1. Introduction
Cotton (
Gossypium L.) is a dicotyledonous, perennial shrub cultivated for its soft fibres that develop around the seeds of the cotton plant [
1]. Grown in over 80 countries and in a range of ecological niches, cotton is the most economically important source of natural fibre worldwide [
2]. The genus
Gossypium contains over 50 species, of which four are cultivated globally as annual crops [
3].
Gossypium hirsutum L., known as upland cotton, is the most widely grown of these species, representing 90% of cotton production globally [
3,
4,
5]. In Australia, upland cotton production comprises an expanding multi-billion-dollar industry that employs over 12,000 people nationwide [
6].
Verticillium dahliae Kleb. is an asexual, soil-borne member of the Ascomycota which acts as a monocyclic phytopathogen to over 400 plant species [
7,
8,
9]. It is speculated that
V. dahliae originated in Europe [
7,
10], and has since spread to major temperate cropping regions worldwide [
11].
V. dahliae is the primary causal agent of Verticillium wilt in many economically important crop species, including upland cotton [
12]. Verticillium wilt is a systemic disease that arises from the colonisation and subsequent occlusion of the host vasculature by the pathogen [
13]. Resulting symptoms include wilting and stunting of the host, dropping of foliage, discolouration of vascular tissue, development of necrotic lesions, and plant death [
14]. In Australia, Verticillium wilt is reported to cause reduction in cotton yields in the range of 10% to 62% under pathogen conducive conditions [
15].
Eradication of
V. dahliae is challenging once it becomes established in the field. The fungus can persist in soils for up to 14 years in the absence of a susceptible host as melanised resting structures called microsclerotia [
16]. The longevity of these microsclerotia makes it difficult to eradicate the fungus through conventional disease management strategies, such as crop rotation or field fallowing [
7]. Microsclerotia remain dormant in soils until they are triggered to germinate by the presence of plant root exudates [
7,
17]. Upon entering the host xylem tissues, hyaline, asexual conidiospores are produced, which disseminate systemically and colonise the above-ground host vasculature [
13]. The accumulation of fungal material and the resulting host defence responses lead to occlusion of the xylem vessels, inducing the Verticillium wilt disease symptoms [
13]. Upon senescence of the plant, the pathogen permeates the surface of the decaying tissues, producing microsclerotia, after which the fungus returns to dormancy in the soil [
18].
Pathogenic strains of
V. dahliae are generally categorised based on pathotype [
19] or race [
20]. In cotton,
V. dahliae pathotypes are described as either defoliating or non-defoliating, depending on the symptoms induced in the host. The defoliating pathotype is generally considered more severe, whilst non-defoliating isolates may vary in disease aggression [
21,
22,
23]. Populations within
V. dahliae can be further classified on a sub-species level based on vegetative compatibility. This refers to the ability of isolates within a fungal species to exchange genetic information through the successful formation of vegetative heterokaryons [
24]. Due to the clonal nature of the
V. dahliae populations, hyphal anastomosis provides the only mechanism of genetic exchange between strains of
V. dahliae [
25]. Consequently, vegetative compatibility groups (VCGs) within
V. dahliae have classically been considered as genetically isolated populations that may differ from each other in physiology, virulence, environmental response, and host range [
25,
26].
Prior to the 2013/14 cropping season, the only
V. dahliae pathotype reported in Australian cotton fields was the mildly virulent VCG 4B, however, this changed following the confirmation of the non-defoliating VCG 2A pathotype in NSW from isolates collected in the 2009/10 season [
27] and the 2011/2012 [
28]. Chapman et al. (2016) used VCG classification with
nit mutant testing and molecular assays performed on a set of eight historical isolates dating back as far as the 2009/2010 growing seasons in NSW Australia detected the presence of both VCG 4B and 2A as contributors of the non-defoliating pathotypes observed in Australian cotton fields [
27]. In the following season, VCG 1A, a defoliating pathotype, was also detected for the first time. Internationally, VCG 1A has been shown to be a highly virulent pathotype [
15,
27] and has been associated with severe yield losses in cotton, as well as in olive production [
21]. However, typical disease presentation and similar crop losses caused by VCG 1A internationally have not been widely observed in Australia [
7]. On the other hand, VCG 2A has been associated with widespread disease and yield losses in Australian cotton fields, despite there being no reports of VCG 2A causing the same damage overseas [
7,
29]. The reasons behind the disparities occurring between these pathotypes are currently unknown.
It has been suggested that the dominating presence of
V. dahliae VCG 2A observed in Australian cotton fields may be attributed to its ability to colonise and maintain inoculum capacity on weedy hosts common to these regions [
7,
30]. Alternate hosts have the potential to act as intermittent reservoirs for
V. dahliae, increasing inoculum levels in the field [
31]. Pathogenic
V. dahliae VCG 2A strains have been reported infecting economically important weed species from cotton fields overseas [
30]. In Australia, susceptibility to
V. dahliae has been described previously in a range of native and non-native weedy hosts [
32]; although these reports do not characterise the VCGs associated with these infections. The study by Evans in 1971 [
32] also suggests the potential for
V. dahliae to endophytically colonise weed species localised to Australian cotton-growing regions. Endophytic infection of hosts within the cotton field would pose an additional challenge to
V. dahliae management by potentially enhancing carry over of inoculum. It is possible that
V. dahliae VCG 2A has become the dominant strain in Australian cotton fields by endophytically adapting to a broad range of local hosts [
7].
A better understanding of how
V. dahliae moves between geographic areas will guide strategies to limit the spread of VCG 1A and 2A in Australian cotton-growing regions. Established modes of transmission for
V. dahliae include movement of infected plant material or soil, irrigation, and use of contaminated equipment [
8]. In cotton, there is evidence suggesting that seed-borne transmission of the pathogen is also possible. A study on commercially available seed lots reported widespread distribution of
V. dahliae in cotton seed from Turkish fields [
33]. The study identified
V. dahliae isolates belonging to the VCGs 1A, 2A, 2B, and 4B from infected seeds. At present, only the defoliating pathotype of
V. dahliae has been reported to infect seed from Australian cotton varieties at a very low incidence. The pathogen was recovered from 0.025% of seed collected from diseased plants [
34]. The potential for the emerging Australian VCGs to be transmitted by Australian cotton seed therefore remains an ongoing concern for the industry.
The implementation of fluorescent reporter technologies can provide novel insights into the complex interactions between
V. dahliae and its hosts. Integration of fluorescent proteins into plant pathogens has facilitated understanding of infection and colonisation patterns in studies on other filamentous fungi [
35,
36,
37]. The Green Fluorescent Protein (GFP), isolated from
Aequorea victoria [
38], and the mCherry fluorescent protein, optimised from
Dicosoma sp. [
39], are two widely used fluorescent proteins suitable for expressing in
V. dahliae [
40,
41].
V. dahliae isolates tagged with GFP or mCherry have been used to understand pathogen intercellular dissemination in cotton [
40], antifungal activities of compounds [
42], infection of seed in sunflower [
43], infection responses in lettuce resistant or susceptible to Verticillium wilt [
44], and pathogenic processes in the model species
Nicotiana benthamiana [
41].
The aim of this study was to use fluorescent proteins to observe infection by the emerging V. dahliae VCGs 1A and 2A in planta, and to investigate what has allowed these strains to persist in Australian cotton-growing regions. For this purpose, VCG 1A and VCG 2A isolates were stably transformed with mCherry or GFP, respectively. Furthermore, the potential for these strains to colonise weed species common to regions of cotton production in Australia were investigated. The results from this study contribute to the understanding of the epidemiology of Verticillium wilt in cotton and shed light on how this economically significant pathogen can survive on common weeds as alternative hosts, allowing disease control and management to be improved.
4. Discussion
Verticillium wilt is a major disease for the Australian cotton industry. In Australia, it is generally considered that the non-defoliating pathotypes of VCG 4B and the more recently detected VCG 2A are the prevalent disease-causing strains on cotton fields in Australia [
7,
27]. Recent field incidences of Verticillium wilt within the last decade have been low but were observed to be rising steadily in successive seasons [
28]. This increased occurrence and the detection of VCG 1A pathotype from the NSW DPI culture collection [
28] has raised some concerns about the cause of increased disease severity in the field. It is known that VCG 2A can infect weeds commonly found in cotton fields [
30]. Whether its adaptation to survive on other plant species is what makes it the dominant pathotype in Australia is not clearly understood. However, the defoliating VCG 1A is not so widespread in Australia as it has been overseas in causing crop losses and complete defoliation of infected cotton plants [
7]. This study addresses the pathogenicity of these two VCGs and paves the way for the evolution of these populations to be dissected and to aid the practical management of this disease in the Australian cotton industry.
Here we have used transformation of V. dahliae strains with gene cassettes to encode the expression of fluorescent proteins. This has allowed not only the pathogen to be tracked within the plant but also facilitated recovery of the pathogens from various hosts, including those that are non-symptomatic. The GFP-expressing VCG 2A and mCherry-expressing VCG 1A inoculated plants showed similar levels of disease severity when compared to their respective wildtype parents, indicating that the transformation did not alter the virulence of these strains on cotton.
The localisation of the GFP-expressing VCG 2A was visualised in Siokra and Sicot cotton plants during a period of 7 days post inoculation to study the early infection process. Conidia were observed on the root tips of both cultivars at 4 hpi. At 24 hpi, germ tubes were visible on approximately 50% of conidia observed on both cultivars. This is comparable to the germination timing of a GFP-expressing
V. dahliae on lettuce, first observed at 12 to 48 h following inoculation [
44]. Germination of conidia as early as 2 hpi on cotton has been reported [
61]. A narrow infection peg was observed on the surface of the root tip at 24 dpi, with hyphal swelling appearing to narrow after penetration. While infection structures of
V. dahliae in the form of appressoria were observed in penetrating the root surface of lettuce and fiber flax [
44,
62], it has not been observed in other plant species such as
Nicotiana benthamiana [
41].
V. dahliae only displayed slight hyphal swelling without a penetration peg observed before infection in oilseed rape and sunflower [
43,
63]. However, a cotton derived
V. dahliae isolate showed slight hyphal swelling, followed by a narrow penetration peg on Arabidopsis roots [
64]. This appears to be required for the isolate to breach the cell wall of cotton root epidermis during the initial colonisation [
65]. This is consistent with our observations in this study.
The root tip was colonised by the fungus and its intercellular movement through the vascular tissues was evident at 5 dpi. This confirmed that the mechanism of
Verticillium spp. infection is through establishing successful colonisation of the vascular tissues, particularly the xylem elements [
61,
66]. This also confirmed that root tips are sites of penetration for
V. dahliae on cotton hosts [
61,
67].
At 7 dpi, advanced mycelia and mycelial networks became apparent.
V. dahliae mycelia was mostly confined to the individual xylem vessels of the vasculature, with longitudinal movement in the xylem and the perforating tracheary elements. Colonisation of lateral root junctions was observed, as reported in a previous study [
61]. Conidia and mycelia were detected in the stem and petiole of Siokra but not in Sicot. Above-ground colonisation by
V. dahliae, specifically in the petiole base has been previously reported [
61], although it was detected at 30 days post inoculation, using a virulent non-defoliating GFP-expressing isolate of
V. dahliae. At 7 dpi, an intense fluorescence signal in the xylem of Sicot was identified as occlusion due to conidia within a xylem vessel. Vascular occlusions by fungal pathogens are often associated with the formation of plant structures such as tyloses to inhibit the movement of the fungus inside the host. Such occlusions were typically observed as densely clustered conidia in the tracheid of oilseed rape [
63]. But sometimes occlusions can lead to the blockage of xylem vessels and instigate the classic wilt symptoms [
67]. Restricting xylem vessels colonised by
V. dahliae in the lateral roots was identified as an important response in wilt resistant lettuce cultivars [
44]. Similarly, the cotton interaction observed here could point toward the restriction of the fungus at the border pit membranes of the xylem [
8].
The typical defoliating symptoms were induced on cotton by the mCherry expressing VCG 1A isolate. The infection process through the vasculature appears similar to the non-defoliating strain. In another study, both defoliating and non-defoliating isolates recovered from the same stem showed comparable levels of virulence when cotton plants were inoculated with either or both isolates in a pot trial [
53]. Although the detection of a defoliating pathotype even at a relatively low frequency compared to the nondefoliating pathotype complicates the landscape for disease management in the Australian cotton fields [
53].
Mycelial networks with mCherry fluorescence were clearly visualised in the xylem vessels of plants inoculated with the defoliating strain. Interestingly, movement of mycelia were observed in the cortex region. However, the pattern of colonisation suggests that it was moving along the surface or in between cell layers of the cortex and endosperm. Unlike other hosts, intracellular colonisation was observed rarely in
V. dahliae localisation studies on cotton and Arabidopsis [
61,
64]. Both intra- and intercellular movement of hyphae through the endoderm were proposed [
68], however the movement from cortical cells into xylem vessels was observed in an intercellular manner [
64].
The emergence and widespread prevalence of the Australian VCG 2A strain has prompted investigation into the capacity for Australian
V. dahliae isolates to colonise common Australian hosts. Prior reports propose that VCG 2A may predominate in Australian cotton fields through its ability to infect and multiply in associated weed species [
7]. The number of weeds colonised and their reisolation frequencies were higher when comparing VCG 2A and VCG 1A from the weeds selected here (
Table 5), suggesting that VCG 2A clonal lineage are potentially better colonisers of weeds than the VCG 1A lineage. However, further analysis with more weed species and replications will need to be performed to confirm these observations. This highlights the need to investigate in much higher detail the local species, along with field cropping history, as potential reservoirs for
V. dahliae VCG 2A in Australian environments.
V. dahliae was isolated from six of the eight species investigated, including the known host,
Nicotiana benthamiana [
41].
N. benthamiana, a native Australian relative of tobacco, has been used as a model species to understand
V. dahliae infection in previous studies [
41,
69,
70]. However, there is limited literature describing
N. benthamiana susceptibilities to different
V. dahliae VCGs. The study at hand re-isolated transformants 81T0069 and 71T0003 from
N. benthamiana plants, suggesting that this species is a host for both
V. dahliae VCGs 1A and 2A. It was also found that the VCG 1A transformant, 81T0069, induced higher severity of disease symptoms and frequency of plant death than VCG 2A transformant, 71T0003. These findings therefore provide a deeper insight into
V. dahliae interactions with the model species
N. benthamiana.
To our knowledge, this is the first report of
Chloris virgata and
Urochloa panicoides as hosts of
V. dahliae. This is also the first description of
V. dahliae infection of
Conyza bariensis and
Echinochloa colona, although closely related members within these genera have been reported as hosts of the pathogen elsewhere [
32,
71]. Reports on the status of
Sonchus oleraceus as a host for
V. dahliae are conflicting [
30,
72,
73,
74,
75]. In the present study,
S. oleraceus was found to be infected with
V. dahliae 71T0003 at low frequencies (8%). This concurs with previous reports of VCG 2A infecting
S. oleraceus internationally [
71], as well as
V. dahliae infecting this species in Australia [
32].
V. dahliae could not be isolated from the native species
Chloris truncata or from the globally distributed species
Avena fatua. Conflictingly,
A. fatua has previously been reported as a host of
V. dahliae at low frequencies in olive orchards in Greece [
73]. Furthermore,
V. dahliae VCG 2A specifically has been associated with
A. fatua in North American regions of potato production and has been observed to produce microsclerotia on host tissue [
71]. However, given the low frequency of
V. dahliae observed in these studies, it is unsurprising that
V. dahliae was not isolated from
A. fatua in the study at hand. Further investigation is needed to understand whether
A. fatua is a susceptible host to
V. dahliae in an Australian context. Indeed, in this study we have only used a single collection of all weed species so have not in any way assessed any standing host variation for interactions with
V. dahliae. Monocotyledonous species are otherwise generally considered to be resistant to Verticillium wilt disease, however they may remain susceptible to endophytic colonisation by the fungus, which is symptomless [
76]. Consistent with the literature, the study at hand found that infection by
V. dahliae was asymptomatic for all weeds belonging to the
Poaceae family. Similar results have been described previously in other grass species [
77]. In
S. oleraceus, a dicotyledonous weed, there was no difference in disease scores between inoculated and uninoculated groups, which also indicates asymptomatic colonisation of this species. This finding was consistent with other studies on
V. dahliae infection of
S. oleraceus in fields overseas [
71].
V. dahliae 71T0003 and 81T0069 were both re-isolated from the dicotyledonous species C. bonariensis. Some of the C. bonariensis seedlings were exhibiting signs of stress throughout the duration of the experiment, which may explain the elevated disease scores of the uninoculated control plants and plants inoculated with 81T0069. However, tissue reisolations detected none or minimal presence of V. dahliae suggesting that V. dahliae was likely not the cause of leaf yellowing observed on these plants. Nevertheless, this warrants further investigation into whether C. bonariensis is susceptible to Verticillium wilt.
It is important to note that whilst this study identifies species that are susceptible to infection by
V. dahliae VCGs 1A and 2A, it does not investigate the capacity of these hosts to increase pathogen inoculum levels in the field.
V. dahliae microsclerotia are the primary fungal propagules that persist in soils and act as carry-over inoculum into subsequent cropping seasons [
78]. Therefore, quantifying microsclerotia production that occurs in plant hosts is important for Verticillium wilt disease prediction and management [
79]. Prior studies have demonstrated the potential for weed species to be infected by
V. dahliae without increasing the microsclerotia inoculum load [
32,
78]. Evans (1971) isolated
V. dahliae from the weed
S. oleraceus in Australian cotton fields, however concluded the species as a non-host to
V. dahliae because infection was confined to the plant roots and did not result in the production of microsclerotia. On the other hand, a study on the monocotyledonous weed
A. fatua found that the species was host to a tomato-pathogenic
V. dahliae strain from North America, and that production of microsclerotia was higher in this species compared to other local weed hosts [
71]. Consequently, further investigations into the capacity of local species to increase inoculum load of
V. dahliae will help to shape future Verticillium wilt management approaches.
Figure 1.
The development and characterisation of a non-defoliating Verticillium dahliae strain of VCG 2A carrying GFP. (A) V. dahliae wild-type strain Vd71171 and the two GFP transformants derived from it, 71T0003 and 71T0006. (B) The green fluorescence of GFP is associated with the mycelia of 71T0003 imaged using BioTek Cytation 1 cell imaging reader. Scale bar = 200 µm. (C) Mean growth rate of the wildtype Vd71171 and the GFP transformants 71T0003 and 71T0006. Colony diameter (mm) is measured after 4 days of growth on potato dextrose plates (PDA). Median value (bar), lower and upper quartiles, minimum and maximum spore counts (whiskers) are shown. (D) Fecundity or spore concentration of Vd71171, 71T0003 and 71T0006 was determined using a hemocytomer in conidia per mL after spores were collected and then resuspended in 15mL of sterile water after 7 days of growth on PDA. (E) Siokra plants inoculated with wildtype Vd71171, 4 weeks post inoculation. Arrows indicate advanced necrosis spreading across the entire plant (left) and sometimes seen as severe wilting amongst healthy plants grown in the same pot (right). (F) Siokra plants inoculated with the GFP transformant 71T0003, 4 weeks post inoculation. Arrows indicate advanced necrosis on symptomatic plants (left) and a top-down view of a plant with necrosis spreading along the leaf margins (right). (G) Disease severity scored on Siokra and Sicot plants at 4 weeks post inoculation with either the wildtype Vd71171 or the GFP transformant 71T0003. Uninoculated plants served as negative controls. Least-squares means were estimated for each comparison using R package ‘emmeans’. Error bars indicate a 95% confidence interval. n = individual plants tested per isolate per cotton variety.
Figure 1.
The development and characterisation of a non-defoliating Verticillium dahliae strain of VCG 2A carrying GFP. (A) V. dahliae wild-type strain Vd71171 and the two GFP transformants derived from it, 71T0003 and 71T0006. (B) The green fluorescence of GFP is associated with the mycelia of 71T0003 imaged using BioTek Cytation 1 cell imaging reader. Scale bar = 200 µm. (C) Mean growth rate of the wildtype Vd71171 and the GFP transformants 71T0003 and 71T0006. Colony diameter (mm) is measured after 4 days of growth on potato dextrose plates (PDA). Median value (bar), lower and upper quartiles, minimum and maximum spore counts (whiskers) are shown. (D) Fecundity or spore concentration of Vd71171, 71T0003 and 71T0006 was determined using a hemocytomer in conidia per mL after spores were collected and then resuspended in 15mL of sterile water after 7 days of growth on PDA. (E) Siokra plants inoculated with wildtype Vd71171, 4 weeks post inoculation. Arrows indicate advanced necrosis spreading across the entire plant (left) and sometimes seen as severe wilting amongst healthy plants grown in the same pot (right). (F) Siokra plants inoculated with the GFP transformant 71T0003, 4 weeks post inoculation. Arrows indicate advanced necrosis on symptomatic plants (left) and a top-down view of a plant with necrosis spreading along the leaf margins (right). (G) Disease severity scored on Siokra and Sicot plants at 4 weeks post inoculation with either the wildtype Vd71171 or the GFP transformant 71T0003. Uninoculated plants served as negative controls. Least-squares means were estimated for each comparison using R package ‘emmeans’. Error bars indicate a 95% confidence interval. n = individual plants tested per isolate per cotton variety.
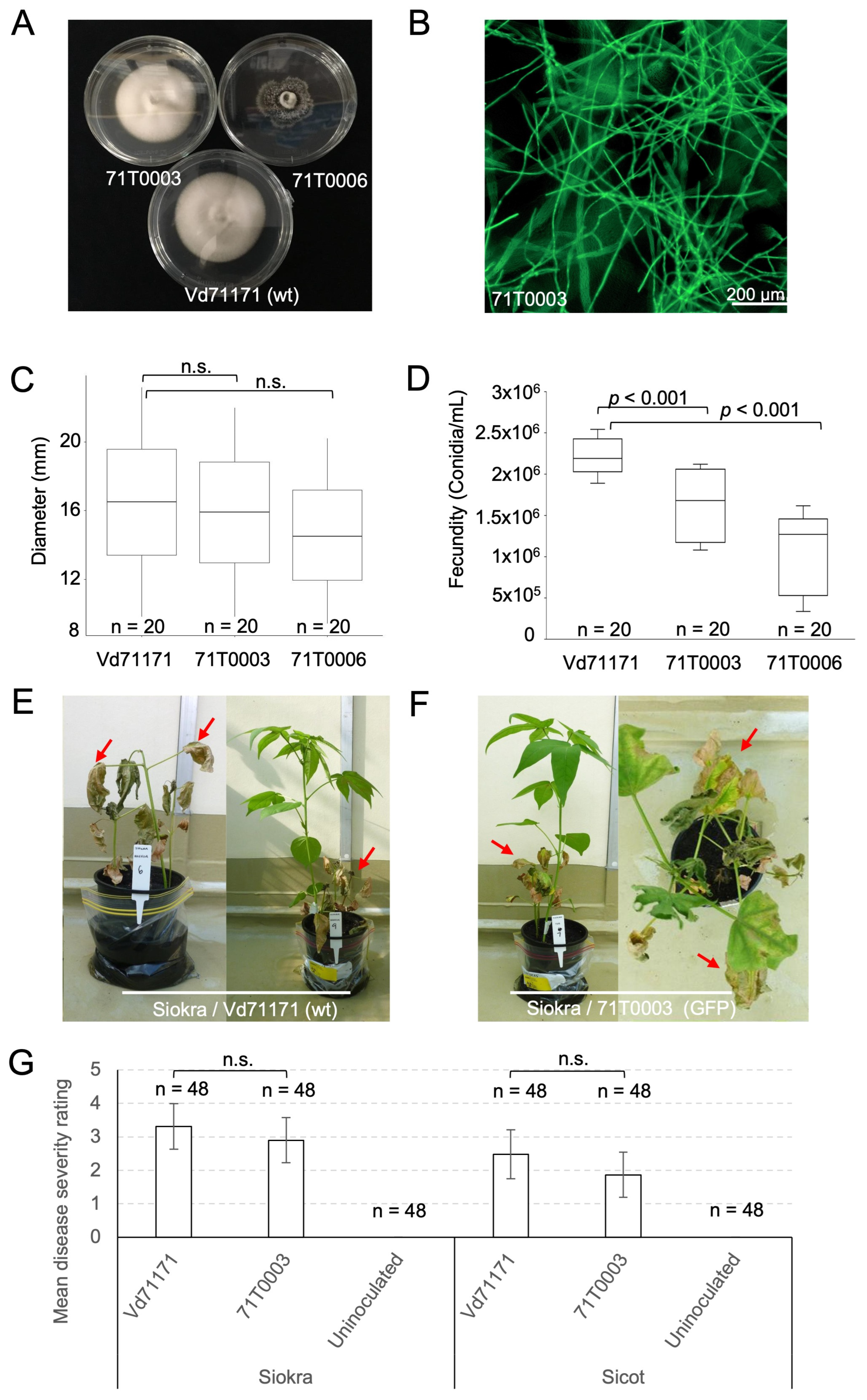
Figure 2.
Confocal laser scanning microscopy performed at 24 hpi, 1 dpi, 5 dpi and 7 dpi on Sicot and Siokra cotton cultivars inoculated with the GFP transformant 71T0003. (A) Conidia with germ tubes and hyphal elongation observed on the root tip of Sicot at 24 hpi. (B) Conidia and an infection peg observed on the root tip of Siokra at 24 hpi. Inset: magnified view of the infection peg under single-channel view. (C) Hyphal elongation and penetration into the root tip epidermis of Siokra at 24 hpi. (D) Mycelia growth on the root tip epidermis of Sicot at 5 dpi. (E) Mycelia visualised in the root cap (left) and at the base of the root tip (right) in Siokra at 5 dpi. (F) Intercellular movement of hyphae through lateral root epidermis on Siokra at 5 dpi. (G) Mycelia visualised in the xylem vessels of the main root in proximity to lateral root junctions in Sicot at 7 dpi. Circled area = free-moving spore observed in the xylem. (H) Mycelia visualised in an entire xylem vessel of the root vasculature in Sicot at 7 dpi. (I) Mycelia and conidia visualised in multiple xylem vessels of the root. Sites of vascular occlusion (o) were observed. (J) Single channel magnified view on the site of vascular occlusion in the xylem vessel densely packaged with conidia. (K) The movement of mycelia into the stem vasculature on Siokra at 7 dpi. (L) Presence of mycelia and free conidia was observed in the petiole of Siokra at 7 dpi. Inset = single channel view of the region containing mycelia and conidia (circled). m = mycelia, c = conidia. Scales are indicated by horizontal bars.
Figure 2.
Confocal laser scanning microscopy performed at 24 hpi, 1 dpi, 5 dpi and 7 dpi on Sicot and Siokra cotton cultivars inoculated with the GFP transformant 71T0003. (A) Conidia with germ tubes and hyphal elongation observed on the root tip of Sicot at 24 hpi. (B) Conidia and an infection peg observed on the root tip of Siokra at 24 hpi. Inset: magnified view of the infection peg under single-channel view. (C) Hyphal elongation and penetration into the root tip epidermis of Siokra at 24 hpi. (D) Mycelia growth on the root tip epidermis of Sicot at 5 dpi. (E) Mycelia visualised in the root cap (left) and at the base of the root tip (right) in Siokra at 5 dpi. (F) Intercellular movement of hyphae through lateral root epidermis on Siokra at 5 dpi. (G) Mycelia visualised in the xylem vessels of the main root in proximity to lateral root junctions in Sicot at 7 dpi. Circled area = free-moving spore observed in the xylem. (H) Mycelia visualised in an entire xylem vessel of the root vasculature in Sicot at 7 dpi. (I) Mycelia and conidia visualised in multiple xylem vessels of the root. Sites of vascular occlusion (o) were observed. (J) Single channel magnified view on the site of vascular occlusion in the xylem vessel densely packaged with conidia. (K) The movement of mycelia into the stem vasculature on Siokra at 7 dpi. (L) Presence of mycelia and free conidia was observed in the petiole of Siokra at 7 dpi. Inset = single channel view of the region containing mycelia and conidia (circled). m = mycelia, c = conidia. Scales are indicated by horizontal bars.
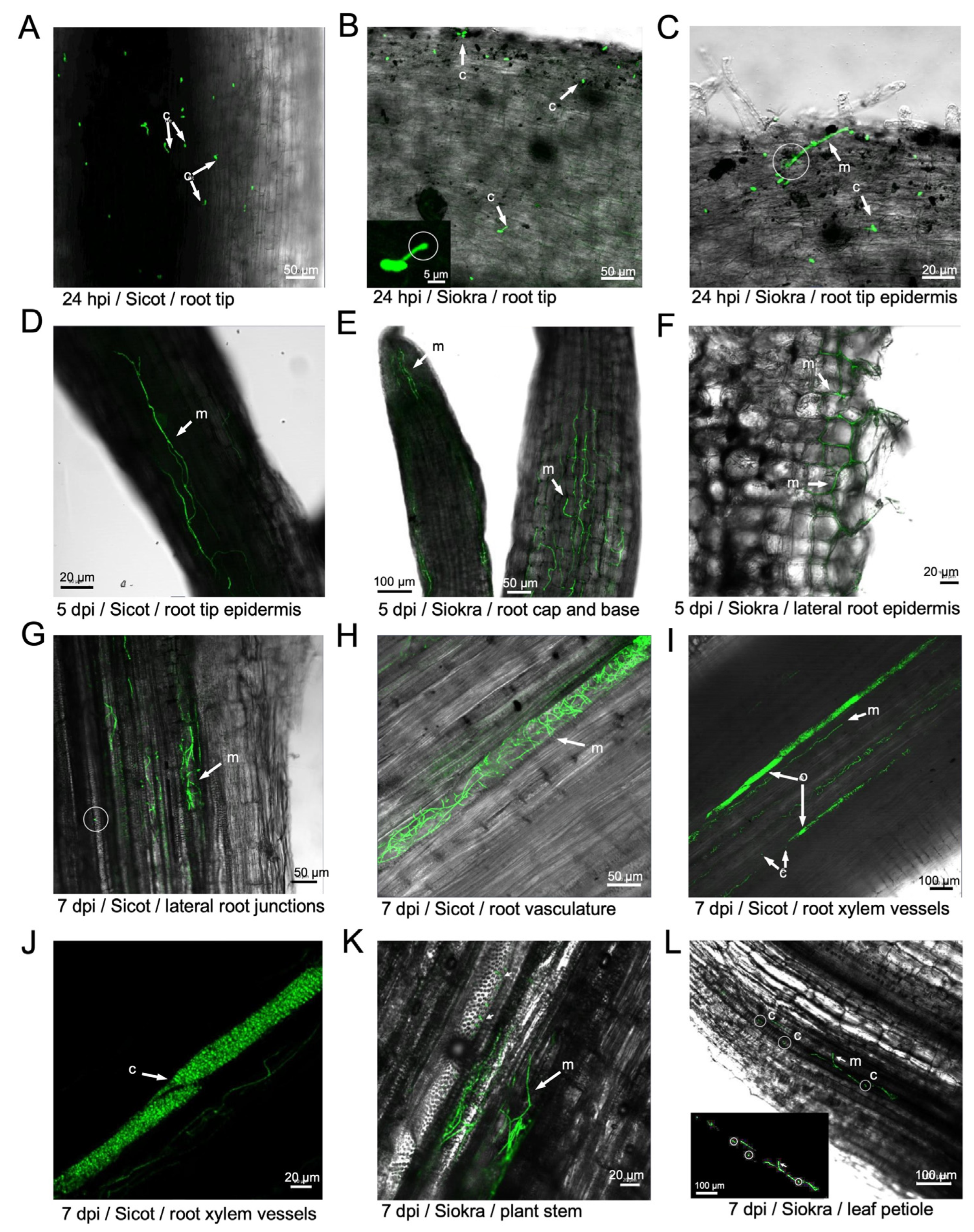
Figure 3.
The development and visualisation of defoliating Verticillium dahliae strains of VCG 1A carrying the mCherry fluorescent protein. (A) Colonies of mCherry transformants compared to the parent Vd71181 after 10 days of growth on half strength PDA. (B) Spores of the parent and the transformants visualised on a Biotek Cytation 1 imager. Scale bar = 200 µm. (C) Fluorescence of spores and hyphae of V. dahliae parent and transformant strains imaged using Biotek Cytation 1 Multi-Reader and Gen5 software. Scale bar = 200 µm. (D) Growth rates (µm2/hr) of the isolates in half-strength PDB media over a 30 h period. No significant differences in growth rates were detected (p = 0.101, one-way ANOVA). (E) mCherry total fluorescence intensity (RF) of the isolates were quantified using Biotek Cytation 1. (F) Fecundity of the isolates measured by spore concentration (spores / mL) after 10 days of growth on half-strength PDA media. Error bars show standard error of the mean (SEM). Statistics performed with a negative binomial GLM. D-E: Medians and IQRs of growth rates are represented by boxes with bars and taken from five replicates (n = 5). Maximum and minimum values are shown as whiskers. E-F: Letters indicate separation of means with significant differences (p < 0.001) detected between groups using One-way ANOVA followed by post-hoc Tukey test.
Figure 3.
The development and visualisation of defoliating Verticillium dahliae strains of VCG 1A carrying the mCherry fluorescent protein. (A) Colonies of mCherry transformants compared to the parent Vd71181 after 10 days of growth on half strength PDA. (B) Spores of the parent and the transformants visualised on a Biotek Cytation 1 imager. Scale bar = 200 µm. (C) Fluorescence of spores and hyphae of V. dahliae parent and transformant strains imaged using Biotek Cytation 1 Multi-Reader and Gen5 software. Scale bar = 200 µm. (D) Growth rates (µm2/hr) of the isolates in half-strength PDB media over a 30 h period. No significant differences in growth rates were detected (p = 0.101, one-way ANOVA). (E) mCherry total fluorescence intensity (RF) of the isolates were quantified using Biotek Cytation 1. (F) Fecundity of the isolates measured by spore concentration (spores / mL) after 10 days of growth on half-strength PDA media. Error bars show standard error of the mean (SEM). Statistics performed with a negative binomial GLM. D-E: Medians and IQRs of growth rates are represented by boxes with bars and taken from five replicates (n = 5). Maximum and minimum values are shown as whiskers. E-F: Letters indicate separation of means with significant differences (p < 0.001) detected between groups using One-way ANOVA followed by post-hoc Tukey test.
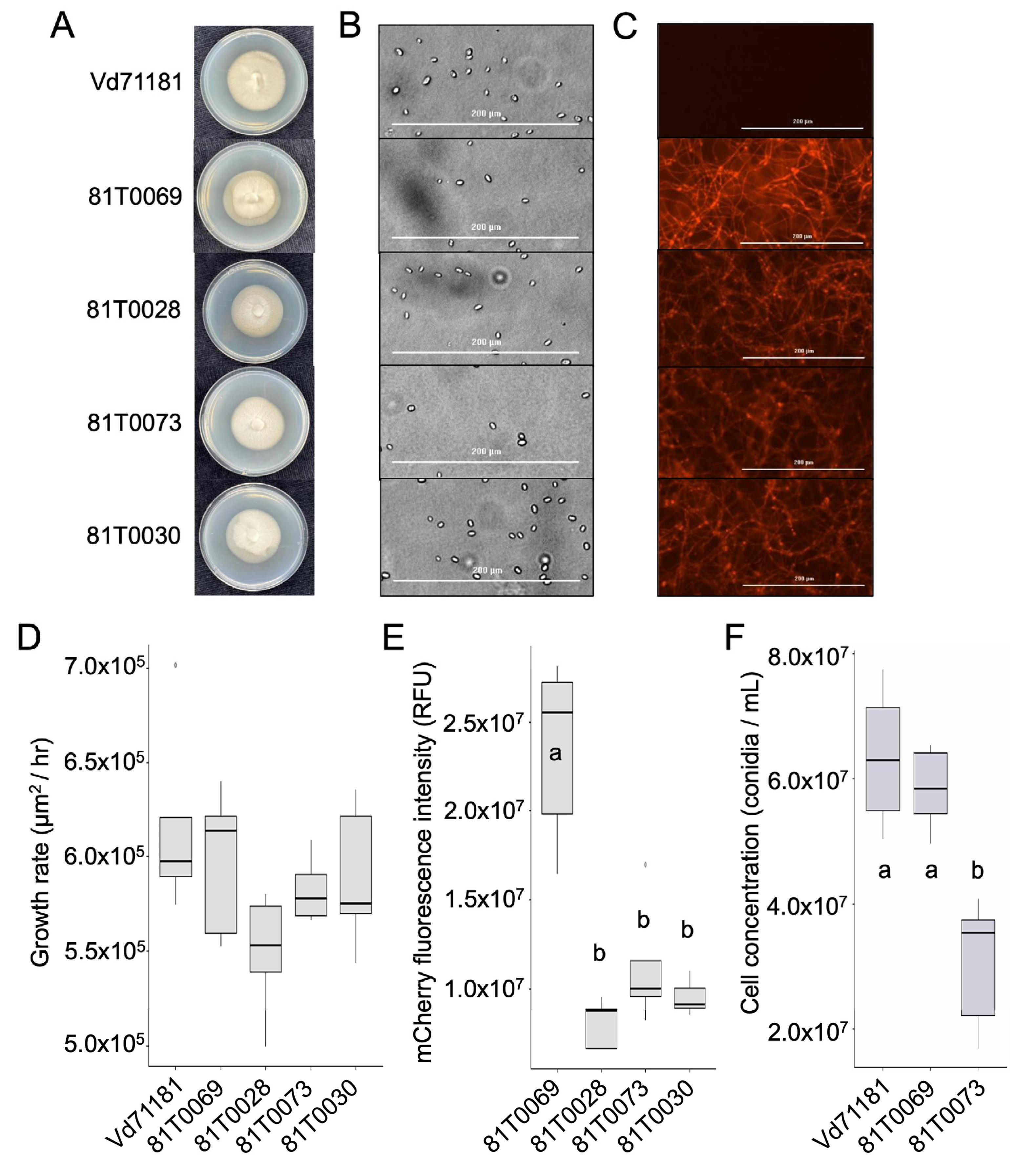
Figure 4.
Representative cotton plants (Sicot) challenged with a defoliating
Verticillium dahliae strain Vd71181 from VCG 1A showing disease symptoms and progression. A scale of 0 to 4 depicts the severity of necrosis, chlorosis, and wilting of leaves, as well as stunting of plant stems when compared to the uninoculated plant. A disease score of 5 indicates a dead plant (not shown). Disease scores are based on a scoring guide adapted from Cirulli et al. (1990) (
Table 3).
Figure 4.
Representative cotton plants (Sicot) challenged with a defoliating
Verticillium dahliae strain Vd71181 from VCG 1A showing disease symptoms and progression. A scale of 0 to 4 depicts the severity of necrosis, chlorosis, and wilting of leaves, as well as stunting of plant stems when compared to the uninoculated plant. A disease score of 5 indicates a dead plant (not shown). Disease scores are based on a scoring guide adapted from Cirulli et al. (1990) (
Table 3).
Figure 5.
Assessing the pathogenicity of mCherry-expressing transformants of Verticillium dahliae 81T0069 and 81T0073 on Sicot to compare with that of the parent strain Vd71181. (A) Assessment of symptoms in Sicot seedlings 4 weeks post inoculation. Plants were rated according to Cirulli et al. (1990) and were scored as the following. Uninoculated plant = 1, parental strain Vd71181 = 3, 81T0069 = 3 and 81T0073 = 4. (B) Transverse (t) and longitudinal (l) stem sections of symptomatic plants showing visible discolouration in the vasculature. Red discolouration is indicative of V. dahliae colonisation in the xylem vessels. (C) V. dahliae colonies recovered from cross-sections of the stem tissues of symptomatic Sicot seedlings after 10 days of incubation on half-strength PDA plates. Individual plates show four samples taken from one symptomatic seedling. (D) Mean disease scores in Sicot plants inoculated with Vd71181 (n = 24), 81T0069 (n = 20) and 81T0073 (n = 21). Sterile distilled water was used as the uninoculated control. Medians and IQRs of the growth rates are represented by bars and boxes. Statistical analysis was performed using the Kruskal-Wallis H test and multiple comparisons made using the post-hoc Dunn test adjusted with Benjamini-Hochberg method. Letters indicate the separation of means between the isolates at p < 0.001.
Figure 5.
Assessing the pathogenicity of mCherry-expressing transformants of Verticillium dahliae 81T0069 and 81T0073 on Sicot to compare with that of the parent strain Vd71181. (A) Assessment of symptoms in Sicot seedlings 4 weeks post inoculation. Plants were rated according to Cirulli et al. (1990) and were scored as the following. Uninoculated plant = 1, parental strain Vd71181 = 3, 81T0069 = 3 and 81T0073 = 4. (B) Transverse (t) and longitudinal (l) stem sections of symptomatic plants showing visible discolouration in the vasculature. Red discolouration is indicative of V. dahliae colonisation in the xylem vessels. (C) V. dahliae colonies recovered from cross-sections of the stem tissues of symptomatic Sicot seedlings after 10 days of incubation on half-strength PDA plates. Individual plates show four samples taken from one symptomatic seedling. (D) Mean disease scores in Sicot plants inoculated with Vd71181 (n = 24), 81T0069 (n = 20) and 81T0073 (n = 21). Sterile distilled water was used as the uninoculated control. Medians and IQRs of the growth rates are represented by bars and boxes. Statistical analysis was performed using the Kruskal-Wallis H test and multiple comparisons made using the post-hoc Dunn test adjusted with Benjamini-Hochberg method. Letters indicate the separation of means between the isolates at p < 0.001.
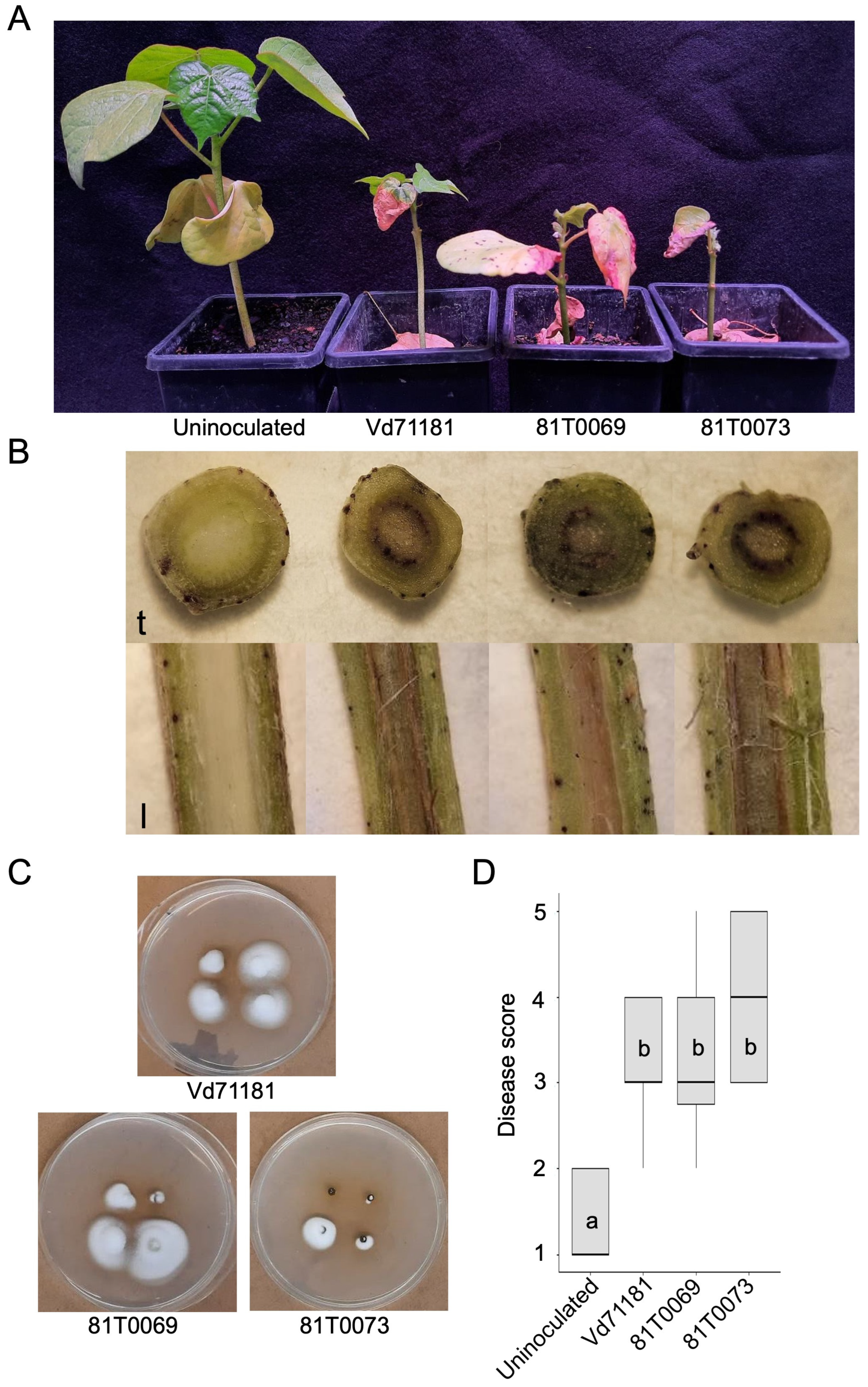
Figure 6.
Localisation of Verticillium dahliae in Sicot cotton seedlings inoculated with the mCherry expressing transformant, 81T0069 at 28 days post inoculation. (A) Confocal microscopy image of longitudinal stem section of Sicot showing the colonisation of host xylem tissues by mCherry expressing mycelia. Viewed at magnification 10x using EC Plan-Neofluar objective. Laser excitation = 555 nm, Master Gain = 809. m = mycelia. c = conidia. mCherry fluorescence is visualised with an overlay of plant tissue in T-PMT transmission illumination mode. (B) mCherry fluorescence visualised in single channel only. (C) T-PMT mode only showing the bright field of plant structure, without the laser scanning mode. (D) magnified view of the cortex region near the xylem and the proliferation of mCherry tagged mycelia in this region. (E) mCherry fluorescence in the cortex region visualised in single channel. (F) T-PMT mode only showing the bright field of cortex region in the stem. Bars indicate the scale used to capture each image.
Figure 6.
Localisation of Verticillium dahliae in Sicot cotton seedlings inoculated with the mCherry expressing transformant, 81T0069 at 28 days post inoculation. (A) Confocal microscopy image of longitudinal stem section of Sicot showing the colonisation of host xylem tissues by mCherry expressing mycelia. Viewed at magnification 10x using EC Plan-Neofluar objective. Laser excitation = 555 nm, Master Gain = 809. m = mycelia. c = conidia. mCherry fluorescence is visualised with an overlay of plant tissue in T-PMT transmission illumination mode. (B) mCherry fluorescence visualised in single channel only. (C) T-PMT mode only showing the bright field of plant structure, without the laser scanning mode. (D) magnified view of the cortex region near the xylem and the proliferation of mCherry tagged mycelia in this region. (E) mCherry fluorescence in the cortex region visualised in single channel. (F) T-PMT mode only showing the bright field of cortex region in the stem. Bars indicate the scale used to capture each image.
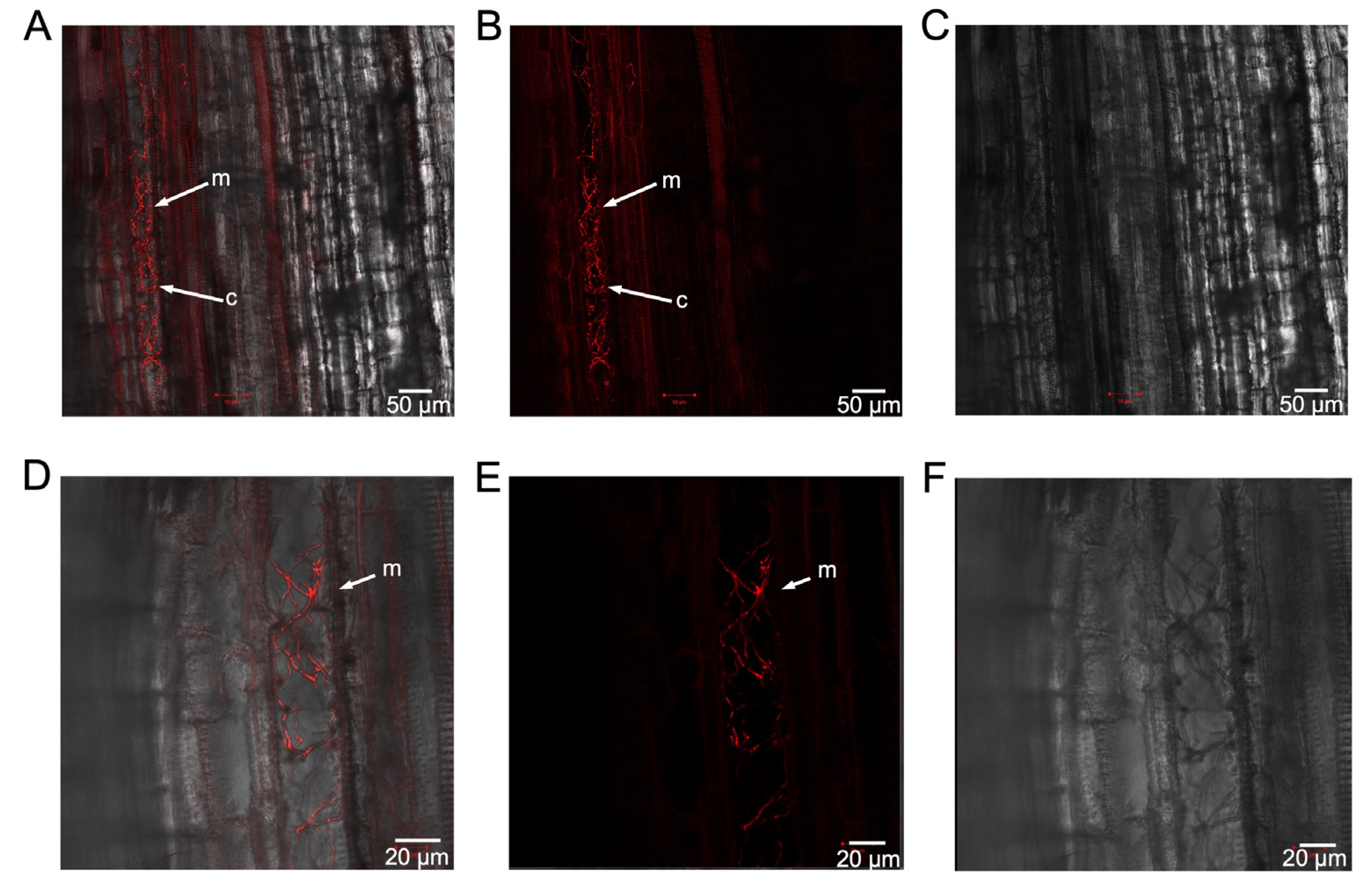
Figure 7.
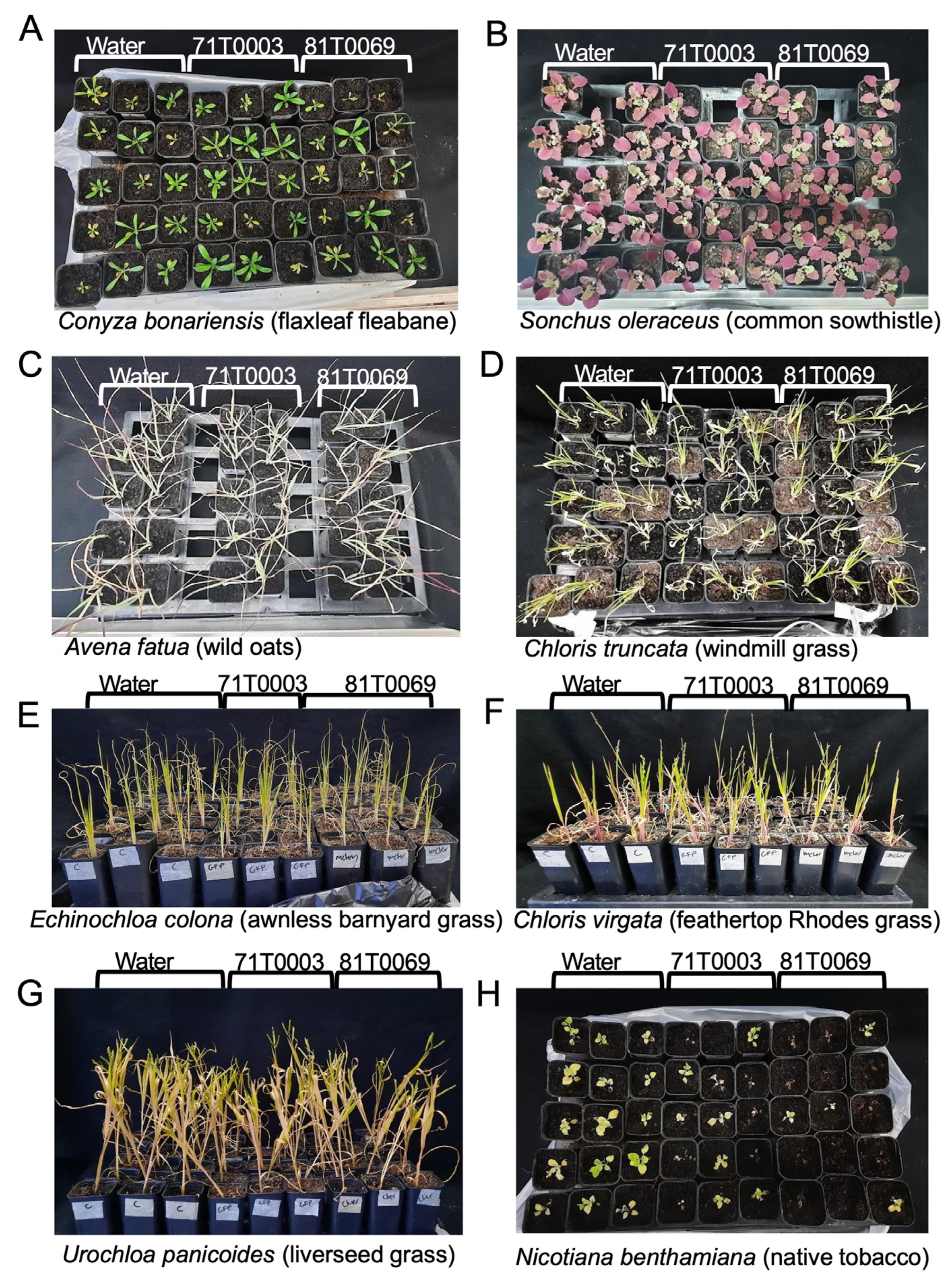
Assessing different weed species and Nicotiana benthamiana for their potentials to house non-defoliating (VCG 1A) and defoliating (VCG 2A) transformant strains as alternative plant hosts. (A) Conyza bonariensis (flaxleaf fleabane) plants. (B) Sonchus oleraceus (common sowthistle) plants. (C) Avena fatua (wild oats) plants. (D) Chloris truncata (windmill grass) plants. (E) Echinochloa colona (awnless barnyard grass). (F) Chloris virgata (feathertop Rhodes grass) plants. (G) Urochloa panicoides (liverseed grass) plants. (H) Nicotiana benthamiana (native tobacco) plants. Plants were root-dipped in either water (uninoculated), GFP-fluorescing transformant 71T0003, or mCherry-expressing transformant 81T0069 and assessed for external symptoms 4 weeks post inoculation. N = 10 to 25 individual plants per treatment group.
Figure 7.
Assessing different weed species and Nicotiana benthamiana for their potentials to house non-defoliating (VCG 1A) and defoliating (VCG 2A) transformant strains as alternative plant hosts. (A) Conyza bonariensis (flaxleaf fleabane) plants. (B) Sonchus oleraceus (common sowthistle) plants. (C) Avena fatua (wild oats) plants. (D) Chloris truncata (windmill grass) plants. (E) Echinochloa colona (awnless barnyard grass). (F) Chloris virgata (feathertop Rhodes grass) plants. (G) Urochloa panicoides (liverseed grass) plants. (H) Nicotiana benthamiana (native tobacco) plants. Plants were root-dipped in either water (uninoculated), GFP-fluorescing transformant 71T0003, or mCherry-expressing transformant 81T0069 and assessed for external symptoms 4 weeks post inoculation. N = 10 to 25 individual plants per treatment group.
Figure 8.
Assessment of symptoms on weed species and Nicotiana benthamiana inoculated with 71T0003 (GFP, VCG 2A), 81T0069 (mCherry, VCG 1A). (A) Comparison of mean disease scores in weed species and N. benthamiana 4 weeks after inoculation. Scoring was performed according to the scale developed by Cirulli et al. (1990). Uninoculated plants were treated with sterile distilled water and served as a negative control. Statistical analyses were performed with a Kruskal-Wallis H test. Multiple comparisons were performed using a post-host Dunn test adjusted with Benjamini-Hochberg method. Letters indicate separation of means amongst treatment groups for Conyza bonariensis and N. benthamiana at p < 0.05. Error bars represent the standard errors of the mean (SEM). (B) Mycelia tagged with GFP fluorescence was observed in a longitudinal section of a leaf petiole from N. benthamiana plants inoculated with 71T0003. (C) Single channel view of GFP fluorescence. (D) Mycelia tagged with mCherry fluorescence was observed in a longitudinal section of roots of N. benthamiana inoculated with 81T0069. (E) Single channel view of mCherry fluorescence. Viewed at magnification 10x using EC Plan-Neofluar objective. Laser excitation = 555 nm (mCherry), 488 nm (GFP).
Figure 8.
Assessment of symptoms on weed species and Nicotiana benthamiana inoculated with 71T0003 (GFP, VCG 2A), 81T0069 (mCherry, VCG 1A). (A) Comparison of mean disease scores in weed species and N. benthamiana 4 weeks after inoculation. Scoring was performed according to the scale developed by Cirulli et al. (1990). Uninoculated plants were treated with sterile distilled water and served as a negative control. Statistical analyses were performed with a Kruskal-Wallis H test. Multiple comparisons were performed using a post-host Dunn test adjusted with Benjamini-Hochberg method. Letters indicate separation of means amongst treatment groups for Conyza bonariensis and N. benthamiana at p < 0.05. Error bars represent the standard errors of the mean (SEM). (B) Mycelia tagged with GFP fluorescence was observed in a longitudinal section of a leaf petiole from N. benthamiana plants inoculated with 71T0003. (C) Single channel view of GFP fluorescence. (D) Mycelia tagged with mCherry fluorescence was observed in a longitudinal section of roots of N. benthamiana inoculated with 81T0069. (E) Single channel view of mCherry fluorescence. Viewed at magnification 10x using EC Plan-Neofluar objective. Laser excitation = 555 nm (mCherry), 488 nm (GFP).
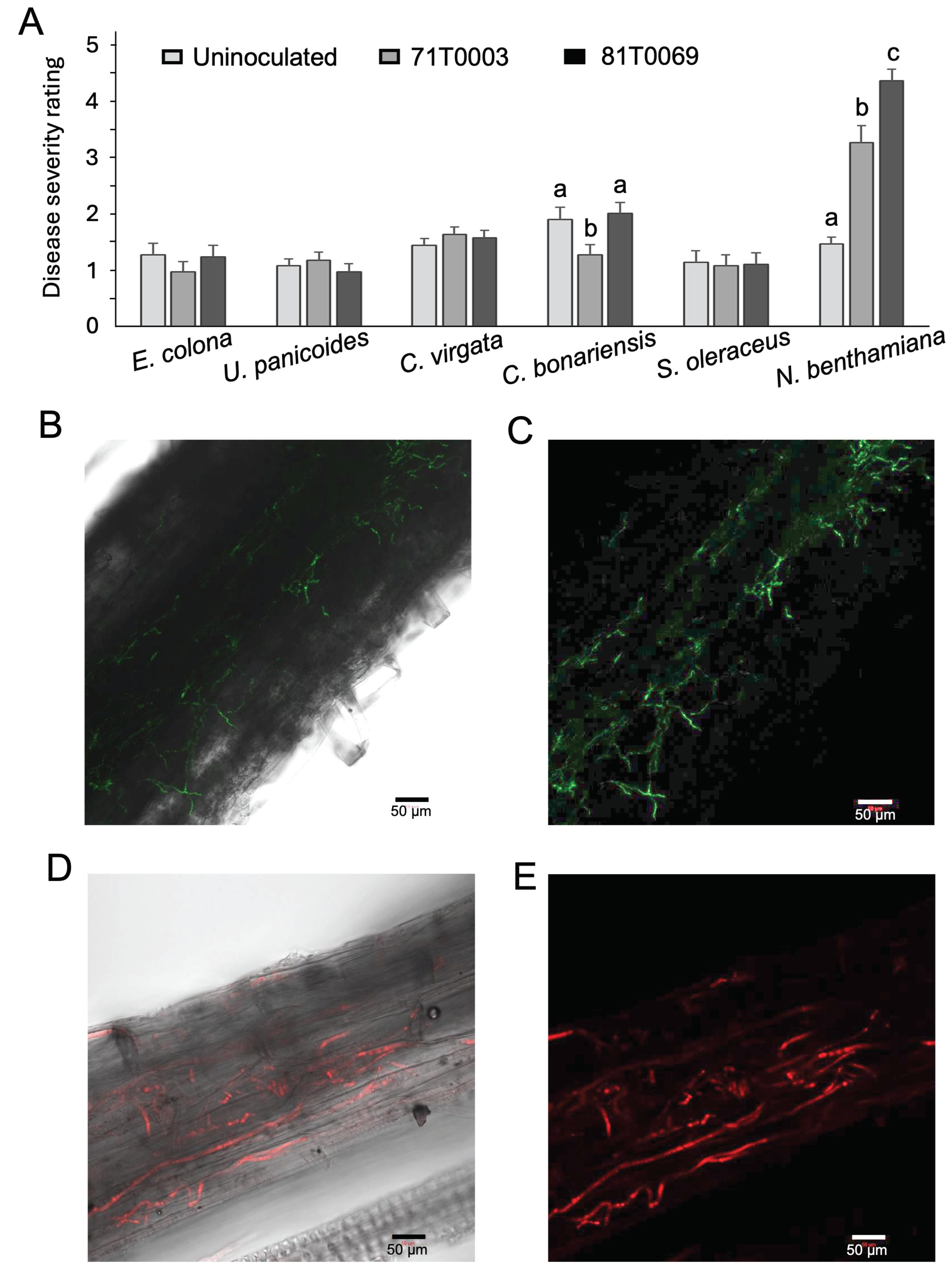
Table 1.
Australian Verticillium dahliae isolates obtained from Gossypium hirsutum (Upland cotton).
Table 1.
Australian Verticillium dahliae isolates obtained from Gossypium hirsutum (Upland cotton).
| Isolate |
Accession |
VCG |
Pathotype |
Locality |
| V. dahliae “Vd71171” |
BRIP71171 |
2A1 |
Non-defoliating |
Namoi Valley, NSW, Australia |
| V. dahliae “Vd71181” |
BRIP71181 |
1A2 |
Defoliating |
Gwydir Valley, NSW, Australia |
Table 2.
Rating scale used to assess disease severity of cotton plants inoculated with a non-defoliating strain of Verticillium dahliae.
Table 2.
Rating scale used to assess disease severity of cotton plants inoculated with a non-defoliating strain of Verticillium dahliae.
| Score |
Description of symptoms |
Score |
| 0 |
Healthy, no symptoms |
0 |
| 1 |
1-20% total leaf area affected |
1 |
| 2 |
21-40% total leaf area affected |
2 |
| 3 |
41-60% total leaf area affected |
3 |
| 4 |
61-80% total leaf area affected |
4 |
| 5 |
81-100% total leaf area affected, and or plant death |
5 |
Table 3.
Rating scale used to assess disease severity of cotton plants, weeds, and tobacco plants inoculated with a non-defoliating strain of
Verticillium dahliae. This scoring system is adapted from a previous study [
52].
Table 3.
Rating scale used to assess disease severity of cotton plants, weeds, and tobacco plants inoculated with a non-defoliating strain of
Verticillium dahliae. This scoring system is adapted from a previous study [
52].
| Symptoms |
Affected leaves (%) |
Degree of stunting compared to control1 |
None or very
slight (<10%) |
Moderate
(11%-50%) |
Severe
(>50%) |
| None. |
0 |
0 |
- |
- |
| Slight leaf chlorosis, flaccidity, necrosis. |
1-9 |
1 |
2 |
3 |
| Moderate leaf chlorosis, flaccidity, necrosis, slight defoliation. |
|
2 |
3 |
4 |
| 10-24 |
|
|
|
| Severe leaf chlorosis, flaccidity, necrosis, moderate defoliation. |
25-50 |
3 |
4 |
4 |
| Plants with severe or complete defoliation. |
>50 |
4 |
4 |
4 |
| Dead plants. |
– |
5 |
5 |
5 |
Table 4.
The percentage of Verticillium dahliae isolates 71T0003and Vd71171 recovered upon termination (4 weeks post inoculation) of the pathogenicity assay.
Table 4.
The percentage of Verticillium dahliae isolates 71T0003and Vd71171 recovered upon termination (4 weeks post inoculation) of the pathogenicity assay.
| |
Siokra |
Siokra |
Sicot |
Sicot |
| Treatment |
Recovery / Total Diseased1 |
Recovery / Total Symptomless2 |
Recovery / Total Diseased1 |
Recovery / Total Symptomless2 |
| Uninoculated |
0 / 0 |
0 / 48 (0 %) |
0 / 0 |
0 / 48 (0 %) |
| Vd711713 |
31 / 33 (94 %) |
1 / 15 (7 %) |
20 / 24 (83 %) |
0 / 24 (0 %) |
| 71T00033 |
24 / 24 (100 %) |
1 / 24 (4 %) |
7 / 18 (39 %) |
1 / 30 (3 %) |
Table 5.
Recovery into culture of Verticillium dahliae transformant isolates 71T0003 and 81T0069 from stem tissue of weed species 4 weeks post-inoculation. Colony identities confirmed on the basis of green or red fluorescence.
Table 5.
Recovery into culture of Verticillium dahliae transformant isolates 71T0003 and 81T0069 from stem tissue of weed species 4 weeks post-inoculation. Colony identities confirmed on the basis of green or red fluorescence.
| Family |
Weed species |
Isolate |
VCG |
Frequency1 |
| Asteraceae |
Conyza bonariensis L. |
71T0003 |
2A |
4/26 (15.4 %) |
| |
|
81T0069 |
1A |
1/26 (3.8 %) |
| |
Sonchus oleraceus L. |
71T0003 |
2A |
2/25 (8 %) |
| |
|
81T0069 |
1A |
0/25 (0 %) |
| Poaceae |
Avena fatua L. |
71T0003 |
2A |
0/10 (0 %) |
| |
|
81T0069 |
1A |
0/10 (0 %) |
| |
Chloris truncata R.Br. |
71T0003 |
2A |
0/26 (0 %) |
| |
|
81T0069 |
1A |
0/26 (0 %) |
| |
Chloris virgata Sw. |
71T0003 |
2A |
3/26 (11.5 %) |
| |
|
81T0069 |
1A |
0/26 (0 %) |
| |
Echinochloa colona L. |
71T0003 |
2A |
2/26 (7.7 %) |
| |
|
81T0069 |
1A |
2/26 (7.7%) |
| |
Urochloa panicoides P.Beauv. |
71T0003
81T0069 |
2A
1A |
6/25 (24 %)
1/25 (4 %) |
| |
|
| Solanaceae |
Nicotiana benthamiana Domin |
71T0003
81T0069 |
2A
1A |
16/26 (61.5 %)
13/26 (50 %) |
| |
|