Submitted:
01 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
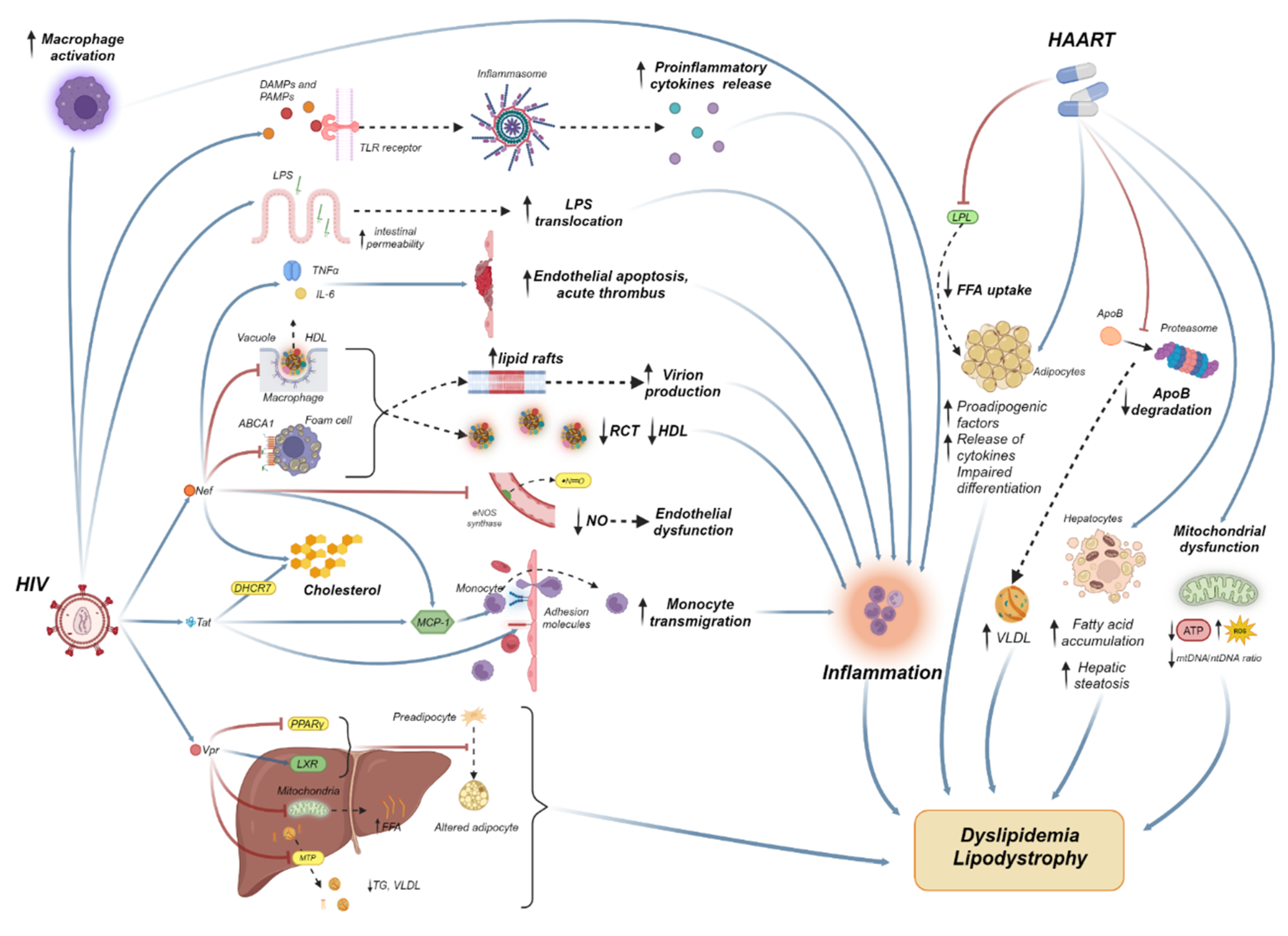
2. The Molecular Mechanisms of HIV-Associated Dyslipidemia
2.1. The Role of HIV Viremia
2.2. The Role of Antiretroviral Treatment
2.2.1. Protease Inhibitors
2.2.2. Nucleoside Reverse Transcriptase Inhibitors
2.2.3. Non-Nucleoside Reverse Transcriptase Inhibitors
2.2.4. Integrase Inhibitors
3. Treatment of Dyslipidemia
3.1. Statins
3.1.1. The Role of Statins in Inflammation
3.1.2. The Role of Statins in Lipid Management
3.1.3. Drug Interactions between HAART and Statins
3.2. Ezetimibe
3.3. PCSK9 Inhibitors
3.3. Bemdedoic Acid
3.4. Fibrates
3.5. Fish Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health, O. Global health sector strategy on HIV 2016-2021. Towards ending AIDS; World Health Organization: Geneva, 2016 2016. [Google Scholar]
- Thaker, H.K.; Snow, M.H. HIV viral suppression in the era of antiretroviral therapy. Postgrad Med J 2003, 79, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, G.R.; Zaunders, J.; Cooper, D.A. Immune reconstitution in HIV-1 infected subjects treated with potent antiretroviral therapy. Sex Transm Infect 1999, 75, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Babiker, A.; Bhaskaran, K.; Darbyshire, J.; Pezzotti, P.; Porter, K.; Walker, A.S.; Collaboration, C. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet 2003, 362, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Enanoria, W.T.; Ng, C.; Saha, S.R.; Colford, J.M., Jr. Treatment outcomes after highly active antiretroviral therapy: a meta-analysis of randomised controlled trials. Lancet Infect Dis 2004, 4, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Brinkman, K.; Geerlings, S.; Smit, C.; Thyagarajan, K.; Sighem, A.; de Wolf, F.; Hallett, T.B.; cohort, A.o. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015, 15, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, E.; Treatment of High Blood Cholesterol in, A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Koethe, J.R. Adipose Tissue in HIV Infection. Compr Physiol 2017, 7, 1339–1357. [Google Scholar] [CrossRef]
- Masenga, S.K.; Elijovich, F.; Koethe, J.R.; Hamooya, B.M.; Heimburger, D.C.; Munsaka, S.M.; Laffer, C.L.; Kirabo, A. Hypertension and Metabolic Syndrome in Persons with HIV. Curr Hypertens Rep 2020, 22, 78. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.H.; Skanderson, M.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Vasan, R.S.; Oursler, K.A.; Gottdiener, J.; et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol 2017, 2, 536–546. [Google Scholar] [CrossRef]
- Gili, S.; Grosso Marra, W.; D'Ascenzo, F.; Lonni, E.; Calcagno, A.; Cannillo, M.; Ballocca, F.; Cerrato, E.; Pianelli, M.; Barbero, U.; et al. Comparative safety and efficacy of statins for primary prevention in human immunodeficiency virus-positive patients: a systematic review and meta-analysis. Eur Heart J 2016, 37, 3600–3609. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.C.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Bidwell Goetz, M.; Leaf, D.; Oursler, K.A.; et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013, 173, 614–622. [Google Scholar] [CrossRef]
- Tseng, Z.H.; Secemsky, E.A.; Dowdy, D.; Vittinghoff, E.; Moyers, B.; Wong, J.K.; Havlir, D.V.; Hsue, P.Y. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012, 59, 1891–1896. [Google Scholar] [CrossRef]
- Ryom, L.; Lundgren, J.D.; El-Sadr, W.; Reiss, P.; Kirk, O.; Law, M.; Phillips, A.; Weber, R.; Fontas, E.; d' Arminio Monforte, A.; et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A:D international prospective multicohort study. Lancet HIV 2018, 5, e291–e300. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Waters, D.D. Time to Recognize HIV Infection as a Major Cardiovascular Risk Factor. Circulation 2018, 138, 1113–1115. [Google Scholar] [CrossRef]
- Feingold, K.R.; Krauss, R.M.; Pang, M.; Doerrler, W.; Jensen, P.; Grunfeld, C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab 1993, 76, 1423–1427. [Google Scholar] [CrossRef]
- Buchacz, K.; Baker, R.K.; Palella, F.J., Jr.; Shaw, L.; Patel, P.; Lichtenstein, K.A.; Chmiel, J.S.; Vellozzi, C.; Debes, R.; Henry, K.; et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013, 18, 65–75. [Google Scholar] [CrossRef]
- Riddler, S.A.; Smit, E.; Cole, S.R.; Li, R.; Chmiel, J.S.; Dobs, A.; Palella, F.; Visscher, B.; Evans, R.; Kingsley, L.A. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003, 289, 2978–2982. [Google Scholar] [CrossRef]
- Sviridov, D.; Mukhamedova, N.; Makarov, A.A.; Adzhubei, A.; Bukrinsky, M. Comorbidities of HIV infection: role of Nef-induced impairment of cholesterol metabolism and lipid raft functionality. AIDS 2020, 34, 1–13. [Google Scholar] [CrossRef]
- Mohseni Ahooyi, T.; Shekarabi, M.; Torkzaban, B.; Langford, T.D.; Burdo, T.H.; Gordon, J.; Datta, P.K.; Amini, S.; Khalili, K. Dysregulation of Neuronal Cholesterol Homeostasis upon Exposure to HIV-1 Tat and Cocaine Revealed by RNA-Sequencing. Sci Rep 2018, 8, 16300. [Google Scholar] [CrossRef]
- Reeds, D.N.; Mittendorfer, B.; Patterson, B.W.; Powderly, W.G.; Yarasheski, K.E.; Klein, S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab 2003, 285, E490–497. [Google Scholar] [CrossRef]
- Duong, M.; Petit, J.M.; Martha, B.; Galland, F.; Piroth, L.; Walldner, A.; Grappin, M.; Buisson, M.; Duvillard, L.; Chavanet, P.; et al. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials 2006, 7, 41–47. [Google Scholar] [CrossRef]
- Mujawar, Z.; Rose, H.; Morrow, M.P.; Pushkarsky, T.; Dubrovsky, L.; Mukhamedova, N.; Fu, Y.; Dart, A.; Orenstein, J.M.; Bobryshev, Y.V.; et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 2006, 4, e365. [Google Scholar] [CrossRef]
- Damouche, A.; Lazure, T.; Avettand-Fenoel, V.; Huot, N.; Dejucq-Rainsford, N.; Satie, A.P.; Melard, A.; David, L.; Gommet, C.; Ghosn, J.; et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog 2015, 11, e1005153. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Mantecon, M.; Atlan, M.; Pourcher, V.; Pourcher, G.; Le Grand, R.; Desjardins, D.; Feve, B.; Lambotte, O.; et al. Impact of HIV/simian immunodeficiency virus infection and viral proteins on adipose tissue fibrosis and adipogenesis. AIDS 2019, 33, 953–964. [Google Scholar] [CrossRef]
- Maurin, T.; Saillan-Barreau, C.; Cousin, B.; Casteilla, L.; Doglio, A.; Penicaud, L. Tumor necrosis factor-alpha stimulates HIV-1 production in primary culture of human adipocytes. Exp Cell Res 2005, 304, 544–551. [Google Scholar] [CrossRef]
- Munier, S.; Borjabad, A.; Lemaire, M.; Mariot, V.; Hazan, U. In vitro infection of human primary adipose cells with HIV-1: a reassessment. AIDS 2003, 17, 2537–2539. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Lake, J.E.; Currier, J.S. Metabolic disease in HIV infection. Lancet Infect Dis 2013, 13, 964–975. [Google Scholar] [CrossRef]
- Anastos, K.; Lu, D.; Shi, Q.; Tien, P.C.; Kaplan, R.C.; Hessol, N.A.; Cole, S.; Vigen, C.; Cohen, M.; Young, M.; et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr 2007, 45, 34–42. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Bukrinsky, M.I. HIV-1 accessory proteins: VpR. Methods Mol Biol 2014, 1087, 125–134. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat Med 2004, 10, 355–361. [Google Scholar] [CrossRef]
- Francis, G.A.; Li, G.; Casey, R.; Wang, J.; Cao, H.; Leff, T.; Hegele, R.A. Peroxisomal proliferator activated receptor-gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3). BMC Med Genet 2006, 7, 3. [Google Scholar] [CrossRef]
- Agarwal, N.; Iyer, D.; Gabbi, C.; Saha, P.; Patel, S.G.; Mo, Q.; Chang, B.; Goswami, B.; Schubert, U.; Kopp, J.B.; et al. HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRalpha and PPARalpha dysregulation: implications for HIV-specific pathogenesis of NAFLD. Sci Rep 2017, 7, 13362. [Google Scholar] [CrossRef]
- Shrivastav, S.; Kino, T.; Cunningham, T.; Ichijo, T.; Schubert, U.; Heinklein, P.; Chrousos, G.P.; Kopp, J.B. Human immunodeficiency virus (HIV)-1 viral protein R suppresses transcriptional activity of peroxisome proliferator-activated receptor gamma and inhibits adipocyte differentiation: implications for HIV-associated lipodystrophy. Mol Endocrinol 2008, 22, 234–247. [Google Scholar] [CrossRef]
- Rice, A.P. The HIV-1 Tat Protein: Mechanism of Action and Target for HIV-1 Cure Strategies. Curr Pharm Des 2017, 23, 4098–4102. [Google Scholar] [CrossRef]
- Liu, Y.; Jones, M.; Hingtgen, C.M.; Bu, G.; Laribee, N.; Tanzi, R.E.; Moir, R.D.; Nath, A.; He, J.J. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med 2000, 6, 1380–1387. [Google Scholar] [CrossRef]
- Weiss, J.M.; Nath, A.; Major, E.O.; Berman, J.W. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol 1999, 163, 2953–2959. [Google Scholar] [CrossRef]
- Zauli, G.; Furlini, G.; Re, M.C.; Milani, D.; Capitani, S.; La Placa, M. Human immunodeficiency virus type 1 (HIV-1) tat-protein stimulates the production of interleukin-6 (IL-6) by peripheral blood monocytes. New Microbiol 1993, 16, 115–120. [Google Scholar]
- van 't Wout, A.B.; Swain, J.V.; Schindler, M.; Rao, U.; Pathmajeyan, M.S.; Mullins, J.I.; Kirchhoff, F. Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells. J Virol 2005, 79, 10053–10058. [Google Scholar] [CrossRef]
- Lin, S.; Nadeau, P.E.; Wang, X.; Mergia, A. Caveolin-1 reduces HIV-1 infectivity by restoration of HIV Nef mediated impairment of cholesterol efflux by apoA-I. Retrovirology 2012, 9, 85. [Google Scholar] [CrossRef]
- Lin, S.; Nadeau, P.E.; Mergia, A. HIV inhibits endothelial reverse cholesterol transport through impacting subcellular Caveolin-1 trafficking. Retrovirology 2015, 12, 62. [Google Scholar] [CrossRef]
- Duffy, P.; Wang, X.; Lin, P.H.; Yao, Q.; Chen, C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res 2009, 156, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Olivetta, E.; Percario, Z.; Fiorucci, G.; Mattia, G.; Schiavoni, I.; Dennis, C.; Jager, J.; Harris, M.; Romeo, G.; Affabris, E.; et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol 2003, 170, 1716–1727. [Google Scholar] [CrossRef]
- Wang, T.; Green, L.A.; Gupta, S.K.; Kim, C.; Wang, L.; Almodovar, S.; Flores, S.C.; Prudovsky, I.A.; Jolicoeur, P.; Liu, Z.; et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS One 2014, 9, e91063. [Google Scholar] [CrossRef]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab 2012, 23, 407–415. [Google Scholar] [CrossRef]
- Dorfmuller, P.; Zarka, V.; Durand-Gasselin, I.; Monti, G.; Balabanian, K.; Garcia, G.; Capron, F.; Coulomb-Lhermine, A.; Marfaing-Koka, A.; Simonneau, G.; et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002, 165, 534–539. [Google Scholar] [CrossRef]
- Freeman, M.L.; Hossain, M.B.; Burrowes, S.A.B.; Jeudy, J.; Bui, R.; Moisi, D.; Mitchell, S.E.; Khambaty, M.; Weiss, R.G.; Lederman, M.M.; et al. Association of Soluble Markers of Inflammation With Peri-coronary Artery Inflammation in People With and Without HIV Infection and Without Cardiovascular Disease. Open Forum Infect Dis 2023, 10, ofad328. [Google Scholar] [CrossRef]
- Couturier, J.; Agarwal, N.; Nehete, P.N.; Baze, W.B.; Barry, M.A.; Jagannadha Sastry, K.; Balasubramanyam, A.; Lewis, D.E. Infectious SIV resides in adipose tissue and induces metabolic defects in chronically infected rhesus macaques. Retrovirology 2016, 13, 30. [Google Scholar] [CrossRef]
- McGillicuddy, F.C.; de la Llera Moya, M.; Hinkle, C.C.; Joshi, M.R.; Chiquoine, E.H.; Billheimer, J.T.; Rothblat, G.H.; Reilly, M.P. Inflammation impairs reverse cholesterol transport in vivo. Circulation 2009, 119, 1135–1145. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Memon, R.A.; Feingold, K.R.; Grunfeld, C. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis 2000, 181 Suppl 3, S462–472. [Google Scholar] [CrossRef]
- Perrotta, I.; Aquila, S. The role of oxidative stress and autophagy in atherosclerosis. Oxid Med Cell Longev 2015, 2015, 130315. [Google Scholar] [CrossRef]
- Ma, R.; Yang, L.; Niu, F.; Buch, S. HIV Tat-Mediated Induction of Human Brain Microvascular Endothelial Cell Apoptosis Involves Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Mol Neurobiol 2016, 53, 132–142. [Google Scholar] [CrossRef]
- Cross, A.R.; Segal, A.W. The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochim Biophys Acta 2004, 1657, 1–22. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Guo, H.; Gao, J.; Taxman, D.J.; Ting, J.P.; Su, L. HIV-1 infection induces interleukin-1beta production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem 2014, 289, 21716–21726. [Google Scholar] [CrossRef]
- Blanc, M.; Hsieh, W.Y.; Robertson, K.A.; Watterson, S.; Shui, G.; Lacaze, P.; Khondoker, M.; Dickinson, P.; Sing, G.; Rodriguez-Martin, S.; et al. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol 2011, 9, e1000598. [Google Scholar] [CrossRef]
- Vyboh, K.; Jenabian, M.A.; Mehraj, V.; Routy, J.P. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res 2015, 2015, 614127. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Vazquez-Castellanos, J.F.; Vallejo, A.; Latorre, A.; Sainz, T.; Ferrando-Martinez, S.; Rojo, D.; Martinez-Botas, J.; Del Romero, J.; Madrid, N.; et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol 2017, 10, 1279–1293. [Google Scholar] [CrossRef]
- Nordell, A.D.; McKenna, M.; Borges, A.H.; Duprez, D.; Neuhaus, J.; Neaton, J.D.; Insight Smart, E.S.G.; Committee, S.S. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014, 3, e000844. [Google Scholar] [CrossRef]
- Villanueva-Millan, M.J.; Perez-Matute, P.; Recio-Fernandez, E.; Lezana Rosales, J.M.; Oteo, J.A. Characterization of gut microbiota composition in HIV-infected patients with metabolic syndrome. J Physiol Biochem 2019, 75, 299–309. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Levi, L.; Alagaratnam, J.; Van Bremen, K.; Mastrangelo, A.; Waalewijn, H.; Molina, J.M.; Guaraldi, G.; Winston, A.; Boesecke, C.; et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med 2023, 24, 1126–1136. [Google Scholar] [CrossRef]
- Okunorobo, M.N.; Nnamah, N.K.; Ude, U.A.; Ude, E.A. Lipids and apolipoproteins C-III and E among treatment-naive and treatment-experienced persons with HIV in Nigeria. Afr J Lab Med 2023, 12, 2018. [Google Scholar] [CrossRef]
- Lu, L.; Yang, Y.; Yang, Z.; Wu, Y.; Liu, X.; Li, X.; Chen, L.; Han, Y.; Song, X.; Kong, Z.; et al. Altered plasma metabolites and inflammatory networks in HIV-1 infected patients with different immunological responses after long-term antiretroviral therapy. Front Immunol 2023, 14, 1254155. [Google Scholar] [CrossRef]
- Mandal, A.; Mukherjee, A.; Lakshmy, R.; Kabra, S.K.; Lodha, R. Dyslipidemia in HIV Infected Children Receiving Highly Active Antiretroviral Therapy. Indian J Pediatr 2016, 83, 226–231. [Google Scholar] [CrossRef]
- Ambisa Lamesa, T.; Getachew Mamo, A.; Arega Berihun, G.; Alemu Kebede, R.; Bekele Lemesa, E.; Cheneke Gebisa, W. Dyslipidemia and Nutritional Status of HIV-Infected Children and Adolescents on Antiretroviral Treatment at the Comprehensive Chronic Care and Training Center of Jimma Medical Center. HIV AIDS (Auckl) 2023, 15, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, X.; Han, Y.; Qiu, Z.; Cao, W.; Li, T. Risk factors and longitudinal changes of dyslipidemia among Chinese people living with HIV receiving antiretroviral therapy. BMC Infect Dis 2023, 23, 598. [Google Scholar] [CrossRef]
- Mallon, P.W.; Cooper, D.A.; Carr, A. HIV-associated lipodystrophy. HIV Med 2001, 2, 166–173. [Google Scholar] [CrossRef]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Feeney, E.R.; Mallon, P.W. HIV and HAART-Associated Dyslipidemia. Open Cardiovasc Med J 2011, 5, 49–63. [Google Scholar] [CrossRef]
- Martini, S.; Pisaturo, M.; Russo, A.; Palamone, M.G.; Russo, M.T.; Zollo, V.; Maggi, P.; Coppola, N. Evaluation of Lipid Profile and Intima Media Thickness in Antiretroviral-Experienced HIV-Infected Patients Treated with Protease Inhibitor-Based Regimens versus Protease Inhibitor-Sparing Regimens. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- den Boer, M.A.; Berbee, J.F.; Reiss, P.; van der Valk, M.; Voshol, P.J.; Kuipers, F.; Havekes, L.M.; Rensen, P.C.; Romijn, J.A. Ritonavir impairs lipoprotein lipase-mediated lipolysis and decreases uptake of fatty acids in adipose tissue. Arterioscler Thromb Vasc Biol 2006, 26, 124–129. [Google Scholar] [CrossRef]
- Caron, M.; Auclair, M.; Sterlingot, H.; Kornprobst, M.; Capeau, J. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS 2003, 17, 2437–2444. [Google Scholar] [CrossRef]
- Caron, M.; Auclair, M.; Vigouroux, C.; Glorian, M.; Forest, C.; Capeau, J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes 2001, 50, 1378–1388. [Google Scholar] [CrossRef]
- Zhou, H.; Gurley, E.C.; Jarujaron, S.; Ding, H.; Fang, Y.; Xu, Z.; Pandak, W.M., Jr.; Hylemon, P.B. HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. Am J Physiol Gastrointest Liver Physiol 2006, 291, G1071–1080. [Google Scholar] [CrossRef]
- Liang, J.S.; Distler, O.; Cooper, D.A.; Jamil, H.; Deckelbaum, R.J.; Ginsberg, H.N.; Sturley, S.L. HIV protease inhibitors protect apolipoprotein B from degradation by the proteasome: a potential mechanism for protease inhibitor-induced hyperlipidemia. Nat Med 2001, 7, 1327–1331. [Google Scholar] [CrossRef]
- Akita, S.; Suzuki, K.; Yoshimoto, H.; Ohtsuru, A.; Hirano, A.; Yamashita, S. Cellular Mechanism Underlying Highly-Active or Antiretroviral Therapy-Induced Lipodystrophy: Atazanavir, a Protease Inhibitor, Compromises Adipogenic Conversion of Adipose-Derived Stem/Progenitor Cells through Accelerating ER Stress-Mediated Cell Death in Differentiating Adipocytes. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Friis-Moller, N.; Weber, R.; Reiss, P.; Thiebaut, R.; Kirk, O.; d'Arminio Monforte, A.; Pradier, C.; Morfeldt, L.; Mateu, S.; Law, M.; et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS 2003, 17, 1179–1193. [Google Scholar] [CrossRef]
- Fontas, E.; van Leth, F.; Sabin, C.A.; Friis-Moller, N.; Rickenbach, M.; d'Arminio Monforte, A.; Kirk, O.; Dupon, M.; Morfeldt, L.; Mateu, S.; et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis 2004, 189, 1056–1074. [Google Scholar] [CrossRef]
- Sadler, B.M.; Piliero, P.J.; Preston, S.L.; Lloyd, P.P.; Lou, Y.; Stein, D.S. Pharmacokinetics and safety of amprenavir and ritonavir following multiple-dose, co-administration to healthy volunteers. AIDS 2001, 15, 1009–1018. [Google Scholar] [CrossRef]
- Purnell, J.Q.; Zambon, A.; Knopp, R.H.; Pizzuti, D.J.; Achari, R.; Leonard, J.M.; Locke, C.; Brunzell, J.D. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS 2000, 14, 51–57. [Google Scholar] [CrossRef]
- Shafran, S.D.; Mashinter, L.D.; Roberts, S.E. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med 2005, 6, 421–425. [Google Scholar] [CrossRef]
- Lee, G.A.; Seneviratne, T.; Noor, M.A.; Lo, J.C.; Schwarz, J.M.; Aweeka, F.T.; Mulligan, K.; Schambelan, M.; Grunfeld, C. The metabolic effects of lopinavir/ritonavir in HIV-negative men. AIDS 2004, 18, 641–649. [Google Scholar] [CrossRef]
- Voigt, E.; Wasmuth, J.C.; Vogel, M.; Mauss, S.; Schmutz, G.; Kaiser, R.; Rockstroh, J.K. Safety, efficacy and development of resistance under the new protease inhibitor lopinavir/ritonavir: 48-week results. Infection 2004, 32, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Badiou, S.; De Boever, C.M.; Dupuy, A.M.; Baillat, V.; Cristol, J.P.; Reynes, J. Small dense LDL and atherogenic lipid profile in HIV-positive adults: influence of lopinavir/ritonavir-containing regimen. AIDS 2003, 17, 772–774. [Google Scholar] [CrossRef]
- Montes, M.L.; Pulido, F.; Barros, C.; Condes, E.; Rubio, R.; Cepeda, C.; Dronda, F.; Antela, A.; Sanz, J.; Navas, E.; et al. Lipid disorders in antiretroviral-naive patients treated with lopinavir/ritonavir-based HAART: frequency, characterization and risk factors. J Antimicrob Chemother 2005, 55, 800–804. [Google Scholar] [CrossRef]
- Gutierrez, F.; Padilla, S.; Navarro, A.; Masia, M.; Hernandez, I.; Ramos, J.; Esteban, A.; Martin-Hidalgo, A. Lopinavir plasma concentrations and changes in lipid levels during salvage therapy with lopinavir/ritonavir-containing regimens. J Acquir Immune Defic Syndr 2003, 33, 594–600. [Google Scholar] [CrossRef]
- Torti, C.; Quiros-Roldan, E.; Regazzi-Bonora, M.; De Luca, A.; Lo Caputo, S.; Di Giambenedetto, S.; Patroni, A.; Villani, P.; Micheli, V.; Carosi, G.; et al. Lipid abnormalities in HIV-infected patients are not correlated with lopinavir plasma concentrations. J Acquir Immune Defic Syndr 2004, 35, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.P.; Qian, D.; Edmondson-Melancon, H.; Sattler, F.R.; Goodwin, D.; Martinez, C.; Williams, V.; Johnson, D.; Buchanan, T.A. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin Infect Dis 2002, 35, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Fisac, C.; Virgili, N.; Ferrer, E.; Barbera, M.J.; Fumero, E.; Vilarasau, C.; Podzamczer, D. A comparison of the effects of nevirapine and nelfinavir on metabolism and body habitus in antiretroviral-naive human immunodeficiency virus-infected patients: a randomized controlled study. J Clin Endocrinol Metab 2003, 88, 5186–5192. [Google Scholar] [CrossRef]
- Squires, K.; Lazzarin, A.; Gatell, J.M.; Powderly, W.G.; Pokrovskiy, V.; Delfraissy, J.F.; Jemsek, J.; Rivero, A.; Rozenbaum, W.; Schrader, S.; et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr 2004, 36, 1011–1019. [Google Scholar] [CrossRef]
- Mobius, U.; Lubach-Ruitman, M.; Castro-Frenzel, B.; Stoll, M.; Esser, S.; Voigt, E.; Christensen, S.; Rump, J.A.; Fatkenheuer, G.; Behrens, G.M.; et al. Switching to atazanavir improves metabolic disorders in antiretroviral-experienced patients with severe hyperlipidemia. J Acquir Immune Defic Syndr 2005, 39, 174–180. [Google Scholar] [PubMed]
- Mills, A.M.; Nelson, M.; Jayaweera, D.; Ruxrungtham, K.; Cassetti, I.; Girard, P.M.; Workman, C.; Dierynck, I.; Sekar, V.; Abeele, C.V.; et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 2009, 23, 1679–1688. [Google Scholar] [CrossRef]
- Clotet, B.; Bellos, N.; Molina, J.M.; Cooper, D.; Goffard, J.C.; Lazzarin, A.; Wohrmann, A.; Katlama, C.; Wilkin, T.; Haubrich, R.; et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 2007, 369, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Aberg, J.A.; Tebas, P.; Overton, E.T.; Gupta, S.K.; Sax, P.E.; Landay, A.; Falcon, R.; Ryan, R.; De La Rosa, G. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses 2012, 28, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Auclair, M.; Lagathu, C.; Lombes, A.; Walker, U.A.; Kornprobst, M.; Capeau, J. The HIV-1 nucleoside reverse transcriptase inhibitors stavudine and zidovudine alter adipocyte functions in vitro. AIDS 2004, 18, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T.N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther 2000, 22, 685–708. [Google Scholar] [CrossRef]
- Maagaard, A.; Kvale, D. Long term adverse effects related to nucleoside reverse transcriptase inhibitors: clinical impact of mitochondrial toxicity. Scand J Infect Dis 2009, 41, 808–817. [Google Scholar] [CrossRef]
- Johnson, A.A.; Ray, A.S.; Hanes, J.; Suo, Z.; Colacino, J.M.; Anderson, K.S.; Johnson, K.A. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem 2001, 276, 40847–40857. [Google Scholar] [CrossRef]
- Zaera, M.G.; Miro, O.; Pedrol, E.; Soler, A.; Picon, M.; Cardellach, F.; Casademont, J.; Nunes, V. Mitochondrial involvement in antiretroviral therapy-related lipodystrophy. AIDS 2001, 15, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Blas-Garcia, A.; Apostolova, N.; Ballesteros, D.; Monleon, D.; Morales, J.M.; Rocha, M.; Victor, V.M.; Esplugues, J.V. Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology 2010, 52, 115–125. [Google Scholar] [CrossRef]
- Cote, H.C. Possible ways nucleoside analogues can affect mitochondrial DNA content and gene expression during HIV therapy. Antivir Ther 2005, 10 Suppl 2, M3–11. [Google Scholar] [CrossRef]
- Mallal, S.A.; John, M.; Moore, C.B.; James, I.R.; McKinnon, E.J. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS 2000, 14, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Shikuma, C.M.; Hu, N.; Milne, C.; Yost, F.; Waslien, C.; Shimizu, S.; Shiramizu, B. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS 2001, 15, 1801–1809. [Google Scholar] [CrossRef]
- Cote, H.C.; Brumme, Z.L.; Craib, K.J.; Alexander, C.S.; Wynhoven, B.; Ting, L.; Wong, H.; Harris, M.; Harrigan, P.R.; O'Shaughnessy, M.V.; et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 2002, 346, 811–820. [Google Scholar] [CrossRef]
- Walker, U.A.; Setzer, B.; Venhoff, N. Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS 2002, 16, 2165–2173. [Google Scholar] [CrossRef]
- Llibre, J.M.; Domingo, P.; Palacios, R.; Santos, J.; Perez-Elias, M.J.; Sanchez-de la Rosa, R.; Miralles, C.; Antela, A.; Moreno, S.; Lipo-Rec Study, G. Sustained improvement of dyslipidaemia in HAART-treated patients replacing stavudine with tenofovir. AIDS 2006, 20, 1407–1414. [Google Scholar] [CrossRef]
- Milinkovic, A.; Martinez, E.; Lopez, S.; de Lazzari, E.; Miro, O.; Vidal, S.; Blanco, J.L.; Garrabou, G.; Laguno, M.; Arnaiz, J.A.; et al. The impact of reducing stavudine dose versus switching to tenofovir on plasma lipids, body composition and mitochondrial function in HIV-infected patients. Antivir Ther 2007, 12, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.D.; Battegay, M.; Behrens, G.; De Wit, S.; Guaraldi, G.; Katlama, C.; Martinez, E.; Nair, D.; Powderly, W.G.; Reiss, P.; et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med 2008, 9, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Q.; Liu, J.Y.; He, Y.; Zhou, Y.; Xu, L.M.; Zhang, L.K.; Zhao, F.; Liu, X.N.; Song, Y.; Cao, T.Z.; et al. Evolution of blood lipids and risk factors of dyslipidemia among people living with human immunodeficiency virus who had received first-line antiretroviral regimens for 3 years in Shenzhen. Chin Med J (Engl) 2020, 133, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Workman, C.; Smith, D.E.; Hoy, J.; Hudson, J.; Doong, N.; Martin, A.; Amin, J.; Freund, J.; Law, M.; et al. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial. JAMA 2002, 288, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.M.; Tierney, C.; Budhathoki, C.; Daar, E.S.; Sax, P.E.; Collier, A.C.; Fischl, M.A.; Zolopa, A.R.; Balamane, M.; Katzenstein, D. Early virologic response to abacavir/lamivudine and tenofovir/emtricitabine during ACTG A5202. HIV Clin Trials 2013, 14, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Jaschinski, N.; Greenberg, L.; Neesgaard, B.; Miro, J.M.; Grabmeier-Pfistershammer, K.; Wandeler, G.; Smith, C.; De Wit, S.; Wit, F.; Pelchen-Matthews, A.; et al. Recent abacavir use and incident cardiovascular disease in contemporary-treated people with HIV. AIDS 2023, 37, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanz, J.; Serrano-Villar, S.; Muriel, A.; Garcia Fraile, L.J.; Orviz, E.; Mena de Cea, A.; Campins, A.A.; Moreno, S. Metabolic-Related Outcomes After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Adults With Human Immunodeficiency Virus (HIV): A Multicenter Prospective Cohort Study. Clin Infect Dis 2023, 76, e652–e660. [Google Scholar] [CrossRef]
- Moschopoulos, C.D.; Protopapas, K.; Thomas, K.; Kavatha, D.; Papadopoulos, A.; Antoniadou, A. Switching from Tenofovir Disoproxil to Tenofovir Alafenamide Fumarate: Impact on Cardiovascular Risk and Lipid Profile in People Living with HIV, an Observational Study. AIDS Res Hum Retroviruses 2023, 39, 68–75. [Google Scholar] [CrossRef]
- Friis-Moller, N.; Thiebaut, R.; Reiss, P.; Weber, R.; Monforte, A.D.; De Wit, S.; El-Sadr, W.; Fontas, E.; Worm, S.; Kirk, O.; et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010, 17, 491–501. [Google Scholar] [CrossRef]
- van der Valk, M.; Kastelein, J.J.; Murphy, R.L.; van Leth, F.; Katlama, C.; Horban, A.; Glesby, M.; Behrens, G.; Clotet, B.; Stellato, R.K.; et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS 2001, 15, 2407–2414. [Google Scholar] [CrossRef]
- Apostolova, N.; Blas-Garcia, A.; Esplugues, J.V. Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-gamma inhibition. Trends Pharmacol Sci 2011, 32, 715–725. [Google Scholar] [CrossRef]
- Apostolova, N.; Gomez-Sucerquia, L.J.; Moran, A.; Alvarez, A.; Blas-Garcia, A.; Esplugues, J.V. Enhanced oxidative stress and increased mitochondrial mass during efavirenz-induced apoptosis in human hepatic cells. Br J Pharmacol 2010, 160, 2069–2084. [Google Scholar] [CrossRef] [PubMed]
- Gwag, T.; Meng, Z.; Sui, Y.; Helsley, R.N.; Park, S.H.; Wang, S.; Greenberg, R.N.; Zhou, C. Non-nucleoside reverse transcriptase inhibitor efavirenz activates PXR to induce hypercholesterolemia and hepatic steatosis. J Hepatol 2019, 70, 930–940. [Google Scholar] [CrossRef]
- Williams, P.; Wu, J.; Cohn, S.; Koletar, S.; McCutchan, J.; Murphy, R.; Currier, J.; Team, A.C.T.G.S. Improvement in lipid profiles over 6 years of follow-up in adults with AIDS and immune reconstitution. HIV Med 2009, 10, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Podzamczer, D.; Andrade-Villanueva, J.; Clotet, B.; Taylor, S.; Rockstroh, J.K.; Reiss, P.; Domingo, P.; Gellermann, H.J.; de Rossi, L.; Cairns, V.; et al. Lipid profiles for nevirapine vs. atazanavir/ritonavir, both combined with tenofovir disoproxil fumarate and emtricitabine over 48 weeks, in treatment-naive HIV-1-infected patients (the ARTEN study). HIV Med 2011, 12, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Haubrich, R.H.; Riddler, S.A.; DiRienzo, A.G.; Komarow, L.; Powderly, W.G.; Klingman, K.; Garren, K.W.; Butcher, D.L.; Rooney, J.F.; Haas, D.W.; et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 2009, 23, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- van Leth, F.; Phanuphak, P.; Stroes, E.; Gazzard, B.; Cahn, P.; Raffi, F.; Wood, R.; Bloch, M.; Katlama, C.; Kastelein, J.J.; et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med 2004, 1, e19. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; Tatarelli, P.; Ricci, E.; Madeddu, G.; Menzaghi, B.; Squillace, N.; De Socio, G.V.; Martinelli, C.; Gulminetti, R.; Maggi, P.; et al. Improvement of lipid profile after switching from efavirenz or ritonavir-boosted protease inhibitors to rilpivirine or once-daily integrase inhibitors: results from a large observational cohort study (SCOLTA). BMC Infect Dis 2018, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Rokx, C.; Verbon, A.; Rijnders, B.J. Short communication: Lipids and cardiovascular risk after switching HIV-1 patients on nevirapine and emtricitabine/tenofovir-DF to rilpivirine/emtricitabine/tenofovir-DF. AIDS Res Hum Retroviruses 2015, 31, 363–367. [Google Scholar] [CrossRef] [PubMed]
- MacInnes, A.; Lazzarin, A.; Di Perri, G.; Sierra-Madero, J.G.; Aberg, J.; Heera, J.; Rajicic, N.; Goodrich, J.; Mayer, H.; Valdez, H. Maraviroc can improve lipid profiles in dyslipidemic patients with HIV: results from the MERIT trial. HIV Clin Trials 2011, 12, 24–36. [Google Scholar] [CrossRef]
- Valenzuela-Rodriguez, G.; Diaz-Arocutipa, C.; Collins, J.A.; Hernandez, A.V. Weight and Metabolic Outcomes in Naive HIV Patients Treated with Integrase Inhibitor-Based Antiretroviral Therapy: A Systematic Review and Meta-Analysis. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Cardoso-Neto, E.C.; Netto, E.M.; Brites, C. Weight gain in patients starting Dolutegravir-based ART according to baseline CD4 count after 48 weeks of follow up. Braz J Infect Dis 2023, 27, 102807. [Google Scholar] [CrossRef]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; McComsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis 2020, 71, 1379–1389. [Google Scholar] [CrossRef]
- Bourgeois, C.; Gorwood, J.; Olivo, A.; Le Pelletier, L.; Capeau, J.; Lambotte, O.; Bereziat, V.; Lagathu, C. Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment. Front Immunol 2021, 12, 670566. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E. The Fat of the Matter: Obesity and Visceral Adiposity in Treated HIV Infection. Curr HIV/AIDS Rep 2017, 14, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Winchester, L.C.; Suliburk, J.W.; Wilkerson, G.K.; Podany, A.T.; Agarwal, N.; Xuan Chua, C.Y.; Nehete, P.N.; Nehete, B.P.; Grattoni, A.; et al. Adipocytes impair efficacy of antiretroviral therapy. Antiviral Res 2018, 154, 140–148. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin Infect Dis 2020, 71, e549–e560. [Google Scholar] [CrossRef] [PubMed]
- Katlama, C.; Assoumou, L.; Valantin, M.A.; Soulie, C.; Martinez, E.; Beniguel, L.; Bouchaud, O.; Raffi, F.; Molina, J.M.; Fellahi, S.; et al. Dual therapy combining raltegravir with etravirine maintains a high level of viral suppression over 96 weeks in long-term experienced HIV-infected individuals over 45 years on a PI-based regimen: results from the Phase II ANRS 163 ETRAL study. J Antimicrob Chemother 2019, 74, 2742–2751. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Moser, C.; Brown, T.T.; Kelesidis, T.; Dube, M.P.; Stein, J.H.; Currier, J.; McComsey, G.A. Changes in Insulin Resistance After Initiation of Raltegravir or Protease Inhibitors With Tenofovir-Emtricitabine: AIDS Clinical Trials Group A5260s. Open Forum Infect Dis 2016, 3, ofw174. [Google Scholar] [CrossRef]
- Ofotokun, I.; Na, L.H.; Landovitz, R.J.; Ribaudo, H.J.; McComsey, G.A.; Godfrey, C.; Aweeka, F.; Cohn, S.E.; Sagar, M.; Kuritzkes, D.R.; et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis 2015, 60, 1842–1851. [Google Scholar] [CrossRef]
- Pantazis, N.; Papastamopoulos, V.; Antoniadou, A.; Adamis, G.; Paparizos, V.; Metallidis, S.; Sambatakou, H.; Psichogiou, M.; Chini, M.; Chrysos, G.; et al. Changes in Body Mass Index after Initiation of Antiretroviral Treatment: Differences by Class of Core Drug. Viruses 2022, 14. [Google Scholar] [CrossRef]
- The, R.S.G. Incidence of dyslipidemia in people with HIV who are treated with integrase inhibitors versus other antiretroviral agents. AIDS 2021, 35, 869–882. [Google Scholar] [CrossRef]
- Baldin, G.; Ciccullo, A.; Lombardi, F.; D'Angelillo, A.; Dusina, A.; Emiliozzi, A.; Farinacci, D.; Moschese, D.; Picarelli, C.; Borghetti, A.; et al. Short Communication: Comparing Lamivudine+Dolutegravir and Bictegravir/Emtricitabine/Tenofovir Alafenamide as Switch Strategies: Preliminary Results from Clinical Practice. AIDS Res Hum Retroviruses 2021, 37, 429–432. [Google Scholar] [CrossRef]
- Adachi, E.; Saito, M.; Otani, A.; Koga, M.; Yotsuyanagi, H. Brief communications: changes in inflammatory biomarkers and lipid profiles after switching to long-acting cabotegravir plus rilpivirine. AIDS Res Ther 2024, 21, 1. [Google Scholar] [CrossRef]
- Achhra, A.C.; Lyass, A.; Borowsky, L.; Bogorodskaya, M.; Plutzky, J.; Massaro, J.M.; D'Agostino, R.B., Sr.; Triant, V.A. Assessing Cardiovascular Risk in People Living with HIV: Current Tools and Limitations. Curr HIV/AIDS Rep 2021, 18, 271–279. [Google Scholar] [CrossRef]
- Triant, V.A.; Perez, J.; Regan, S.; Massaro, J.M.; Meigs, J.B.; Grinspoon, S.K.; D'Agostino, R.B., Sr. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation 2018, 137, 2203–2214. [Google Scholar] [CrossRef]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Harrington, R.A. Statins-Almost 30 Years of Use in the United States and Still Not Quite There. JAMA Cardiol 2017, 2, 66. [Google Scholar] [CrossRef]
- Moore, R.D.; Bartlett, J.G.; Gallant, J.E. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One 2011, 6, e21843. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Xia, H.; Zhang, J. Influence of Statin Therapy on the Incidence of Cardiovascular Events, Cancer, and All-Cause Mortality in People Living With HIV: A Meta-Analysis. Front Med (Lausanne) 2021, 8, 769740. [Google Scholar] [CrossRef] [PubMed]
- Schonbeck, U.; Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 2004, 109, II18–26. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.P.; Blumberg, N. Statin islands and PPAR ligands in platelets. Arterioscler Thromb Vasc Biol 2009, 29, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, S.; Jensen, K.S.; Liao, J.K. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol 2003, 23, 729–736. [Google Scholar] [CrossRef]
- Laufs, U.; Liao, J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 1998, 273, 24266–24271. [Google Scholar] [CrossRef] [PubMed]
- Loppnow, H.; Zhang, L.; Buerke, M.; Lautenschlager, M.; Chen, L.; Frister, A.; Schlitt, A.; Luther, T.; Song, N.; Hofmann, B.; et al. Statins potently reduce the cytokine-mediated IL-6 release in SMC/MNC cocultures. J Cell Mol Med 2011, 15, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Kosmidou, I.; Moore, J.P.; Weber, M.; Searles, C.D. Statin treatment and 3' polyadenylation of eNOS mRNA. Arterioscler Thromb Vasc Biol 2007, 27, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Lorido, J.C. Clinical relevance for lowering C-reactive protein with statins. Ann Med 2016, 48, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Takahashi, A.; Nagao, K.; Hirayama, A. Contribution of apolipoprotein A-I to the reduction in high-sensitivity C-reactive protein levels by different statins: comparative study of pitavastatin and atorvastatin. Heart Vessels 2015, 30, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Steinman, L.; Zamvil, S.S. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006, 6, 358–370. [Google Scholar] [CrossRef]
- Zivkovic, S.; Maric, G.; Cvetinovic, N.; Lepojevic-Stefanovic, D.; Bozic Cvijan, B. Anti-Inflammatory Effects of Lipid-Lowering Drugs and Supplements-A Narrative Review. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Nixon, D.E.; Bosch, R.J.; Chan, E.S.; Funderburg, N.T.; Hodder, S.; Lake, J.E.; Lederman, M.M.; Klingman, K.L.; Aberg, J.A.; Team, A.C.T.G.S.A. Effects of atorvastatin on biomarkers of immune activation, inflammation, and lipids in virologically suppressed, human immunodeficiency virus-1-infected individuals with low-density lipoprotein cholesterol <130 mg/dL (AIDS Clinical Trials Group Study A5275). J Clin Lipidol 2017, 11, 61–69. [Google Scholar] [CrossRef]
- Calza, L.; Colangeli, V.; Borderi, M.; Beci, G.; Esposito, F.; Bon, I.; Re, M.C.; Viale, P. Rosuvastatin decreases serum inflammatory markers and slows atherosclerosis progression rate in treated HIV-infected patients with metabolic syndrome. Infect Dis (Lond) 2021, 53, 81–88. [Google Scholar] [CrossRef]
- Mora, S.; Glynn, R.J.; Hsia, J.; MacFadyen, J.G.; Genest, J.; Ridker, P.M. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010, 121, 1069–1077. [Google Scholar] [CrossRef]
- Funderburg, N.T.; Jiang, Y.; Debanne, S.M.; Storer, N.; Labbato, D.; Clagett, B.; Robinson, J.; Lederman, M.M.; McComsey, G.A. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis 2014, 58, 588–595. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists, C.; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Calza, L.; Manfredi, R.; Colangeli, V.; Pocaterra, D.; Pavoni, M.; Chiodo, F. Rosuvastatin, pravastatin, and atorvastatin for the treatment of hypercholesterolaemia in HIV-infected patients receiving protease inhibitors. Curr HIV Res 2008, 6, 572–578. [Google Scholar] [CrossRef]
- Lo, J.; Lu, M.T.; Ihenachor, E.J.; Wei, J.; Looby, S.E.; Fitch, K.V.; Oh, J.; Zimmerman, C.O.; Hwang, J.; Abbara, S.; et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015, 2, e52–63. [Google Scholar] [CrossRef]
- Rahman, A.P.; Eaton, S.A.; Nguyen, S.T.; Bain, A.M.; Payne, K.D.; Bedimo, R.; Busti, A.J. Safety and efficacy of simvastatin for the treatment of dyslipidemia in human immunodeficiency virus-infected patients receiving efavirenz-based highly active antiretroviral therapy. Pharmacotherapy 2008, 28, 913–919. [Google Scholar] [CrossRef]
- Sabin, C.A.; Yee, T.T.; Devereux, H.; Griffioen, A.; Loveday, C.; Phillips, A.N.; Lee, C.A. Two decades of HIV infection in a cohort of haemophilic individuals: clinical outcomes and response to highly active antiretroviral therapy. AIDS 2000, 14, 1001–1007. [Google Scholar] [CrossRef]
- Aberg, J.A.; Sponseller, C.A.; Ward, D.J.; Kryzhanovski, V.A.; Campbell, S.E.; Thompson, M.A. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV 2017, 4, e284–e294. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.K.; Fitch, K.V.; Zanni, M.V.; Fichtenbaum, C.J.; Umbleja, T.; Aberg, J.A.; Overton, E.T.; Malvestutto, C.D.; Bloomfield, G.S.; Currier, J.S.; et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. N Engl J Med 2023, 389, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Myerson, M.; Malvestutto, C.; Aberg, J.A. Management of lipid disorders in patients living with HIV. J Clin Pharmacol 2015, 55, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, C.; Riva, A.; Rizzardini, G.; Clementi, E.; Galli, M.; Cattaneo, D. Potential association between rosuvastatin use and high atazanavir trough concentrations in ritonavir-treated HIV-infected patients. Antivir Ther 2015, 20, 449–451. [Google Scholar] [CrossRef]
- Chauvin, B.; Drouot, S.; Barrail-Tran, A.; Taburet, A.M. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet 2013, 52, 815–831. [Google Scholar] [CrossRef]
- Ieiri, I.; Tsunemitsu, S.; Maeda, K.; Ando, Y.; Izumi, N.; Kimura, M.; Yamane, N.; Okuzono, T.; Morishita, M.; Kotani, N.; et al. Mechanisms of pharmacokinetic enhancement between ritonavir and saquinavir; micro/small dosing tests using midazolam (CYP3A4), fexofenadine (p-glycoprotein), and pravastatin (OATP1B1) as probe drugs. J Clin Pharmacol 2013, 53, 654–661. [Google Scholar] [CrossRef]
- Aberg, J.A.; Rosenkranz, S.L.; Fichtenbaum, C.J.; Alston, B.L.; Brobst, S.W.; Segal, Y.; Gerber, J.G.; team, A.A. Pharmacokinetic interaction between nelfinavir and pravastatin in HIV-seronegative volunteers: ACTG Study A5108. AIDS 2006, 20, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Fichtenbaum, C.J.; Gerber, J.G.; Rosenkranz, S.L.; Segal, Y.; Aberg, J.A.; Blaschke, T.; Alston, B.; Fang, F.; Kosel, B.; Aweeka, F.; et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS 2002, 16, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Hare, C.B.; Vu, M.P.; Grunfeld, C.; Lampiris, H.W. Simvastatin-nelfinavir interaction implicated in rhabdomyolysis and death. Clin Infect Dis 2002, 35, e111–112. [Google Scholar] [CrossRef]
- Courlet, P.; Decosterd, L.A.; Alves Saldanha, S.; Cavassini, M.; Stader, F.; Stoeckle, M.; Buclin, T.; Marzolini, C.; Csajka, C.; Guidi, M.; et al. Influence of Drug-Drug Interactions on the Pharmacokinetics of Atorvastatin and Its Major Active Metabolite ortho-OH-Atorvastatin in Aging People Living with HIV. Clin Pharmacokinet 2020, 59, 1037–1048. [Google Scholar] [CrossRef]
- Gibert, C.L. Treatment Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents: An Update. Fed Pract 2016, 33, 31S–36S. [Google Scholar] [PubMed]
- Custodio, J.M.; Wang, H.; Hao, J.; Lepist, E.I.; Ray, A.S.; Andrews, J.; Ling, K.H.; Cheng, A.; Kearney, B.P.; Ramanathan, S. Pharmacokinetics of cobicistat boosted-elvitegravir administered in combination with rosuvastatin. J Clin Pharmacol 2014, 54, 649–656. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Nirmala, N.; Avendano, E.E.; Morin, R.A. Effectiveness of ezetimibe in human immunodeficiency virus patients treated for hyperlipidaemia: a systematic review and meta-analysis. Infect Dis (Lond) 2022, 54, 99–109. [Google Scholar] [CrossRef]
- Saeedi, R.; Johns, K.; Frohlich, J.; Bennett, M.T.; Bondy, G. Lipid lowering efficacy and safety of Ezetimibe combined with rosuvastatin compared with titrating rosuvastatin monotherapy in HIV-positive patients. Lipids Health Dis 2015, 14, 57. [Google Scholar] [CrossRef]
- Alvarez-Sala, L.A.; Cachofeiro, V.; Masana, L.; Suarez, C.; Pinilla, B.; Plana, N.; Trias, F.; Moreno, M.A.; Gambus, G.; Lahera, V.; et al. Effects of fluvastatin extended-release (80 mg) alone and in combination with ezetimibe (10 mg) on low-density lipoprotein cholesterol and inflammatory parameters in patients with primary hypercholesterolemia: a 12-week, multicenter, randomized, open-label, parallel-group study. Clin Ther 2008, 30, 84–97. [Google Scholar] [CrossRef]
- Ghanim, H.; Green, K.; Abuaysheh, S.; Patel, R.; Batra, M.; Chaudhuri, A.; Makdissi, A.; Kuhadiya, N.D.; Dandona, P. Ezetimibe and simvastatin combination inhibits and reverses the pro-inflammatory and pro-atherogenic effects of cream in obese patients. Atherosclerosis 2017, 263, 278–286. [Google Scholar] [CrossRef]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Dalla Man, C.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am J Physiol Endocrinol Metab 2021, 321, E105–E121. [Google Scholar] [CrossRef]
- Qin, L.; Yang, Y.B.; Yang, Y.X.; Zhu, N.; Li, S.X.; Liao, D.F.; Zheng, X.L. Anti-inflammatory activity of ezetimibe by regulating NF-kappaB/MAPK pathway in THP-1 macrophages. Pharmacology 2014, 93, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Scherer, D.J.; Nelson, A.J.; Psaltis, P.J.; Nicholls, S.J. Targeting low-density lipoprotein cholesterol with PCSK9 inhibitors. Intern Med J 2017, 47, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Leucker, T.M.; Gerstenblith, G.; Schar, M.; Brown, T.T.; Jones, S.R.; Afework, Y.; Weiss, R.G.; Hays, A.G. Evolocumab, a PCSK9-Monoclonal Antibody, Rapidly Reverses Coronary Artery Endothelial Dysfunction in People Living With HIV and People With Dyslipidemia. J Am Heart Assoc 2020, 9, e016263. [Google Scholar] [CrossRef]
- Boccara, F.; Caramelli, B.; Calmy, A.; Kumar, P.; Lopez, J.A.G.; Bray, S.; Cyrille, M.; Rosenson, R.S.; investigators of the, B.s. Long-term effects of evolocumab in participants with HIV and dyslipidemia: results from the open-label extension period. AIDS 2022, 36, 675–682. [Google Scholar] [CrossRef]
- Bernelot Moens, S.J.; Neele, A.E.; Kroon, J.; van der Valk, F.M.; Van den Bossche, J.; Hoeksema, M.A.; Hoogeveen, R.M.; Schnitzler, J.G.; Baccara-Dinet, M.T.; Manvelian, G.; et al. PCSK9 monoclonal antibodies reverse the pro-inflammatory profile of monocytes in familial hypercholesterolaemia. Eur Heart J 2017, 38, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, L.; Peng, J.; Ren, Z.; Wei, D.; Wu, C.; Pan, L.; Jiang, Z.; Liu, L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kappaB activation in THP-1-derived macrophages. Int J Mol Med 2012, 30, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.M.; Jones, P.H.; Ballantyne, C.M.; Virani, S.S.; Nambi, V. Greater than expected reduction in low-density lipoprotein-cholesterol (LDL-C) with bempedoic acid in a patient with heterozygous familial hypercholesterolemia (HeFH). J Clin Lipidol 2021, 15, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.C.; Telford, D.E.; Sutherland, B.G.; Edwards, J.Y.; Sawyez, C.G.; Barrett, P.H.R.; Newton, R.S.; Pickering, J.G.; Huff, M.W. Bempedoic Acid Lowers Low-Density Lipoprotein Cholesterol and Attenuates Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient (LDLR(+/-) and LDLR(-/-)) Yucatan Miniature Pigs. Arterioscler Thromb Vasc Biol 2018, 38, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of Bempedoic Acid Administration With Atherogenic Lipid Levels in Phase 3 Randomized Clinical Trials of Patients With Hypercholesterolemia. JAMA Cardiol 2020, 5, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N Engl J Med 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020, 27, 593–603. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Cincione, I. Evaluating pharmacokinetics of bempedoic acid in the treatment of hypercholesterolemia. Expert Opin Drug Metab Toxicol 2021, 17, 1031–1038. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Kytikova, O.Y.; Perelman, J.M.; Novgorodtseva, T.P.; Denisenko, Y.K.; Kolosov, V.P.; Antonyuk, M.V.; Gvozdenko, T.A. Peroxisome Proliferator-Activated Receptors as a Therapeutic Target in Asthma. PPAR Res 2020, 2020, 8906968. [Google Scholar] [CrossRef] [PubMed]
- Busse, K.H.; Hadigan, C.; Chairez, C.; Alfaro, R.M.; Formentini, E.; Kovacs, J.A.; Penzak, S.R. Gemfibrozil concentrations are significantly decreased in the presence of lopinavir-ritonavir. J Acquir Immune Defic Syndr 2009, 52, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi-Hagihara, R.; Nakai, D.; Tokui, T.; Abe, T.; Ikeda, T. Gemfibrozil and its glucuronide inhibit the hepatic uptake of pravastatin mediated by OATP1B1. Xenobiotica 2007, 37, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Leyden, W.; Hurley, L.; Go, A.S.; Quesenberry, C.P., Jr.; Klein, D.; Horberg, M.A. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med 2009, 150, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.A.; Liu, W.; Delaney, J.A.; Brown, E.; Mugavero, M.J.; Mathews, W.C.; Napravnik, S.; Willig, J.H.; Eron, J.J.; Hunt, P.W.; et al. Comparative effectiveness of fish oil versus fenofibrate, gemfibrozil, and atorvastatin on lowering triglyceride levels among HIV-infected patients in routine clinical care. J Acquir Immune Defic Syndr 2013, 64, 254–260. [Google Scholar] [CrossRef]
- Grandi, A.M.; Nicolini, E.; Rizzi, L.; Caputo, S.; Annoni, F.; Cremona, A.M.; Marchesi, C.; Guasti, L.; Maresca, A.M.; Grossi, P. Dyslipidemia in HIV-positive patients: a randomized, controlled, prospective study on ezetimibe+fenofibrate versus pravastatin monotherapy. J Int AIDS Soc 2014, 17, 19004. [Google Scholar] [CrossRef] [PubMed]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N Engl J Med 2022, 387, 1923–1934. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Effects of Icosapent Ethyl on Total Ischemic Events: From REDUCE-IT. J Am Coll Cardiol 2019, 73, 2791–2802. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; MacFadyen, J.; Glynn, R.J.; Jiao, L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Tardif, J.C.; et al. Effects of Randomized Treatment With Icosapent Ethyl and a Mineral Oil Comparator on Interleukin-1beta, Interleukin-6, C-Reactive Protein, Oxidized Low-Density Lipoprotein Cholesterol, Homocysteine, Lipoprotein(a), and Lipoprotein-Associated Phospholipase A2: A REDUCE-IT Biomarker Substudy. Circulation 2022, 146, 372–379. [Google Scholar] [CrossRef]
- Peters, B.S.; Wierzbicki, A.S.; Moyle, G.; Nair, D.; Brockmeyer, N. The effect of a 12-week course of omega-3 polyunsaturated fatty acids on lipid parameters in hypertriglyceridemic adult HIV-infected patients undergoing HAART: a randomized, placebo-controlled pilot trial. Clin Ther 2012, 34, 67–76. [Google Scholar] [CrossRef]
- Gerber, J.G.; Kitch, D.W.; Fichtenbaum, C.J.; Zackin, R.A.; Charles, S.; Hogg, E.; Acosta, E.P.; Connick, E.; Wohl, D.; Kojic, E.M.; et al. Fish oil and fenofibrate for the treatment of hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: results of ACTG A5186. J Acquir Immune Defic Syndr 2008, 47, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Strocchi, E.; Veronesi, M.; Borghi, C.; Cicero, A.F.G. Effect of Omega-3 Polyunsaturated Fatty Acids Treatment on Lipid Pattern of HIV Patients: A Meta-Analysis of Randomized Clinical Trials. Mar Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
| Drug class | Antiretroviral drug | Total Cholesterol | LDL-C | HDL-C | Triglycerides | |||
|---|---|---|---|---|---|---|---|---|
| Protease Inhibitors (PIs) |
Atazanavir/Ritonavir Darunavir/Ritonavir Indinavir Lopinavir/Ritonavir Nelfinavir |
↔ ↔ ↑ ↑↑ ↑ |
↑ ↑ ↑ ↑ ↑↑ |
↔ ↔ ↑ ↔ ↔ |
↑ ↑ ↑ ↑↑ ↑ |
|||
| Nucleotide reverse transcriptase inhibitors (NRTIs) |
Abacavir Zidovudine Emtricitabine Lamivudine Stravudine Tenofovir alafenamide Tenofovir disoproxil |
↑ ↑ ↔ ↔ ↑ ↔ ↓ |
↑ ↑ ↔ ↔ ↑ ↑ ↔ |
↔ ↔ ↔ ↔ ↓ ↑ ↓ |
↑ ↑ ↔ ↔ ↑ ↑ ↔ |
|||
| Non-nucleotide reverse transcriptase inhibitors (NNRTIs) |
Efavirenz Etravirine Nevirapine Rilpivirine |
↑ ↔ ↑ ↑ |
↑ ↔ ↑ ↑ |
↑ ↔ ↑↑ ↔ |
↑ ↔ ↑ ↔ |
|||
| Integrase strand transfer inhibitors (INSTIs) |
Raltegravir Dolutegravir Bictegravir Cabotegravir |
↔ ↔ ↑ ↓ |
↔ ↔ ↓ ↔ |
↑ ↑ ↑ ↑ |
↓ ↓ ↓ ↓ |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





