1. Introduction
The emergence of cannabinoid-based products, particularly those containing tetrahydrocannabinol (THC) and cannabidiol (CBD), has witnessed a surge in popularity and availability in the market. THC, the primary psychoactive component of
Cannabis sativa [
1], is accompanied by the non-psychoactive CBD. In recent years, the synthetic production of THC variants [
2,
3,
4,
5,
6,
7,
8,
9], such as D8-THC and D9-THC, has become a focal point within the industry, presenting both opportunities and challenges.
While the potential therapeutic benefits of cannabinoids are widely acknowledged, the unregulated landscape has allowed for the rapid proliferation of D8-THC products. This surge in availability, however, raises concerns about the quality, safety, and legality of these products. The synthesis of D8-THC and D9-THC involves various techniques, from batch and flow chemistry to total and semi-synthetic routes. This diversity in production methods, coupled with inadequate oversight and testing, has paved the way for an influx of products into the market that may not adhere to established regulations.
The lack of stringent regulation, oversight, and standardized laboratory testing has led to an alarming prevalence of contaminated D8-THC products in various retail outlets. These products, sold outside the confines of regulated cannabis spaces, are frequently found in gas stations, smoke shops, and convenience stores. The consequences of consuming such products can be severe, with potential health risks stemming from contaminants, impurities, and, notably, illegal concentrations of D9-THC – a compound restricted by the 2018 Farm Bill to concentrations below 0.3% [
10,
11].
This concerning trend underscores the pressing need for accurate validation of cannabinoid concentrations within product batches. Notably, the validation process is complicated by the co-elution of D8-THC and D9-THC during testing, leading to potential mischaracterization [
12]. Internal and external testing procedures must be refined to accurately identify and quantify these compounds within samples. The evolving landscape of testing methodologies, such as adjusting HPLC parameters, provides a promising avenue for achieving precise results. Such advancements are crucial not only for regulatory compliance but also for ensuring consumer safety and preventing adverse health effects associated with contaminated or mislabeled cannabinoid products [
13]. In this context, the development of more precise testing methods stands as a critical step towards maintaining industry integrity and safeguarding public health.
2. Materials and Methods
All compounds were dissolved in chloroform (CDCl3), and
1H/
13C data were acquired on a 500 MHz Bruker AVANCE II system at 25 °C. 1H and 13C data sets were analyzed using MNova software to for 1H and 13C peak assignments. The HPLC used was the Agilent 1100 series with Diode Array Detector equipped with a RPC18 Shimadzu Next Leaf CBX for Potency (150x4.6mm). Solvent A: H2O + 0.1% H3PO4. Solvent B: ACN + 0.1% H3PO4. Cannabinoid CRMs were purchased from Cayman Chemical Company, (Ann Arbor, MI) and utilized as references for HPLC data collection. Solvents were purchased from Sigma Aldrich (Burlington, MA). Deuterated solvents were purchased from Cambridge Isotope Laboratories (Andover, MA). CBD was purchased from GVB Biopharma, Oregon, and was converted to D8-THC [
10].
2.1. Synthesis of D8 THC
To a round bottom flask equipped with a magnetic stir bar, CBD (5g, 15.91 mmol, 1 equiv.) was added. Hexane (10 mL) was added. Para-toluene sulfonic acid-hexahydrate (0.151g, 0.08 equiv) was added and the reaction stirred until the reaction was completed by HPLC. Upon completion, the reaction mixture was washed with water. Following the wash, the organic layer was separated. This was repeated with washes of brine and sodium bicarbonate. After the washes are complete, the mixture is concentrated in vacuo and yields a mixture of D8 and D9 THC as a red oil and matched literature spectroscopy [
10].
HPLC (C18): [(D9-THC) 20.463 min], [(D8-THC) 19.557 min], 1H NMR (400 MHz, CDCl3) δ 6.33 (d, J = 1.6 Hz, 1H), 6.12 (d, J = 1.6 Hz, 1H), 5.47 (d, J = 12.4 Hz, 2H), 3.32 – 3.22 (m, 1H), 2.75 (td, J = 10.9, 4.5 Hz, 1H), 2.44 (td, J = 7.4, 1.9 Hz, 2H), 2.23 – 2.10 (m, 1H), 1.94 – 1.83 (m, 2H), 1.83 (s, 1H), 1.57 (p, J = 7.2 Hz, 2H), 1.43 (s, 3H), 1.38 – 1.24 (m, 4H), 1.14 (s, 3H), 0.91 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 155.02, 154.73, 142.80, 134.92, 119.43, 110.87, 110.09, 108.16, 108.07, 77.07, 45.09, 36.14, 35.60, 31.75, 30.92, 30.74, 28.04, 27.64, 23.63, 22.68, 18.60, 14.18.
2.2. HPLC method for the Separation of THC Isomers
Table 1.
HPLC method for the separation of D9 THC from D8 THC. Solvent A: H2O + 0.1% H3PO4. Solvent B: ACN + 0.1% H3PO4.
Table 1.
HPLC method for the separation of D9 THC from D8 THC. Solvent A: H2O + 0.1% H3PO4. Solvent B: ACN + 0.1% H3PO4.
| Time (min) |
Solvent A |
Solvent B |
Solvent C |
Solvent D |
Flow (mL/min) |
Pressure (bar) |
| 0.00 |
45 |
55 |
0 |
0 |
1.5 |
375 |
| 5.50 |
43 |
57 |
0 |
0 |
1.5 |
375 |
| 6.51 |
40 |
60 |
0 |
0 |
1.5 |
375 |
| 11.00 |
40 |
60 |
0 |
0 |
1.5 |
375 |
| 25.00 |
40 |
60 |
0 |
0 |
1.5 |
375 |
Table 2.
Typical HPLC method. Solvent A: H2O + 0.1% H3PO4. Solvent B: ACN + 0.1% H3PO4.
Table 2.
Typical HPLC method. Solvent A: H2O + 0.1% H3PO4. Solvent B: ACN + 0.1% H3PO4.
| Time (min) |
Solvent A |
Solvent B |
Solvent C |
Solvent D |
Flow (mL/min) |
Pressure (bar) |
| 0.00 |
30 |
70 |
0 |
0 |
1.60 |
375 |
| 3.00 |
30 |
70 |
0 |
0 |
1.60 |
375 |
| 7.00 |
15 |
85 |
0 |
0 |
1.60 |
375 |
| 7.01 |
5 |
95 |
0 |
0 |
1.60 |
375 |
| 8.00 |
5 |
95 |
0 |
0 |
1.60 |
375 |
| 8.01 |
30 |
70 |
0 |
0 |
1.60 |
375 |
| 10.00 |
30 |
70 |
0 |
0 |
1.60 |
375 |
3. Results and Discussion
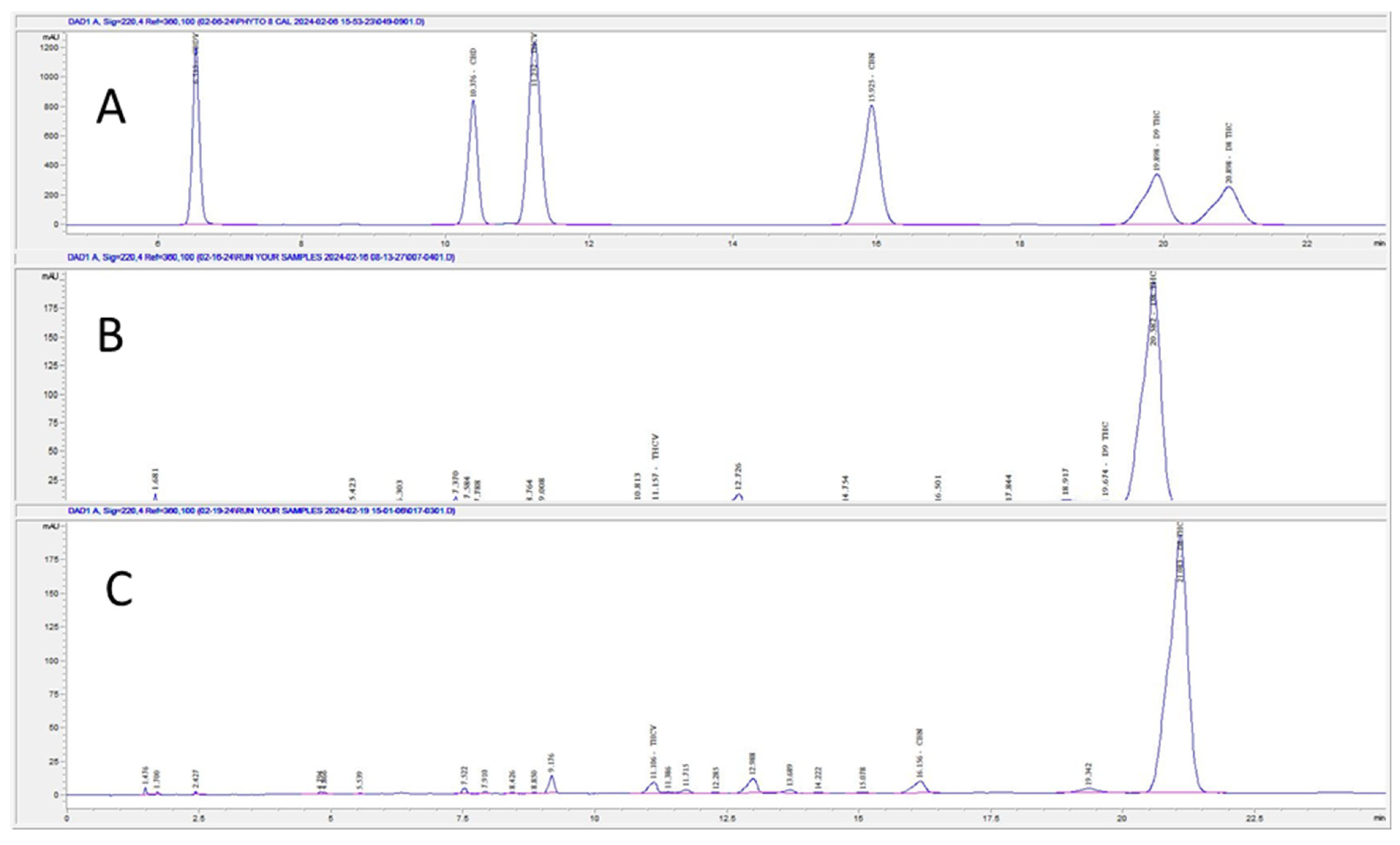
The developed method for the separation of THC isomers was utilized on the following samples as shown below in
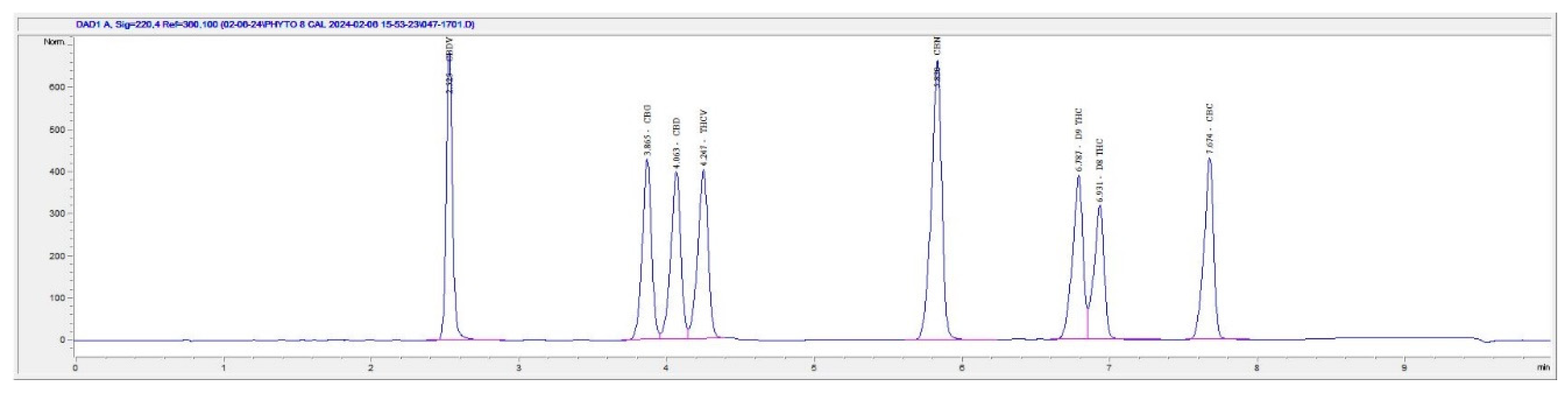
Figure 1: CRM (Sample 1), a synthesized THC reaction mixture of isomers (Sample 2), and synthesized THC reaction following removal of D9 THC from the reaction mixture (Sample 3). The method was able to resolve D9 THC (19.898 min) and D8 THC (20.898 min) using Sample 1. Sample 2 led to separation of D9 THC (19.557 min) at 3% potency and D8 THC (20.463 min) at 80.7% potency. Sample 3 showed non-detect D9 THC and only D8 THC (21.083 min) with a potency of 75.4%. An outdated method was ran using the 8-cannabinoid CRM as shown below in
Figure 2 shows D9-THC eluting at (6.787 min), co-eluting and poor resolution with D8-THC eluting at (6.931 min). This method was developed for the sole purpose of quantifying D8 and D9 THC, peak shape and method length were sacrificed in the name of better separation. In comparison to the newly developed method, the typical method had a shorter run time but did not separate the co-eluting THC isomer peaks. With a longer run time and a more stable solvent gradient the separation of THC isomer peaks is possible allowing for true characterization and percentage identification of the troublesome D9-THC peak to keep products compliant.
Companies selling “compliant” D8-THC can truly characterize batches and identify the true D8-THC content, where an ever-growing demand for such products is increasing, in a consumer scene where reliability and true cognizance of product makeup is necessary [
14,
15,
16].
4. Conclusion
In conclusion, the developed method for the separation of THC isomers proves to be a valuable tool for accurate characterization and quantification of D9 THC and D8 THC in various cannabis samples. The application of this method to different samples, including a synthesized THC reaction mixture and its derivatives, demonstrates its effectiveness in resolving and quantifying the isomers.
The comparison with an outdated method underscores the significance of the newly developed approach, which, despite a longer run time, successfully separates co-eluting THC isomer peaks. This improvement is crucial for companies in the cannabis industry, especially those selling D8-THC products, as it enables them to provide reliable and compliant products. The ability to accurately identify and quantify D9-THC, a regulatory concern, ensures that products meet compliance standards and provides consumers with trustworthy information about the product makeup.
Author Contributions
Conceptualization: G.A.R., W.C., T.T.T. Methodology: G.A.R., M.K.P., T.T.T., W.C. Formal Analysis: G.A.R., W.C. Writing Original Draft: G.A.R., M.K.P., W.C. Writing Review & Editing: G.A.R., M.K.P., W.C. Supervision: K.P.R., W.C. Project Administration: K.P.R., W.C. All authors have read and approved this manuscript for submission.
Funding
There is no funding to report.
Acknowledgements
Authors gratefully acknowledge NMR spectroscopy support from Dr. Jin Hong at Custom NMR Services, Inc. from Woburn.
Author Disclosure
GAR, MKP, and TTT are employees of Colorado Chromatography Labs. WC and KPR are founders of Colorado Chromatography Labs.
Abbreviations
| ACN |
Acetonitrile |
| CBD |
Cannabidiol |
| CPG |
Consumer Product Good |
| CRM |
Certified Reference Material |
| HPLC |
High Performance Liquid Chromatography |
| THC |
Tetrahydrocannabinol |
References
- Schilling, S.; Melzer, R.; McCabe, P.F. Quick Guide Cannabis Sativa, Current Biology 2020, 30, R1-R9.
- Bassetti, B.; Hone, C. A.; Kappe, C. O. Continuous-Flow Synthesis of Δ9-Tetrahydrocannabinol and Δ8-Tetrahydrocannabinol from Cannabidiol. J. Org. Chem. 2023, 88 (9), 6227–6231. [CrossRef]
- Giorgi, P. D.; Liautard, V.; Pucheault, M.; Antoniot-ti, S. Biomimetic Cannabinoid Synthesis Revisit-ed: Batch and Flow All-Catalytic Synthesis of (±)-Ortho -Tetrahydrocannabinols and Analogues from Natural Feedstocks: Biomimetic Canna-binoid Synthesis Revisited: Batch and Flow All-Catalytic Synthesis of (±)-Ortho-Tetrahydrocannabinols and Analogues from Natural Feeds. European J. Org. Chem. 2018, 2018 (11), 1307–1311. [CrossRef]
- Chiurchiù, E.; Sampaolesi, S.; Allegrini, P.; Ciceri, D.; Ballini, R.; Palmieri, A. A Novel and Practical Continuous Flow Chemical Synthesis of Canna-bidiol (CBD) and Its CBDV and CBDB Ana-logues. European J. Org. Chem. 2021, 2021 (8), 1286–1289. [CrossRef]
- Bloemendal, V. R. L. J.; Spierenburg, B.; Boltje, T. J.; van Hest, J. C. M.; Rutjes, F. P. J. T. One-Flow Synthesis of Tetrahydrocannabinol and Cannabidiol Using Homo- and Heterogeneous Lewis Acids. J. Flow Chem. 2021, 11 (2), 99–105. [CrossRef]
- Marzullo, P.; Foschi, F.; Coppini, D. A.; Fanchini, F.; Magnani, L.; Rusconi, S.; Luzzani, M.; Passarella, D. Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization. J. Nat. Prod. 2020, 83 (10), 2894–2901. [CrossRef]
- Collins, A.; Ramirez, G.; Tesfatsion, T.; Ray, K. P.; Caudill, S.; Cruces, W. Synthesis and Characterization of the Diastereomers of HHC and H4CBD. Nat. Prod. Commun. 2023, 18 (3), 1934578X2311589. [CrossRef]
- Ramirez, G. A., Docampo-Palacios, M. L., Tesfatsion, T. T., Cruces, I., Hellmann, A. J., Okhovat, A., Pittiglio, M., Ray, K. P., Cruces, W., Ultrasonic or Microwave modified continuous flow chemistry for the synthesis of tetrahydrocannabinol: Observing the effects of various solvents and acids, Accepted, ACS Omega, 2024, Preprint. [CrossRef]
- Tesfatsion, T. T., Ramirez, G. A., Docampo, M. L., Collins, A. C., Mzannar, Y., Khan, H. Y., Aboukameel, O., Azmi, A. S., Jagtap, P. G., Ray, K. P., Cruces, W., Antineoplastic Properties of THCV, HHC and their anti-Proliferative effects on HPAF-II, MIA-paca2, Aspc-1, and PANC-1 PDAC Pancreatic Cell Lines, 2022, Submitted to J. Cannabis Res., Preprint. [CrossRef]
- Gong X, Sun C, Abame MA, et al. Synthesis of CBD and its derivatives bearing various C4′-side chains with a late-stage diversification method. J Org Chem 2020;85(4):2704–2715. [CrossRef]
- Erickson BE. Delta-8-THC craze concerns chemists. Chem Eng News. 2021;99:25-28.
- Docampo-Palacios, M. L., Ramirez, G. A., Tesfatsion, T. T., Pittiglio, M. K., Ray, K. P., Cruces, Comprehensive Safety Assessment of Diverse Cannabinoids: A Scientific Inquiry, 2024. [CrossRef]
- Segall, B. 13News investigation finds some Delta-8 products exceed state’s THC limit, underscoring need for regulation, 13WTHR, accessed February 23, 2024 https://www.wthr.com/article/news/investigations/13-investigates/13news-investigation-shows-some-delta-8-products-exceed-legal-delta-9-thc-limit-posing-risks-consumers-retailers/531-755951a1-981c-446d-b5f5-995370a9c397.
- Geci M, Scialdone M, Tishler J. The dark side of cannabidiol: The unanticipated social and clinical implications of synthetic Δ8-THC. Cannabis Cannabinoid Res. Published online 2022. [CrossRef]
- Babalonis S, Raup-Konsavage WM, Akpunonu PD, Balla A, Vrana KE. Δ8-THC: Legal status, widespread availability, and safety concerns. Cannabis Cannabinoid Res. 2021;6(5):362-365. [CrossRef]
- Livne O, Budney A, Borodovsky J, Walsh C, Shmulewitz D, Fink DS, et al. Delta-8 THC use in US adults: Sociodemographic characteristics and correlates. Addict Behav. 2022;133(107374):107374. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).