1. Introduction
The therapeutic use of apoptotic cells is an emerging modality and is currently being assessed in clinical studies [
1,
2,
3]. Apoptotic cells exert immunomodulatory effects [
4] by physically interacting with macrophages and dendritic cells and by secreting several paracrine factors; the clearance process of these cells drives an immune homeostasis state [
5,
6,
7,
8,
9,
10,
11,
12]. Apoptosis, or programmed cell death, is a natural and critical process in tissue homeostasis and results in immediate removal of a dying cell by macrophages and dendritic cells (DCs). DCs are the professional antigen-presenting cells (APC) in mammals; they mature from highly phagocytic precursors in response to ‘danger’ signals that are present in damaged or infected tissue. At the same time, immature DCs are capable of large-scale phagocytosis of apoptotic cells and mature alternatively into a homeostatic state [
13]. In a similar way, macrophages may produce a large variety of inflammatory cytokines in response to danger signals. However, apoptotic cells removal by macrophages downregulated NF-Kb [
14] and inflammatory responses in macrophages [
15,
16]. The combined effect was shown to induce several changes and functional activities in the engulfing APC involving the regulation of immune responses. Clearance of apoptotic cells allows immune homeostasis, which can lead to a non-inflammatory state for both macrophages and DCs and contribute to maintenance of peripheral homeostasis and tolerance in some clinical settings [
12,
17]. Conversely, under certain conditions, such as the killing of the tumor cells by specific cell-death inducers, the recognition of apoptotic tumor cells can promote an immunogenic response and anti-tumor immunity. Complex factors such as cell type, the conditions leading to apoptosis, the apoptotic stage of the cells and others, may lead to various pro-homeostatic effects mediated by macrophages and other APCs. Thus, apoptotic cells may deliver complex information that regulates different immunological responses in a context-dependent manner. The cell type, the cause of cell death, the microenvironment, quantity, and other factors may result in different immunomodulating signals, thus allowing the modulation of immune processes in different clinical settings [
18]. As many as 3x10
8 cells undergo apoptosis every hour in our bodies, and one of the primary “eat me” signals expressed by apoptotic cells is phosphatidylserine (PtdSer). Apoptotic cells themselves are major contributors to the “non-inflammatory” nature of the engulfment process, some by secreting thrombospondin-1 (TSP-1) or adenosine monophosphate and possibly other immune modulating “calm-down” signals that interact with macrophages and DCs. Apoptotic cells also produce “find me” and “tolerate me” signals to attract and immune modulate macrophages and DCs that express specific receptors for some of these signals [
19,
20]. Among emerging cell-based therapies, the use of dying cells defined as apoptotic cells as novel therapeutic strategies appears to hold significant promise. These apoptotic cell-based therapies may be limited not only to transplantation settings, but also to other inflammatory diseases. The immunomodulatory mechanisms associated with physiological apoptotic cell removal (called efferocytosis) were reported in different experimental models of inflammatory diseases or transplantation settings using apoptotic cell infusion [
21,
22,
23,
24,
25,
26]. Apoptotic cells have been suggested as a clinical therapeutic modality in a variety of immune-mediated disorders [
27,
28,
29,
30,
31], including autoimmune and autoinflammatory conditions (e.g.
, arthritis [
32], colitis [
33]), graft rejection [
34], post-ischemic injury and conditions characterized by a cytokine storm such as septic shock [
35] and COVID-19 [
36]. The properties of apoptotic cells may promote an immunogenic response and anti-tumor activity by restoring organ macrophage homeostasis [
37].
Cell-based human products may contain cells that are autologous, or allogeneic, and may or may not include cells that have been genetically modified, or which may or may not be combined with a device, scaffold, or mesh. Cell-based human products are aimed at repairing, restoring, replacing, or regenerating the structure and function of a damaged organ in order to ameliorate or cure previously untreatable injury or disease. Although there has been an increase and rapid progression in recent years in the development of new cellular therapies, more particularly the development of apoptotic cells, for clinical use, very limited data is available regarding their toxicity and tolerability in preclinical studies and few guidelines have been published to assist in the design of appropriate preclinical studies [
38].
Enlivex Therapeutics Ltd. (Ness-Ziona, Israel) is developing Allocetra-OTS, an off-the-shelf macrophage reprogramming cell therapy produced by the induction and stabilization of apoptotic cells derived from peripheral blood mononuclear cells (PBMCs) of healthy donors. The stable apoptotic state of these cells is verified by staining with annexin V and propidium iodide, thus avoiding potential adverse effects from necrotic cells. The robust manufacturing process of Allocetra-OTS enables the production of stable apoptotic cells with a predefined quality attribute profile. A comprehensive nonclinical Good Laboratory Practice (GLP) biodistribution and persistence study demonstrated that Allocetra-OTS levels in the blood, lungs, liver, spleen, and additional organs peaked 10−60 min following the injection, with rapid clearance of Allocetra-OTS cells from most of the organs within 24 h (
Supplementary Figure S1). The Allocetra-OTS cell suspension is transfused intravenously (IV) for systemic indications such as sepsis and COVID-19, intraperitoneally (IP) for the treatment of peritoneal metastasis, and intra-articularly for the treatment of osteoarthritis. It has shown a favorable safety profile in solid tumors (ClinicalTrials.gov identifiers NCT05431907 and NCT05581719, unpublished data). In addition, Allocetra-OTS has shown promising safety and preliminary efficacy in Phase 1 [
3] and phase 2 studies of sepsis (NCT03925857 and NCT04612413, respectively) as well as in COVID-19in humans (NCT04922957) [
2]. In addition, a previously conducted preclinical toxicology study in rats (using the liquid formulation of the product) showed that Allocetra-OTS was well tolerated, and that no serious adverse effects were observed (unpublished data).
To extend the shelf life of Allocetra-OTS, Enlivex Therapeutics Ltd. has developed a new cryopreserved formulation for the product. Here we present a recently conducted toxicology study, aimed at establishing the safety of repeated IV administrations of the newly formulated Allocetra-OTS in Sprague Dawley (SD) rats. Allocetra-OTS was shown to be safe and well tolerated, with a major finding of spleen enlargement. These data, by showing similar safety profiles for both formulations, strengthens the safety profile of Allocetra-OTS. This finding is expected to be of significant importance for future clinical studies performed with similar treatment modalities.
2. Materials and Methods
Investigational Product
Allocetra-OTS (Enlivex Therapeutics, Ness Ziona, Israel) is manufactured from a mononuclear-enriched cell fraction collected from the peripheral blood of healthy unrelated donors via leukapheresis. Cells and plasma are transported to Enlivex’s manufacturing facility under temperature-controlled conditions. Upon arrival, the cells are processed and cryopreserved according to common practice for the cryopreservation of donor lymphocyte infusion (DLI) cells. Following thawing and removal of the freezing medium, the cells are resuspended and incubated in apoptosis induction medium containing methylprednisolone, which is subsequently removed using an automated process of media replacement and volume reduction. The apoptotic cells are suspended in Plasma-Lyte, irradiated (x-ray, 4000 cGy), diluted 1:1 with CryoStor® CS5 to a final targeted concentration in a solution containing 2.5% DMSO, and stored in liquid nitrogen. On each dosing day, vehicle (Allocetra-OTS suspension medium) is thawed in a 37±1°C water bath for up to 5 min, transferred to a sterile bottle under aseptic conditions, and kept at ambient conditions. Allocetra-OTS cells are thawed (37±1°C water bath for approx. 2 min), centrifuged (300g, 10 min at 2−8°C), and resuspended using the vehicle. The final cell concentration is adjusted using the vehicle, according to the required injected dose. Cells re administered not later than 2 h after thawing.
Animal Husbandry and Maintenance
SD rats were chosen as the animal species since they are commonly used in safety studies in accordance with international recommendations and published literature. The SD strain is a well-known laboratory model with adequate historical data. A total of 120 SD rats (60 males (M) and 60 females (F)), aged 8 weeks at study initiation, were obtained from Envigo CRS Ltd. (Ness-Ziona, Israel) and were housed and treated at Pharmaseed Ltd. (Ness-Ziona, Israel). Animals were provided with a commercial rodent diet ad libitum and allowed free access to drinking water. Temperature was maintained at 18−24°C with 30−70% relative humidity and a 12-h light/dark cycle. The study was performed in compliance with the Israel Animal Welfare Act and was approved by the Israel Board for Animal Experiments Ethics Committee (#NPC-Ph-IL-2111-113-5).
Experimental Design
Allocetra-OTS was studied at three doses (140×106 [group 2M/2F], 700×106 [group 3M/3F] and 1260×106 Allocetra-OTS cells/kg [group 4M/4F]) and compared to the vehicle group (in suspension media; group 1M/1F). Allocetra-OTS or vehicle was administered IV (tail vain) via three consecutive administrations (Days 1-3). Animals of the Main study were sacrificed on Day 4, and the animals of the recovery groups (Allocetra-OTS high dose and vehicle) were kept for two or four weeks of recovery after the last dosing day (see
Table 1). Allocetra-OTS or vehicle were administered to 5-10 animals per group at a rate of 2 mL/min, at a dose volume of 10 mL/kg (
Table 1). The study was conducted in compliance with the principles of Good Laboratory Practice (GLP). The details regarding the design of the experiment, the groups allocation across the study phases (main study, 14 days recovery phase and 28 days recovery phase) and the treatment doses are described in
Table 1.
Observations and Examinations
All animals were observed for clinical signs prior to each dose, and for the first 3 h post first dosing, for 1 h post the second and the third dose, twice a week thereafter for the Recovery phases, and before termination. Body weight and food consumption were monitored, along with ophthalmic examinations. Prior to termination, urine was collected from animals of both the Main and Recovery phases, and analyzed for pH, glucose, specific gravity, bilirubin, urine total protein, ketones, urobilinogen, and microscopic sediment. Upon termination, and following at least 2 h of food restriction, blood was drawn from all animals for the following analyses:
Hematology: 300 µL whole blood in K3 EDTA-containing tubes were run on an Advia 2120i blood analyzer (automatic differential count) or 50 µL whole blood using the Sysmex blood analyzer (manual differential count);
Clinical Chemistry: approx. 0.5 mL whole blood per animal was collected into cup tubes with clotting activator gel. The tubes were kept at room temperature for at least 30 min for clotting, and then centrifuged at 4°C for 10 min at 1790g. Separated serum (180 µL) was analyzed with a Cobas 6000 chemistry analyzer.
Coagulation: citrated blood (approx. 450 µL blood + 50 µL sodium citrate or approx. 900 µL blood + 100 µL sodium citrate) per animal was collected into sodium citrate tubes. The samples were centrifuged at4°C for 10 min at 1790g for separation of plasma. Citrated plasma (300 µL) was analyzed for prothrombin time (PT), activated partial thromboplastin time (aPTT) and fibrinogen (FIB) using a Sysmex CS2500 analyzer. Group mean values were compared to the normal references (strain- and sex-related values) provided by the clinical pathology laboratory and analyzed by t-test for statistical differences from the relevant vehicle control group.
Urine Analysis: prior to termination, urine was collected from both the Main and Recovery phases during 14±2 hours using metabolic cages. The urine samples were analyzed for the following parameters: pH, glucose, specific gravity, bilirubin, urine total protein, ketones, urobilinogen – qualitative determination, and microscopic examination of sediment.
Gross Pathology: gross pathology was thoroughly examined, and organs were wet weighed. All groups were subjected to histopathology analysis of the following tissues: adrenals, aorta, brain, cecum, colon, ileum with Peyer’s patches, prostate, seminal vesicles and coagulating glands, duodenum, stomach, epididymides, esophagus, thyroid with parathyroids, trachea, eyes, femoral muscle with sciatic nerve, femur with joint, heart, jejunum, kidneys, liver, lung including main bronchi, skin with mammary glands, mandibular lymph nodes, ovaries and oviducts, pituitary, pancreas, rectum, urinary bladder, salivary glands, spinal cord, spleen, sternum with marrow, testes, thymus, uterus with cervix, vagina, mesentery and tail (injection site).
Histopathological Assessment: Histology slide preparation included tissue decalcification (as applicable), trimming, dehydration and embedding. The resulting blocks were sectioned to a thickness of approx.4 microns, mounted, and stained with hematoxylin and eosin. Histopathology changes were described and scored using a semi-quantitative grading (0 = normal, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe0 [
39]
3. Results
3.1. Morbidity and Mortality, Clinical Signs, and Body Weights
No animal died or was found in morbid condition during the study, and no significant differences in the food consumption were observed between the tested groups. No differences in average body weight were observed between the vehicle (1F) and the Allocetra-OTS-treated groups (2F, 3F and 4F) in female rats of the Main and Recovery phases. However, on days 2 and 3 in male rats in both the Main and the 14-day Recovery phase, a slight (<3%) but statistically significant decrease was observed in the relative average body weight (% of Day 1 weight) of group 4M compared to the vehicle group (1M). This decrease was also observed on day 3 in group 3M of the Main study. Full recovery of body weight was seen in the 28-day Recovery phase.
All animals in both the Main and the Recovery phases of the study demonstrated piloerection, swelling of the feet and face, edema and cyanosis during the first 3 h after initial administration of both vehicle and Allocetra-OTS. These phenomena were also observed after the second and third administrations. Most animals demonstrated full recovery from all symptoms by 24 h post administration. These clinical signs had disappeared by the end of the injection days (on day 4) in all cases, both in the Main and in the Recovery studies. As it was seen in the vehicle as well, it was considered not Allocetra-OTS-related. Furthermore, no Allocetra-OTS-related ophthalmologic symptoms were observed in any of the animals.
3.2. Clinical Pathology
The comprehensive findings from the hematology, clinical chemistry and coagulation analysis are summarized in tables presented in the
Supplementary information (Table S1-S15) as values of averages per group. A distinct table is provided for the treatment groups categorized by gender (male or female groups) and for both the Main study and Recovery phases, for each clinical pathology analysis. For each treatment group, t-test analyses were used to compare the result to the normal range of each parameter, as well as to the vehicle-treated group.
3.2.1. Hematology
White blood cells (WBCs): Among the male rat groups in the Main study, a significant dose-related increase in WBC concentration was observed in all treated groups (2M, p<0.01; 3M and 4M, p<0.001) compared to the vehicle group (1M), although WBC values remained within the normal range for all groups. This increase was not observed in any of the Recovery phases.
Lymphocytes: Among the male rats in the Main study, a significant dose-related increase in the absolute lymphocyte count was observed in all treated groups (2M, p<0.01; 3Mand 4M, p<0.001) compared to the vehicle group (1M). A significant increase (p<0.05) was also observed in the female group (3F) of the Main study compared to the vehicle group (1F). This increase was not observed in any of the Recovery phases. All of the increased values were within their normal ranges.
Monocytes: Among the female rat groups in the Main study, both the absolute count and the average percentage of monocyte values were significantly increased in groups 3F (p<0.05) and 4F (p<0.01) compared to the vehicle group (1F). A significant increase] in the absolute monocyte count was also observed in the male rat groups (3M, p<0.01 and 4M, p<0.001), whereas an increase in monocyte percentage values was observed only in group 4M (p<0.05). Both the absolute and the percentage values of monocytes in all groups remained within normal ranges. No such increases in monocyte values were observed in any of the Recovery phases.
Basophils: In both the male and the female treated groups (2M/2F, 3M/3F and 4M/4F) of the Main study, a significant dose-related increase in both the absolute counts and the percentage values of basophils was observed compared to the vehicle group (1M/1F). Moreover, basophil percentage values were higher than the normal range in both male and female groups of the Main study. This increase was not observed in any of the Recovery phases.
All of the abovementioned changes in hematology were related to Allocetra-OTS and were already resolved by the end of] the 14-day Recovery phase. Most remained within their normal ranges and were therefore not considered to be adverse events (see
Supplementary Information).
3.2.2. Clinical Chemistry
Albumin and globulin: In both the male and the female groups of the Main study, a significant dose-related decrease in albumin concentration, all within the normal range, was observed in all treated groups (2M/2F, 3M/3F and 4M/4F) compared to the vehicle groups (1M/1F). No such decrease was observed in any of the Recovery phases. Among female rats in the Main study, a significant dose-related increase in globulin concentration was observed in all treated groups (2F, 3F and 4F) compared to the vehicle group (1F). All observed changes remained within the normal range. A similar increase, again within the normal range, was also observed in the male rat group 4M of the Main study compared to the vehicle group (1M) and was not observed in any of the Recovery phases. Among female rat groups of the Main study, the dose-related albumin/globulin ratio was significantly decreased in all treated groups (2F, 3F, 4F) compared to the vehicle group (1F). This decrease was also observed in the male rat group (4M) of the Main study compared to the vehicle group (1M), but not in any of the Recovery phases.
LDH: Among the female rat groups within the Main study, LDH concentration was significantly decreased (within normal range) in two groups (2F, p<0.05; 4F, p<0.01) compared to the vehicle group (1F). A similar decrease (within normal range) was also observed in the treated female 14-day Recovery group (4F), but not in the 28-day Recovery phase.
SGOT and SGPT: Among the female rat groups within the Main study, a significant decrease in serum glutamic-oxaloacetic transaminase (SGOT) concentration (though within their normal ranges) was observed in groups 2F and 3F compared to the vehicle group (1F). This decrease was not observed in any of the Recovery phases. In group 3F of the Main study, serum glutamate pyruvate transaminase (SGPT) concentration was also significantly decreased (also within its normal range) compared to the vehicle group (1F), but no such decrease was seen in any of the Recovery phases. In addition, in the Recovery phase the SGPT values among treated female rats (4F) were higher than their normal range but did not differ statistically from values in the vehicle group.
Calcium, phosphorus and potassium: In group 3M of the Main study, a slight but significant increase (within its normal range) in calcium concentration was observed compared to the vehicle group (1M). This increase was not observed in any of the Recovery phases. In group 4M of the Main study, a significant increase (within normal range) in phosphorus concentration was observed compared to the vehicle group (1M). No such increase was observed in any of the Recovery phases. In group 3M of the Main study potassium concentration was significantly decreased (within normal range) in comparison to the vehicle group (1M). A similar decrease was observed in treated males (4) in the 14-day Recovery group (4M).
Creatinine: In the female vehicle group of the Main study, creatinine values were slightly lower than their normal range. In group 3F of the Main study, creatinine concentration was slightly (within normal range) but significantly increased compared to the vehicle group (1F). This slight increase was not observed in any of the Recovery phases.
Total protein: Among the male rat groups of the Main study, a significant dose-related decrease, within its normal range, in total protein concentration was observed in all treated groups (2M, 3M, 4M) compared to the vehicle group (1M). This decrease was not observed in any of the Recovery phases.
3.2.3. Coagulation
Fibrinogen: Among the male rat groups (3M and 4M) of the Main study, fibrinogen concentration was significantly increased compared to the vehicle group (1M), with full recovery attained by day 14.
aPTT: In the male rat high-dose treatment group (4M), both in the Main study and in the 28-day Recovery phase, a slight (within normal range) but significant increase in aPTT was observed in comparison to the vehicle group (1M).
In general, some significant differences in clinical chemistry parameters were observed in both male and female rat groups of the Main study. No such differences were observed in either the 14-day or the 28-day Recovery phases, except for the decrease in LDH concentration observed in the 14-day (but not in the 28-day) Recovery phase in female rats, and the decrease in potassium concentration that was also observed in the males of the 14-day Recovery phase, but not in the 28-day Recovery phase. In general, male rats seemed to be more susceptible to the transient recorded effects than females.
3.3. Urinalysis
There was no difference in urine parameters between the vehicle and the drug treated groups in the main study and the recovery phases.
3.4. Organ Weight
A significant dose-related increase (p<0.001) in absolute and relative spleen weights was observed in all treated groups of the Main study compared to the vehicle groups. A significant increase in absolute and in relative spleen weight was also observed in male treated rats (4M) of the 28-dayRecovery phase (p<0.05). The relative spleen weights were calculated as a percent (%) of the total body weight of each animal. The average of the relative spleen weight results across all treatment groups during both the main study and the recovery phases are presented in
Table 2.
3.5. Gross Pathology
The most prominent abnormality was the enlarged spleen observed in most of the treated male and female rat groups of the Main study, including those in the vehicle groups. This phenomenon was not observed in the 28-day Recovery phase.
3.6. Histopathological Evaluation
The histopathological individual findings are presented in the
Supplementary tables as follows:
Table S16- S19, group 1M-4M (male) respectively, Main Study.
Table S20- S23, group 1F-4F respectively (female), Main Study.
Table S24 1M & 4M (male), Recovery phase 14 days.
Table S25 1F & 4F (female), Recovery phase 14 days.
Table S26 1M & 4M (male), Recovery phase 28 days.
Table S27 1F & 4F (female), Recovery phase 28 days.
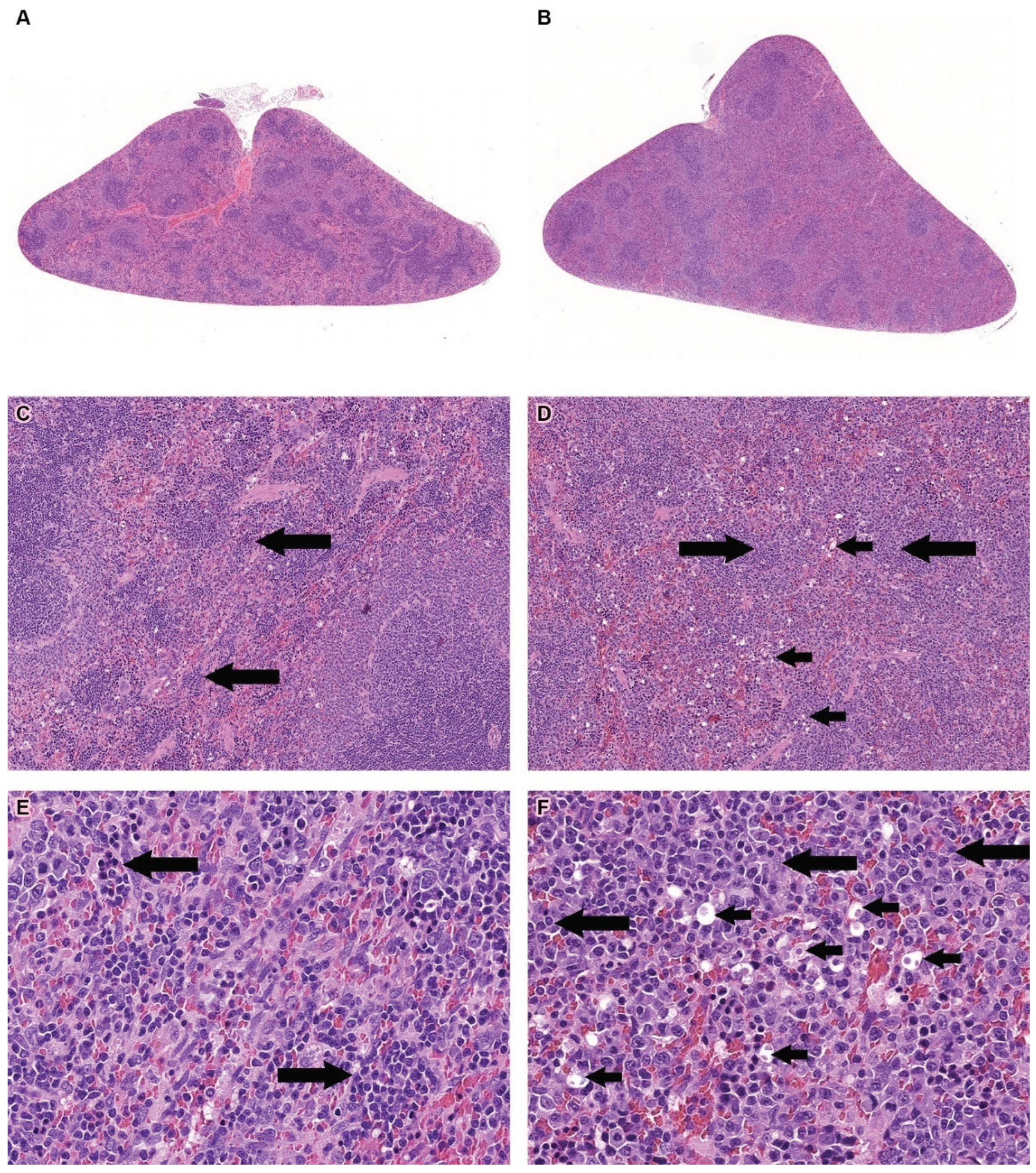
Treatment-related changes were observed only in the red pulp of the spleen. These changes included the presence of round, relatively small hyperchromatic dark-blue nuclear remnants, and/or round empty cavities with or without remaining nuclear and cell eosinophilic ghosts (dead cells) (
Figure 1, A−F, and
Tables S16-S27). These changes were consistent with a diagnosis of apoptotic cells [
40]. The degree of change was mild in groups 3 and 4, and minimal in group 2. In addition, extramedullary hematopoiesis (EMH), manifested as a diffuse increase in the number of cells with a large irregular nucleus, prominent nucleoli, and scant cytoplasm (compared to controls) was observed in the Allocetra-OTS-treated rats. The degree of change was usually moderate in groups 3 and 4 and mild in group 2, and minimal in vehicle groups (
Figure 1, A-F, and
Tables S16-S27). The Extramedullary Hematopoiesis (EMH) in the red pulp of the spleen were observed, with no adverse events, and were considered to be a normal and expected reaction for the removal of degenerated cells, following IV administration of cell-based therapies [
41]. No treatment-related changes were seen in the Recovery phases of the study.
4. Discussion
Here we present the results of a detailed safety evaluation of 3 repeated doses of Allocetra-OTS (140×106, 700×106 and 1260×106cells/kg), administered IV in SD rats and compared to a control vehicle group. The dosages used in this toxicology study were at least 9-fold higher than those of the equivalent human doses administered in a previously conducted Phase 1b clinical study of Allocetra-OTS in sepsis [
3], as well as in an ongoing sepsis Phase 2 study.
All dose levels were well tolerated, and no serious adverse or toxicologically meaningful effects were observed with regard to body weight, clinical signs, food consumption, urinalysis, or ophthalmologic evaluation.
Piloerection, swelling, edema, and cyanosis were observed in all animals during the first 3 h after dosage administration in both vehicle- and Allocetra-OTS-treated groups. These changes were fully resolved in all cases. These changes were considered to be vehicle-related, as also confirmed in a follow-up experiment (data not shown) and are probably caused by the hypersensitivity of rats to dextran, a component of the media formulation. This reaction is well-known in rats and is not expected in humans [
42,
43,
44,
45].
Some significant differences in Hematology analysis parameters were observed in both male and female rat groups of the Main and Recovery phases, while comparing treated and vehicle groups. Since most of these differences were not related to dosage, or disappeared during Recovery phases, they were not considered to be toxicologically adverse events. The high WBC levels observed in male groups in the Main study are an expected phenomenon, since -OTS consists of foreign (human) cells that lead to leukocyte proliferation as part of the physiological immune response [
46]. Significant differences were observed in several of the clinical chemistry parameters in both the male and the female rat groups of the Main study. These differences were not considered clinically relevant, besides decreased in LDH concentration and potassium that were not seen in the 28-dayRecovery phase.
Significant dose-related increases in absolute and relative spleen weights were observed in all Main-treated compared to vehicle-treated groups, including in the 28-day Recovery phase in male rats. These findings correlated with enlarged spleens; the most prominent abnormality observed during evaluation of gross pathology in most of the Allocetra-OTS-treated groups of the Main study. Notably, differences in Recovery phases between the treated and vehicle groups were substantially smaller and were not observed during the gross pathology evaluation after 28 days of recovery. IV-injected cells have a well-known tendency to become trapped in the spleen. This organ serves as a common homing location for immune cells when infused systemically [
47,
48,
49] and was shown to be the major target for accumulation of PBMCs following IV injection in rats [
50]. This phenomenon is also observed in other cell-based treatments such as stem-cell therapies [
51,
52]. It is therefore not surprising to observe the presence of PBMC-derived apoptotic cells in the spleen in this study. Nevertheless, other than the expected splenomegaly, this did not lead to any observed adverse events in the spleen.
This phenomenon was further investigated, moreover, in an in-vivo study in mice, aimed at determining whether the observed splenomegaly is restricted to the xenogeneic source of the apoptotic cells (namely, human cells injected into BALB/c mouse). That study compared the impact of repeated injections of apoptotic cells using xenogeneic, allogeneic (C57BL/6 cells to BALB/c mice, reflecting the clinical administration of allogeneic Allocetra-OTS to human), and syngeneic (BALB/c cells to BALB/c mice) apoptotic cells. The results demonstrated splenomegaly in the human Allocetra-OTS-treated mice, but not in the syngeneic or allogeneic treated mice. Despite several differences in the immune response between Wistar rats and BALB/c mice to foreign antigens (human cells), the central ‘eat me’ signal pathways for apoptotic-cell engulfment from C. elegans to humans are well conserved [
53,
54]. We therefore believe that the findings of that in-vivo mouse study adequately support our splenomegaly finding obtained in this toxicology study.
Since the injection of Allocetra-OTS into humans in a clinical setting is the equivalent of allogeneic apoptotic cell injection into mice, it can be hypothesized that the changes observed in the spleens of Allocetra-OTS treated mice are related to their xenogeneic origin and are not expected in an allogeneic clinical setting.
An additional observed finding is EMH, i.e., the presence of hematopoiesis in locations other than the bone marrow [
55,
56,
57], which has been observed in all animals in thus study and was more pronounced in the Allocetra-OTS-treated rats. EMH is an additional factor contributing to the splenomegaly and the changes seen in the WBC counts observed here in the Allocetra-OTS-treated animals. Commonly observed in rats [
58], EMH is not limited to hematotoxic insults and can also occur in various conditions such as general stress, inflammation and systemic anemia. In all the animals in our study, including those receiving the highest dose of Allocetra-OTS and experiencing the highest degree of EMH and accumulation of apoptotic cells, the findings were completely resolved during the 14-day Recovery phase. Together with the fact that EMH was also observed in the vehicle group, the observed EMH was not regarded as related to Allocetra-OTS treatment. This conclusion is in line with results from a previous GLP toxicology study in which Allocetra-OTS was given at the same doses and the dose regimen applied in the current study, to SD rats with a former (liquid) formulation. In that study, the spleens of animals in both the control and high-dose groups (1260 cells × 106/kg) demonstrated the presence of apoptotic cells and EMH, which were both more] pronounced in the Allocetra-OTS-treated rats (unpublished data). Based on those previous results, it can be concluded that the EMH observed in all animals in the present study indeed reflects the presence of Allocetra-OTS cells in the spleen.
Given that the spleen was the only target organ, and that the changes noted in this organ are considered to reflect the expected pharmacological accumulation of IV-administered Allocetra-OTS cells (associated with reactive EMH, which recovered completely following the 14 days of drug withdrawal), the observed changes are judged to be not adverse according to the criteria of the Society of Toxicologic Pathology [
59,
60,
61,
62,
63,
64].
In general, Allocetra-OTS was well tolerated in our SD rat model, and no serious adverse or toxicologically meaningful effects were observed. It was therefore concluded that Allocetra-OTS is safe, with the highest dose administered determined as No Observed Adverse Effect Level (NOAEL). This safety profile is comparable to a previously conducted GLP toxicology study evaluating the safety of a liquid formulation of Allocetra-OTS in SD rats using identical doses and dose regimen, in which the highest administered dose was also determined as NOAEL (unpublished data). Since this toxicity preclinical examination revealed no signs of abnormal cell proliferation in any organ, and no threshold for adverse effects on the host was detected [
38,
65], we believe the present study confirms the favorable safety profile of Allocetra-OTS as a possible treatment modality for humans in clinical trials settings. It also provides critical information on the expected changes in animal models when injected IV with PBMC-derived apoptotic cells, thus facilitating the interpretation of histopathology findings in future studies of such treatments.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, C.A., O.H., and L.H.; Methodology, M.B., H.S, R.L., and E.S.; Formal Analysis, M.B., H.S., and A.N.; Investigation, C.A., D.M., B.R., and V.A.B.; Resources, V.A.B., B.R., and D.M.; Data Curation, M.B., H.S., A.N, B.R., and C.A.; Writing – Original Draft Preparation, C.A., M.B., H.S., and A.N ; Writing – Review & Editing, O.H, L.H., D.M., and E.S.; Supervision, C.A., and O.H.; All authors have read and approved the published version of the manuscript.
Funding
This study was funded by Enlivex Therapeutics Ltd. Ness-Ziona, Israel.
Institutional Review Board Statement
The animal study protocol was approved by the Israel Board for Animal Experiments Ethics Committee (#NPC-Ph-IL-2111-113-5, date of approval 22, December 2021).
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author’s contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
C.A., O.H., B.R., V.A-B and D.M. are employees of Enlivex Therapeutics Ltd. The study described in this manuscript received funding support from Enlivex Therapeutics Ltd. Although representatives of Enlivex Therapeutics Ltd. participated in the principal design of the study, writing and approving the decision to publish the results, they had no involvement in the study execution, data collection, analyses, or interpretation of the findings. Also in this study, L.H. served as toxicology and preclinical safety consultant for Enlivex, she had no involvement in the study execution, data collection, analyses, or interpretation of the findings or approving the decision to publish the results. M.B, R.L, H.S, E.S. and A.N. served as service providers, they were involved in planning the study, gathering and analyzing data, and writing the manuscript. However, none of the above owns shares or has any financial stake in Enlivex Therapeutics Ltd.
References
- Mevorach D, Zuckerman T, Reiner I, et al. Single Infusion of Donor Mononuclear Early Apoptotic Cells as Prophylaxis for Graft-versus-Host Disease in Myeloablative HLA-Matched Allogeneic Bone Marrow Transplantation: A Phase I/IIa Clinical Trial. Biology of Blood and Marrow Transplantation. 2014;20:58-65. [CrossRef]
- Mevorach D. Immunotherapy: TREATMENT WITH ALLOCETRA-OTS APOPTOTIC CELLS IN 21 SEVERELY/CRITICALLY ILL PATIENTS WITH COVID-19. Cytotherapy. 2022;24(5):S121-S122. [CrossRef]
- van Heerden PV, Abutbul A, Sviri S, et al. Apoptotic Cells for Therapeutic Use in Cytokine Storm Associated With Sepsis– A Phase Ib Clinical Trial. Front Immunol. 2021;12(718191). [CrossRef]
- Hutchinson, James A. MD, PhD1; Benazzo, Alberto MD, PhD2,3. Extracorporeal Photopheresis Suppresses Transplant Fibrosis by Inducing Decorin Expression in Alveolar Macrophages. Transplantation 107(5):p 1010-1012, May 2023. [CrossRef]
- Beer L, Mildner M, Gyöngyösi M, Ankersmit HJ. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis. 2016;21(12):1336-1353. [CrossRef]
- Bonnefoy F, Gauthier T, Vallion R, et al. Factors produced by macrophages eliminating apoptotic cells demonstrate pro-resolutive properties and terminate ongoing inflammation. Front Immunol. 2018;9(2586). [CrossRef]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: Implications in health and disease. Journal of Cell Biology. 2010;189(7):1059-1070. [CrossRef]
- Krispin A, Bledi Y, Atallah M, et al. Apoptotic cell thrombospondin-1 and heparin-binding domain lead to dendritic-cell phagocytic and tolerizing states. Blood. 2006;108(10):3580-3589. [CrossRef]
- Mevorach D, Zuckerman T, Reiner I, et al. Single Infusion of Donor Mononuclear Early Apoptotic Cells as Prophylaxis for Graft-versus-Host Disease in Myeloablative HLA-Matched Allogeneic Bone Marrow Transplantation: A Phase I/IIa Clinical Trial. Biology of Blood and Marrow Transplantation. 2014;20:58-65. [CrossRef]
- Poon IKH, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat Rev Immunol. 2014;14(3):166-180. [CrossRef]
- Saas P, Daguindau E, Perruche S. Concise Review: Apoptotic Cell-Based Therapies-Rationale, Preclinical Results and Future Clinical Developments. Stem Cells. 2016;34(6):1464-1473. [CrossRef]
- Trahtemberg U, Mevorach D. Apoptotic cells induced signaling for immune homeostasis in macrophages and dendritic cells. Front Immunol. 2017;8(OCT). [CrossRef]
- Verbovetski, Inna, Hila Bychkov, Uriel Trahtemberg, Itzhak Shapira, Mara Hareuveni, Ofira Ben-Tal, Ina Kutikov, Oranit Gill, and Dror Mevorach. 2002. ‘Opsonization of Apoptotic Cells by Autologous IC3b Facilitates Clearance by Immature Dendritic Cells, down-Regulates DR and CD86, and up-Regulates CC Chemokine Receptor 7.’ The Journal of Experimental Medicine 196(12):1553–61. [CrossRef]
- Gordon S, Plüddemann A. Macrophage clearance of apoptotic cells: a critical assessment. Front Immunol. 2018;9:127. [CrossRef]
- Amarilyo, Gil, Inna Verbovetski, Mizhir Atallah, Amir Grau, Giora Wiser, Oranit Gil, Ynon Ben-Neriah, and Dror Mevorach. 2010. ‘IC3b-Opsonized Apoptotic Cells Mediate a Distinct Anti-Inflammatory Response and Transcriptional NF-ΚB-Dependent Blockade’. European Journal of Immunology 40(3):699–709. [CrossRef]
- Grau, Amir, Adi Tabib, Inna Grau, Inna Reiner, and Dror Mevorach. 2015. ‘Apoptotic Cells Induce NF-ΚB and Inflammasome Negative Signaling’. PLoS ONE 10(3):1–17. [CrossRef]
- Das, Amitava et al. “Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation.” Journal of immunology (Baltimore, Md.: 1950) vol. 192,3 (2014): 1120-9. [CrossRef]
- Poon, Ivan K. H., Christopher D. Lucas, Adriano G. Rossi, and Kodi S. Ravichandran. 2014. ‘Apoptotic Cell Clearance: Basic Biology and Therapeutic Potential’. Nature Reviews Immunology 14(3):166–80. [CrossRef]
- Arandjelovic, S., Ravichandran, K. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16, 907–917 (2015). [CrossRef]
- Fadok, V.A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992). [CrossRef]
- Perruche S, Saas P, Chen W. Apoptotic cell-mediated suppression of streptococcal cell wall-induced arthritis is associated with alteration of macrophage function and local regulatory T-cell increase: A potential cell-based therapy? Arthritis Res Ther 2009;11:R104. [CrossRef]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 2002;109:41–50. [CrossRef]
- Kushwah R, Oliver JR, Zhang J et al. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J Immunol 2009;183:7104–7118. [CrossRef]
- Sun E, Gao Y, Chen J et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ 2004;11:1258–1264. [CrossRef]
- Kleinclauss F, Perruche S, Masson E et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ 2006;13:41–52. [CrossRef]
- Lichtenauer M, Mildner M, Baumgartner A et al. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol 2011;106:645–655. [CrossRef]
- Barten MJ, Sax B, Schopka S, Amarelli C, Epailly E, Natali B, Teszák T, Gökler J, Borchert K, Theil J, Ingram A, Zuckermann A. European multicenter study on the real-world use and clinical impact of extracorporeal photopheresis after heart transplantation. J Heart Lung Transplant. 2023 Aug;42(8):1131-1139. Epub 2023 Mar 15. PMID: 37037751. [CrossRef]
- Gregorini M, Del Fante C, Pattonieri EF, Avanzini MA, Grignano MA, Cassaniti I, Baldanti F, Comolli G, Nocco A, Ramondetta M, Viarengo G, Sepe V, Libetta C, Klersy C, Perotti C, Rampino T. Photopheresis Abates the Anti-HLA Antibody Titer and Renal Failure Progression in Chronic Antibody-Mediated Rejection. Biology (Basel). 2021 Jun 18;10(6):547. PMID: 34207225; PMCID: PMC8234140. [CrossRef]
- Delbrück C, Gambichler T, Susok L, Peinemann F. Extracorporeal photopheresis for systemic sclerosis: A meta-analysis of randomized clinical trials. Dermatol Ther. 2022 Jul;35(7):e15530. Epub 2022 Apr 29. PMID: 35445504. [CrossRef]
- Gandelman JS, Song DJ, Chen H, Engelhardt BG, Chen YB, Clark WB, Giver CR, Waller EK, Jung DK, Jagasia M. A Prospective Trial of Extracorporeal Photopheresis for Chronic Graft-versus-Host Disease Reveals Significant Disease Response and No Association with Frequency of Regulatory T Cells. Biol Blood Marrow Transplant. 2018 Dec;24(12):2373-2380. [CrossRef]
- Saas, P., Daguindau, E. and Perruche, S. (2016), Concise Review: Apoptotic Cell-Based Therapies–Rationale, Preclinical Results and Future Clinical Developments. Stem Cells, 34: 1464-1473. [CrossRef]
- Toussirot E, Bonnefoy F, Vauchy C, Perruche S, Saas P. Mini-Review: The Administration of Apoptotic Cells for Treating Rheumatoid Arthritis: Current Knowledge and Clinical Perspectives. Front Immunol. 2021;12. [CrossRef]
- Grau A, Tabib A, Grau I, Reiner I, Mevorach D. Apoptotic cells induce NF-κB and inflammasome negative signaling. PLoS One. 2015;10(3):1-17. [CrossRef]
- Kleinclauss F, Perruche S, Masson E, et al. Intravenous apoptotic spleen cell infusion induces a TGF-β-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13(1):41-52. [CrossRef]
- Karbian N, Abutbul A, el-Amore R, et al. Apoptotic cell therapy for cytokine storm associated with acute severe sepsis. Cell Death Dis. 2020;11(7). [CrossRef]
- Michalski A, Wójcicka P, Bielawska-Drózd A, Bartoszcze M. Review of studies on SARS-CoV-2 infection inhibitors. Annals of Agricultural and Environmental Medicine. 2021;28(4):541-550. [CrossRef]
- Shin SA, Moon SY, Park D, Park JB, Lee CS. Apoptotic cell clearance in the tumor microenvironment: a potential cancer therapeutic target. Arch Pharm Res. 2019;42(8):658-671. [CrossRef]
- Bradley AE, Black L. Evaluation of Stem Cell-Derived Cellular Therapy Products. Toxicol Pathol. 2021 Oct;49(7):1288-1293. Epub 2020 Jan 10. PMID: 31918631. [CrossRef]
- Schafer KA, Eighmy J, Fikes JD, et al. Use of Severity Grades to Characterize Histopathologic Changes. Toxicol Pathol. 2018;46(3):256-265. [CrossRef]
- Elmore SA, Dixon D, Hailey JR, et al. Recommendations from the INHAND Apoptosis/Necrosis Working Group. ToxicolPathol. 2016;44(2):173-188. [CrossRef]
- Suttie AW. Histopathology of the spleen. Toxicol Pathol. 2006;34(5):466-503. [CrossRef] [PubMed]
- Blazsó G, Koltai M, Ottlecz A, Minker E. Dextran anaphylactoid reaction in Sprague-Dawley CFY rats. Acta PhysiolAcad Sci Hung. 1979;54(3):281-286.
- De Brito FB, Hanahoe THP, Shah P, West GB. Delayed Hypersensitivity Reactions in Rats and Their Response to Clinical Dextran. Archs Allergy appl Immun. 1982;69:109-112. [CrossRef]
- Delitheos A, Nanahoe T, West G. A Comparison of the Anaphylactoid Actions of a Synthetic Linear Dextran and a Natural Branched Dextran. Archs Allergy appl Immun. 1976;50:436-445. [CrossRef]
- Edlund T, Lofgren B, Vali L. Toxicity of dextran in rats. Nature. 1952;170:125. [CrossRef]
- Ashton N. Physiology of red and white blood cells. Anaesthesia and Intensive Care Medicine. 2013;14(6):261-266. [CrossRef]
- Patel SS, Thiagarajan R, Willerson JT, Yeh ETH. Inhibition of 4 Integrin and ICAM-1 Markedly Attenuate Macrophage Homing to Atherosclerotic Plaques in ApoE-Deficient Mice. Circulation. 1998;97(1):75-81. [CrossRef]
- Iezzi G, Scheidegger D, Lanzavecchia A. Brief Definitive Report Migration and Function of Antigen-primed Nonpolarized T Lymphocytes In Vivo. J Exp Med. 2001;193(8):987-993. http://www.jem.org/cgi/content/full/193/8/987. [CrossRef]
- Hendrikx P, Martens C, Hagenbeek A, Keij J, Visser J. Homing of fluorescently labeled murine hematopoietic stem cells. Exp Hematol. 1996;24(2):129-140.
- Ankersmit HJ, Hoetzenecker K, Dietl W, et al. Irradiated cultured apoptotic peripheral blood mononuclear cells regenerate infarcted myocardium. Eur J Clin Invest. 2009;39(6):445-456. [CrossRef]
- Kurpisz M, Czepczyński R, Grygielska B, et al. Bone marrow stem cell imaging after intracoronary administration. Int J Cardiol. 2007;121(2):194-195. [CrossRef]
- Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107(16):2134-2139. [CrossRef]
- Elliott MR, Ravichandran KS. The Dynamics of Apoptotic Cell Clearance. Dev Cell. 2016;38(2):147-160. [CrossRef]
- Nagata S. Apoptosis and Clearance of Apoptotic Cells. Annu Rev Immunol. 2018;36:489-517. [CrossRef]
- Kumari D, Nair N, Bedwal RS. Morphological changes in spleen after dietary zinc deficiency and supplementation in Wistar rats. Pharmacological Reports. 2019;71(2):206-217. [CrossRef]
- Kim C. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med. Published online March 2010:13. [CrossRef]
- Zhang HZ, Li Y, Liu X, Chen BR, Yao GH, Peng YN. Extramedullary hematopoiesis: A report of two cases. Exp Ther Med. 2016;12(6):3859-3862. [CrossRef]
- Raval SH, Joshi DV, Patel BJ, Patel JG, Patel P. Extramedullary haematopoiesis in spleen of Wistar rat: A case report. Indian J Vet Pathol. 2014;38(2):131. [CrossRef]
- Palazzi X, Burkhardt JE, Caplain H, et al. Characterizing “adversity” of Pathology Findings in Nonclinical Toxicity Studies: Results from the 4th ESTP International Expert Workshop. ToxicolPathol. 2016;44(6):810-824. [CrossRef]
- Kerlin R, Bolon B, Burkhardt J, et al. Scientific and Regulatory Policy Committee: Recommended (“Best”) Practices for Determining, Communicating, and Using Adverse Effect Data from Nonclinical Studies. ToxicolPathol. 2016;44(2):147-162. [CrossRef]
- Baldrick P, Cosenza ME, Alapatt T, Bolon B, Rhodes M, Waterson I. Toxicology Paradise: Sorting Out Adverse and Non-adverse Findings in Animal Toxicity Studies. Int J Toxicol. 2020;39(5):365-378. [CrossRef]
- Pandiri AR, Kerlin RL, Mann PC, et al. Is It Adverse, Nonadverse, Adaptive, or Artifact? ToxicolPathol. 2017;45(1):238-247. [CrossRef]
- Lewis RW, Billington R, Debryune E, Gamer A, Lang B, Carpanini F. Recognition of Adverse and Nonadverse Effects in Toxicity Studies. Vol 30.; 2002. [CrossRef]
- Engelhardt JA, Dorato MA. Perspective on Adversity in Toxicology Evaluations. ToxicolPathol. 2021;49(2):408-410. [CrossRef]
- Jensen NK, Ingvorsen C, Petersen DR, Pereira MJ, Lu TTH, Alsted TJ, Kirkegaard JS, Keane KA. Characterization of the Nonendocrine Cell Populations in Human Embryonic Stem Cell-Derived (hESC) Islet-Like Clusters Posttransplantation. Toxicol Pathol. 2021 Oct;49(7):1269-1287. Epub 2021 Sep 23. PMID: 34555946. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).