Submitted:
29 February 2024
Posted:
01 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Coagulation Cascade in Non-Alcoholic Fatty Liver Disease
3. Role of Platelet in Masld (NAFLD)
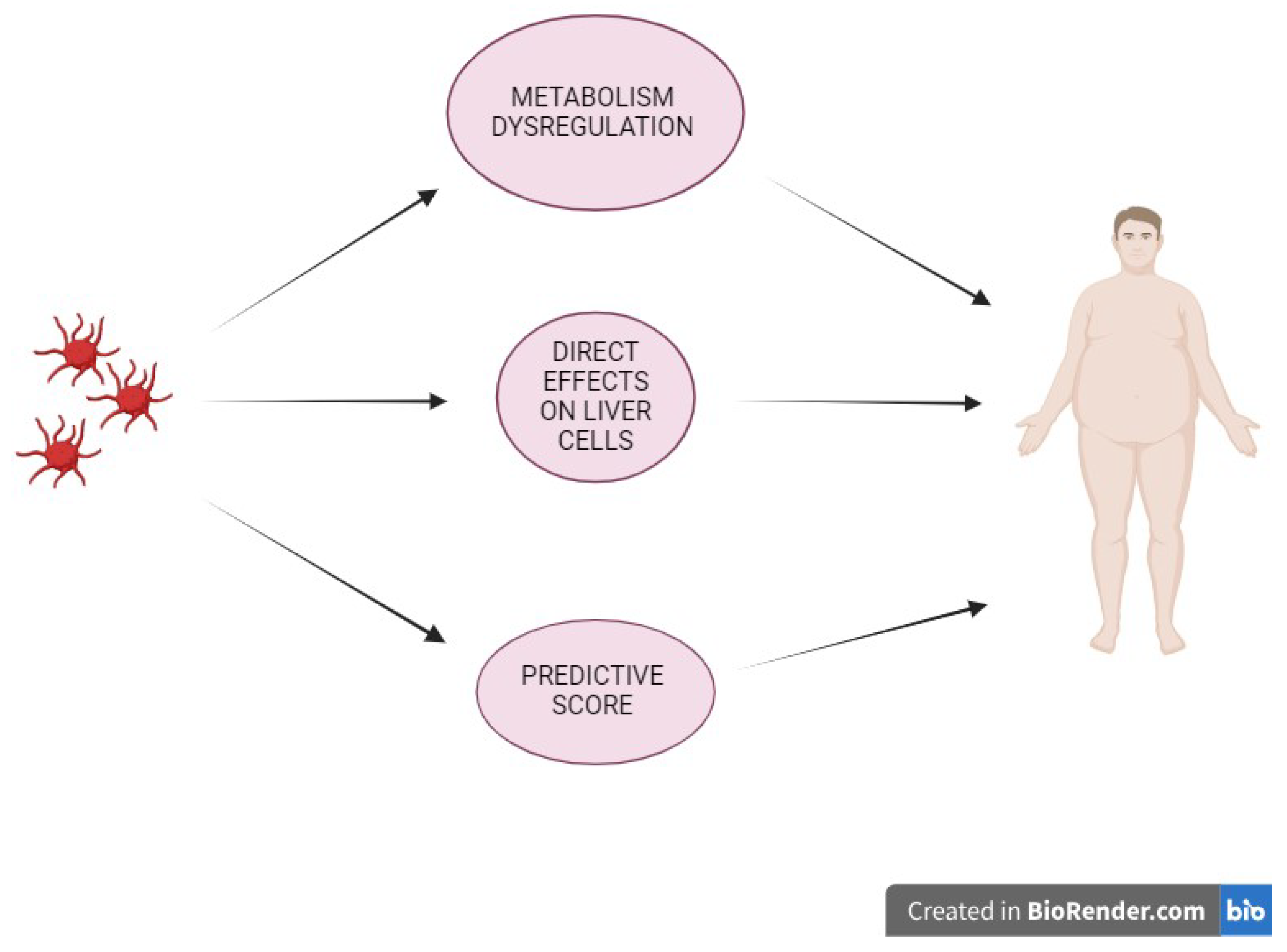
3.1. Platelet, Metabolism Dysregulation & Liver Disease
3.2. Platelet & Liver
4. Platelet and Predictive Score of Fibrosis
5. Antiplatelet Therapy in NAFLD
6. Antiplatelet Therapy and Cancer
Molecular Mechanisms
7. Implications for Antiplatelet Therapy in Primary Prevention
8. Conclusions
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef]
- Mantovani, A.; Csermely, A.; Petracca, G.; Beatrice, G.; Corey, K.E.; Simon, T.G.; et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021, 6, 903–913. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, H.A.; Kim, E.J.; Kim, H.Y.; Kim, H.C.; Ahn, S.H.; et al. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut. 2023. [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; D'Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; et al. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Miele, L.; Zoppini, G.; Pichiri, I.; Muggeo, M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009, 35, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Sundaram, V.; Fallon, M.B.; Reddy, K.R.; Balogun, R.A.; Sanyal, A.J.; et al. Hypercoagulation and thrombophilia in liver disease. J Thromb Haemost. 2008, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Argo, C.K.; Shah, N.; Caldwell, S.H. Hypercoagulation and thrombophilia in nonalcoholic fatty liver disease: mechanisms, human evidence, therapeutic implications, and preventive implications. Semin Liver Dis. 2012, 32, 39–48. [Google Scholar] [CrossRef]
- Wanless, I.R.; Wong, F.; Blendis, L.M.; Greig, P.; Heathcote, E.J.; Levy, G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995, 21, 1238–1247. [Google Scholar]
- Anstee, Q.M.; Wright, M.; Goldin, R.; Thursz, M.R. Parenchymal extinction: coagulation and hepatic fibrogenesis. Clin Liver Dis. 2009, 13, 117–126. [Google Scholar] [CrossRef]
- Lisman, T.; Kamphuisen, P.W.; Northup, P.G.; Porte, R.J. Established and new-generation antithrombotic drugs in patients with cirrhosis - possibilities and caveats. J Hepatol. 2013, 59, 358–366. [Google Scholar] [CrossRef]
- Kotronen, A.; Joutsi-Korhonen, L.; Sevastianova, K.; Bergholm, R.; Hakkarainen, A.; Pietiläinen, K.H.; et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2011, 31, 176–183. [Google Scholar] [CrossRef]
- Verrijken, A.; Francque, S.; Mertens, I.; Prawitt, J.; Caron, S.; Hubens, G.; et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014, 59, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Fracanzani, A.L.; Primignani, M.; Chantarangkul, V.; Clerici, M.; Mannucci, P.M.; et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J Hepatol. 2014, 61, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Lallukka, S.; Luukkonen, P.K.; Zhou, Y.; Isokuortti, E.; Leivonen, M.; Juuti, A.; et al. Obesity/insulin resistance rather than liver fat increases coagulation factor activities and expression in humans. Thromb Haemost. 2017, 117, 286–294. [Google Scholar] [CrossRef]
- Potze, W.; Siddiqui, M.S.; Boyett, S.L.; Adelmeijer, J.; Daita, K.; Sanyal, A.J.; et al. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J Hepatol. 2016, 65, 980–987. [Google Scholar] [CrossRef]
- Assy, N.; Bekirov, I.; Mejritsky, Y.; Solomon, L.; Szvalb, S.; Hussein, O. Association between thrombotic risk factors and extent of fibrosis in patients with non-alcoholic fatty liver diseases. World J Gastroenterol. 2005, 11, 5834–5839. [Google Scholar] [CrossRef] [PubMed]
- Zanetto, A.; Campello, E.; Senzolo, M.; Simioni, P. The evolving knowledge on primary hemostasis in patients with cirrhosis: A comprehensive review. Hepatology. 2023. [CrossRef]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Fricker, Z.P.; Pedley, A.; Massaro, J.M.; Vasan, R.S.; Hoffmann, U.; Benjamin, E.J.; et al. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin Gastroenterol Hepatol. 2019, 17, 1157–1164. [Google Scholar] [CrossRef]
- Santilli, F.; Blardi, P.; Scapellato, C.; Bocchia, M.; Guazzi, G.; Terzuoli, L.; et al. Decreased plasma endogenous soluble RAGE, and enhanced adipokine secretion, oxidative stress and platelet/coagulative activation identify non-alcoholic fatty liver disease among patients with familial combined hyperlipidemia and/or metabolic syndrome. Vascul Pharmacol. 2015, 72, 16–24. [Google Scholar] [CrossRef]
- Santilli, F.; Vazzana, N.; Bucciarelli, L.G.; Davì, G. Soluble forms of RAGE in human diseases: clinical and therapeutical implications. Curr Med Chem. 2009, 16, 940–952. [Google Scholar] [CrossRef]
- Vazzana, N.; Santilli, F.; Cuccurullo, C.; Davì, G. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009, 4, 389–401. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Zoppini, G.; Zenari, L.; Falezza, G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005, 22, 1354–1358. [Google Scholar]
- Chávez-Tapia, N.C.; Rosso, N.; Uribe, M.; Bojalil, R.; Tiribelli, C. Kinetics of the inflammatory response induced by free fatty acid accumulation in hepatocytes. Ann Hepatol. 2013, 13, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.W.; Hsu, Y.C.; Lee, T.F.; Lin, Y.; Chiu, Y.T.; Yang, K.C.; et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Gardemann, A.; Keilhoff, G.; Peter, D.; Wiswedel, I.; Schild, L. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine. 2012, 19, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010, 59, 347–357. [Google Scholar] [CrossRef]
- Beavers, C.J.; Heron, P.; Smyth, S.S.; Bain, J.A.; Macaulay, T.E. Obesity and Antiplatelets-Does One Size Fit All? Thromb Res. 2015, 136, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, L.; Persaud, A.; Feurdean, M.; Ahlawat, S.; Kim, H.S. The Association of leptin with severity of non-alcoholic fatty liver disease: A population-based study. Clin Mol Hepatol. 2018, 24, 392–401. [Google Scholar] [CrossRef]
- Corsonello, A.; Perticone, F.; Malara, A.; De Domenico, D.; Loddo, S.; Buemi, M.; et al. Leptin-dependent platelet aggregation in healthy, overweight and obese subjects. Int J Obes Relat Metab Disord. 2003, 27, 566–573. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Mao, J.; Li, H.; Wang, M.; Zhang, H.; et al. Genistein Ameliorates Non-alcoholic Fatty Liver Disease by Targeting the Thromboxane A(2) Pathway. J Agric Food Chem. 2018, 66, 5853–5859. [Google Scholar] [CrossRef]
- Russo, I.; Traversa, M.; Bonomo, K.; De Salve, A.; Mattiello, L.; Del Mese, P.; et al. In central obesity, weight loss restores platelet sensitivity to nitric oxide and prostacyclin. Obesity (Silver Spring). 2010, 18, 788–797. [Google Scholar] [CrossRef]
- Grande, R.; Dovizio, M.; Marcone, S.; Szklanna, P.B.; Bruno, A.; Ebhardt, H.A.; et al. Platelet-Derived Microparticles From Obese Individuals: Characterization of Number, Size, Proteomics, and Crosstalk With Cancer and Endothelial Cells. Front Pharmacol. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Horigome, H.; Tanaka, K.; Nakata, Y.; Ohkawara, K.; Katayama, Y.; et al. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb Res. 2007, 119, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Goubran, H.A.; Chou, M.L.; Devos, D.; Radosevic, M. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014, 28, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Barry, O.P.; Pratico, D.; Lawson, J.A.; FitzGerald, G.A. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997, 99, 2118–2127. [Google Scholar] [CrossRef]
- Smith, C.W.; Marlin, S.D.; Rothlein, R.; Toman, C.; Anderson, D.C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989, 83, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Forlow, S.B.; McEver, R.P.; Nollert, M.U. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood. 2000, 95, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes. EMBO Mol Med. 2013, 5, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Braig, D.; Nero, T.L.; Koch, H.G.; Kaiser, B.; Wang, X.; Thiele, J.R.; et al. Transitional changes in the CRP structure lead to the exposure of proinflammatory binding sites. Nat Commun. 2017, 8, 14188. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Bonomo, K.; Frascaroli, C.; Morotti, A.; Guerrasio, A.; Cavalot, F.; et al. Platelet function and activation markers in primary hypercholesterolemia treated with anti-PCSK9 monoclonal antibody: A 12-month follow-up. Nutr Metab Cardiovasc Dis. 2020, 30, 282–291. [Google Scholar] [CrossRef]
- Yamazaki, M.; Uchiyama, S.; Xiong, Y.; Nakano, T.; Nakamura, T.; Iwata, M. Effect of remnant-like particle on shear-induced platelet activation and its inhibition by antiplatelet agents. Thromb Res. 2005, 115, 211–218. [Google Scholar] [CrossRef]
- Gerrits, A.J.; Gitz, E.; Koekman, C.A.; Visseren, F.L.; van Haeften, T.W.; Akkerman, J.W. Induction of insulin resistance by the adipokines resistin, leptin, plasminogen activator inhibitor-1 and retinol binding protein 4 in human megakaryocytes. Haematologica. 2012, 97, 1149–1157. [Google Scholar] [CrossRef]
- Simon, T.G.; Henson, J.; Osganian, S.; Masia, R.; Chan, A.T.; Chung, R.T.; et al. Daily Aspirin Use Associated With Reduced Risk For Fibrosis Progression In Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2019, 17, 2776–2784. [Google Scholar] [CrossRef]
- Davì, G.; Catalano, I.; Averna, M.; Notarbartolo, A.; Strano, A.; Ciabattoni, G.; et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990, 322, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Stitham, J.; Gleim, S.; Di Febbo, C.; Porreca, E.; Fava, C.; et al. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J Clin Invest. 2011, 121, 4462–4476. [Google Scholar] [CrossRef] [PubMed]
- Watala, C. Blood platelet reactivity and its pharmacological modulation in (people with) diabetes mellitus. Curr Pharm Des. 2005, 11, 2331–2365. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Massucco, P.; Mattiello, L.; Doronzo, G.; De Salve, A.; et al. Impaired synthesis and action of antiaggregating cyclic nucleotides in platelets from obese subjects: possible role in platelet hyperactivation in obesity. Eur J Clin Invest. 2004, 34, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Marchisio, M.; Lanuti, P.; Boccatonda, A.; Miscia, S.; Davì, G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost. 2016, 116, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Davì, G.; Consoli, A.; Cipollone, F.; Mezzetti, A.; Falco, A.; et al. Thromboxane-dependent CD40 ligand release in type 2 diabetes mellitus. J Am Coll Cardiol. 2006, 47, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Castaño, G.O.; Burgueño, A.L.; Rosselli, M.S.; Gianotti, T.F.; Mallardi, P.; et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis. 2010, 209, 585–591. [Google Scholar] [CrossRef]
- Poggi, M.; Engel, D.; Christ, A.; Beckers, L.; Wijnands, E.; Boon, L.; et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler Thromb Vasc Biol. 2011, 31, 2251–2260. [Google Scholar] [CrossRef]
- Tang, W.H.; Stitham, J.; Jin, Y.; Liu, R.; Lee, S.H.; Du, J.; et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation. 2014, 129, 1598–1609. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Taus, F.; Meneguzzi, A.; Castelli, M.; Minuz, P. Platelet-Derived Extracellular Vesicles as Target of Antiplatelet Agents. What Is the Evidence? Front Pharmacol. 2019, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Bai, J.; Xia, M.; Xue, Y.; Ma, F.; Cui, A.; Sun, Y.; et al. Thrombospondin 1 improves hepatic steatosis in diet-induced insulin-resistant mice and is associated with hepatic fat content in humans. EBioMedicine 2020, 57, 102849. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B., Jr.; Hunninghake, D.B.; et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef]
- Werner, M.; Driftmann, S.; Kleinehr, K.; Kaiser, G.M.; Mathé, Z.; Treckmann, J.W.; et al. All-In-One: Advanced preparation of Human Parenchymal and Non-Parenchymal Liver Cells. PLoS One. 2015, 10, e0138655. [Google Scholar] [CrossRef]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014, 26, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J Clin Invest. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.; Karimian, G.; Adelmeijer, J.; Giepmans, B.N.; Porte, R.J.; Lisman, T. Horizontal RNA transfer mediates platelet-induced hepatocyte proliferation. Blood. 2015, 126, 798–806. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Liang, G.; Peng, J.; Xu, X. Platelet microparticles-derived miR-25-3p promotes the hepatocyte proliferation and cell autophagy via reducing B-cell translocation gene 2. J Cell Biochem. 2020, 121, 4959–4973. [Google Scholar] [CrossRef]
- Kurokawa, T.; Ohkohchi, N. Platelets in liver disease, cancer and regeneration. World J Gastroenterol. 2017, 23, 3228–3239. [Google Scholar] [CrossRef]
- Ikeda, N.; Murata, S.; Maruyama, T.; Tamura, T.; Nozaki, R.; Kawasaki, T.; et al. Platelet-derived adenosine 5'-triphosphate suppresses activation of human hepatic stellate cell: In vitro study. Hepatol Res. 2012, 42, 91–102. [Google Scholar] [CrossRef]
- Salem, N.A.; Hamza, A.; Alnahdi, H.; Ayaz, N. Biochemical and Molecular Mechanisms of Platelet-Rich Plasma in Ameliorating Liver Fibrosis Induced by Dimethylnitrosurea. Cell Physiol Biochem. 2018, 47, 2331–2339. [Google Scholar] [CrossRef]
- Zaldivar, M.M.; Pauels, K.; von Hundelshausen, P.; Berres, M.L.; Schmitz, P.; Bornemann, J.; et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010, 51, 1345–1353. [Google Scholar] [CrossRef]
- Mahmoud, N.I.; Messiha, B.A.S.; Salehc, I.G.; Abo-Saif, A.A.; Abdel-Bakky, M.S. Interruption of platelets and thrombin function as a new approach against liver fibrosis induced experimentally in rats. Life Sci. 2019, 231, 116522. [Google Scholar] [CrossRef] [PubMed]
- Kinnman, N.; Francoz, C.; Barbu, V.; Wendum, D.; Rey, C.; Hultcrantz, R.; et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003, 83, 163–173. [Google Scholar] [CrossRef]
- Joshi, N.; Kopec, A.K.; Ray, J.L.; Cline-Fedewa, H.; Groeneveld, D.J.; Lisman, T.; et al. Von Willebrand factor deficiency reduces liver fibrosis in mice. Toxicol Appl Pharmacol. 2017, 328, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, T.; Maceyka, M.; Spiegel, S. Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Crit Rev Biochem Mol Biol. 2017, 52, 543–553. [Google Scholar] [CrossRef]
- Ghafoory, S.; Varshney, R.; Robison, T.; Kouzbari, K.; Woolington, S.; Murphy, B.; et al. Platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018, 2, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ikenaga, N.; Liu, S.B.; Peng, Z.W.; Chung, J.; Sverdlov, D.Y.; et al. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. 2014, 147, 1378–1392. [Google Scholar] [CrossRef]
- Miele, L.; Alberelli, M.A.; Martini, M.; Liguori, A.; Marrone, G.; Cocomazzi, A.; et al. Nonalcoholic fatty liver disease (NAFLD) severity is associated to a nonhemostatic contribution and proinflammatory phenotype of platelets. Transl Res. 2021, 231, 24–38. [Google Scholar] [CrossRef]
- Chauhan, A.; Adams, D.H.; Watson, S.P.; Lalor, P.F. Platelets: No longer bystanders in liver disease. Hepatology. 2016, 64, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic Alempijevic, T.; Stojkovic Lalosevic, M.; Dumic, I.; Jocic, N.; Pavlovic Markovic, A.; Dragasevic, S.; et al. Diagnostic Accuracy of Platelet Count and Platelet Indices in Noninvasive Assessment of Fibrosis in Nonalcoholic Fatty Liver Disease Patients. Can J Gastroenterol Hepatol. 2017, 2017, 6070135. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhao, C.; Shen, C.; Wang, Y. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS One. 2013, 8, e82092. [Google Scholar] [CrossRef]
- Ozhan, H.; Aydin, M.; Yazici, M.; Yazgan, O.; Basar, C.; Gungor, A.; et al. Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010, 21, 29–32. [Google Scholar] [CrossRef]
- Shin, W.Y.; Jung, D.H.; Shim, J.Y.; Lee, H.R. The association between non-alcoholic hepatic steatosis and mean platelet volume in an obese Korean population. Platelets. 2011, 22, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.A.; John, F.; Pitchumoni, C.S. Nonalcoholic Fatty Liver Disease and Mean Platelet Volume: A Systemic Review and Meta-analysis. J Clin Gastroenterol. 2016, 50, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Potze, W.; Siddiqui, M.S.; Sanyal, A.J. Vascular Disease in Patients with Nonalcoholic Fatty Liver Disease. Semin Thromb Hemost. 2015, 41, 488–493. [Google Scholar] [CrossRef]
- Papanas, N.; Symeonidis, G.; Maltezos, E.; Mavridis, G.; Karavageli, E.; Vosnakidis, T.; et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004, 15, 475–478. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Rasmussen, J.W.; Bekker, C.; Madsen, P.E. Kinetics and in vivo distribution of 111-In-labelled autologous platelets in chronic hepatic disease: mechanisms of thrombocytopenia. Scand J Haematol. 1985, 34, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Fujii, H.; Sumida, Y.; Hyogo, H.; Itoh, Y.; Ono, M.; et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011, 46, 1300–1306. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, Y.S.; Park, H.W.; Seo, S.H.; Jang, B.G.; Hwang, J.Y.; et al. Factors associated or related to with pathological severity of nonalcoholic fatty liver disease. Korean J Intern Med. 2004, 19, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, H.; Hashimoto, E.; Yatsuji, S.; Tokushige, K.; Shiratori, K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2006, 21, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Fierbinteanu-Braticevici, C.; Dina, I.; Petrisor, A.; Tribus, L.; Negreanu, L.; Carstoiu, C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol. 2010, 16, 4784–4791. [Google Scholar] [CrossRef]
- Nakamura, S.; Konishi, H.; Kishino, M.; Yatsuji, S.; Tokushige, K.; Hashimoto, E.; et al. Prevalence of esophagogastric varices in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008, 38, 572–579. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Duran-Bertran, J.; Rusu, E.C.; Barrientos-Riosalido, A.; Bertran, L.; Mahmoudian, R.; Aguilar, C.; et al. Platelet-associated biomarkers in nonalcoholic steatohepatitis: Insights from a female cohort with obesity. Eur J Clin Invest. 2023, 54, e14123. [Google Scholar] [CrossRef]
- Liu, Y.; Nong, L.; Jia, Y.; Tan, A.; Duan, L.; Lu, Y.; et al. Aspirin alleviates hepatic fibrosis by suppressing hepatic stellate cells activation via the TLR4/NF-κB pathway. Aging (Albany NY). 2020, 12, 6058–6066. [Google Scholar] [CrossRef]
- Li, C.J.; Yang, Z.H.; Shi, X.L.; Liu, D.L. Effects of aspirin and enoxaparin in a rat model of liver fibrosis. World J Gastroenterol. 2017, 23, 6412–6419. [Google Scholar] [CrossRef]
- Börgeson, E.; Johnson, A.M.; Lee, Y.S.; Till, A.; Syed, G.H.; Ali-Shah, S.T.; et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 2015, 22, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Paik, Y.H.; Kim, J.K.; Lee, J.I.; Kang, S.H.; Kim, D.Y.; An, S.H.; et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009, 58, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nozaki, Y.; Wada, K.; Yoneda, M.; Endo, H.; Takahashi, H.; et al. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008, 57, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.G.; Feldbrügge, L.; Tapper, E.B.; Popov, Y.; Ghaziani, T.; Afdhal, N.; et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016, 43, 734–743. [Google Scholar] [CrossRef]
- Shen, H.; Shahzad, G.; Jawairia, M.; Bostick, R.M.; Mustacchia, P. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: a cross-sectional study from the Third National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2014, 40, 1066–1073. [Google Scholar] [CrossRef]
- Thongtan, T.; Deb, A.; Vutthikraivit, W.; Laoveeravat, P.; Mingbunjerdsuk, T.; Islam, S.; et al. Antiplatelet therapy associated with lower prevalence of advanced liver fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Indian J Gastroenterol. 2022, 41, 119–126. [Google Scholar] [CrossRef]
- Karahan, O.I.; Kurt, A.; Yikilmaz, A.; Kahriman, G. New method for the detection of intraperitoneal free air by sonography: scissors maneuver. J Clin Ultrasound. 2004, 32, 381–385. [Google Scholar] [CrossRef]
- Murohara, T.; Horowitz, J.R.; Silver, M.; Tsurumi, Y.; Chen, D.; Sullivan, A.; et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998, 97, 99–107. [Google Scholar] [CrossRef]
- Sánchez de Miguel, L.; de Frutos, T.; González-Fernández, F.; del Pozo, V.; Lahoz, C.; Jiménez, A.; et al. Aspirin inhibits inducible nitric oxide synthase expression and tumour necrosis factor-alpha release by cultured smooth muscle cells. Eur J Clin Invest. 1999, 29, 93–99. [Google Scholar] [CrossRef]
- Campbell, J.S.; Hughes, S.D.; Gilbertson, D.G.; Palmer, T.E.; Holdren, M.S.; Haran, A.C.; et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005, 102, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Prattali, R.R.; Barreiro, G.C.; Caliseo, C.T.; Fugiwara, F.Y.; Ueno, M.; Prada, P.O.; et al. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in growth hormone treated animals. FEBS Lett. 2005, 579, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Caputi, A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J Gastroenterol. 2011, 17, 3785–3794. [Google Scholar] [CrossRef]
- Clària, J.; Planagumà, A. Liver: the formation and actions of aspirin-triggered lipoxins. Prostaglandins Leukot Essent Fatty Acids 2005, 73, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Kozakova, M.; Højlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009, 49, 1537–1544. [Google Scholar] [CrossRef]
- Han, Y.M.; Lee, Y.J.; Jang, Y.N.; Kim, H.M.; Seo, H.S.; Jung, T.W.; et al. Aspirin Improves Nonalcoholic Fatty Liver Disease and Atherosclerosis through Regulation of the PPARδ-AMPK-PGC-1α Pathway in Dyslipidemic Conditions. Biomed Res Int. 2020, 2020, 7806860. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; El-Serag, H.B. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015, 13, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018, 155, 1828–1837. [Google Scholar] [CrossRef]
- Mittal, S.; Sada, Y.H.; El-Serag, H.B.; Kanwal, F.; Duan, Z.; Temple, S.; et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015, 13, 594–601. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019, 17, 748–755. [Google Scholar] [CrossRef]
- Haldar, D.; Kern, B.; Hodson, J.; Armstrong, M.J.; Adam, R.; Berlakovich, G.; et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019, 71, 313–322. [Google Scholar] [CrossRef]
- Lee, M.; Chung, G.E.; Lee, J.H.; Oh, S.; Nam, J.Y.; Chang, Y.; et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017, 66, 1556–1569. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hsu, Y.C.; Tseng, H.C.; Yu, S.H.; Lin, J.T.; Wu, M.S.; et al. Association of Daily Aspirin Therapy With Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis B. JAMA Intern Med. 2019, 179, 633–640. [Google Scholar] [CrossRef]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N Engl J Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
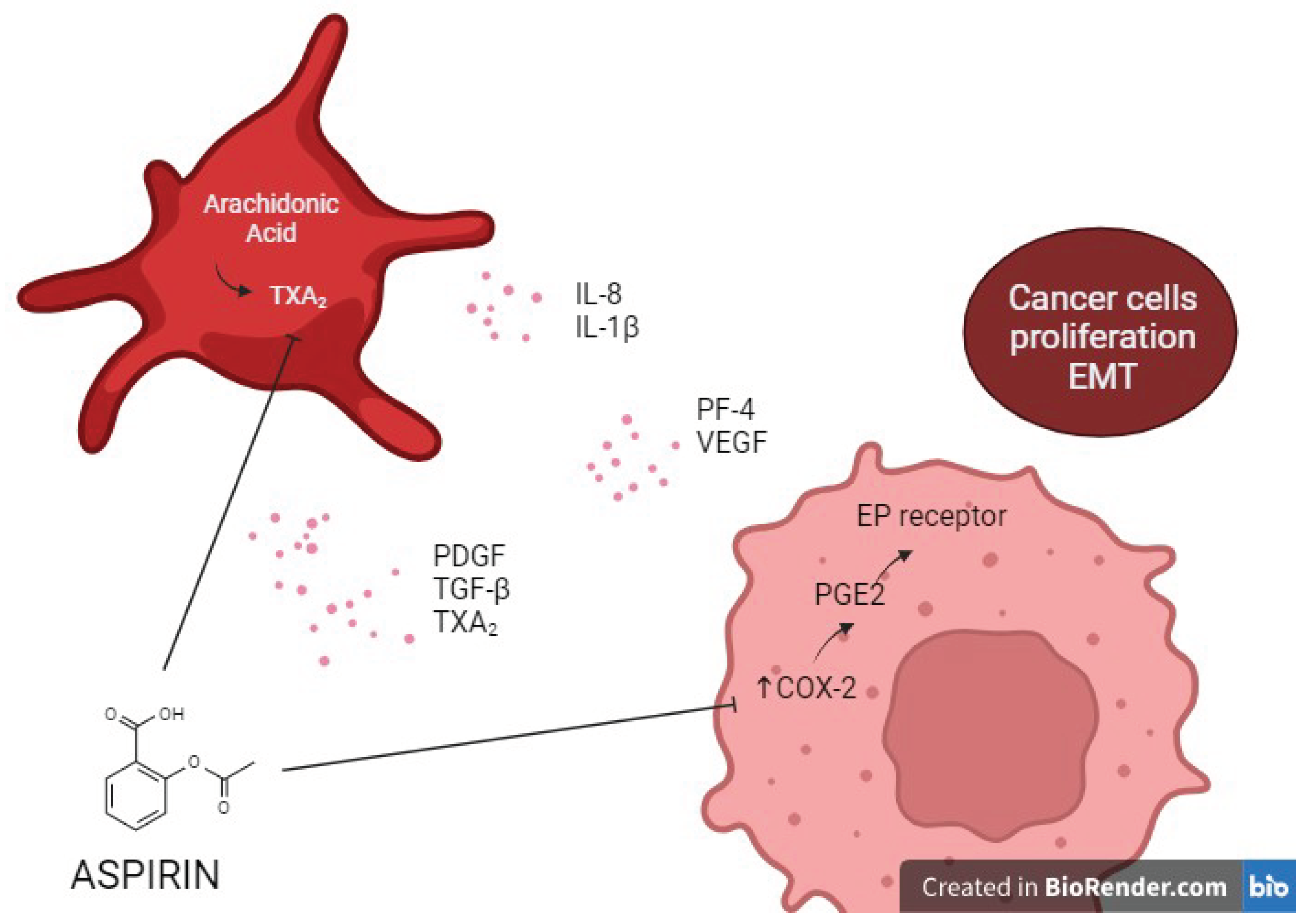
- Santilli, F.; Boccatonda, A.; Davì, G. Aspirin, platelets, and cancer: The point of view of the internist. 2016, 34:11-20. Eur J Intern Med. 2016, 34, 11–20. [Google Scholar] [CrossRef]
- Lai, Q.; De Matthaeis, N.; Finotti, M.; Galati, G.; Marrone, G.; Melandro, F.; et al. The role of antiplatelet therapies on incidence and mortality of hepatocellular carcinoma. Eur J Clin Invest. 2023, 53, e13870. [Google Scholar] [CrossRef]
- Sahasrabuddhe, V.V.; Gunja, M.Z.; Graubard, B.I.; Trabert, B.; Schwartz, L.M.; Park, Y.; et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012, 104, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsu, C.Y.; Yen, T.H.; Wu, T.H.; Yu, M.C.; Hsieh, S.Y. Daily Aspirin Reduced the Incidence of Hepatocellular Carcinoma and Overall Mortality in Patients with Cirrhosis. Cancers (Basel) 2023, 15, 2946. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Min, E.K.; Lee, J.G.; Joo, D.J.; Kim, M.S.; Kim, D.G. Antiplatelet Drugs on the Recurrence of Hepatocellular Carcinoma after Liver Transplantation. Cancers (Basel) 2022, 14, 5329. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Hsu, Y.C.; Ho, H.J.; Lin, J.T.; Chen, Y.J.; Wu, C.Y. Daily aspirin associated with a reduced risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: a population-based cohort study. EClinicalMedicine 2023, 61, 102065. [Google Scholar] [CrossRef]
- Tan, R.Z.H.; Lockart, I.; Abdel Shaheed, C.; Danta, M. Systematic review with meta-analysis: The effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021, 54, 356–367. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Aspirin, platelet inhibition and cancer prevention. Platelets. 2018, 29, 779–785. [Google Scholar] [CrossRef]
- Lloyd, K.E.; Hall, L.H.; Ziegler, L.; Foy, R.; Green, S.M.C.; MacKenzie, M.; et al. Acceptability of aspirin for cancer preventive therapy: a survey and qualitative study exploring the views of the UK general population. BMJ Open. 2023, 13, e078703. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. Cyclooxygenase Inhibitors and Cancer: The Missing Pieces. J Pharmacol Exp Ther. 2023, 386, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Boccatonda, A.; Davì, G.; Cipollone, F. The Coxib case: Are EP receptors really guilty? Atherosclerosis. 2016, 249, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Joharatnam-Hogan, N.; Hatem, D.; Cafferty, F.H.; Petrucci, G.; Cameron, D.A.; Ring, A.; et al. Thromboxane biosynthesis in cancer patients and its inhibition by aspirin: a sub-study of the Add-Aspirin trial. Br J Cancer. 2023, 129, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Fu, Q.; Diggs, L.P.; McVey, J.C.; McCallen, J.; Wabitsch, S.; et al. Platelets control liver tumor growth through P2Y12-dependent CD40L release in NAFLD. Cancer Cell. 2022, 40, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.; Sitia, G.; Isogawa, M.; Marchese, P.; Castro, M.G.; Lowenstein, P.R.; et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005, 11, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Vonderheide, R.H. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007, 13, 1083–1088. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef]
- Vogt, A.; Sadeghlar, F.; Ayub, T.H.; Schneider, C.; Möhring, C.; Zhou, T.; et al. Alpha-Fetoprotein- and CD40Ligand-Expressing Dendritic Cells for Immunotherapy of Hepatocellular Carcinoma. Cancers (Basel) 2021, 13, 3375. [Google Scholar] [CrossRef]
- Hermann, A.; Rauch, B.H.; Braun, M.; Schrör, K.; Weber, A.A. Platelet CD40 ligand (CD40L)--subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001, 12, 74–82. [Google Scholar] [CrossRef]
- Sutti, S.; Jindal, A.; Locatelli, I.; Vacchiano, M.; Gigliotti, L.; Bozzola, C.; et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014, 59, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; David, E.; Ramadori, P.; Pfister, D.; Safran, M.; Li, B.; et al. XCR1(+) type 1 conventional dendritic cells drive liver pathology in non-alcoholic steatohepatitis. Nat Med. 2021, 27, 1043–1054. [Google Scholar] [CrossRef]
- Ezzaty Mirhashemi, M.; Shah, R.V.; Kitchen, R.R.; Rong, J.; Spahillari, A.; Pico, A.R.; et al. The Dynamic Platelet Transcriptome in Obesity and Weight Loss. Arterioscler Thromb Vasc Biol. 2021, 41, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017, 551, 340–345. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, P.B.; et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018, 392, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med. 2018, 379, 1529–1539. [Google Scholar] [PubMed]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Simeone, P. Aspirin in primary prevention: the triumph of clinical judgement over complex equations. Intern Emerg Med. 2019, 14, 1217–1231. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, L.; Na, S.H.; Santilli, F.; Shi, Z.; Blaha, M. Implications of the heterogeneity between guideline recommendations for the use of low dose aspirin in primary prevention of cardiovascular disease. Am J Prev Cardiol. 2022, 11, 100363. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, Y.; Anderson, J.E.; Bakris, G.L.; Ballantyne, C.M.; Beckman, J.A.; Bhatt, D.L.; et al. DCRM Multispecialty Practice Recommendations for the management of diabetes, cardiorenal, and metabolic diseases. J Diabetes Complications. 2022, 36, 108101. [Google Scholar] [CrossRef] [PubMed]
- Merat, S.; Jafari, E.; Radmard, A.R.; Khoshnia, M.; Sharafkhah, M.; Nateghi Baygi, A.; et al. Polypill for prevention of cardiovascular diseases with focus on non-alcoholic steatohepatitis: the PolyIran-Liver trial. Eur Heart J. 2022, 43, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Rocca, B.; De Cristofaro, R.; Lattanzio, S.; Pietrangelo, L.; Habib, A.; et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin "resistance". J Am Coll Cardiol. 2009, 53, 667–677. [Google Scholar] [CrossRef]
- Rocca, B.; Santilli, F.; Pitocco, D.; Mucci, L.; Petrucci, G.; Vitacolonna, E.; et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. J Thromb Haemost. 2012, 10, 1220–1230. [Google Scholar] [CrossRef]
- Simeone, P.; Liani, R.; Tripaldi, R.; Ciotti, S.; Recchiuti, A.; Abbonante, V.; et al. Reduced platelet glycoprotein Ibα shedding accelerates thrombopoiesis and COX-1 recovery: implications for aspirin dosing regimen. Haematologica. 2023, 108, 1141–1157. [Google Scholar] [CrossRef]

| CD40 pathway ANTI-TUMOR ACTIVITY | CD40 pathway PRO-TUMOR ACTIVITY |
|---|---|
| CD40L is related to a strong CD8+ T cells responses through CD40 licensing of dendritic cell (61, 122, 124, 136, 137, 138, 146). | Platelet-derived CD40L is greater released in both NAFLD mouse models and patients with NASH [136] |
| Co-stimulation with CD40L-expressing dendritic cells (DC) significantly improves vaccination by inducing an early and strong Th1-shift in the tumor environment as well as higher tumor apoptosis [141]. | In NAFLD patients CD40L protein production is induced in megakaryocytes rather than NAFLD or tumors causing a transfer of CD40L pre-mRNA or mRNA into platelets [145]. |
| Platelet-derived CD40L can increase CD8+ T cell activation and their recruitment to liver in NAFLD (61, 122, 124, 136, 137, 138, 146). | IL-12 dependent increase of CD40L production in bone marrow megakaryocytes in NAFLD models [136,143] |
| Megakaryocytes harbored in lung can produce higher CD40L amount [136,144] | |
| Inhibition of the P2Y12 receptor on platelets can promote tumor growth via CD40L in mice with NAFLD [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





