Submitted:
04 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Line and Animal Model
2.2. Pharmaceuticals
2.3. Treatment Regimens
2.4. Immunohistochemical Analysis
2.5. Gene Expression Analyses
2.6. Statistical Analyses
3. Results
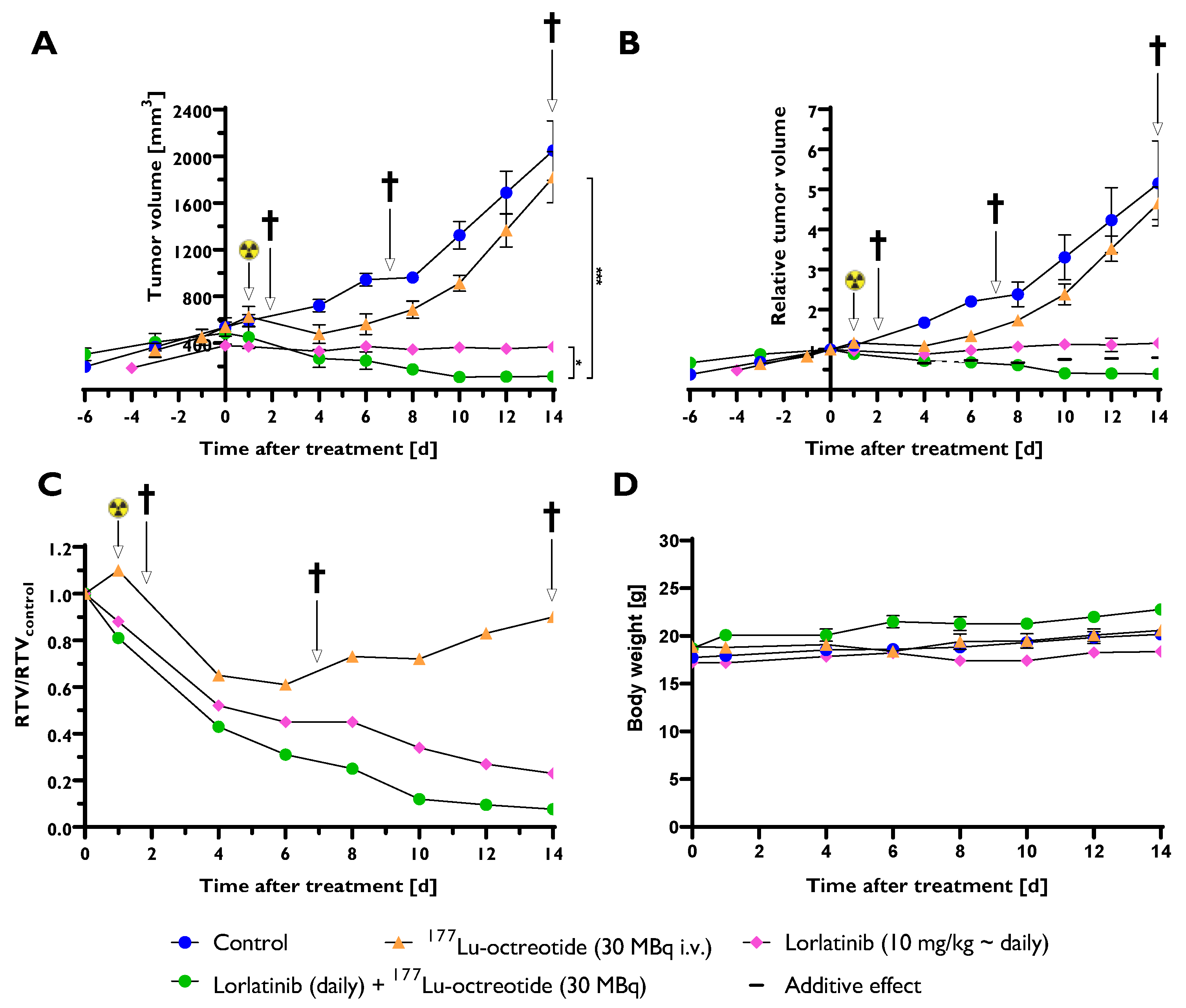
3.1. Combination Therapy Gave the Largest Tumor Volume Reduction
3.2. Changes in the Expression of Genes Involved in Apoptosis
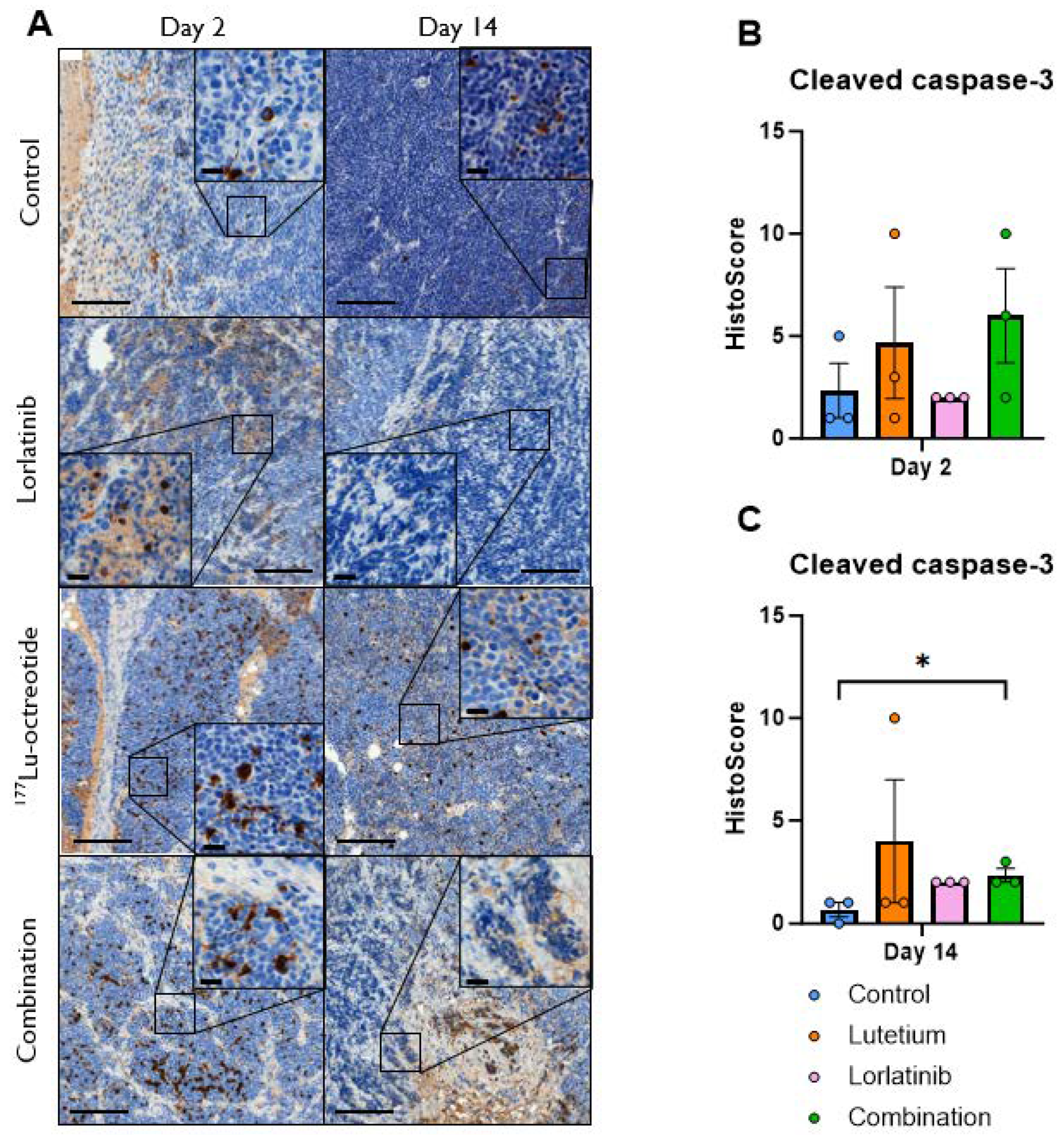
3.3. Immunohistochemical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Umapathy:, G.; Mendoza-Garcia, P.; Hallberg, B.; Palmer, R.H. Targeting anaplastic lymphoma kinase in neuroblastoma. APMIS 2019, 127, 288–302. [Google Scholar] [CrossRef]
- De Brouwer, S.; De Preter, K.; Kumps, C.; Zabrocki, P.; Porcu, M.; Westerhout, E.M.; Lakeman, A.; Vandesompele, J.; Hoebeeck, J.; Van Maerken, T.; et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin Cancer Res 2010, 16, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Colmet Daage, L.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 2015, 47, 864–871. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013, 45, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A. The ALKF1174L mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer cell 2012, 22, 117–130. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, J.-S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer cell 2012, 21, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Schonherr, C.; Ruuth, K.; Kamaraj, S.; Wang, C.L.; Yang, H.L.; Combaret, V.; Djos, A.; Martinsson, T.; Christensen, J.G.; Palmer, R.H.; et al. Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 2012, 31, 5193–5200. [Google Scholar] [CrossRef]
- Morgenstern, D.A.; Bagatell, R.; Cohn, S.L.; Hogarty, M.D.; Maris, J.M.; Moreno, L.; Park, J.R.; Pearson, A.D.; Schleiermacher, G.; Valteau-Couanet, D.; et al. The challenge of defining "ultra-high-risk" neuroblastoma. Pediatr Blood Cancer 2019, 66, e27556. [Google Scholar] [CrossRef]
- Johnson, T.W.; Richardson, P.F.; Bailey, S.; Brooun, A.; Burke, B.J.; Collins, M.R.; Cui, J.J.; Deal, J.G.; Deng, Y.-L.; Dinh, D. Discovery of (10 R)-7-Amino-12-fluoro-2, 10, 16-trimethyl-15-oxo-10, 15, 16, 17-tetrahydro-2H-8, 4-(metheno) pyrazolo [4, 3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. Journal of medicinal chemistry 2014, 57, 4720–4744. [Google Scholar]
- Syed, Y.Y. Lorlatinib: first global approval. Drugs 2019, 79, 93–98. [Google Scholar] [CrossRef]
- Zou, H.Y.; Friboulet, L.; Kodack, D.P.; Engstrom, L.D.; Li, Q.; West, M.; Tang, R.W.; Wang, H.; Tsaparikos, K.; Wang, J. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer cell 2015, 28, 70–81. [Google Scholar] [CrossRef]
- Guan, J.; Tucker, E.R.; Wan, H.; Chand, D.; Danielson, L.S.; Ruuth, K.; El Wakil, A.; Witek, B.; Jamin, Y.; Umapathy, G.; et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN. Disease Models & Mechanisms 2016, 9, 941–952. [Google Scholar] [CrossRef]
- Liu, T.; Merguerian, M.D.; Rowe, S.P.; Pratilas, C.A.; Chen, A.R.; Ladle, B.H. Exceptional response to the ALK and ROS1 inhibitor lorlatinib and subsequent mechanism of resistance in relapsed ALK F1174L-mutated neuroblastoma. Molecular Case Studies 2021, 7, a006064. [Google Scholar] [CrossRef]
- Goldsmith, K.C.; Park, J.R.; Kayser, K.; Malvar, J.; Chi, Y.Y.; Groshen, S.G.; Villablanca, J.G.; Krytska, K.; Lai, L.M.; Acharya, P.T.; et al. Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: phase 1 trial results. Nat Med 2023, 29, 1092–1102. [Google Scholar] [CrossRef]
- Redaelli, S.; Ceccon, M.; Zappa, M.; Sharma, G.G.; Mastini, C.; Mauri, M.; Nigoghossian, M.; Massimino, L.; Cordani, N.; Farina, F.; et al. Lorlatinib Treatment Elicits Multiple On- and Off-Target Mechanisms of Resistance in ALK-Driven Cancer. Cancer Research 2018, 78, 6866–6880. [Google Scholar] [CrossRef]
- Mizuta, H.; Okada, K.; Araki, M.; Adachi, J.; Takemoto, A.; Kutkowska, J.; Maruyama, K.; Yanagitani, N.; Oh-Hara, T.; Watanabe, K. Gilteritinib overcomes lorlatinib resistance in ALK-rearranged cancer. Nature communications 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Okada, K.; Araki, M.; Sakashita, T.; Ma, B.; Kanada, R.; Yanagitani, N.; Horiike, A.; Koike, S.; Oh-Hara, T.; Watanabe, K. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine 2019, 41, 105–119. [Google Scholar] [CrossRef]
- Xie, B.; Qiu, Y.; Zhou, J.; Du, D.; Ma, H.; Ji, J.; Zhu, L.; Zhang, W. Establishment of an acquired lorlatinib-resistant cell line of non-small cell lung cancer and its mediated resistance mechanism. Clinical and Translational Oncology 2022, 24, 2231–2240. [Google Scholar] [CrossRef]
- Lamberts, S.W.J.; Krenning, E.P.; Reubi, J.-C. The Role of Somatostatin and Its Analogs in the Diagnosis and Treatment of Tumors. Endocrine Reviews 1991, 12, 450–482. [Google Scholar] [CrossRef]
- Baum, R.P.; Kluge, A.W.; Kulkarni, H.; Schorr-Neufing, U.; Niepsch, K.; Bitterlich, N.; van Echteld, C.J. [177Lu-DOTA] 0-D-Phe1-Tyr3-octreotide (177Lu-DOTATOC) for peptide receptor radiotherapy in patients with advanced neuroendocrine tumours: a phase-II study. Theranostics 2016, 6, 501. [Google Scholar] [CrossRef]
- Esser, J.-P.; Krenning, E.; Teunissen, J.; Kooij, P.; Van Gameren, A.; Bakker, W.; Kwekkeboom, D.J. Comparison of [177Lu-DOTA0, Tyr3] octreotate and [177Lu-DOTA0, Tyr3] octreotide: which peptide is preferable for PRRT? European journal of nuclear medicine and molecular imaging 2006, 33, 1346–1351. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. European journal of nuclear medicine 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Uusijärvi, H.; Bernhardt, P.; Ericsson, T.; Forssell-Aronsson, E. Dosimetric characterization of radionuclides for systemic tumor therapy: influence of particle range, photon emission, and subcellular distribution. Medical physics 2006, 33, 3260–3269. [Google Scholar] [CrossRef]
- Uusijärvi, H.; Bernhardt, P.; Rösch, F.; Maecke, H.R.; Forssell-Aronsson, E. Electron- and positron-emitting radiolanthanides for therapy: aspects of dosimetry and production. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2006, 47, 807–814. [Google Scholar]
- Swärd, C.; Bernhardt, P.; Ahlman, H.; Wängberg, B.; Forssell-Aronsson, E.; Larsson, M.; Svensson, J.; Rossi-Norrlund, R.; Kölby, L. [177Lu-DOTA0-Tyr3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: the value of measuring absorbed dose to the kidney. World journal of surgery 2010, 34, 1368–1372. [Google Scholar] [CrossRef]
- Romiani, A.; Spetz, J.; Shubbar, E.; Lind, D.E.; Hallberg, B.; Palmer, R.H.; Forssell-Aronsson, E. Neuroblastoma xenograft models demonstrate the therapeutic potential of 177Lu-octreotate. BMC Cancer 2021, 21, 950. [Google Scholar] [CrossRef]
- Gains, J.E.; Bomanji, J.B.; Fersht, N.L.; Sullivan, T.; D'Souza, D.; Sullivan, K.P.; Aldridge, M.; Waddington, W.; Gaze, M.N. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. Journal of Nuclear Medicine 2011, 52, 1041–1047. [Google Scholar] [CrossRef]
- Kong, G.; Hofman, M.S.; Murray, W.K.; Wilson, S.; Wood, P.; Downie, P.; Super, L.; Hogg, A.; Eu, P.; Hicks, R.J. Initial experience with gallium-68 DOTA-octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. Journal of pediatric hematology/oncology 2016, 38, 87–96. [Google Scholar] [CrossRef]
- Gains, J.E.; Moroz, V.; Aldridge, M.D.; Wan, S.; Wheatley, K.; Laidler, J.; Peet, C.; Bomanji, J.B.; Gaze, M.N. A phase IIa trial of molecular radiotherapy with 177-lutetium DOTATATE in children with primary refractory or relapsed high-risk neuroblastoma. European Journal of Nuclear Medicine and Molecular Imaging 2020, 47, 2348–2357. [Google Scholar] [CrossRef]
- Sundquist, F.; Georgantzi, K.; Jarvis, K.B.; Brok, J.; Koskenvuo, M.; Rascon, J.; van Noesel, M.; Grybäck, P.; Nilsson, J.; Braat, A. A Phase II Trial of a Personalized, Dose-Intense Administration Schedule of 177Lutetium-DOTATATE in Children With Primary Refractory or Relapsed High-Risk Neuroblastoma–LuDO-N. Frontiers in pediatrics 2022, 167. [Google Scholar] [CrossRef]
- Fransson, S.; Hansson, M.; Ruuth, K.; Djos, A.; Berbegall, A.; Javanmardi, N.; Abrahamsson, J.; Palmer, R.H.; Noguera, R.; Hallberg, B.; et al. Intragenic anaplastic lymphoma kinase (ALK) rearrangements: translocations as a novel mechanism of ALK activation in neuroblastoma tumors. Genes Chromosomes Cancer 2015, 54, 99–109. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nature protocols 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bliss, C.I. The toxicity of poisons applied jointly 1. Annals of applied biology 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Sandblom, V.; Spetz, J.; Shubbar, E.; Montelius, M.; Ståhl, I.; Swanpalmer, J.; Nilsson, O.; Forssell-Aronsson, E. Gemcitabine potentiates the anti-tumour effect of radiation on medullary thyroid cancer. Plos one 2019, 14, e0225260. [Google Scholar] [CrossRef]
- Cazes, A.; Louis-Brennetot, C.; Mazot, P.; Dingli, F.; Lombard, B.; Boeva, V.; Daveau, R.; Cappo, J.; Combaret, V.; Schleiermacher, G.; et al. Characterization of Rearrangements Involving the ALK Gene Reveals a Novel Truncated Form Associated with Tumor Aggressiveness in Neuroblastoma. Cancer Research 2013, 73, 195–204. [Google Scholar] [CrossRef]
- Romiani. Comparison of 177Lu-octreotate and 177Lu-octreotide for treatment in human neuroblastoma-bearing mice (submitted)
- Kölby, L.; Bernhardt, P.; Johanson, V.; Schmitt, A.; Ahlman, H.; Forssell-Aronsson, E.; Mäcke, H.; Nilsson, O. Successful receptor-mediated radiation therapy of xenografted human midgut carcinoid tumour. British journal of cancer 2005, 93, 1144–1151. [Google Scholar] [CrossRef]
- Swärd, C.; Bernhardt, P.; Johanson, V.; Schmitt, A.; Ahlman, H.; Stridsberg, M.; Forssell-Aronsson, E.; Nilsson, O.; Kölby, L. Comparison of [177Lu-DOTA0, Tyr3]-octreotate and [177Lu-DOTA0, Tyr3]-octreotide for receptor-mediated radiation therapy of the xenografted human midgut carcinoid tumor GOT1. Cancer biotherapy & radiopharmaceuticals 2008, 23, 114–120. [Google Scholar] [CrossRef]
- Elvborn, M.; Shubbar, E.; Forssell-Aronsson, E. Hyperfractionated Treatment with 177Lu-Octreotate Increases Tumor Response in Human Small-Intestine Neuroendocrine GOT1 Tumor Model. Cancers 2022, 14, 235. [Google Scholar] [CrossRef]
- Schmitt, A.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Kölby, L.; Forssell-Aronsson, E. Differences in biodistribution between 99mTc-depreotide, 111In-DTPA-octreotide, and 177Lu-DOTA-Tyr3-octreotate in a small cell lung cancer animal model. Cancer biotherapy & radiopharmaceuticals 2005, 20, 231–236. [Google Scholar] [CrossRef]
- Ducray, S.P.; Natarajan, K.; Garland, G.D.; Turner, S.D.; Egger, G. The transcriptional roles of ALK fusion proteins in tumorigenesis. Cancers 2019, 11, 1074. [Google Scholar] [CrossRef]
- Borenas, M.; Umapathy, G.; Lind, D.E.; Lai, W.Y.; Guan, J.; Johansson, J.; Jennische, E.; Schmidt, A.; Kurhe, Y.; Gabre, J.L.; et al. ALK signaling primes the DNA damage response sensitizing ALK-driven neuroblastoma to therapeutic ATR inhibition. Proc Natl Acad Sci U S A 2024, 121, e2315242121. [Google Scholar] [CrossRef]
- Szydzik, J.; Lind, D.E.; Arefin, B.; Kurhe, Y.; Umapathy, G.; Siaw, J.T.; Claeys, A.; Gabre, J.L.; Van den Eynden, J.; Hallberg, B.; et al. ATR inhibition enables complete tumour regression in ALK-driven NB mouse models. Nat Commun 2021, 12, 6813. [Google Scholar] [CrossRef]
- Dolman, M.E.M.; Van Der Ploeg, I.; Koster, J.; Bate-Eya, L.T.; Versteeg, R.; Caron, H.N.; Molenaar, J.J. DNA-dependent protein kinase as molecular target for radiosensitization of neuroblastoma cells. PLoS One 2015, 10, e0145744. [Google Scholar] [CrossRef]
- Toulany, M.; Kehlbach, R.; Florczak, U.; Sak, A.; Wang, S.; Chen, J.; Lobrich, M.; Rodemann, H.P. Targeting of AKT1 enhances radiation toxicity of human tumor cells by inhibiting DNA-PKcs-dependent DNA double-strand break repair. Molecular cancer therapeutics 2008, 7, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Stronach, E.A.; Chen, M.; Maginn, E.N.; Agarwal, R.; Mills, G.B.; Wasan, H.; Gabra, H. DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired platinum resistance. Neoplasia 2011, 13, 1069–IN1035. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ren, Y.; Zhang, T.; Wang, Z.; Ling, C.C.; Li, G.C.; He, F.; Wang, C.; Wen, B. Inactivation of DNA-PK by knockdown DNA-PKcs or NU7441 impairs non-homologous end-joining of radiation-induced double strand break repair. Oncology reports 2018, 39, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Lundsten, S.; Berglund, H.; Jha, P.; Krona, C.; Hariri, M.; Nelander, S.; Lane, D.P.; Nestor, M. p53-Mediated Radiosensitization of (177)Lu-DOTATATE in Neuroblastoma Tumor Spheroids. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Berglund, H.; Salomonsson, S.L.; Mohajershojai, T.; Gago, F.J.F.; Lane, D.P.; Nestor, M. p53 stabilisation potentiates [(177)Lu]Lu-DOTATATE treatment in neuroblastoma xenografts. Eur J Nucl Med Mol Imaging 2024, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nowak, K.A.; Zaorsky, N.G.; Winchester, C.-L.; Dalal, K.; Giacalone, N.J.; Liu, N.; Werner-Wasik, M.; Wasik, M.A.; Dicker, A.P. ALK Inhibitor PF02341066 (Crizotinib) Increases Sensitivity to Radiation in Non–Small Cell Lung Cancer Expressing EML4-ALKPF02341066 Sensitizes EML4-ALK NSCLC Cells to Radiation Treatment. Molecular cancer therapeutics 2013, 12, 696–704. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, Q.; Schwager, C.; Moustafa, M.; Zhou, C.; Lipson, K.E.; Weichert, W.; Debus, J.; Abdollahi, A. Synergistic effects of crizotinib and radiotherapy in experimental EML4–ALK fusion positive lung cancer. Radiotherapy and Oncology 2015, 114, 173–181. [Google Scholar] [CrossRef]
- Liu, Q.; Turner, K.M.; Alfred Yung, W.K.; Chen, K.; Zhang, W. Role of AKT signaling in DNA repair and clinical response to cancer therapy. Neuro-Oncology 2014, 16, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Harari, P.M.; Wheeler, D.L.; Toulany, M. Targeting AKT/PKB to improve treatment outcomes for solid tumors. Mutat Res 2020, 819-820, 111690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhan, Y.; Zhang, Y.; Liu, S.; Lu, J.; Yang, Y.; Wen, Q.; Fan, S. Elevated expression of G3BP1 associates with YB1 and p-AKT and predicts poor prognosis in nonsmall cell lung cancer patients after surgical resection. Cancer Medicine 2019, 8, 6894–6903. [Google Scholar] [CrossRef] [PubMed]
- Opel, D.; Poremba, C.; Simon, T.; Debatin, K.-M.; Fulda, S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer research 2007, 67, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Berlak, M.; Tucker, E.; Dorel, M.; Winkler, A.; McGearey, A.; Rodriguez-Fos, E.; da Costa, B.M.; Barker, K.; Fyle, E.; Calton, E.; et al. Mutations in ALK signaling pathways conferring resistance to ALK inhibitor treatment lead to collateral vulnerabilities in neuroblastoma cells. Molecular Cancer 2022, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Riely, G.J.; Shaw, A.T. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discovery 2017, 7, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, Y.; Hayashi, H.; Haratani, K.; Tanizaki, J.; Tanaka, K.; Takeda, M.; Sakai, K.; Shimizu, S.; Ito, A.; Nishio, K. A case of ALK-rearranged non–small cell lung cancer that responded to ceritinib after development of resistance to alectinib. Oncotarget 2018, 9, 23315. [Google Scholar] [CrossRef]
- Sharma, G.G.; Cortinovis, D.; Agustoni, F.; Arosio, G.; Villa, M.; Cordani, N.; Bidoli, P.; Bisson, W.H.; Pagni, F.; Piazza, R. A compound L1196M/G1202R ALK mutation in a patient with ALK-positive lung cancer with acquired resistance to brigatinib also confers primary resistance to lorlatinib. Journal of Thoracic Oncology 2019, 14, e257–e259. [Google Scholar] [CrossRef]
- Takahashi, K.; Seto, Y.; Okada, K.; Uematsu, S.; Uchibori, K.; Tsukahara, M.; Oh-hara, T.; Fujita, N.; Yanagitani, N.; Nishio, M. Overcoming resistance by ALK compound mutation (I1171S+ G1269A) after sequential treatment of multiple ALK inhibitors in non-small cell lung cancer. Thoracic Cancer 2020, 11, 581–587. [Google Scholar] [CrossRef]
- Recondo, G.; Mezquita, L.; Facchinetti, F.; Planchard, D.; Gazzah, A.; Bigot, L.; Rizvi, A.Z.; Frias, R.L.; Thiery, J.P.; Scoazec, J.-Y. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung CancerResistance to Lorlatinib in ALK-Rearranged Lung Cancer. Clinical Cancer Research 2020, 26, 242–255. [Google Scholar] [CrossRef]
- Shaw, A.T.; Friboulet, L.; Leshchiner, I.; Gainor, J.F.; Bergqvist, S.; Brooun, A.; Burke, B.J.; Deng, Y.-L.; Liu, W.; Dardaei, L. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. New England Journal of Medicine 2016, 374, 54–61. [Google Scholar] [CrossRef]
- Gomez, R.L.; Ibragimova, S.; Ramachandran, R.; Philpott, A.; Ali, F.R. Tumoral heterogeneity in neuroblastoma. Biochim Biophys Acta Rev Cancer 2022, 1877, 188805. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews Molecular cell biology 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nature reviews Molecular cell biology 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Spetz, J.K.E.; Florido, M.H.C.; Fraser, C.S.; Qin, X.; Choiniere, J.; Yu, S.J.; Singh, R.; Friesen, M.; Rubin, L.L.; Salem, J.E.; et al. Heightened apoptotic priming of vascular cells across tissues and life span predisposes them to cancer therapy-induced toxicities. Sci Adv 2022, 8, eabn6579. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Yu, S.; Osman, M.; Inde, Z.; Fraser, C.; Cleveland, A.H.; Almanzar, N.; Lim, C.B.; Joshi, G.N.; Spetz, J.; et al. Radiotherapy-Induced Neurocognitive Impairment Is Driven by Heightened Apoptotic Priming in Early Life and Prevented by Blocking BAX. Cancer Res 2023, 83, 3442–3461. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-m. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell research 2000, 10, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Aksoy, E.; Cingoz, A.; Senbabaoglu, F.; Seker, F.; Sur-Erdem, I.; Kayabolen, A.; Lokumcu, T.; Sahin, G.N.; Karahuseyinoglu, S.; Bagci-Onder, T. The pro-apoptotic Bcl-2 family member Harakiri (HRK) induces cell death in glioblastoma multiforme. Cell death discovery 2019, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-J.; Han, L.-H.; Cong, R.-S.; Liang, J. Caspase family proteases and apoptosis. Acta biochimica et biophysica Sinica 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Dostert, C.; Grusdat, M.; Letellier, E.; Brenner, D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiological reviews 2019, 99, 115–160. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M. A role for p53 in the frequency and mechanism of mutation. Mutation Research/Reviews in Mutation Research 2002, 511, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Zawacka-Pankau, J.E. The Role of p53 Family in Cancer. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Spetz, J.; Rudqvist, N.; Langen, B.; Parris, T.Z.; Dalmo, J.; Schüler, E.; Wängberg, B.; Nilsson, O.; Helou, K.; Forssell-Aronsson, E. Time-dependent transcriptional response of GOT1 human small intestine neuroendocrine tumor after 177Lu [Lu]-octreotate therapy. Nuclear Medicine and Biology 2018, 60, 11–18. [Google Scholar] [CrossRef]
- Rassol, N.; Andersson, C.; Pettersson, D.; Al-Awar, A.; Shubbar, E.; Kovacs, A.; Akerstrom, B.; Gram, M.; Helou, K.; Forssell-Aronsson, E. Co-administration with A1M does not influence apoptotic response of (177)Lu-octreotate in GOT1 neuroendocrine tumors. Sci Rep 2023, 13, 6417. [Google Scholar] [CrossRef] [PubMed]
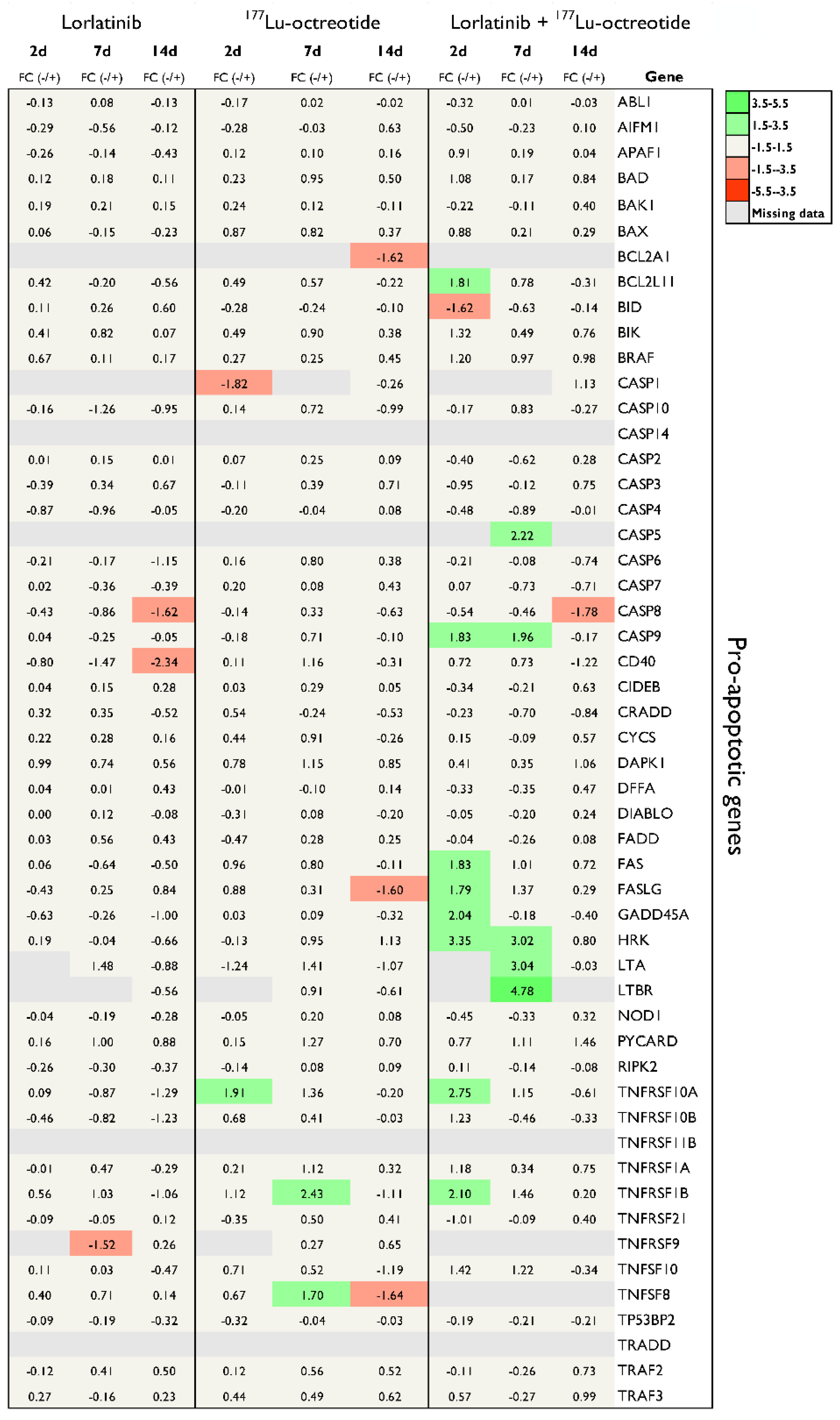
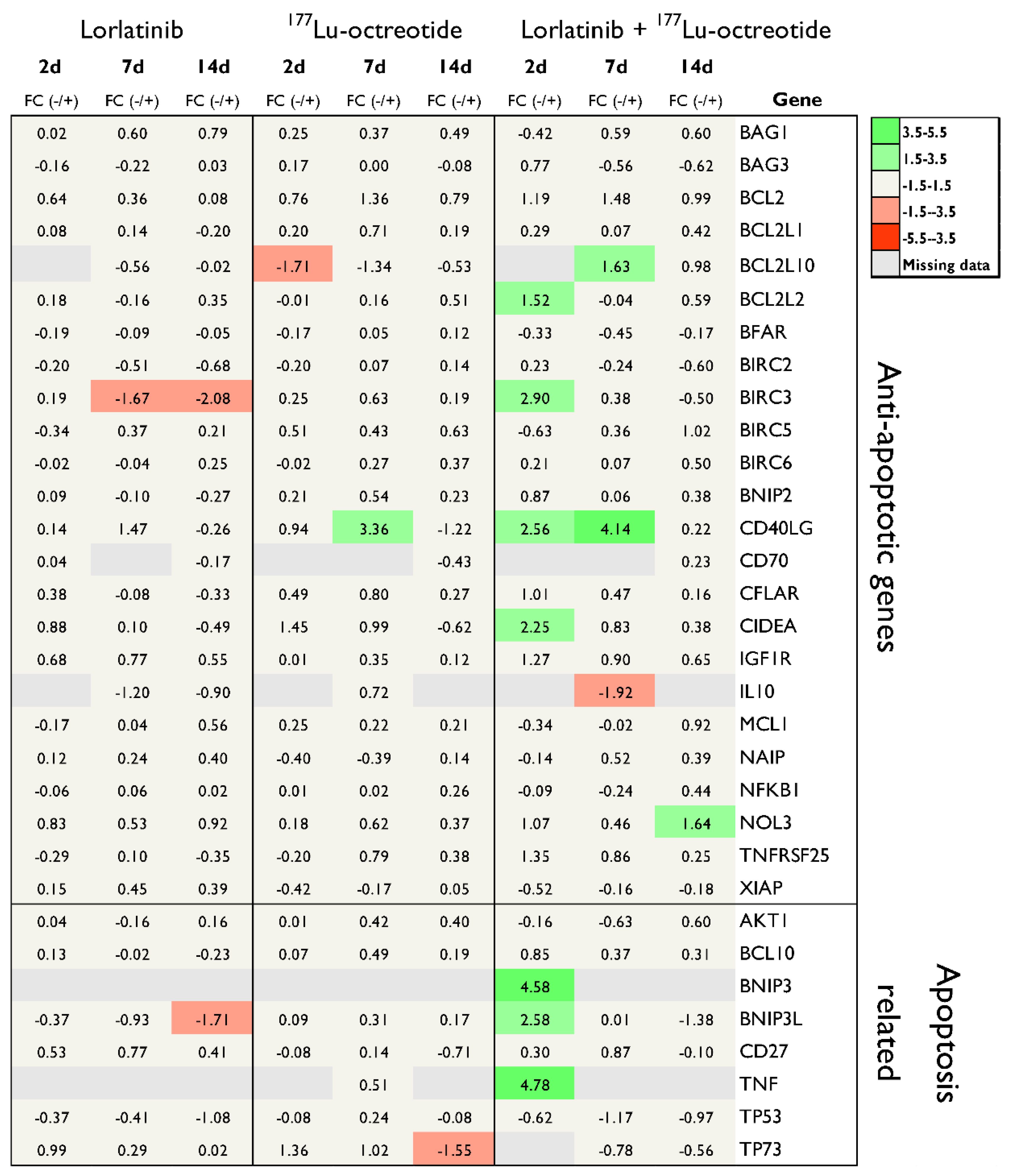
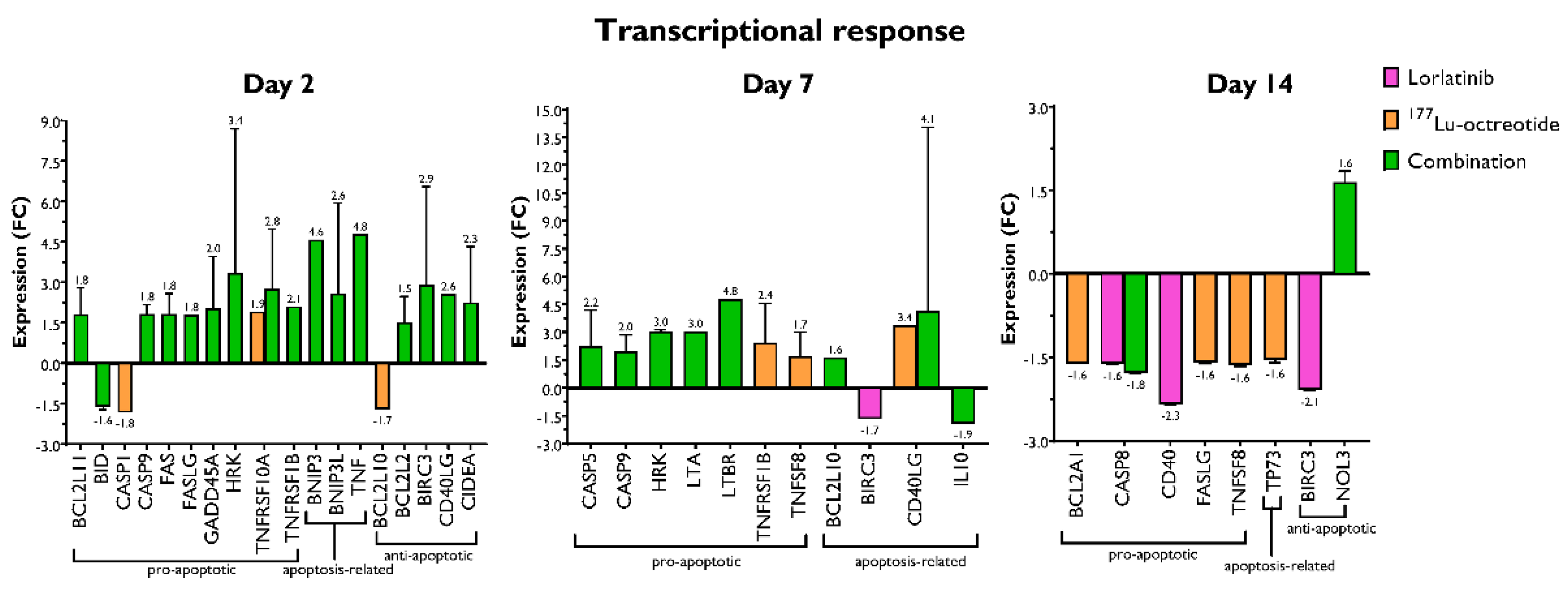
| Gene | Description | Protein family | FC | p | Time after treatment |
|---|---|---|---|---|---|
| CASP8 | Caspase 8, apoptosis-related cysteine peptidase | Caspase family | -1.62 | 0.0003 | 14 days, Lorlatinib |
| CD40 | CD40 molecule, TNF receptor superfamily member 5 | TNF-receptor superfamily | -2.34 | 0.0001 | 14 days, Lorlatinib |
| BNIP3L | BCL2/adenovirus interacting protein 3-like | Pro-apoptotic subfamily within the Bcl-2 family | -1.71 | 0.0001 | 14 days, Lorlatinib |
| BIRC3 | Baculoviral IAP repeat containing 3 | Inhibition of apoptosis (IAP) family | -1.67-2.08 | 0.02180.0005 | 7 days, Lorlatinib14 days, Lorlatinib |
| FASLG | Fas ligand (TNF superfamily, member 6) | TNF superfamily | -1.60 | 0.0036 | 14 days, 177Lu-octreotide |
| TNFRSF10A | TNF receptor superfamily, member 10a | TNF-receptor superfamily | 1.91 | 0.0004 | 2 days, 177Lu-octreotide |
| TP73 | Tumor protein p73 | TP53 family | -1.55 | 0.0380 | 14 days, 177Lu-octreotide |
| BID | BH3 interacting domain death agonist | Bcl–2 family | -1.62 | 0.0356 | 2 days, Combination |
| CASP8 | Caspase 8, apoptosis-related cysteine peptidase | Caspase family | -1.78 | 0.0003 | 14 days, Combination |
| CASP9 | Caspase 9, apoptosis-related cysteine peptidase | Caspase family | 1.83 1.96 | 0.00110.0103 | 2 days, Combination7 days, Combination |
| HRK | Harakiri, BCL2 interacting protein (contains only BH3 domain) | Bcl-2 family | 3.02 | 0.0014 | 7 days, Combination |
| TNFRSF10A | TNF receptor superfamily, member 10a | TNF-receptor superfamily | 2.75 | 0.0254 | 2 days, Combination |
| NOL3 | Nucleolar protein 3 (apoptosis repressor with CARD domain) | Down-regulates activities of caspase 2, 8 and p53 | 1.64 | 0.0011 | 14 days, Combination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





