S, Yoshimura S, Sawada M, Naganawa T, Iwama T, Nakashima S, et al. Role of ceramide during cisplatin-induced apoptosis in C6 glioma cells. J Neurooncol 2002; 52(1):11-21. [CrossRef]
- Tayyaba Afsar, Suhail Razak, Ali Almajwal. Reversal of cisplatin triggered neurotoxicity by Acacia hydaspica ethyl acetate fraction via regulating brain acetylcholinesterase activity, DNA damage, and pro-inflammatory cytokines in the rodent model. BMC Complement Med Ther 2022; 22(1):179. [CrossRef]
- Vasilia Tamamouna, Evangelia Pavlou, Christiana M Neophytou, Panagiotis Papageorgis, Paul Costeas. Regulation of metastatic tumor dormancy and emerging opportunities for therapeutic intervention. Int J Mol Sci, 2022; 23(22):13931. [CrossRef]
- Aida Karachi, Farhad Dastmalchi, Duane A Mitchell, Maryam Rahman. Temozolamide for immunomodulation in the treatment of gioblastoma. NeuroOncol 2018; 20(12):1566-1572. [CrossRef]
- Herrera-Silva JC, Treviño-Moore A, López-Beltran AL. Metabolic syndrome in cancer patients during chemotherapy treatment [Síndrome metabólico en pacientes con cáncer durante el tratamiento con quimioterapia] Bol Med Hosp Infant Mex 2008; 65:110-120.
- Dósa E, Heltai K, Radovits T, Molnár G, Kapocsi J, Merkely B, et al. Dose escalation study of intravenous and intra-arterial N-acetylcysteine for the prevention of oto- and nephrotoxicity of cisplatin with a contrast-induced nephropathy model in patients with renal insufficiency. Fluids Barriers CNS 2017; 14(1):26. [CrossRef]
- Germain E, Chajès V, Cognault S, Lhuillery C, Bougnoux P. Enhancement of doxorubicin cytotoxicity by polyunsaturated fatty acids in the human breast tumor cell line MDA-MB-231: relationship to lipid peroxidation. Int J Cancer 1998; 75(4):578-83. [CrossRef]
- Hogg N, Singh RJ, Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Let 1996; 382:223-228. [CrossRef]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxides. Proc Natl Acad Sci USA 1990; 87:1624-1629. [CrossRef]
- Gutteridge JM, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci 1990; 15:129-135. [CrossRef]
- Driver AS, Kodavanti PR, Mundy WR. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 2000; 22:175-181. [CrossRef]
- Shamitko-Klingensmith N, Molchanoff KM, Burke KA, Magnone GJ, Legleiter J. Mapping the mechanical properties of cholesterol-containing supported lipid bilayers with nanoscale spatial resolution. Langmuir 2012; 28(37):13411-13422. [CrossRef]
- Swapna I, Sathya KV, Murthy CR, Senthilkumaran B. Membrane alterations and fluidity changes in cerebral cortex during ammonia intoxication. Neuro Toxicol 2005; 335:700- 704. [CrossRef]
- Stefanello FM, Chiarani F, Kurek AG. Methionine alters Na+, K+ ATPase activity, lipid peroxidation and nonenzymatic antioxidant defenses in rat hippocampus. Int J Dev Neurosc 2005; 23:651-656. [CrossRef]
- Calderon GD, Juarez OH, Hernandez GE, Labra RN, Barragan MG, Trujillo JF, et al. Effect of an antiviral and vitamins A,C,D on dopamine and some oxidative stress markers in rat brain exposed to ozone. Arch Biol Sci Belgrade 2013; 65(4):1371-1379. [CrossRef]
- Barragán MG, Castilla SL, Calderón GD, Hernández IJL, Labra RN, Rodríguez PRA, et al. Effect of nutritional status and ozone exposure on rat brain serotonin. Arch Med Res 2002; 33:15-19. [CrossRef]
- Beck O, Palmskog G, Hultman E. Quantitative determination of 5-hydroxyindole- 3-acetic acid in body fluids by HPLC. Clin Chim Acta 1977; 79:149-154. [CrossRef]
- Peraza AV, Guzmán DC, Brizuela NO, Herrera MO, Olguín HJ, Silva ML, et al. Riboflavin and pyridoxine restore dopamine levels and reduce oxidative stress in brain of rats. BMC Neurosci 2018; 19:71. [CrossRef]
- Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissue. Anal Biochem 1974; 4:214-226. [CrossRef]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem 2018, 19, 7. [Google Scholar] [CrossRef]
- Sinha, K.A. Colorimetric assay of catalase. Anal Biochem 1971, 47, 389–394. [Google Scholar] [CrossRef]
- Guzmán DC, Brizuela NO, Herrera MO, Olguín HJ, García EH, Peraza AV, et al. Oleic acid protects against oxidative stress exacerbated by cytarabine and doxorubicin in rat brain. Mini-Revi Med Chem 2016; 16:1491-1495. [CrossRef]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem 1972; 66:375-400. [CrossRef]
- Kelvin K Hui, Maya Latif, Chesa Dojo Soeandy, Shudi Huang, Christopher E Rodgers, Andrew J Elia, et al. Cisplatin toxicity in the developing brain displays an absolute requirement for caspase-3. Exp Neurol 2022; 351:114010. [CrossRef]
- Tseng-Ting Kao, Chia-Yi Chu, Gang-Hui Lee, Tsun-Hsien Hsiao, Nai-Wei Cheng, Nan-Shan Chang, et al. Folate deficiency-induced oxidative stress contributes to neuropathy in young and aged zebrafish. Iimplication in neural tube defects and Alzheimer’s diseases. Neurobiol Dis 2014; 71:234-44. [CrossRef]
- Backway KL, McCulloch EA, Chow S, Hedley DW. Relationships between the mitochondrial permeability transition and oxidative stress during ara-C toxicity. Cancer Res 1977; 57(12):2446-2451.
- Tchantchou F, Graves M, Rogers E, Ortiz D, Shea TB. N-acteyl cysteine alleviates oxidative damage to central nervous system of ApoE-deficient mice following folate and vitamin E-deficiency. J Alzheimers Dis 2005; 7(2):135-138. [CrossRef]
- Dickey DT, Muldoon LL, Doolittle ND, Peterson DR, Kraemer DF, Neuwelt EA. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin- induced toxicity in rat models. Cancer Chemother Pharmacol 2007; 62(2):235-241. [CrossRef]
- Li J, Wang H, Stoner GD, Bray TM. Dietary supplementation with cysteine prodrugs selectively restores tissue glutathione levels and redox status in protein- malnourished mice. J Nutr Biochem 2002; 13(10):625-633. [CrossRef]
- Lautermann L, Schacht J. Reduced nutritional status enhances ototoxicity. Laryngorhinootologie 1995; 74(12):724-7. [CrossRef]
- Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev 2013; 963520. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).