Submitted:
06 March 2024
Posted:
08 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Theoretical Background and Comments on Experimental Data
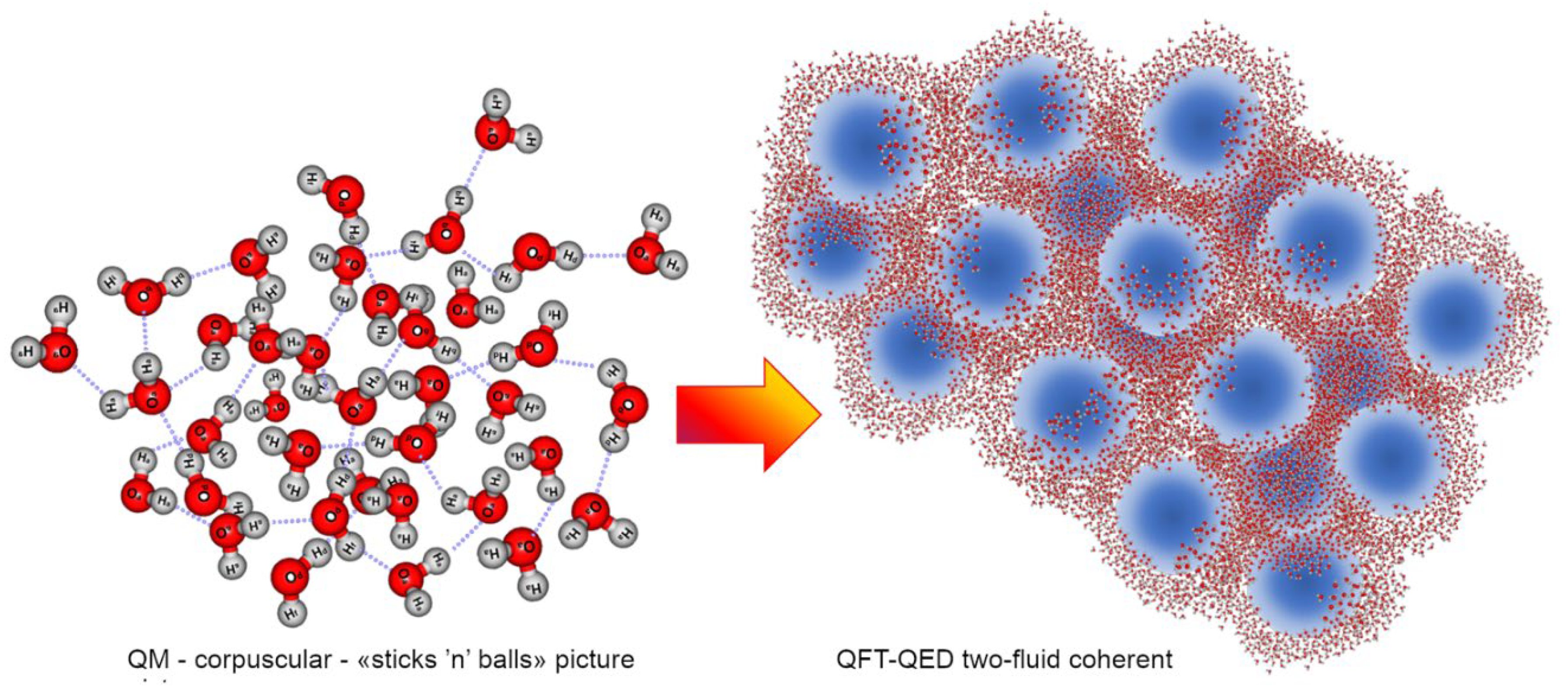
2.1. Resuming Some of the Problems within the Corpuscular QM View (1st Quantization)
- 0.05 eV for direct dipole-dipole Keesom interactions (Equation (2))
- 0.03 eV for Debye interactions between permanent and induced dipoles (Equation (3))
- 0.12 eV for dispersive London interactions between two induced dipoles (Equation (4))
-
1HOMO-1, HOMO-2, HOMO-3, …, HOMO-N or LUMO+1, LUMO+2, LUMO+3, …, LUMO+N denote electronic levels, among the several molecular orbitals, placed at the Nth level below (-) the HOMO or at the Nth level above (+) the LUMO.
-
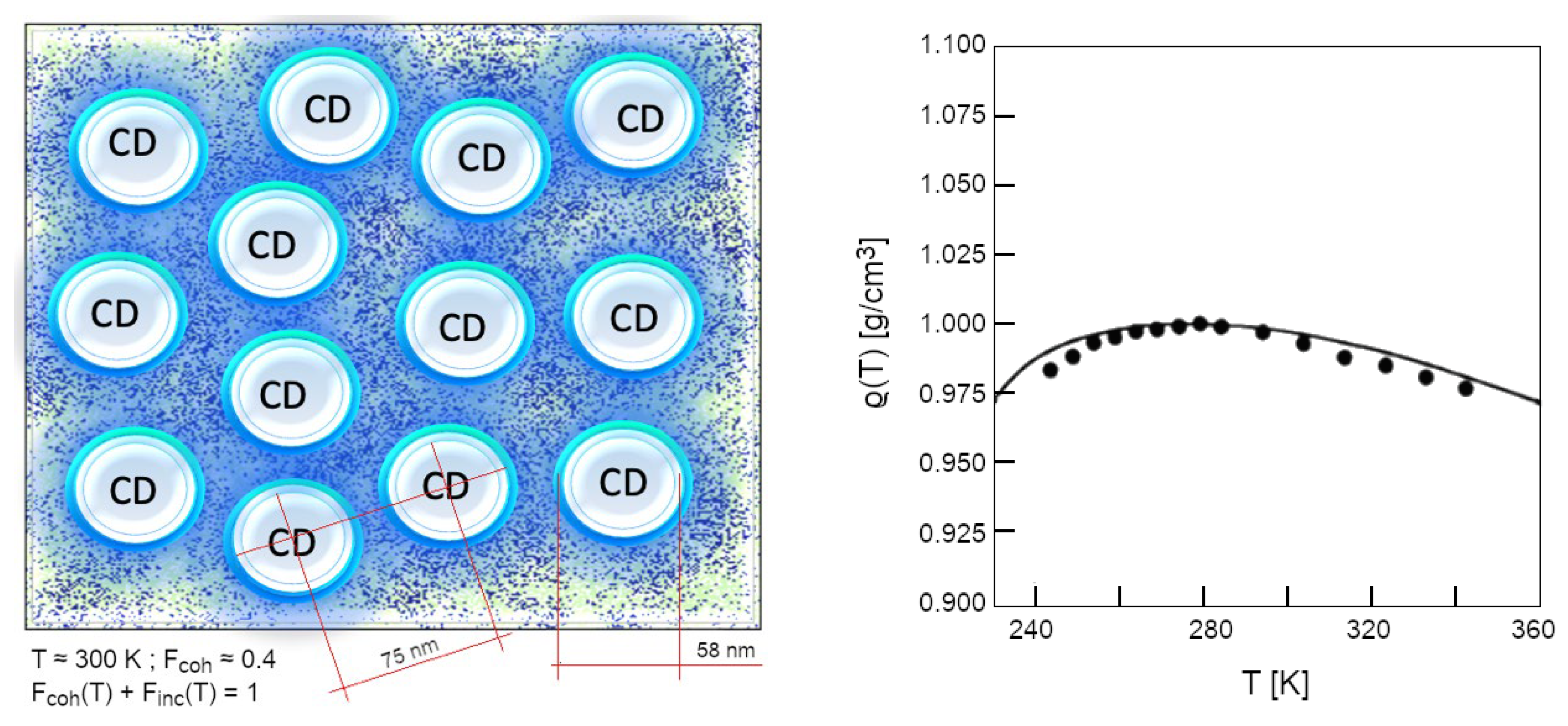
2The problem with X-ray scattering, indeed, is that it tends to give a static image of water, whereas it is a dynamic medium. Neutron scattering, on the contrary, has revealed the existence of two relaxation times in liquid water (Teixeira, Bellissent-Funel, Chen, & Dianoux, 1985). Thus, the first time close to 1-2 ps at room temperature corresponds to the fluctuation of the network of hydrogen bonds following the rotations of the water molecules. This relaxation time follows an Arrhenius law τLH= τ0 ∙ exp(U#/kBT) with τ0=0.0485 ps and an activation energy U# = 7.7 kJ/mol. As for the second relaxation time, it varies very strongly with temperature, from 1.25 ps at 20 °C to 22.7 ps at −20 °C. This indicates that two fraction exists, and one of the two has intrinsic dynamics independent on temperature (the one which is coherent, as it will be discussed deeply in the following).
2.1. Synthesis of the Theoretical Background in QFT-QED for Liquid Water
- The first case refers to infrared (IR) and near IR (NIR) analysis of water or water solutions spectra (of O-H stretch mode range, IR, or of its first harmonic, NIR) taken at different temperatures (De Ninno, Del Giudice, Gamberale, & Castellano, 2014), (Renati, Kovacs, De Ninno, & Tsenkova, 2019) whose trends showed the clear existence of an isosbestic points that expresses the existence of two populations of molecules which depend reciprocally on T. This, of course, is not a novelty, but what it’s worth to look at are the resulting van’t Hoff plots (i.e.,: the Log (equilibrium constant of the passage from one population to the other) vs 1/T) is linear, revealing that (i) the energy difference between the two states does not depend on T and (ii) that its slope is in good agreement with the energy gap predicted by QED theory. Moreover, in (Renati, Kovacs, De Ninno, & Tsenkova, 2019) it has been shown how the plot of the logarithm of the ratios between the spectral intensity of one population (distinguished from the other one by the isosbestic point) with respect to the total, taken at each temperature, plotted as a function of log T yields a straight line. This accounts for a scale-free behaviour, revealing the underlying coherent dynamics for the demonstrated isomorphism existing between self-similar (fractal) topologies and squeezed quantum coherent states (Celeghini, De Martino, De Siena, Rasetti, & Vitiello, 1995) (Celeghini, Rasetti, & Vitiello, 1992) (Vitiello, 2009).
- The second case deals with the fit of dielectric permittivity of pure water and electrolytes water solutions in the range 0.2-1.5 THz (De Ninno, Nikollari, Missori, & Frezza, 2020), (Nikollari, De Ninno, & Frezza, 2023). The fit to the experimental data requires a two-fluid Debye model that mimics the electrical permittivity (both for the real and the imaginary part). However, in order to be effective over the whole spectral range, it requires an additional linear term (ξω, where ξ ≈ 0.47 ps) to the imaginary part of the dielectric function. This fact has a profound physical meaning because implies the violation of Kramers-Kronig (KK) relations (Toll, 1956) within the time span ξ. The KK relations express the causal relation between the forcing field and the charge displacement. This tiny violation, within a time scale right of the order of magnitude of the renormalized oscillation period of the coherent field within the CDs (which excite and relax in a few hundreds of femtoseconds, τr ≈ 1/ωr ~ 300-500 fs) witnesses temporally non-local correlations in the medium (i.e., phase correlations), possible if the system is in an entangled coherent state (a phase eigenstate). As Ke-Hsueh Li pointed out (Li, 1994) (Li, 1992a), the concept of coherence is strictly linked to Heisenberg’s uncertainty principle, i.e., coherence space-time being actually equivalent to the uncertainty space-time. This is the range of space and time within which particles lose their classical features as individuality and countability (the operator become undefined). The particles and fields within coherence space-time range must be considered as an indivisible whole where phase is well-defined: thus, what occurs to “a part” of a CD, within its coherent space-time range, is occurring to the whole CD (Li, 1994). This is a noteworthy point also for overcoming the prevailing naïve picture of the HBs (Del Giudice, Galimberti, Gamberale, & Preparata, 1995) conceived still as forces among “particles”. As described, this classic idea originated from the 1st quantization can be fruitfully replaced by the QFT perspective (2nd quantization) where the apparent (non-directional) force is the emergent property deriving from an energy gradient which is NOT primarily tied to the bonding among molecules, but established is routed on the ground energy level (vacuum) (Preparata G. , 1995) as a consequence of the “em-field + matter-field” coupling over the whole high-numbered system, (Bono, Del Giudice, Gamberale, & Henry, 2012).
- Another crucial topic is the one concerning ions and their solvation in water. Within an electrostatic conception of dissolution of electrolytes in water, the initial dynamics has no physical consistency, since few layers of water molecules should be able to keep some Na+ and Cl- ions apart from their crystal lattice when the energy barrier to be overcome in order to brake ion bonds is in the order of 5 eV and a single water layer could produce at most a dielectric drop of the Coulomb force equal to 13 ( εr = 13 and not εr = 80 which holds for the bulk). Again, only by abandoning an ingenuous “stics’n’balls” interpretation of condensed matter, and by taking into account the quantum electrodynamic nature of objects like ions and their coupling with vacuum, it is possible to describe consistently the spontaneous process of solvation showing that ions establish in the incoherent fraction of water their own coherence domains, with their energy gaps (bigger than the ion-bond energy), dissolving in the liquid phase of the solvent without collisions (Del Giudice & Preparata, 2000). This explains (i) why by increasing temperature the solvent power of water increases (despite the net value of bulk dielectric permittivity decreases), (ii) why there is no emission of bremsstrahlung radiation from an electrolyte solution and (iii) why the phenomenon of ion-cyclotron resonance occurs (Del Giudice, Fleischmann, Preparata, & Talpo, 2002).
- There are numerous other cases, which we will only briefly mention here, as they go beyond the scope of this topic and will therefore be dealt with in future papers. These regard the morphogenic role of water in biological matter (Henry M. , 2020), interfacial water (Pollack, 2013), dispersion properties of biologically bound water upon exposure in the 10 Hz to 100 GHz range (Schwan, 1977), burning salt water upon RF-exposure (Roy, Rao, & Kanzius, 2008), branching chain reaction of water (Voeikov, 2010), coherent water and cellular information processing (Henry M. , 2015) as well as stable water mixtures of both hydrophobic/hydrophilic liquids (Germany Patent No. DE 1.557.213, 1966) by Viktor Schauberger.
-
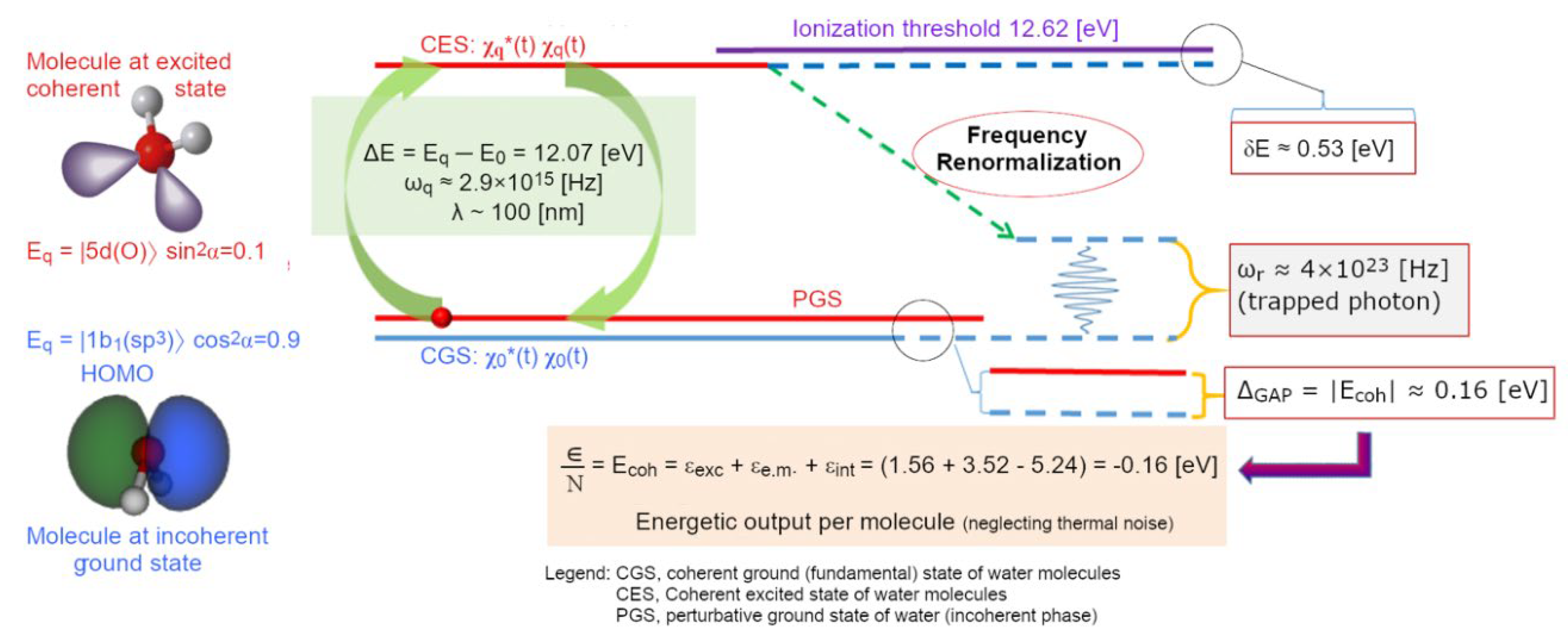
3According to the slowly varying envelope (SVE) approximation the frequency spectrum of the “envelope amplitudes” of the em-field is concentrated only on one mode, |ω| ≪ ωk = |k| (in natural units). Doing so means to neglect the third order time-derivative term in the equations of motion, which shows an instability of the perturbative ground state (PGS) in the matter-em field coupling, and is responsible for a departure from it towards a non-trivial solution of the equation of motion: a coherent state (see (Bono, Del Giudice, Gamberale, & Henry, 2012) for further details).
3. Discussion
- water is necessarily a two-fluid system, like already Röntgen proposed over a century ago (Röntgen, 1892);
- the two phases in liquid water differentiate from one another for much deeper physical reasons than “different arrangements” (furthermore unjustifiable) of the classical “HB-networks”;
- the short-range (electrostatic or perturbatively electrodynamic) forces – such van der Waals interactions – act mainly in the non-coherent fraction and do not change their typicality in dependence on the aggregation state (clusters, normal liquid, supercooled liquid, kinds of ice, etc.) and together with the long-range forces, they determine the maximum reachable close-packing level in coherent fraction;
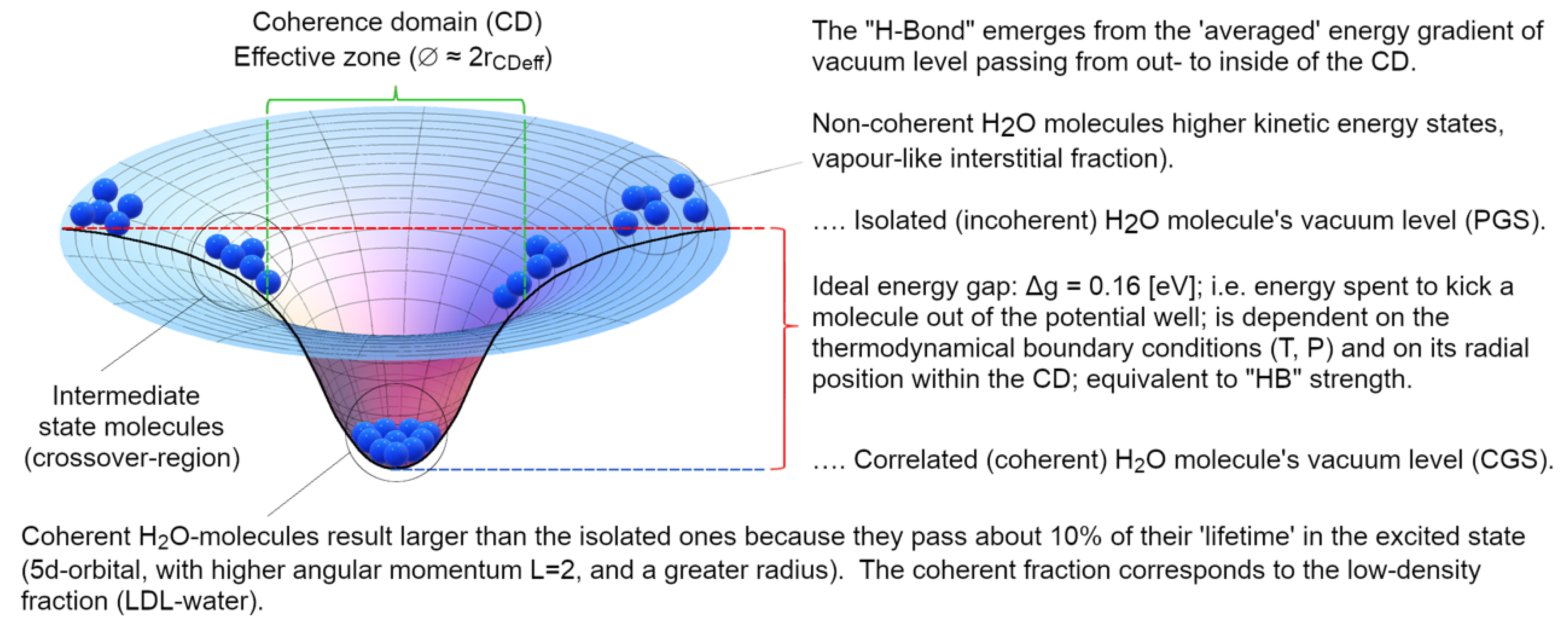
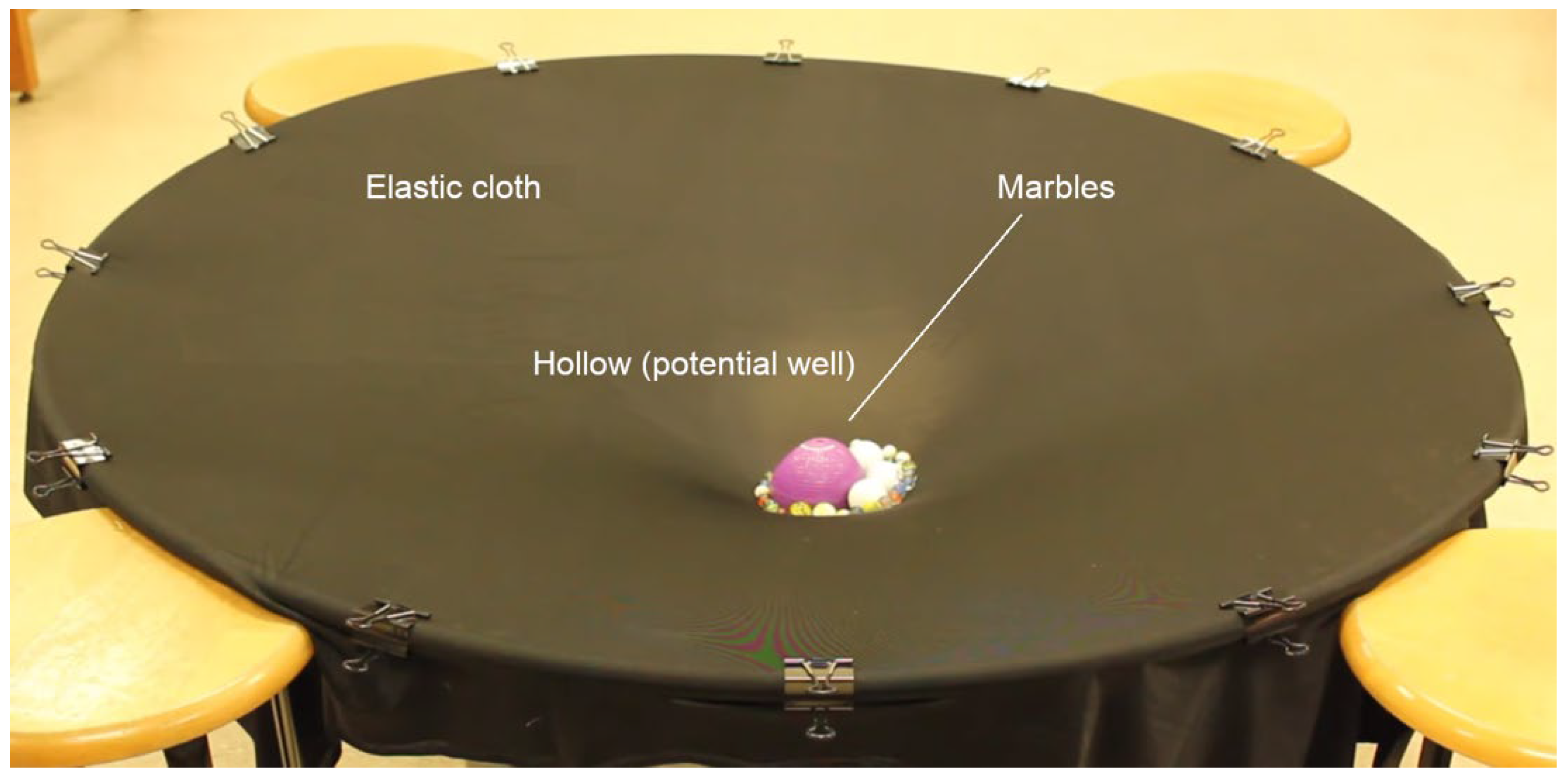
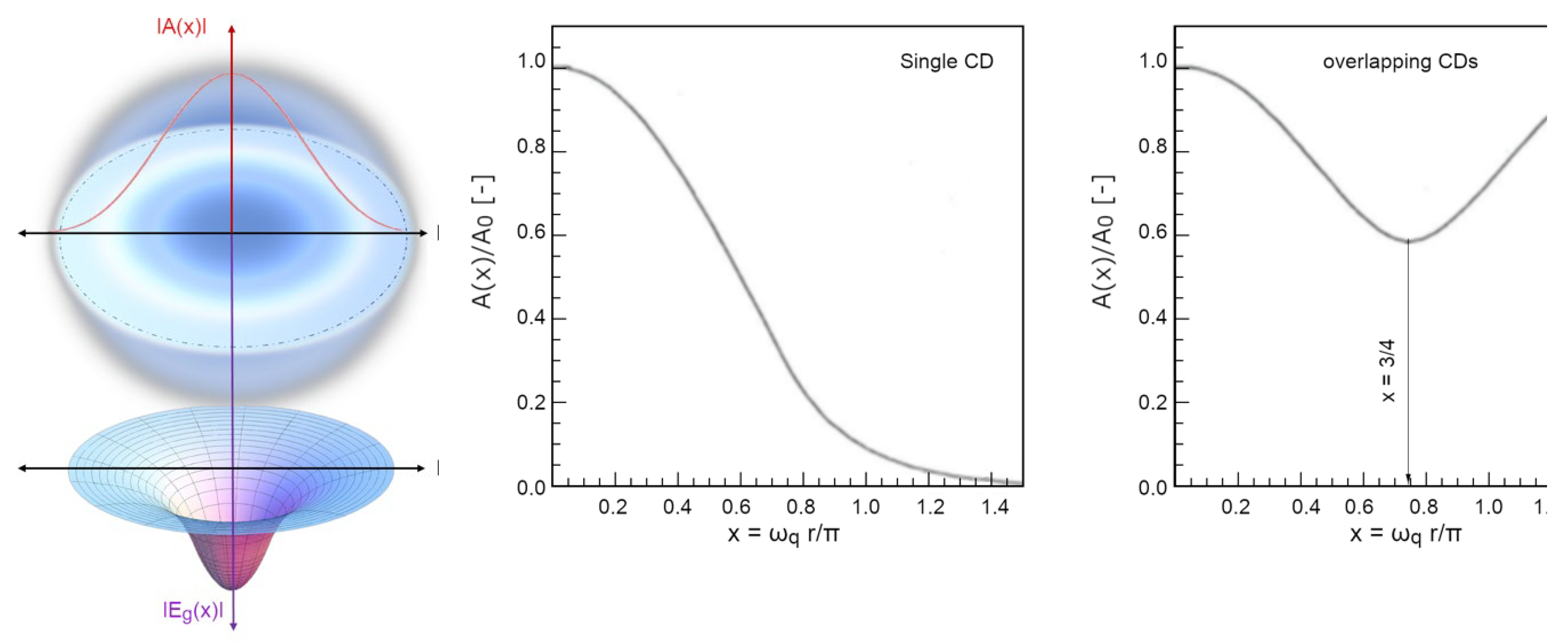
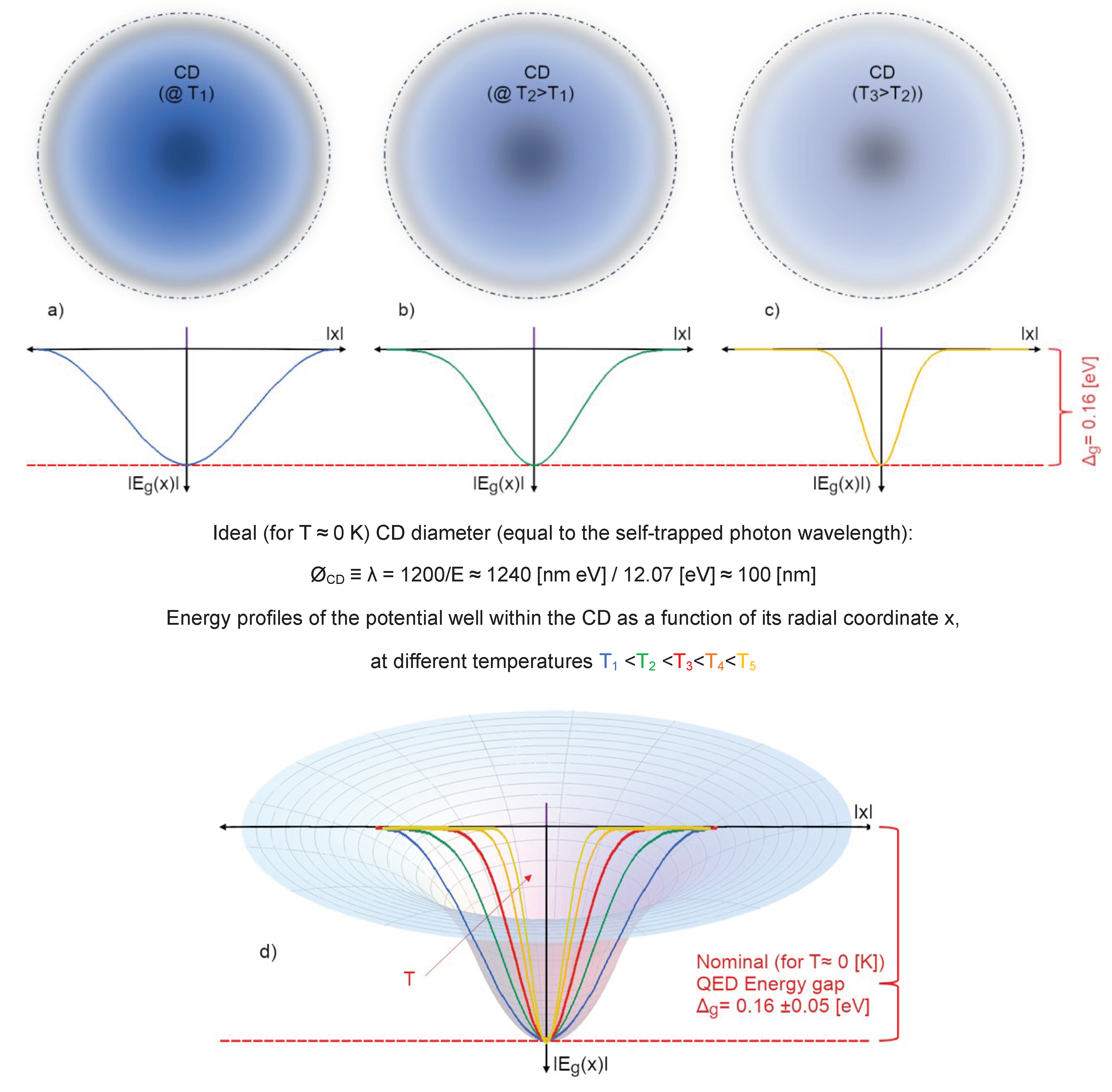
- the main agent for the cohesion of the system cannot be primarily assigned to local, directional, short-range forces among molecules (which, if attractive, would not be sufficient at room temperature (Del Giudice, Galimberti, Gamberale, & Preparata, 1995), (Bono, Del Giudice, Gamberale, & Henry, 2012), (Henry M. , 2015)). Instead, the emergence of a coherent field matter constituted of in-phase oscillating electric charges and photons, produces potential wells (namely as large as the volume of the photons) at the ground level (vacuum) that is experienced by nearby molecules. An analogy can be made with marbles placed on an elastic cloth that cluster next to one another in the hollow produced by their own weight (if they’re sufficiently close to one another, i.e.,: enough dense), and not because of the existence of a net attractive force between them. Due to coherence, water molecules flip into such a minimum potential energy well, see Figure 7;
- the differences retrieved experimentally in the emergent intermolecular “attraction”, called in a QM-corpuscular perspective “Hydrogen Bonds” derive from the dependence on the energy-well profile within the CD, thus we can understand why this apparent “intermolecular” force depends on the thermodynamic boundary conditions and on the kind of aggregation experienced by the molecules (see Figure 8).
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
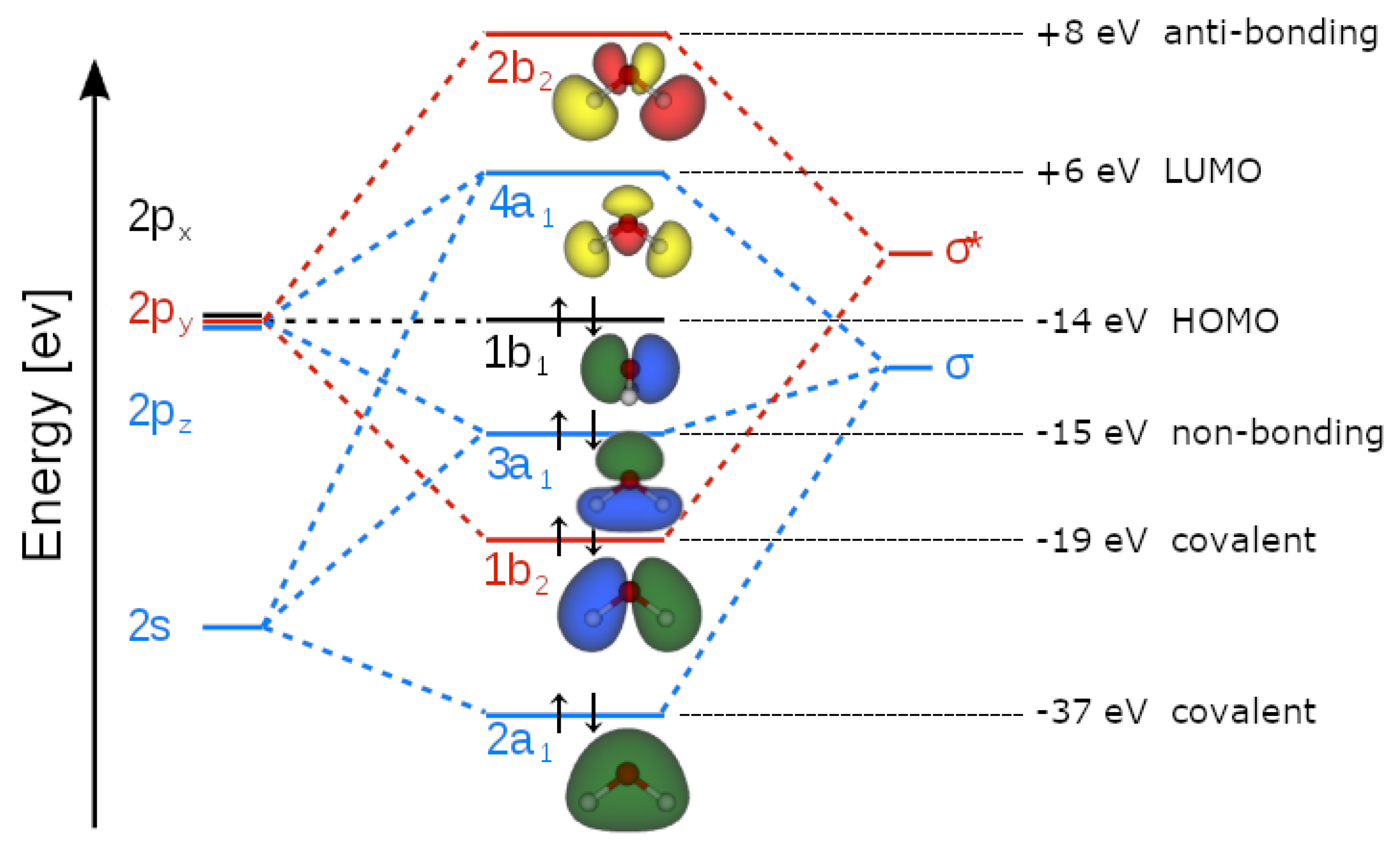
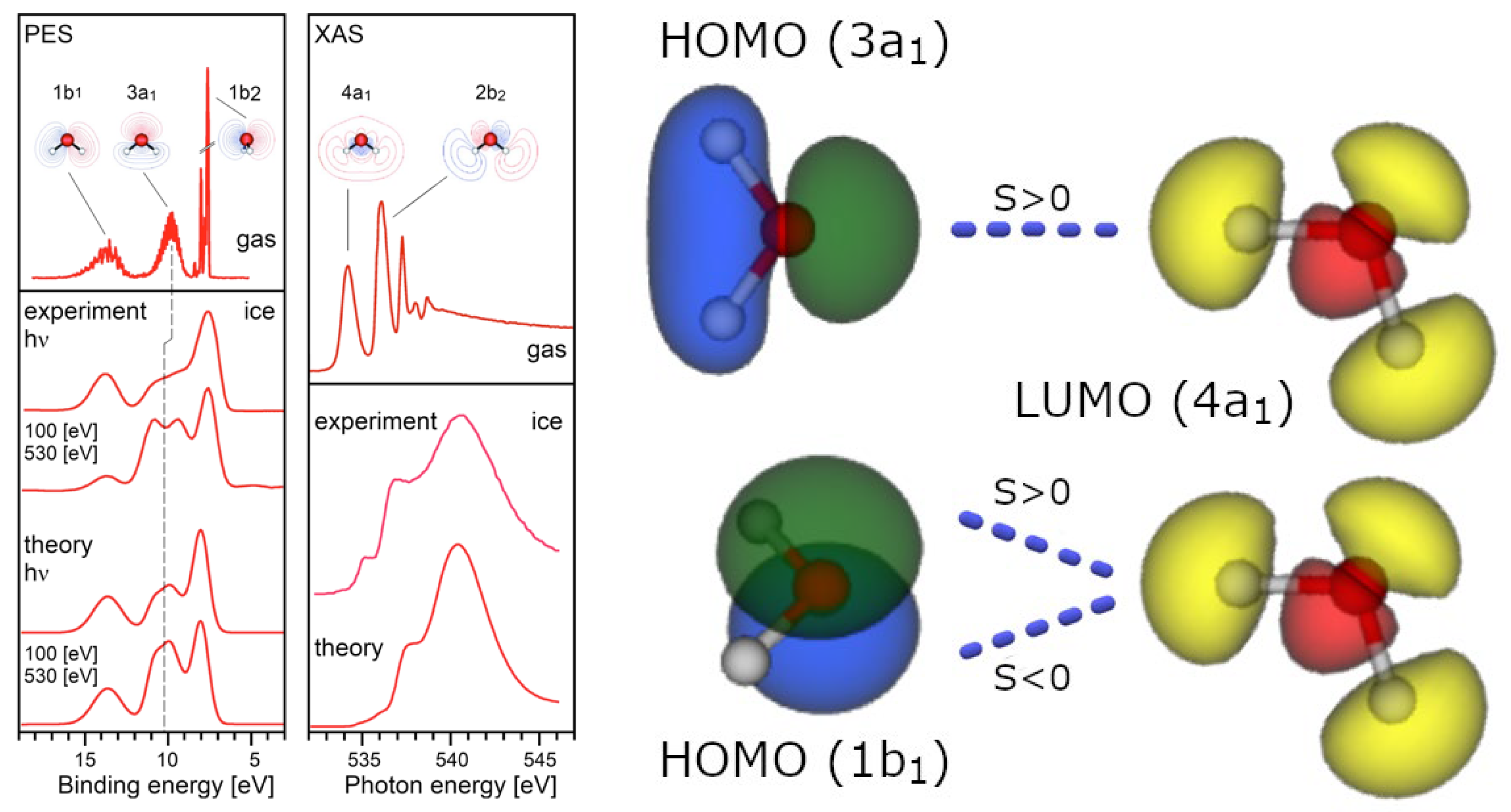
Appendix A. Definition of the Hydrogen Bond within 1st Quantization
References
- Anderson, P. Coherent Excited States in the Theory of Superconductivity: Gauge Invariance and the Meissner Effect. Phys Rev, 1958 110(4), 827–835. [CrossRef]
- Anderson, P. Basic Notions Of Condensed Matter Physics. Basic Books. XXXX, 1984. [CrossRef]
- Arani, R.; Bono, I.; Del Giudice, E.; Preparata, G. QED coherence and the thermodynamics of water. International Journal of Modern Physics B, 1995, 9, 1813–1841. [CrossRef]
- Bader, v.R. Atoms in Molecules. A Quantum Theory (Vol. 22). Clanderson Press. Oxford, 1990. [CrossRef]
- Becke, A.; Edgecombe, K. A simple measure of electron localization in atomic and molecular systems. JOurnal of Chemical Physics, 1990, 92(9), 5397. [CrossRef]
- Blasone, M.; Jizba, J.; Vitiello, G. Quantum field theory and its macroscopic manifestations. Imperial College Press. London, 2011. [CrossRef]
- Bono, I., Del Giudice, E., Gamberale, L., & Henry, M. (2012). Emergence of the Coherent Structure of Liquid Water. Water, 4, 510–532. [CrossRef]
- Bragg, W. The Crystal Structure of Ice. Proc Phys Soc London, 1922, 34(1), 98–103. [CrossRef]
- Bukowski, R.; Szalewicz, K.; Groenenboom, G. C.; van der Avoird, A. Predictions of the Properties of Water from First Principles. Science, 2007, 315(5816), 1249–1252. [CrossRef]
- Buzzacchi, M.; Del Giudice, E.; Preparata, G. Glasses: a new view from QED. MITH, 1999, 1-15. arXiv:cond-mat/9906395.
- Buzzacchi, M.; Del Giudice, E.; Preparata, G. Coherence of the Glassy State. Int J Mod Phys B, 2002, 16(25), 3771-3786. [CrossRef]
- Celeghini, E.; De Martino, S.; De Siena, S.; Rasetti, M.; Vitiello, G. Quantum Groups, Coherent States, Squeezing and Lattice Quantum Mechanics. Annals of Physics, 1995, 241, 50-67. [CrossRef]
- Celeghini, E.; Rasetti, M.; Vitiello, G. Quantum dissipation. Ann Phys, 1992, 215, 156–170. [Google Scholar] [CrossRef]
- De Ninno, A.; Del Giudice, E.; Gamberale, L.; Castellano, C. The structure of liquid water emerging from the vibrational spectroscopy interpretation with QED theory. Water, 2014, 6, 13–25. [Google Scholar] [CrossRef]
- De Ninno, A.; Nikollari, E.; Missori, M.; Frezza, F. Dielectric permittivity of aqueous solutions of electrolytes probed by THz time-domain and FTIR spectroscopy. Phys Lett A, 2020, 384(34), 126865. [CrossRef]
- Del Giudice, E.; Preparata, G. QED coherence and electrolyte solutions. J Electroanal Chem, 2000, 482(2), 110–116. [CrossRef]
- Del Giudice, E.; Tedeschi, A. Water and Autocatalysis in Living Matter. Electromag Biol Med, 2009, 28, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, E.; Vitiello, G. The Role of the electromagnetic field in the formation of domains in the process of symmetry-breaking phase transitions. Phys Rev A, 2006, 74, 022105. [CrossRef]
- Del Giudice, E.; Galimberti, U.; Gamberale, L.; Preparata, G. Electrodynamic Coherence in water: a possible origin of the tetrahedral coordination. Mod Phys Lett B, 1995, 9(15), 953-961. [CrossRef]
- Del Giudice, E.; Spinetti, P. R.; Tedeschi, A. Water Dynamics at the Root of Metamorphosis in Living Organisms. Water, 2012, 566-586. [CrossRef]
- Del Giudice, E.; Voeikov, V.; Tedeschi, A.; Vitiello, G. The origin and the special role of coherent water in living systems. In M. C. D. Fels, Fields of the Cell (pp. 95-111). Research Signpost, Kerala, 2015. [CrossRef]
- Elangannan, A.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; Kjaergaard, H.G.; Legon, A.C. ; Mennucci, B;. Nesbitt, D.J. Definition of the hydrogen bond, Pure Applied Chemistry, 2011, 83(8), 1637–1641. [CrossRef]
- Flurry, R.L. Symmetry Groups: Theory and Chemical Applications. Prentice-Hall, XXXX, 1980. ISBN 0-13-880013-8.
- Franks, F. Water a comprehensive treatise (7 volumes). Plenum Press. New York:.
- Vol.1 (1974, 2nd ed. [CrossRef]
- Vol 2 (1973):. [CrossRef]
- Vol 3 (1977), 2nd ed.):. [CrossRef]
- Vol 4 (1975):. [CrossRef]
- Vol 5 (1975):. [CrossRef]
- Vol.6 (1979):. [CrossRef]
- Vol 7 (1982):. [CrossRef]
- Garbelli, A. Proprietà termodinamiche e dielettriche dell’acqua alla luce della teoria complessa delle interazioni molecolari rlettrodinamiche ed elettrostatiche (Thermodynamic and Dielectric Properties of Water in the Light of the Complex Theory of Electrodinamic and Electrostatic Molecular Interactions). Ph.D. Thesis, University of Milan, Italy, 2000. Tesi Laurea 2000, 1–89. [Google Scholar]
- Ghanty, T.K.; Staroverov, V.N.; Koren, P.R.; Davidson, E.R. Is the Hydrogen Bond in Water Dimer and Ice Covalent? J Am Cheml Soc, 2000, 122(6), 1210. [CrossRef]
- Guo, J.H.; Luo, Y.; Augustsson, A.; Rubensson, J.E.; Såthe, C.; Ågren, H.; Siegbahn, H.; Nordgren, J. X-Ray Emission Spectroscopy of Hydrogen Bonding and Electronic Structure of Liquid Water. Phys Rev Lett, 2002, 89(13), 137402. [CrossRef]
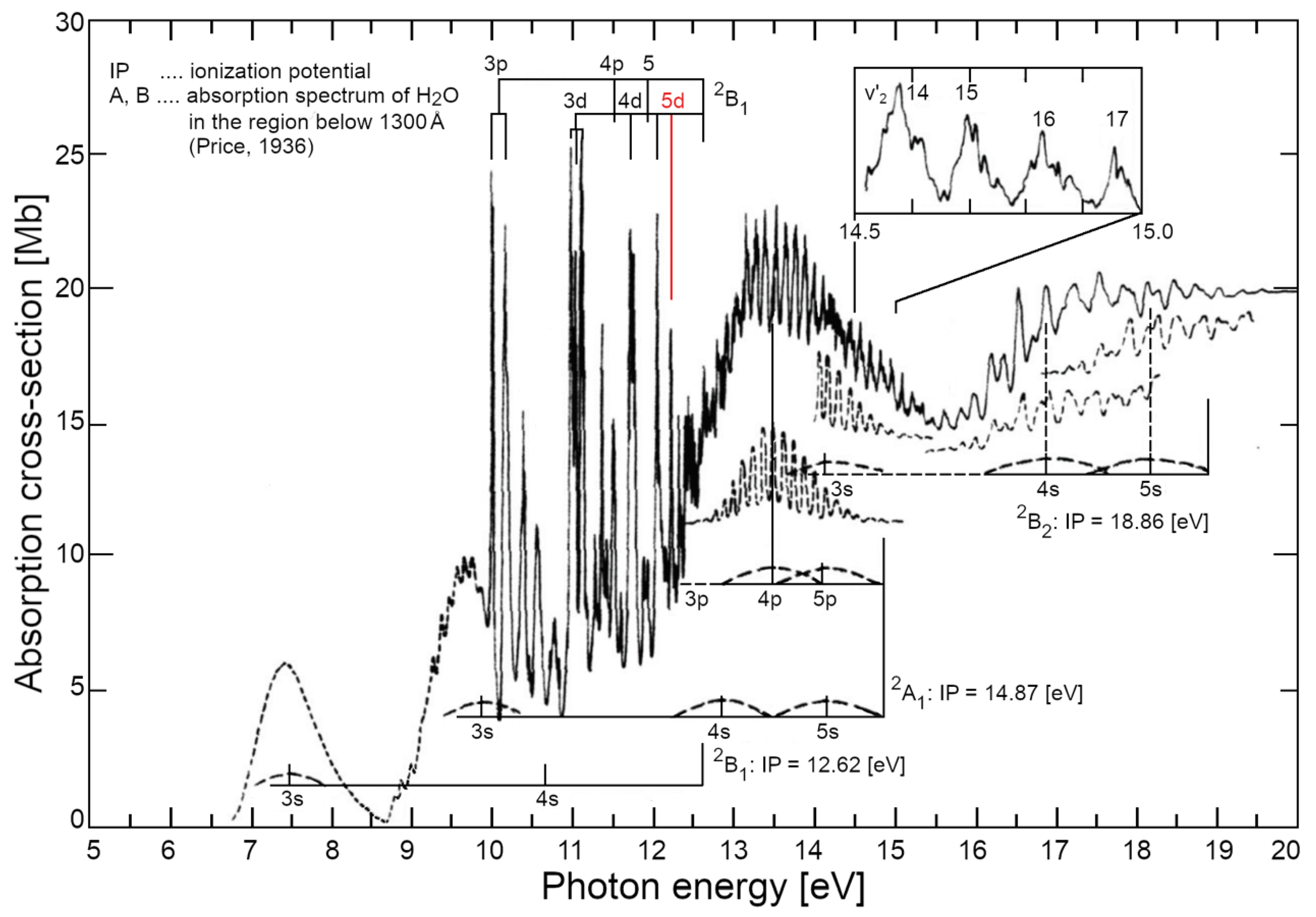
- Gürtler, P.; Saile, V.; Koch, E. Rydberg series in the absorption spectra of H2O and D2O in the vacuum ultraviolet. Chem Phys Lett, 1977, 51(2), 386-391. [CrossRef]
- Henry, M. The topological and quantum structure of zoemorphic water. In: Aqua Incognita: Why Ice Floats on Water and Galileo 400 Years on. (P. N. LoNostro, Ed.) Connor Court Publisher, Ballarat, 2014. ISBN 978-1-925138-21-4.
- Henry, M. The Hydrogen Bond. Inference Review, 2015, 1(2). [CrossRef]
- Henry, M. L’eau Morphogenique—Sante, Information Et Champs de Coscience, Escalquens; Éditions Dangles, 2020. ISBN: 978-2-7033-1269-7.
- Henry, M. The topological and quantum structure of zoemorphic water. In Aqua Incognita: Why Ice Floats on Water and Galileo 400 Years on; LoNostro, P.N.B.E., Ed.; Connor Court Publisher: Ballarat, Australia, 2014; pp. 197–239. ISBN 978-1925138214. [Google Scholar]
- Henry, M. L’Eau et la physique quantique, Dangles Editions. Escalquens, 2016. ISBN 978-2703311478.
- Henry, M. De l’information a l’exformation - une historie de vide, d’eau ou d’AND? (From Information to Exformation – a story of vacuum, water or DNA?). Ed. NAQ Strassbourg, ISBN 10-95620-03-7.
- Huang, C.; Wikfeldt, K.; Tokushima, T.; Nordlund, D.; Harada, Y.; Bergmann, U.; Niebuhr, M.; Weiss, T.M.; Horikawa, Y.; Leetmaa, M.; Ljungberg, M.P.; Takahashi, O.; Lenz, A.; Ojamäe, L.; Lyubartsev, A.P.; Shin, S.; Pettersson, L.G.; Nilsson, A. The inhomogeneous structure of water at ambient conditions. PNAS, 2009, 106, 15214-15218. [CrossRef]
- Huggins, M.L. 50 Years of Hydrogen Bond Theory. Ang Chem Int Ed, 1971, 10(3), 147–152. [CrossRef]
- Isaacs, E. D.; Shukla, A.; Platzman, P. M.; Hamann, D.R.; Barbiellini, B.; Tulk, C. Covalency of the Hydrogen Bond in Ice: A Direct X-Ray Measurement. Phys Rev Lett, 1999, 82(3), 600-603. [CrossRef]
- Kumar, A.; Gadre, S.R.; Mohan, N.; Suresh, C.H. Lone Pairs: An Electrostatic Viewpoint. J Phys Chem, 2014, 118(2), 526-532. [CrossRef]
- Li, K. Coherence in physics and biology. In Popp, F.; Li, K.; Gu, Q (eds), Recent Advances in Biophoton Research and its Applications. World Scientific Publishing. Sinngapore, 1992. [CrossRef]
- Li, K. Uncertainty Principle, Coherence and Structures. Springer Series in Synergetics, 1994, 61, 113-155. [CrossRef]
- Madl, P.; Renati, P. Quantum Electrodynamics Coherence and Hormesis: Foundations of Quantum Biology. Int J Molec Scie, 2023, 24(18), 14003. [CrossRef]
- Matcha, R.L. : King, S.C. Theory of the chemical bond. 1. Implicit perturbation theory and dipole moment model for diatomic molecules. J Am Chem Soc, 1976, 98(12), 3415-3420. [CrossRef]
- Moore, T.; Winmill, T. The States of Amines in Aqueous Solutions. J Chem Soc Trans, 1912, 101, 1635. [CrossRef]
- Nernst, W. Verteilung eines Stoffes zwischen zwei Lösungsmitteln und zwischen Lösungsmittel und Dampfraum. Zeitschr Physik Chem, 1891, 8(1), 110-139. [CrossRef]
- Nilsson, A.; Ogasawara, H.; Cavalleri, M.; Nordlund, D.; Nyberg, M.; Wernet, P.; Pettersson, L.G.M. The hydrogen bond in ice probed by soft x-ray spectroscopy and density functional theory. J Chem Phys, 2005, 122(15), 154505. [CrossRef]
- Pauling, L. The Shared-Electron Chemical Bond. PNAS, 1928, 14(4), 359-362. [CrossRef]
- Pauling, L. The Nature of the Chemical Bond. Application of Results Obtained from the Quantum Mechanics and from a Theory of Paramagnetic Susceptibility to the Structure of Molecules. J Am Chem Soc, 1931, 53(4), 1367-1400. [CrossRef]
- Pauling, L. The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement. J Am Chem Soc, 1935, 57(12), 2680-2684. [CrossRef]
- Pauling, L.; Brockway, L. The Structure of the Carboxyl Group: I. The Investigation of Formic Acid by the Diffraction of Electrons. PNAS, 1934, 20(6), 336-340. [CrossRef]
- Pollack, G.H. The Fourth Phase of Water - Beyond Solid, Liquid, and Vapor. Ebner & Sons, Seatle), 2013. ISBN 978-0962-6895-4-3.
- Preparata, G. QED Coherence in Matter; World Scientific. Singapore, 1995. [CrossRef]
- Preparata, G. An Introduction to a Realistic Quantum Physics; World Scientific: Singapore, 2002. [Google Scholar] [CrossRef]
- Preparata, G.; Del Giudice, E.; Vitiello, G. Water as a Free Electric Dipole Laser. Phys Rev Lett, 1988, 61(9), 1085-1088. [CrossRef]
- Price, W.C. The Far Ultraviolet Absorption Spectra and Ionization Potentials of H2O and H2S. Journal of Chem Phys 4(3), 1936, 147-153. [CrossRef]
- Renati, P. Electrodynamic Coherence as a Physical Basis for Emergence of Perception, Semantics, and Adaptation in Living Systems. J. Genet. Mol. Cell. Biol. 2020, 7, 1–34. [Google Scholar] [CrossRef]
- Renati, P.; Kovacs, Z.; De Ninno, A.; Tsenkova, R. Temperature dependence analysis of the NIR spectra of liquid water confirms the existence of two phases, one of which is in a coherent state. J Mol Liq, 019, 292, 111449. [CrossRef]
- Robinson, G.W.; Cho, C.H.; Urquidi, J. Isosbestic points in liquid water: Further strong evidence for the two-state mixture model. J Chem Phys, 1999, 111(2), 698. [CrossRef]
- Romero, A.; Silvestrelli, P.; Parrinello, M. Compton scattering and the character of the hydrogen bond in ice Ih. J Chem Phys, 2001, 115(1), 115. [CrossRef]
- Röntgen, W. Über die Constitution des flüssigen Wassers. Ann Phys, 1892, 281(1), 91-97. [CrossRef]
- Roy, R.; Rao, M.L.; Kanzius, J. Observations of polarised RF radiation catalysis of dissociation of H2O–NaCl solutions, Mat Res Innov, 2008, 12(1): 3-6. [CrossRef]
- Shank, A.; Wang, Y.M.; Kaledin, A.; Braams, B.J.; Bowman, J.M. Accurate ab initio and “hybrid” potential energy surfaces, intramolecular vibrational energies, and classical ir spectrum of the water dimer. J Chem Phys, 2009, 130(14), 144314. [CrossRef]
- Schauberger W (1966) Verfahren und Vorrichtung zur Herstellung von Gemischen, Lösungen, Emulsionen, Suspensionen u. dgl. sowie zur biologischen Reinigung von freien Gewässern (Process and apparatus for the preparation of mixtures, solutions, emulsions, suspensions and the like and for the biological purification of open waters) Patent AT00000025991B; https://depatisnet.dpma.de/DepatisNet/depatisnet?action=pdf&docid=AT000000265991B&xxxfull=1 (accessed:Jan.
- Schwan, H.P. Field interaction with biological matter. Ann N Y Acad Sci, 1977, 303: 198-216. [CrossRef]
- Simons, J. Hydrogen Fluoride and its Solutions. Chem Rev, 1931, 8(2), 213-235. [CrossRef]
- Taschin, A.; Bartolini, P.; Eramo, R.; Righini, R.; Torre, R. Evidence of two distinct local structures of water from ambient to supercooled conditions. Nature Comm, 2013, 4, 2401. [CrossRef]
- Teixeira, J. Experimental determination of the nature of diffusive motions of water molecules at low temperatures. Phys Rev A, 1985, 31(3), 1913–1917. [CrossRef]
- Teixeira, J.; Luzar, A. Physics of liquid water: Structure and dynamics. In M. Bellissent-Funel (ed), Hydration Processes in Biology: Theoretical and Experimental Approaches. IOS Press, Amsterdam, 1999. ISBN 9-051-9943-9-7.
- Tokushima, T.; Harada, Y.; Takahashi, O.; Senba, Y.; Ohashi, H.; Pettersson, Nilsson, A.; L.; Shin, S. High resolution X-ray emission spectroscopy of liquid water: The observation of two structural motifs. Chem Physcs Lett, 2008, 460. [CrossRef]
- Toll, J.S. Causality and the Dispersion Relation: Logical Foundations. Phys Rev J Arch, 1956, 104(6), 1760–1770. [CrossRef]
- Vitiello, G. Coherent states, fractals and brain waves. New Math Nat Comp, 2009, 5(1), 245-264. [CrossRef]
- von Neumann, J. Mathematical Foundations of Quantum Theory. Princeton University Press, Princeton, 1955. ISBN XXXX.
- Wernet, P.; Nordlund, D.; Bergmann, U.; Cavalleri, M.; Odelius, M.; Ogasawara, H.; Näslund, L.A.; Hirsch, T.K.; Ojamäe, L.; Glatzel, P.; Pettersson, L.G.M.; Nilsson, A. The Structure of the First Coordination Shell in Liquid Water. Science, 2004, 304(5673), 995-999. [CrossRef]
- Voeikov, V. Reactive oxygen species, water, photons and life. Riv Biol. 2010, 103(2-3): 321-342. PMID: 21384328. [PubMed]
- Anderson, P. (1958). Coherent Excited States in the Theory of Superconductivity: Gauge Invariance and the Meissner Effect. Physical Review, 110(4), 827-835. [CrossRef]
- Anderson, P. (1984). Basic Notions Of Condensed Matter Physics. Basic Books.
- Arani, R., Bono, I., Del Giudice, E., & Preparata, G. (1995). QED coherence and the thermodynamics of water. International Journal of Modern Physics B, 9, 1813-1841.
- Bader, v. R. (1990). Atoms in Molecules. A Quantum Theory, (Vol. 22). Oxford: Clanderson Press. [CrossRef]
- Becke, A., & Edgecombe, K. (1990). A simple measure of electron localization in atomic and molecular systems. Journal of Chemical Physics, 92(9), 5397. [CrossRef]
- Bernal, J., & Fowler, R. (1933). A Theory of Water and Ionic Solution, with Particular Reference to Hydrogen and Hydroxyl Ions. Journal of Chemical Physics, 1(8), 515. [CrossRef]
- Blasone, M. , Jizba, J., & Vitiello, G. (2011). Quantum field theory and its macroscopic manifestations. London: Imperial College Press.
- Bono, I., Del Giudice, E., Gamberale, L., & Henry, M. (2012). Emergence of the Coherent Structure of Liquid Water. Water, 4, 510-532. [CrossRef]
- Bragg, W. (1922). The Crystal Structure of Ice. Proceedings of the Physical Society of London, 34(1), 98-103. Retrieved from https://iopscience.iop.org/article/10.1088/1478-7814/34/1/322.
- Bukowski, R. , Szalewicz, K., Groenenboom, G. C., & van der Avoird, A. (2007, March). Predictions of the Properties of Water from First Principles. Science, 315(5816), 1249-1252. [CrossRef]
- Buzzacchi, M. , Del Giudice, E., & Preparata, G. (1999, June). Glasses: a new view from QED. MITH-98/9, 1-15. arXiv:cond-mat/9906395.
- Buzzacchi, M. , Del Giudice, E., & Preparata, G. (2002). COHERENCE OF THE GLASSY STATE. International Journal of Modern Physics B, 16(25), 3771–3786. [CrossRef]
- Celeghini, E., De Martino, S., De Siena, S., Rasetti, M., & Vitiello, G. (1995). Quantum Groups, Coherent States, Squeezing and Lattice Quantum Mechanics. Annals of Physics, 241, 50-67.
- Celeghini, E., Rasetti, M., & Vitiello, G. (1992). Quantum dissipation. Annals of Physics, 215, 156–170.
- Chaplin, M. (2023, January 18). Water Absorption Spectrum. Retrieved from Water structure and science:. http://www1.lsbu.ac.uk/water/water_anomalies.html.
- De Ninno, A., Del Giudice, E., Gamberale, L., & Castellano, C. (2014). The structure of liquid water emerging from the vibrational spectroscopy interpretation with QED theory. Water, 6, 13-25. [CrossRef]
- De Ninno, A. , Nikollari, E., Missori, M., & Frezza, F. (2020). Dielectric permittivity of aqueous solutions of electrolytes probed by THz time-domain and FTIR spectroscopy. Physics Letters A, 384(34), 126865. [CrossRef]
- Del Giudice, E., & Preparata, G. a. (2000, January). QED coherence and electrolyte solutions. Journal of Electroanalytical Chemistry, 482(2), 110-116. [CrossRef]
- Del Giudice, E., & Tedeschi, A. (2009). Water and Autocatalysis in Living Matter. Electromagnetic Biology and Medicine, 28, 46–52. [CrossRef]
- Del Giudice, E. , & Vitiello, G. (2006). The Role of the electromagnetic field in the formation of domains in the process of symmetry-breaking phase transitions. Physical Review A, 74, 022105.
- Del Giudice, E. , Fleischmann, M., Preparata, G., & Talpo, G. (2002, October). On the “unreasonable” effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics, 23(7), 522-530. [CrossRef]
- Del Giudice, E. , Galimberti, U., Gamberale, L., & Preparata, G. (1995). Electrodynamic Coherence in water: a possible origin of the tetrahedral coordination. Modern Physics Letters B, 9(15), 953-961. [CrossRef]
- Del Giudice, E., Spinetti, P. R., & Tedeschi, A. (2012). Water Dynamics at the Root of Metamorphosis in Living Organisms. Water, 566-586.
- Del Giudice, E. , Voeikov, V., Tedeschi, A., & Vitiello, G. (2015). The origin and the special role of coherent water in living systems. In M. C. D. Fels, Fields of the Cell (pp. 95-111). Kerala, India: Research Signpost. Retrieved from https://www.emmind.net/openpapers_repos/Endogenous_Fields-Mind/Water_EMF/Exclusion_Zones/2014_The_origin_and_the_special_role_of_coherent_water_in_living_systems.pdf.
- Dirac, P. (1929). Quantum Mechanics of Many-Electron Systems. Proceedings of the Royal Society of London. Containing Papers of a Mathematical and Physical Character 123. Series A, p. 714. London: Royal Society of London.
- Elangannan, A., & al., e. (2011, July). Pure Applied Chemistry, 83(8), 1637–1641. [CrossRef]
- Flurry, R. L. (1980). Symmetry Groups: Theory and Chemical Applications. Prentice-Hall.
- Franks, F. (1972-1982). Water a comprehensive treatise (7 volumes). New York: Plenum Press. Retrieved from https://www.springer.com/gp/book/9781468429602.
- Garbelli, A. (2000, May). Proprietà termodinamiche e dielettriche dell’acqua alla luce della teoria complessa delle interazioni molecolari rlettrodinamiche ed elettrostatiche. (G. Preparata, & E. Del Giudice, Eds.) Tesi di Laurea, 1-89.
- Ghanty, T. K. , Staroverov, V. N., Koren, P. R., & Davidson, E. R. (2000). Is the Hydrogen Bond in Water Dimer and Ice Covalent? Journal of American Chemical Society, 122(6), 1210. [CrossRef]
- Guo, J.-H. , Luo, Y., Augustsson, A., Rubensson, J.-E., Såthe, C., Ågren, H., … Nordgren, J. (2002, September). X-Ray Emission Spectroscopy of Hydrogen Bonding and Electronic Structure of Liquid Water. Physical Review Letters, 89(13), 137402. [CrossRef]
- Gürtler, P., Saile, V., & Koch, E. (1977, October). Rydberg series in the absorption spectra of H2O and D2O in the vacuum ultraviolet. Chemical Physics Letters, 51(2), 386-391. [CrossRef]
- Henry, M. (2014). The topological and quantum structure of zoemorphic water. In: Aqua Incognita: Why Ice Floats on Water and Galileo 400 Years on. (P. N. LoNostro, Ed.) Ballarat, Australia: Connor Court Publisher.
- Henry, M. (2015). De l’information a l’exformation - une historie de vide, d’eau ou d’ADN” (From Information to Exformation – a story of vacuum, water or DNA?). Strassbourg, France: NAQ Strassbourg.
- Henry, M. (2015, March). The Hydrogen Bond. Inference Review, 1(2). Retrieved from https://inferencereview.com/article/the-hydrogen-bond.
- Henry, M. (2016). L’Eau et La Physique Quantique – Vers une révolution de la médecine (Water and Quantum Physics – progres-sing towards a medical revolution, in French). Escalquens, France: Éditions Dangles.
- Henry, M. (2020). L’eau Morphogenique – Sante, Information Et Champs de Coscience. Escalquens, France: Éditions Dangles.
- Huang, C., Wikfeldt, K., Tokushima, T., Nordlund, D., Harada, Y., Bergmann, U., … Nilsson, A. (2009). The inhomogeneous structure of water at ambient conditions. PNAS, 106, 15214-15218. [CrossRef]
- Huggins, M. (1971). 50 Years of Hydrogen Bond Theory. Angewandte Chemie International (Edition in English), 10(3), 147-152. [CrossRef]
- Isaacs, E. D. , Shukla, A., Platzman, P. M., Hamann, D. R., Barbiellini, B., & Tulk, C. (1999, November). Covalency of the Hydrogen Bond in Ice: A Direct X-Ray Measurement. Phyical Review Letters, 82(3), 600-603. [CrossRef]
- Kumar, A. , Gadre, S. R., Mohan, N., & Suresh, C. H. (2014). Lone Pairs: An Electrostatic Viewpoint. Journal of Physical Chemistry, 118(2), 526-532. [CrossRef]
- Lamb, W. E., & Retherford, R. C. (1947). Fine structure of the hydrogen atom by a microwave method. Physical Review Letter(72), 241-243.
- Li, K. (1992a). Coherence in physics and biology. In F. Popp, K. Li, & Q. Gu, Recent Advances in Biophoton Research and its Applications (pp. 113-155). Singapore: World Scientific Publishing.
- Li, K. (1994). Uncertainty Principle, Coherence and Structures. Springer Series in Synergetics, 61, 113-155.
- Madl, P. , & Renati, P. (2023). Quantum Electrodynamics Coherence and Hormesis: Foundations of Quantum Biology. International Journal of Molecular Sciences, 24(18), 14003. [CrossRef]
- Matcha, R. L., & King, S. C. (1976). Theory of the chemical bond. 1. Implicit perturbation theory and dipole moment model for diatomic molecules. Journal of the American Chemical Society, 98(12), 3415-3420. [CrossRef]
- Moore, T. , & Winmill, T. (1912). The States of Amines in Aqueous Solutions. Journal of the Chemical Society Transactions, 101, 1635. Retrieved from https://pubs.rsc.org/en/content/articlelanding/1912/ct/ct9120101635#!divAbstract.
- Nernst, W. (1891, January). Verteilung eines Stoffes zwischen zwei Lösungsmitteln und zwischen Lösungsmittel und Dampfraum. Zeitschrift für Physikalische Chemie, 8(1), 110-139. [CrossRef]
- Nikollari, E. , De Ninno, A., & Frezza, A. (2023). Dielectric response of liquid water and aqueous solutions: Two-fluid behaviour. 3rd European Aquaphotomics Conference (02-04 September 2023). Rome, Italy.
- Nilsson, A. , & al., e. (2005). The hydrogen bond in ice probed by soft x-ray spectroscopy and density functional theory. The Journal of Chemical physics, 122(15), 154505. [CrossRef]
- Nilsson, A., & Petterson, L. (2015). The structural origin of anomalous properties of liquid water. Nature Communications, 6, 8998. [CrossRef]
- Pauling, L. (1928, Aprile). The Shared-Electron Chemical Bond. Proceedings of the National Academi of Sciences of the United Sates of America (PNAS), 14(4), 359-362. [CrossRef]
- Pauling, L. (1931). The Nature of the Chemical Bond. Application of Results Obtained from the Quantum Mechanics and from a Theory of Paramagnetic Susceptibility to the Structure of Molecules. Journal of the American Chemical Society, 53(4), 1367-1400. [CrossRef]
- Pauling, L. (1935). The Structure and Entropy of Ice and of Other Crystals with Some Randomness of Atomic Arrangement. Journal of the American Chemical Society, 57(12), 2680-2684. [CrossRef]
- Pauling, L., & Brockway, L. (1934). THE STRUCTURE OF THE CARBOXYL GROUP. I. THE INVESTIGATION OF FORMIC ACID BY THE DIFFRACTION OF ELECTRONS. Proceedings of The National Academy of Sciences of The uNited Sates of America (PNAS), 20(6), 336-340. [CrossRef]
- Pauling, L. , Corey, R., & Branson, H. (1951). The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proceedings of the National Academy of Sciences (PNAS), 37(4), 205-211. [CrossRef]
- Pollack, G. H. (2013). The Fourth Phase of Water. Seattle: Ebner and Sons.
- Preparata, G. (1995). QED Coherence in Matter, World Scientific.
- Preparata, G. (2002). An Introduction to a Realistic Quantum Physics. World Scientific.
- Preparata, G., Del Giudice, E., & Vitiello, G. (1988, August). Water as a Free Electric Dipole Laser. Physical Review Letters, 61(9), 1085-1088.
- Price, W. (1936). he Far Ultraviolet Absorption Spectra and Ionization Potentials of H2O and H2S. Journal of Chemical Physics, 4(3), 147-153. [CrossRef]
- Quane, D. (1990). The Reception of Hydrogen Bonding by the Chemical Community: 1920-1937. Bulletin for the History of Chemistry, 7, 3-13. Retrieved from http://acshist.scs.illinois.edu/bulletin_open_access/num7/num7%20p3-13.pdf.
- Renati, P. (2020). Electrodynamic Coherence as a Physical Basis for Emergence of Perception, Semantics, and Adaptation in Living Systems. Journal of Genetic, Molecular and Cellular Biology, 7(2), 1-34. [CrossRef]
- Renati, P., Kovacs, Z., De Ninno, A., & Tsenkova, R. (2019). Temperature dependence analysis of the NIR spectra of liquid water confirms the existence of two phases, one of which is in a coherent state. Journal of Molecular Liquids, 292, 111449. [CrossRef]
- Robinson, G. W. , Cho, C. H., & Urquidi, J. (1999, April). Isosbestic points in liquid water: Further strong evidence for the two-state mixture model. The Journal of Chemical Physics, 111(2), 698. [CrossRef]
- Romero, A. , Silvestrelli, P., & Parrinello, M. (2001). Compton scattering and the character of the hydrogen bond in ice Ih. The Journal of Chemical Physics, 115(1), 115. [CrossRef]
- Röntgen, W. (1892). Ueber die Constitution des flüssigen Wassers Annalen der Physik. Annalen der Physik, 281 (1), 91-97. [CrossRef]
- Roy, R. , Rao, M., & Kanzius, J. (2008). Observations of polarised RF radiation catalysis of dissociation of H2O–NaCl solutions. Materials Research Innovations, 12(1), 3-6. [CrossRef]
- Schauberger, V. (1966). Germany Patent No. DE 1.557.213. Retrieved January 29, 2024, from http://pks.or.at/wp-content/uploads/PAT_265_991_DE.pdf.
- Schwan, H. (1977). Field interaction with biological matter. Annals of New York Academy of Sciences, 198-216. [CrossRef]
- 151. Shank, Wang, Kaledin, Braams, & Bowman. (2009). Accurate ab initio and “hybrid” potential energy surfaces, intramolecular vibrational energies, and classical ir spectrum of the water dimer. Journal of Chemical Physics, 130(14), 144314. [CrossRef]
- Simons, J. (1931, April). Hydrogen Fluoride and its Solutions. Chemical Reviews, 8(2), 213-235. [CrossRef]
- Taschin, A., Bartolini, P., Eramo, R., Righini, R., & Torre, R. (2013). Evidence of two distinct local structures of water from ambient to supercooled conditions. Nature Communications, 4, 2401. [CrossRef]
- Teixeira, J. , & Luzar, A. (1999). Physics of liquid water: Structure and dynamics. In M. Bellissent-Funel, Hydration Processes in Biology: Theoretical and Experimental Approaches (pp. 35-65). Amsterdam: IOS Press.
- Teixeira, J. , Bellissent-Funel, M., Chen, S. H., & Dianoux, A. J. (1985, March). Experimental determination of the nature of diffusive motions of water molecules at low temperatures. Physical Review A, 31(3), 1913-1917. [CrossRef]
- Tokushima, T. , Harada, Y., Takahashi, O., Senba, Y., Ohashi, H., Pettersson, L., … Shin, S. (2008). High resolution X-ray emission spectroscopy of liquid water: The observation of two structural motifs. Chemical Physics Letters, 460. [CrossRef]
- Toll, J. S. (1956, December). Causality and the Dispersion Relation: Logical Foundations. Physical Review Journals Archive, 104(6), 1760-1770. [CrossRef]
- Vitiello, G. (2009). Coherent states, fractals and brain waves. New Mathematics and Natural Computing, 5(1), 245-264. [CrossRef]
- Voeikov, V. (2010). Reactive oxygen species, water, photons and life. Biology Forum, 321-342. Retrieved from https://www.researchgate.net/publication/50305420_Reactive_Oxygen_Species_Water_Photons_and_Life.
- von Neumann, J. (1955). Mathematical Foundations of Quantum Theory. Princeton: Princeton University Press.
- Wernet, P. e. (2004). The Structure of the First Coordination Shell in Liquid Water. Science, 304(5673), 995-999. [CrossRef]
- Wernet, P., Nordlund, D., Bergmann, U., Cavalleri, M., Odelius, M., Ogasawara, H., … Nilsson, A. (2004, May). The Structure of the First Coordination Shell in Liquid Water. Science, 304(5673), 995-999. [CrossRef]
- Zachariasen, W. H. (1935). The Liquid “Structure” of Methyl Alcohol. The Journal of Chemical Physics, 3(3), 158–161. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




