1. Introduction
Since the initial description of the epithelial-mesenchymal transition (EMT) effect as an event regulating embryonic developmental process by Elizabeth Dexter “Betty” Hay (1927–2007, Harvard Medical School) (Hay, 1995), EMT has been reported as a universal cellular event involved in many different aspect of life process, including organogenesis, tissue repair, wound healing, inflammation, fibrosis, cancer progression, and even COVID-19 (Haensel and Dai, 2018; Kalluri and Neilson, 2003; Kalluri and Weinberg, 2009; Lamouille et al., 2014; Marconi et al., 2021; Pandolfi et al., 2021; Stone et al., 2016; Suarez-Carmona et al., 2017; Vincent and Fuxe, 2017). Particularly, EMT was employed to explain cancer metastasis initially but now has been implicated in every feature of cancer cells, including stemness, proliferation, evasion of death and immunosurveillance, dysregulated epigenetics, dysregulated metabolism, resistance to therapies, cancer heterogeneity, etc. (Bakir et al., 2020; Brabletz et al., 2021; Celià-Terrassa and Jolly, 2020; Dongre and Weinberg, 2019; Lu and Kang, 2019; Mittal, 2018; Nieto et al., 2016; Pastushenko and Blanpain, 2019; Polyak and Weinberg, 2009; Romano et al., 2020; Sato et al., 2016; Sciacovelli and Frezza, 2017; Shibue and Weinberg, 2017). EMT research has become a large research field and generated about 46,000 papers so far, and the number of publications is still growing rapidly. This makes EMT appearing as a mainstream concept (Sheng et al., 2022). Nevertheless, behind the prosperous EMT research are essential flaws in the rationale of EMT concept. The flaws might make it a groundless concept rather than a universal dogma that dictates developmental biology and pathology.
2. Outline of the History of EMT Research
2.1. Hay and the Initiation of EMT Research
It is generally credited that the American cellular and developmental biologist Elizabeth D. Hay played the pioneering role of EMT research (Sheng et al., 2022; Yang et al., 2020). She observed initially that cartilage cells of limbs of Ambystoma larvae are able to dedifferentiate and re-differentiate again into cartilage cells, thereby contributing to limb regeneration (Hay, 1958). Later, she found that regeneration of newt amputated limb needs the migration of epidermal cells over the wound surface of the limb (Hay and Fischman, 1961). These EMT-like processes implied that they may play important roles in wound healing and tissue regeneration, and led her to study the differentiation of epithelial cells and embryonic development. Elizabeth D. Hay and co-workers observed that extracellular matrix can influence differentiation of corneal epithelial cells (Meier and Hay, 1974). Using chicken embryos as a model and optical and electronic microscopy, she could identify different cellular phenotypes in chicken embryos. At the 18th Hahnemann symposium in Baltimore, she reported how mesenchymal cells are transformed from epithelial cells during the migration of neural crest cells in neural tube formation. This was considered as a description of EMT effect before the term “EMT” was created. The 18th Hahnemann symposium was therefore considered as the birthplace of EMT research.
Around the 1970s, studies by other groups demonstrated epithelial-mesenchymal interactions during tissue formation and organogenesis, including heart, neural crest, Mullerian duct, intestinal brush border membrane, embryonic lungs, etc. (Bluemink et al., 1976; Dyche, 1979; Kedinger et al., 1981; Markwald et al., 1977; Newgreen et al., 1979). In a publication reporting adult cells undergoing EMT in 1982, Hay and colleague used the term “epithelial-mesenchymal transformation” for the first time. They demonstrated that chicken lens epithelial cells cultured in vitro looked like mesenchymal cells and were able to move in collagen matrix (Greenburg and Hay, 1982). Different term or phrase were also devised by other groups to represent the EMT-like effect during the same period. Dulbecco and colleagues used “cuboid-to-fusiform transition” to describe their observation that cuboid epithelial cells of rat mammary tumors changed to fibroblast-like cells with fusiform morphology (Dulbecco et al., 1981). The phrase “rapid change from epithelial to mesenchymal character” was used by Illmensee’s group to represent an EMT-like effect observed during mouse embryogenesis (Franke et al., 1982).
In subsequent studies, Hay continued to described the morphological changes during EMT, and tried to delineate EMT with molecular changes. For instances, her team showed cultured embryonic lens epithelial cells undergoing an EMT-like phenotypic change, and lose type IV collagen expression and γ-crystallin while expressing type I collagen (characteristic of mesenchymal cells) (Greenburg and Hay, 1986). They also showed that thyroid epithelial cells undergoing EMT lose thyroglobulin but gain vimentin expression, suggestive of a dedifferentiation effect (Greenburg and Hay, 1988). The term “epithelial-mesenchymal transition” appeared for the first time in a cited literature in a review by Hay and Zuk (1995) (Hay and Zuk, 1995). It became the official term after the first TEMTIA (The EMT International Association) meeting in 2003. “Epithelial-mesenchymal transition” was used instead of “epithelial-mesenchymal transformation” to distinguish it from the neoplastic transformation commonly used by cancer researchers (Yang et al., 2020).
2.2. Transition from Morphological to Molecular Description of EMT
After extensive phenotypic description of EMT, EMT research began to shift to molecular analysis. Hepatocyte growth factor (HGF) was observed to dissolve the junction proteins between epithelial cells, causing transformation of epithelial cells into migratory fibroblasts (Stoker and Perryman, 1985; Stoker et al., 1987). Thiery’s group found that fibroblast growth factor 1 (FGF1) induced an EMT effect in rat bladder carcinoma cells, linking EMT to cancer (Vallés et al., 1990). Epidermal growth factor (EGF) was also shown to promote EMT in rat neonatal hepatocytes (Pagan et al., 1997). TGF family proteins were more extensively investigated for their roles in EMT. It was shown that TGFalpha was able to induce a mesenchymal and invasive phenotype in rat prostate cancer cell (Gavrilović et al., 1990). TGFbeta proteins were shown to play an important role in embryonic heart endothelial cells (Potts et al., 1991) and embryonic palatal cells undergoing EMT (Sun et al., 1998), and mammary epithelial cells treated with TGFbeta can undergo EMT (Miettinen et al., 1994). TGFbeta mediated EMT effect involves activation of TGFbeta receptor and Smad signal transducers (Piek et al., 1999). TGFbeta receptor also activates Rho-GTPase, PI3K/AKT and MAPK pathways that can induce an EMT effect in embryonic chick heart, lens epithelial cells, renal epithelial cells, in vitro cultured tumor and non-tumor mammary epithelial cells (Bakin et al., 2002; Cho and Yoo, 2007; Kattla et al., 2008; Tavares et al., 2006; Xie et al., 2004). In the pursuit of molecular mechanisms of EMT, Hay’s group demonstrated in 2008 that the Snail family of EMT activated transcription factors can induce TGFbeta3 expression in cancer cell lines (Medici et al., 2008).
Studies of identifying molecular regulators of EMT increased dramatically in the 1990s. These led to the identification of EMT transcription factors (EMT-TFs), the first of which were Snail (Snai1) and Slug (Snai2) (Nieto et al., 1992; Nieto et al., 1994; Smith et al., 1992). Nieto et al. (1994) showed for the first time that knockdown of Snail or Slug impaired EMT and subsequent cell migration during mesoderm and neural crest formation in chicken embryos (Nieto et al., 1994). Later, they were shown to promote an EMT effect in cancer cells (Batlle et al., 2000; Cano et al., 2000; Savagner et al., 1997). In 2001, E12/E47 basic helix-loop-helix transcription factor (also called TCF3) was shown to evoke an EMT effect in MDCK kidney cells (Perez-Moreno et al., 2001), and the ZEB family transcription factors, ZEB1 and ZEB2, were reported to induce an invasive phenotype in cancer cells (Comijn et al., 2001), linking their function in regulating EMT. Later on, Weinberg’s group revealed that TWIST1 plays an essential role in cancer metastasis via promoting EMT (Yang et al., 2004). These EMT-TFs were capable of inducing EMT-associated morphological and molecular changes, particularly transcriptional repression of the typical epithelial gene E-cadherin (Batlle et al., 2000; Bolós et al., 2003; Cano et al., 2000; Comijn et al., 2001; Eger et al., 2005; Hajra et al., 2002; Perez-Moreno et al., 2001).
Jean Paul Thiery in the 1980s clutched “the gospel of developmental EMT to bravely jump from development to oncology, and finally grab cancer biologists by the scruff of the neck and force them to see the light. It was really from this point that the EMT field commenced its exponential growth” (Sheng et al., 2022). Since then, a large number of studies have demonstrated that EMT-TFs regulate not only cancer metastasis but eventually every aspect of cancer initiation and progression, and every feature of cancer cells, including stemness, unlimited cell proliferation, evasion of cell death and immunosuppression, chemoresistance, genomic instability, metabolic reprogramming, etc. Correspondingly, molecular mechanisms underlying regulation of cancer by EMT-TFs and regulation of EMT-TFs in cancer have also been extensively investigated (Bakir et al., 2020; Brabletz et al., 2021; Celià-Terrassa and Jolly, 2020; Dongre and Weinberg, 2019; Fischer et al., 2015; Haerinck et al., 2023; Lamouille et al., 2014; Lu and Kang, 2019; Mittal, 2018; Nieto et al., 2016; Pastushenko and Blanpain, 2019; Romano et al., 2020; Saitoh, 2023; Sato et al., 2016; Sciacovelli and Frezza, 2017; Shibue and Weinberg, 2017; Thiery et al., 2009). Due to their central role in EMT and extensive studies in cancer, ZEB1, ZEB2, SNAI1, SNAI2 and TWIST1 are considered as the core EMT-TFs (Kalluri and Weinberg, 2009; Nieto et al., 2016; Yang et al., 2020). Besides these core factors, a number of additional EMT-TFs have been identified, including FOXC2 (Mani et al., 2007), GSC (Hartwell et al., 2007), KLF8 (Wang et al., 2007), PRRX (Ocaña et al., 2012; Takano et al., 2016), RUNX2 (Tavares et al., 2018), SIX1 (McCoy et al., 2009), TCF3 (also known as E47 or ITF1) (Perez-Moreno et al., 2001), and TCF4 (also known as E2-2 or ITF2) (Sobrado et al., 2009).
3. Mesenchymal-Epithelial Transition (MET) and Endothelial-Mesenchymal Transition (EndMT)
Other two EMT-related cellular state transitions that have been extensively investigated are MET and EndMT. It is believed that transition from epithelial to mesenchymal state is reversible. The reversed process is known as mesenchymal-epithelial transition (MET) (Hay and Zuk, 1995; Pei et al., 2019; Polyak and Weinberg, 2009). This means that mesenchyme derived from epithelium can sometimes reverts back to the epithelial phenotype. At the molecular level, MET is characterized by the decreased expression of mesenchymal factors and increased expression in epithelial markers, particularly E-cadherin (Polyak and Weinberg, 2009; Bakir et al., 2020). Putative roles of MET in embryogenesis and cancer have also been widely reported or proposed (Bakir et al., 2020; Hay and Zuk, 1995; Pei et al., 2019; Polyak and Weinberg, 2009).
The inner surface of all vessels in the body, including capillaries, arterioles, arteries, veins, and lymphatic vessels, is lined by a thin membrane-like structure, the endothelium. It plays the primary roles in regulating and maintaining vessel wall permeability (Piera-Velazquez and Jimenez, 2019). EndMT is cellular differentiation process by which resident endothelial cells delaminate and migrate away from the endothelium, progressively lose their endothelial features and acquire mesenchymal features. Accordingly, there is a tendency of decreased expression of endothelial markers and gain of mesenchymal marker expression in cells undergoing EndMT (Bischoff, 2019; Clere et al., 2020; Piera-Velazquez and Jimenez, 2019; Potenta et al., 2008). The molecular pathways regulating EndMT have been extensively investigated (Xu and Kovacic, 2023), which substantially overlap with those regulating EMT. Endothelial cells can be considered as a special type of epithelial cells. Therefore, EndMT is often considered as a special form of EMT. EndMT has been reported or proposed to play essential roles in many normal developmental and pathological processes, including cancer (Bischoff, 2019; Clere et al., 2020; Piera-Velazquez and Jimenez, 2019; Potenta et al., 2008; Simons, 2023; Xu and Kovacic, 2023)
4. EMT and Development, Fibrosis and Cancer
It seems that EMT and its reversed process MET are employed generally throughout embryogenesis to organogenesis. EMT was first observed during gastrulation in vertebrate embryos when some cells of the epiblast (the definitive ectoderm) undergo EMT and move between the epiblast and hypoblast (the definitive endoderm) to form the third germ layer: the mesoderm (Akhurst, 2023; Lim and Thiery, 2012; Nakaya and Sheng, 2008; Pérez-Pomares and Muñoz-Chápuli, 2002; Solnica-Krezel and Sepich, 2012; Tam and Beddington, 1987), from which the embryonic and adult mesenchymal cells are derived. Conversely, MET turns early mesoderm into somites (Nakaya et al., 2004), which differentiate into dermamyotome, cartilage and bone by subsequent EMT (Noden, 1988). It is believed that EMT drives the formation of migratory neural crest cells from neuroectoderm, leading to loss of the original neuroepithelial morphology and gain of migratory phenotype with a fibroblast-like shape (Bronner, 2012; Duband et al., 1995; Nieto et al., 1994; Mancilla and Mayor, 1996; Strobl-Mazzulla and Bronner, 2012; Theveneau and Mayor, 2012). During organogenesis, EMT has been reported to involve in the formation of many different types of cells or tissues in an animal, such as fetal liver stroma (Chagraoui et al., 2003), the cardiac cushion tissue (Markwald et al., 1977; Markwald et al., 1996; Person et al., 2005), and oral palatal shelves (Ferguson, 1988; Fitchett and Hay, 1989; Sweney and Shapiro, 1970).
Many reports have shown that EMT is involved in fibrosis or scarring in different organs, including liver, lung, kidney, and heart. During normal wound healing, myofibroblasts, which are mesenchymal cells, undergo apoptosis and disappear once upon the completion of re-epithelialization. Pathologically prolonged myofibroblast activity leads to fibrogenesis. In fact, persistent myofibroblast activation is a common feature of fibrogenesis, in which EMT is believed to play an essential role (Stone et al., 2016; Nieto et al., 2016; Yang et al., 2020). Myofibroblasts can be derived from a variety of sources. However, many lines of evidence showed that a major part of them are generated through EMT during organ fibrosis (Stone et al., 2016). During kidney fibrosis, tubular epithelial cells turn into myofibroblasts via EMT and adopt fibroblast morphology, as evidenced by studies with animal models, human kidney biopsies, epithelial and mesenchymal marker stainings, and lineage tracing with the mesenchymal marker FSP1 (also known as S100A4) (Kriz et al., 2011; Loeffler and Wolf, 2015; Stone et al., 2016). It is believed that in lungs, epithelial cells experience repeated injury and persistent inflammation could undergo EMT, leading to fibrosis. The origin of myofibroblasts in lung fibrosis is not certain. Some studies showed that alveolar epithelial cells undergo EMT or partial EMT and contribute to fibrotic pathology. In a TGFβ1 murine model of lung fibrosis, beta-galactosidase (β-gal)-labeling epithelial cells also expressed mesenchymal markers, indicating epithelial cells as the progenitors for the fibroblasts (Bartis et al., 2014; Jolly et al., 2018; Rout-Pitt et al., 2018; Stone et al., 2016). Origin of activated myofibroblasts during liver fibrosis is also not clear, but epithelial cells undergoing EMT has been proposed as the source. Lineage-tracing studies with mouse models demonstrated hepatocytes underwent EMT, thereby contributing to the population of cells with the morphology of fibroblasts or expression mesenchymal markers (Stone et al., 2016; Xie and Diehl, 2013; Munker et al., 2017; Taura et al., 2016). EMT regulated fibrogenesis following heart injury has been reported. Adult epicardial cells undergo EMT, and migrate into the injured myocardium where they generate different types of cells, including cardiac interstitial fibroblasts and coronary smooth muscle cells, to help tissue repair (Stone et al., 2016). The role of EndMT during heart fibrosis has been more widely investigated because fibroblasts are derived from endothelial cells via EndMT (Anbara et al., 2020; Chua et al., 2011; Li et al., 2018). During fibrogenesis of different organs, TGFbeta signaling seems to play a general role in mediating EMT or EndMT.
Cancer has been the primary focus of EMT research. At the time of writing, 36,700 out of all ~46,000 EMT papers are studies dealing with cancer according to Pubmed. Among 4,246 EMT papers published in 2023, 3,325 are related with cancer. Since the initial studies about the link of EMT-TFs with cancer cell metastasis, EMT programme mediated by EMT-TFs has been reported to endow nearly all malignant features to cancer cells, including stemness, fast cell cycle/proliferation, evasion of cell death and immunosuppression, therapy resistance, etc., and involve in nearly all aspects of carcinogenesis. Molecular mechanisms underlying how EMT-TFs regulates carcinogenesis or how EMT-TFs are regulated by other factors at gene, transcriptional and translational levels in cancer have been extensively reviewed (Bakir et al., 2020; Brabletz et al., 2021; Celià-Terrassa and Jolly, 2020; Dongre and Weinberg, 2019; Haerinck et al., 2023; Lambert et al., 2017; Lambert and Weinberg, 2021; Lu and Kang, 2019; Mittal, 2018; Nieto et al., 2016; Pastushenko and Blanpain, 2019; Polyak and Weinberg, 2009; Romano et al., 2020; Saitoh, 2023; Sato et al., 2016; Sciacovelli and Frezza, 2017; Shibue and Weinberg, 2017; Tam and Weinberg, 2013).
Based on EMT functions, EMT is classified into three subtypes. Type I is associated with implantation, embryonic gastrulation and organogenesis during embryonic development; type II plays roles in inflammation and fibrosis; and type III is involved in cancer (Kalluri and Weinberg, 2009).
5. The Controversies Over EMT Research on Fibrosis and Cancer
5.1. The Earliest Argument against EMT
Although EMT has become a formidable research discipline and a mainstream concept (Sheng et al., 2022), it has been under intense debate since its early stage of study. When EMT effects were increasingly observed during tissue formation and organogenesis around the 1970s (Bluemink et al., 1976; Dyche, 1979; Kedinger et al., 1981; Markwald et al., 1977; Newgreen et al., 1979), studies from two groups demonstrated the co-existence of differentiated and undifferentiated cell types, including epithelial and mesenchymal cells, in mesodermal mixed uterus tumors (Böcker and Stegner, 1975; Ishikawa et al., 1979). Contrary to the view that mesenchymal cells are derived directly from epithelial cells, these studies considered that it was not possible for epithelial cells to acquire a mesenchymal shape or vice versa, and concluded that epithelial and mesenchymal cells share a common cancer stem cell origin (Böcker and Stegner, 1975; Ishikawa et al., 1979). This viewpoint was not considered by mainstream research, of course. Nevertheless, it might reflect the truth (see text below).
5.2. The Controversies Over EMT in Fibrosis
With the progress of EMT research, the disputes over EMT have been also growing but primarily concentrated on the EMT effects in fibrosis of different organs. In a study in which double transgenic mice Alb-Cre × ROSA26-floxSTOPflox-LacZ were bred with transgenic mice expressing green fluorescent protein (GFP) driven by the collagen 1α1 promoter to generate triple transgenic mice in which β-galactosidase was expressed in “hepatocyte-derived” cells and GFP was expressed in “collagen-expressing” cells, transition of LacZ-positive (hepatocyte-derived) cells into GFP-positive (collagen-expressing) myofibroblasts in induced fibrotic liver was not detected (Taura et al., 2010). By using Alfp-Cre × Rosa26-YFP mice in which the epithelial cells of the liver (hepatocytes, cholangiocytes, and their bipotential progenitors) are heritably labeled at high efficiency with yellow fluorescent protein (YFP), the study by Chu et al. (2011) showed that in induced liver fibrosis in Alfp-Cre × Rosa26-YFP mice, EMT did not occur because no evidence of colocalization of YFP with the mesenchymal markers S100A4, vimentin, α-SMA, or procollagen 1α2 was found (Chu et al., 2011). Moreover, there was also no evidence for cholangiocyte EMT during hepatic fibrosis (Chu et al., 2011; Scholten et al., 2010). These elaborate lineage-tracing studies argue against EMT in liver fibrosis, and thus, it was suggested that the term EMT should be abandoned in cholangiocyte biology (Fabris et al., 2015; Kisseleva and Brenner, 2011; Munker et al., 2017; Popov and Schuppan, 2010; Taura et al., 2016; Wells, 2010; Xie and Diehl, 2013). The role of EMT in kidney and lung fibrosis are controversial, too. Cell fate tracing studies and absence of cells with mesenchymal morphology do not support EMT as an in vivo process in kidney and lung fibrosis (Bartis et al., 2014; Bielesz et al., 2010; Humphreys et al., 2008; Humphreys et al., 2010; Koesters et al., 2010; Kriz et al., 2011; Loeffler and Wolf, 2015; Rock et al., 2011; Taura et al., 2016). Involvement of EndMT in fibroblast contribution during cardiac fibrosis is also not certain. Evidence of lineage-tracing studies showed that the majority of myofibroblasts after injury was derived from resident fibroblasts, but not from EndMT (Li et al., 2018). The reasons for the inconsistencies in the involvement of EMT or EndMT in organ fibrosis might be the unreliability fibroblast-specific protein-1 (FSP1/S100A4) as a mesechymal-specific marker to identify fibroblasts and cells undergoing EMT, and the unreliability of the detection of β-galactosidase colocalizing with FSP1 (Taura et al., 2016). Absence of solid evidence raised the serious concern why EMT has become so deeply ingrained into fibrosis research (Kriz et al., 2011).
5.3. The Disputes Over EMT in Cancer
As EMT was questioned intensely in fibrosis research, studies of EMT in cancer have been flourishing, and the number of published papers had kept growing dramatically each year (Yang et al., 2020). A few controversies over EMT in cancer, including the two earliest arguments against EMT in mesodermal mixed uterus tumors (Böcker and Stegner, 1975; Ishikawa et al., 1979). In 2005, Tarin pointed out that EMT is a misconception due to some reasons (Tarin, 2005). Firstly, the EMT concept was conceived based on the highly ordered series of events occurring during normal embryonic development. It is inappropriate to extrapolate and generalize from these highly ordered events to the chaotic events during cancer progression in different adult tissues/organs. Moreover, identification of cells as epithelial or mesenchymal based on shape and morphology or a few epithelial and mesenchymal markers is just subjective and unreliable. The earliest EMT effect is believed to occur during mesodermal formation in gastrulating embryos. However, the invaginating mesodermal cells in amphibian gastrulae are not spindle-shaped and do not lose cohesion with each other. Importantly, evidence of EMT in cancer metastasis is lacking (Tarin, 2005). Nevertheless, Tarin suggested that EMT in neural crest is of particular interests (Tarin, 2005). It is not surprising that EMT advocates did not agree with these points (Cardiff, 2005; Thompson et al., 2005). One major piece of evidence supporting EMT in cancer is the downregulation of epithelial marker E-cadherin and upregulation of mesenchymal markers, particularly the core EMT-TFs, which predict invasiveness and metastatic potential and are negatively correlated with overall survival. Paradoxically, carcinoma cells within primary and metastatic lesions with well-differentiated epithelial morphology were also reported. Key epithelial markers, particularly E-cadherin, are expressed in invasive carcinomas (Christiansen and Rajasekaran, 2006), and E-cadherin is required for metastasis in multiple models of breast cancer (Padmanaban et al., 2019). The paradox is reconciled by MET in metastatic outgrowth, but the mechanism underlying activation of MET in metastatic cancer cells remains largely unknown (Bakir et al., 2020; Sun and Ma, 2024; Williams et al., 2019). It is a rather interesting but incomprehensible situation that both EMT and its reversed process contribute to metastasis. Despite these disputes, EMT studies in cancer grow and proliferate quickly, showing EMT as the endower of nearly all malignant features to cancer cells, as mentioned above. However, two studies in 2015, one using lineage tracing with Fsp1 or Vimentin promoter driving Cre recombinase (Fsp1-Cre or Vim-Cre) and the other using genetically engineered mouse models with deletion of Snail or Twist gene, demonstrated that EMT is not required for cancer metastasis but contributes to chemoresistance (Fischer et al., 2015; Zheng et al., 2015). Later, another two studies fought back by indicating that the markers used in the previous two studies are not universal markers for EMT programs or are not reliable as EMT markers (Aiello et al., 2017; Ye et al., 2017).
5.4. Compromising the Discrepancies in EMT Studies
An appealing feature of EMT to cancer researchers is that EMT can convert adhesive and stationary state of epithelial cells into non-adhesive and individually migratory state of mesenchymal cells. Cell migration is fundamental for setting up and maintaining the correct organization during animal development. In adults, cell migration is required for immune response, wound repair, and tissue homeostasis. Many cell types exhibit active migration, including collective migration of epithelial cells during gastrulation or lateral line primordium cells during development of fish, and single-cell migration of neural stem/progenitor cells during the development of the nervous system (Mayor and Etienne-Manneville, 2016; Trepat et al., 2012). Therefore, single-cell migration is not specific to mesenchymal cells, and epithelial cells are also not just stationary. Interestingly, mesenchymal cells also migrate collectively (Theveneau and Mayor, 2013; Campbell and Casanova, 2016). This means that there is no clear-cut distinction in the migratory feature of epithelial and mesenchymal cells. The complex issue in migratory feature of epithelial and mesenchymal cells is compromised that EMT should not be interpreted as a binary switch from one cellular state to the other but should be interpreted as graded processes with a range of intermediate effects (Campbell and Casanova, 2016). Meanwhile, tumor cells with co-expression of various epithelial and mesenchymal markers were frequently observed, meaning that transition from epithelial to mesenchymal state is a multi-step, multi-state, and dynamic process, ranging from a completely epithelial to a completely mesenchymal phenotype, as represented by the expression levels of epithelial and mesenchymal markers. Therefore, new terms ‘EMT-like’, ‘partial EMT’, ‘intermediate EMT’, ‘hybrid EMT’, or ‘dynamic EMT’, etc., were introduced (Brabletz et al., 2021; Grigore et al., 2016; Jolly et al., 2015; Nieto, 2013; Nieto et al., 2016; Ye and Weinberg, 2015), and the EMT concept itself was recommended to be elastic to compromise the discrepancies and complexity of EMT effect in cancer (Savagner, 2015; Williams et al., 2019; Yang et al., 2020; Ye and Weinberg, 2015). Thus, ‘EMT plasticity (EMP)’ was suggested to replace EMT to reflect the high heterogeneity of EMT phenotypes (Yang et al., 2020; Haerinck et al., 2023). With these improvements, the EMT concept can now fit smoothly with any situation encountered in EMT research.
5.5. Neural Stemness as the Core Property of Cancer Cells Suggests that EMT in Cancer Represented by EMT-TFs Is a Misinterpretation
In 2017, co-workers and I published a humble paper, reporting that cancer cells are characteristic of neural stem cells or embryonic neural cells (Zhang et al., 2017). One reason is that inhibition of endogenous cancer promoting factors in cells of different cancer types led to neuronal-like differentiation in vitro, suggestive of the property of neural stem/embryonic neural cells, i.e., neural stemness. After comprehensive analysis on more than 3,000 cancer related genes, we found that most (if not all) cancer promoting genes or genes upregulated/activated in different cancer cells are neural stemness genes, or are specifically expressed or at least enriched in embryonic neural cells. By contrast, a major part of cancer suppressor genes or genes downregulated/silenced in cancer cells are non-neural genes in embryos. Therefore, cancer cells share the regulatory networks with neural stem/embryonic neural cells, thereby acquiring neural stemness in cancer cells (Zhang et al., 2017). In this study, it was noticed that core EMT-TF genes, which are upregulated in cancer cells and promote cancer, are embryonic neural genes, whereas the typical epithelial gene E-cadherin, a tumor suppressor gene (Birchmeier, 1995; Semb and Christofori, 1998), is expressed in epidermis only, excluding embryonic neural tissues (Zhang et al., 2017). These patterns of EMT gene expression match very well with the rules about cancer promoting or suppressor genes mentioned above, and suggest that the EMT effects observed in cancer should be a misinterpretation. The EMT-TFs are a few components of neural regulatory networks that confer cancer cells with neural stemness, rather than mesenchymal state. In-depth analysis revealed that, unfortunately, the so-called epithelial and mesenchymal states in the EMT concept have remained unclear or undefined in spite of large scales of EMT research. In combination with other studies in cancer and developmental biology, I proposed that cancer initiation and progression represent a process of progressive loss of original cell identity and gain of neural stemness. Meanwhile, the plausibility of EMT concept itself, but not merely its roles in cancer, was put into question because what are the general epithelial and mesenchymal states is still unknown (Cao, 2017). In 2020, after two years of discussion, TEMTIA published a consensus statement about the guidelines and definitions for EMT research due to discrepancies in data interpretation and persistent disagreements about whether the process studied is EMT (Yang et al., 2020). The consensus statement listed some critical problems about EMT and EMT research. Firstly, “while the characteristics of fully epithelial cells are relatively clearly defined, our current knowledge does not allow us to define the mesenchymal state with specific cellular characteristic or molecular markers that are universal end-products of all EMT programmes”, indicating that the epithelial state is relatively known but the mesenchymal state is unknown. Most EMT studies have been concentrated on a few EMT factors/markers. However, “EMT status cannot be assessed on the basis of one or a small number of molecular markers”. Therefore, “the primary criteria for defining EMT status should be changes in cellular properties together with a set of molecular markers, rather than relying solely on molecular markers” (Yang et al., 2020).
Subsequent studies of mine revealed that neural stemness is the key cellular property determining and unifying tumorigenicity and pluripotency, which govern tumorigenesis and embryogenesis, respectively. Such a superiority of neural stemness is predestined by the evolutionary advantage of neural genes and neural cell state (Cao, 2022; Cao, 2023; Chen et al., 2021; Lei et al., 2019; Xu et al., 2021; Zhang et al., 2022). Characterization of neural stemness and its regulatory networks revealed that they determine malignant features and tumorigenicity of cancer cells. It is hard to know what the undefined mesenchymal state shares in common with cancer cells (Cao, 2022; Cao, 2023).
6. Reassessing the Rationale of EMT Concept
Analysis above indicates many discrepancies and defects in EMT research, it also casts doubts on the plausibility of the EMT concept.
6.1. The Epithelial and Mesenchymal States Have Not Been Clearly Defined
According to the consensus statement on the guidelines and definitions for research on epithelial–mesenchymal transition by TEMTIA, “Epithelial–mesenchymal transition (EMT) is a cellular process during which epithelial cells acquire mesenchymal phenotypes and behavior following the downregulation of epithelial features” (Yang et al., 2020). This means that the plausibility of EMT concept depends entirely on the understanding of epithelial and mesenchymal cells or phenotypes. In fact, epithelial and mesenchymal cells are highly heterogeneous populations of cells with diverse phenotypes and functions. In general, epithelial cells are tightly packed together in cell sheets, form covering on all internal and external surfaces of animal body, and make up lining of hollow organs. During early embryogenesis, pluripotent epiblast cells are considered as the earliest epithelial cells. Later, there are epithelial cells, including neuroepithelial cells, that give rise to neural crest cells and palatal epithelial cells, etc. Types of epithelial cells are more diverse in adult animals, since each organ is covered by epithelial cells specific to the type of organ, such as those in skin, lung, kidney, etc. This means that epithelial cells of different tissues/organs have different intrinsic regulatory networks to define cell properties including tissue or organ-specific functions. For example, epithelial cells of lung, which is derived from endoderm, must be different in function and regulatory networks from those of kidney or skin, which are derived from mesoderm and ectoderm, respectively. During embryogenesis, mesenchymal cells are derived from mesoderm and form multipotential embryonic connective tissue, and give rise to all adult connective tissues, as well as the lymphatic and circulatory systems. In adulthood, mesenchymal cells are commonly described as non-epithelial, non-hematopoietic and non-endothelial cells that support and connect tissues, including muscle, tendon, and fat tissues, and encompass diverse populations of fibroblasts, stromal cells, pericytes, perivascular smooth muscle cells and mesenchymal progenitors.
Heterogeneity of the types of epithelial and mesenchymal cells raises the question whether there exist the general states or properties of all epithelial cells and mesenchymal cells based on which EMT can be a scientifically meaningful concept and serves as a general rule to explain developmental and pathological effects. Although stable epithelial cell–cell junctions, apical–basal polarity and interactions with basement membrane are recognized as the common features of epithelial state (Yang et al., 2020), however, these features are not independent of other features in a particlular type of epithelial cells. For example, when epiblast cells turn into embryonic mesenchyme via EMT during gastrulation, not only do they lose their apical–basal polarity, change their cytoskeleton and show decreased cell–cell adhesion, but their regulatory networks defining epiblast pluripotency are also changed overall to the networks defining non-pluripotent mesodermal cells. During carcinogenesis of the lung, epithelial cells lose not only cell adhesion, but also their function in respiration. Correspondingly, the regulatory networks change in addition to the decreased expression of epithelial markers, e.g., E-cadherin. Moreover, epithelial cells show a wide range of differentiation potential, from pluripotent epiblast cells to terminally differentiated epithelial cells in different organs. Therefore, focus on the loss of epithelial state only in the EMT concept is an oversimplification and biased interpretation of the change in cellular properties of epithelial cells. The mesenchymal state is more confusing, because there has been no way to define this cellular state with specific cellular characteristic or molecular markers (Cao, 2017; Cao, 2023; Yang et al., 2020). Therefore, EMT means a transition from an almost unknown cellular state to an unknown cellular state. It is incomprehensible how an unknown cellular state can be used as standard reference for the properties of other cells or endow different cellular properties to cancer cells, and how EMT can be a scientifically meaningful concept. No matter whether EMT is interpreted as a binary switch from one cellular state to the other or as graded processes with a range of different outcomes, ‘EMT-like’, ‘partial EMT’, ‘intermediate EMT’, ‘hybrid EMT’, and ‘dynamic EMT’, and ‘EMT plasticity’, express no essential difference from EMT because they all depend on the understanding of mesenchymal state. Classification of the three subtypes of EMT seems to be superfluous when what is EMT is unknown. Similarly, MET and EndMT are also groundless concepts without knowing the mesenchymal state. It was claimed that a pressing issue for EMT is to resolve the controversy on the contribution played by EMT in metastasis (Sheng et al., 2022; Williams et al., 2019). A more pressing issue to resolve seems to be whether EMT is a plausible concept.
6.2. Interpretation of EMT and the Functions of EMT-TFs in the Context of Embryonic Development
It remains an essential question how to interpret the ‘EMT’ effects. In the context of developmental biology, embryonic development is a progressive process of differentiation from the original totipotent unicellular state of a fertilized egg to the pluripotent state of inner cell mass and epiblast cells, which further differentiate into multipotent/oligopotent/unipotent progenitor/precursor cells of tissues/organs of different lineages. These cells finally differentiate into different types of mature and functional cells of tissues/organs. However, in the context of EMT/MET, the progressive differentiation of embryonic cells and the change in cellular properties and corresponding regulatory networks are described only as transitions between the undefined epithelial and mesenchymal states. This is not helpful but a confusion for understanding embryogenesis. The renowned example of EMT, in which epiblast cells turns into embryonic mesenchymal cells, is the differentiation of epiblast cells into mesodermal cells induced by signals from hypoblast cells. The commonality of mesoderm induction in different vertebrate embryos has been extensively investigated (Kimelman, 2006). But EMT is not suggested to play a role in mesoderm induction. Neuroepithelial cells turning into migratory neural crest cells is also considered as an EMT effect. According to definition, mesenchymal cells are cells from mesodermal lineage. However, neural crest cells are precursors of the peripheral nervous system, which belongs to the neural lineage, and the general epithelial marker E-cadherin is not expressed in neuroepithelium (Stemmler et al., 2005; Zhang et al., 2017). Neuroepithelial and neural crest cells are of particular interest, which will be discussed later. The true meaning of the ‘EMT’ effects observed during organogenesis and fibrosis based on marker expression or lineage-tracing studies is unclear and needs re-evaluation.
Core EMT-TFs have been extensively studied for their roles in EMT. Nevertheless, numerous other studies revealed their functions beyond EMT. Zeb2 is critical for exit from the epiblast state in mouse ESCs and for neural and general differentiation (Stryjewska et al., 2017). Mice with homozygous mutation of
Zeb2 display defects in neural tube closure, early arrest of neural crest cell migration, and absence of neural crest cells. Meanwhile,
E-cadherin expression domain extends to the neuroepithelium in mutant mice. By contrast, homozygous
Zeb1-deficient mice exhibit multiple skeletal defects but no distinctive phenotypic change in the central nervous system (Vandewalle et al., 2009). Latest studies showed that ZEB1 is required for the mesodermal-to-myogenic specification but ZEB2 promotes neural fate specification of human embryonic stem cells. Moreover, ZEB1 functions as an inhibitor rather than an inducer of EMT (Ninfali et al., 2023; Sánchez-Tilló et al., 2023). It can be seen that ZEB2 is mainly involved in regulation of neural development, while ZEB1 is principally in mesodermal tissue differentiation. The functional difference corresponds to their expression patterns during embryogenesis.
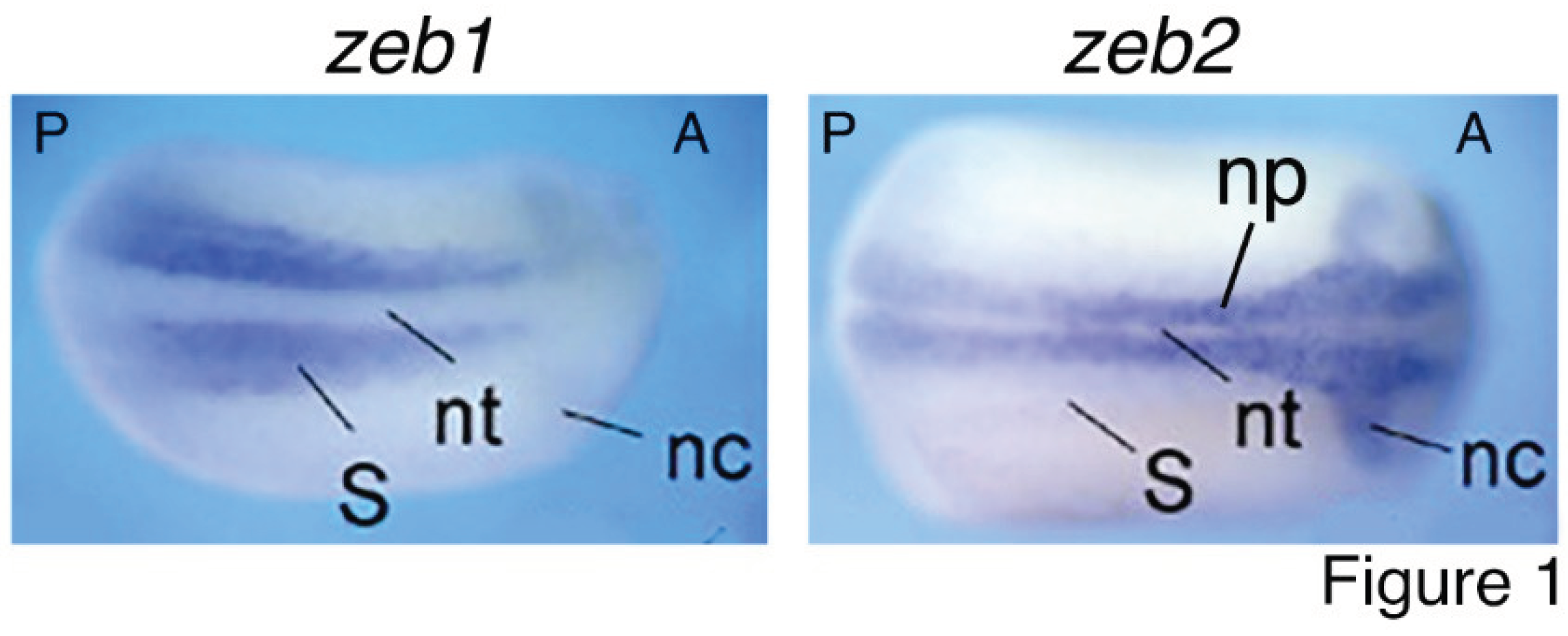
zeb1 expression is localized to the paraxial mesoderm, which gives rise to somites, the precursor of muscle and skeleton; whereas
zeb2 is selectively expressed in the precursor tissues of the nervous system during embryogenesis, including neural plate and neural crest (van Grunsven et al., 2006) (
Figure 1). Similar expression patterns of
Zeb1 and
Zeb2 are also present during mouse embryonic development (Vandewalle et al., 2009). TWIST1 and its orthologues are involved in regulation of gastrulation and body axis patterning of Drosophila embryos (Simpson, 1983), pluripotency and differentiation of embryonic stem cells (Fan et al., 2020), mesoderm differentiation, differentiation of embryonic hematopoietic stem/progenitor cells (Kulkeaw et al., 2017), and particularly, cell fate decision of neural crest and development of neural crest derived structures (Bertol et al., 2022; Fan et al., 2021; O’Rourke and Tam, 2002; Soldatov et al., 2019). Snai1 and Snai2 were intensely studied in the specification and migration of neural crest in vertebrates (Aybar et al., 2003; LaBonne and Bronner-Fraser, 2000; Nieto et al., 1994; Tríbulo et al., 2004). Similar to ZEB2, SNAI1, SNAI2 and TWIST1 are mainly involved in regulation of neural development. Correspondingly, expression of
snai1,
snai2 and
twist1 is localized or at least enriched in neural plate and neural crest at the neurodevelopmental stage (Zhang et al., 2017; Cao, 2023) (
Figure 2). Therefore, the contrasting roles of ZEB1 and ZEB2, together with the functions of other EMT-TFs, match exactly with their localized expression patterns that reflect their endogenous functions in different tissue differentiation or specification during embryonic development but not EMT. These functional studies demonstrated that the EMT-TFs are simply developmental factors. A key piece of evidence for these proteins functioning as EMT-TFs is their repression of
E-cadherin transcription. Such a regulatory relationship is also reflected by that
E-cadherin is specifically expressed in epidermis, excluding from the expression domains of EMT-TF genes (
Figure 1 and
Figure 2).
EMT-TFs are generally upregulated or activated in cancer cells and promote cancer progression. By contrast, E-cadherin is generally downregulated in cancer cells and functions as a cancer suppressor. This fashion of expression change in EMT genes is actually within a much broader range of gene expression change in cancer cells. Detailed investigations on cancer genes and the basic property of cancer cells suggest that it is neural stemness, but not the unfathomable mesenchymal state, that is the endower of not only malignant features and tumorigenicity but also pluripotent differentiation potential to cancer cells.
6.3. EMT and EMT-TFs in Cancer: The Tail Wagging the Dog
EMT, which is symbolized by EMT factors, is believed to be a driving force for cancer progression. However, neural specific or enriched expression of EMT-TFs during embryogenesis implies otherwise. My studies revealed that most cancer promoting genes, including those for EMT-TFs, are neural stemness genes or genes with specific or at least enriched expression in embryonic neural cells, and the embryonic neural regulatory networks confer neural stemness to cancer cells (Zhang et al., 2017). Neural stemness contributes to and is required for both tumorigenic and differentiation potentials of tumorigenic cells. Embryonic pluripotent stem cells and induced pluripotent stem cells have been well characterized for their tumorigenicity and pluripotency, and cancer cells are well known for their tumorigenicity. However, increasing data showed that neural stem cells are both pluripotent and tumorigenic, and cancer cells are characteristic of neural stemness and display pluripotent differentiation potential because of differentiation of tissues/cells derived from all three germ layers in xenograft tumors and their capability of contributing to formation of chimeric embryos (Brinster, 1974; Clarke et al., 2000; Chen et al., 2021; Cooper and Pinkus, 1977; Gerschenson et al., 1986; Gootwine et al., 1982; Hendrix et al., 2007; Illmensee and Mintz, 1976; Kulesa et al., 2006; Papaioannou et al., 1975; Podesta et al., 1984; Tropepe et al., 2001; Webb et al., 1984; Wells and Miotto, 1986; Xu et al., 2021; Zhang et al., 2017; Zhang et al., 2022). Moreover, loss of pro-differentiation genes leads to acquirement of tumorigenicity and neural stemness in differentiated or tissue stem cells (Li et al., 2020; Southall et al., 2014; Xu et al., 2021). A latest study using genetic mouse models demonstrated that metaplastic tuft cells turn into neural-like progenitor cells in the progression of pancreatic cancer (Salas-Escabillas et al., 2024). Vice versa, loss of neural stemness in cancer cells and neural stem cells via differentiation leads to the loss of both tumorigenicity and pluripotency (Zhang et al., 2017; Chen et al., 2021; Yang et al., 2021; Zhang et al., 2022). It may be argued why neural stemness but not the stemness of embryonic pluripotent cells plays the key role in tumorigenicity and pluripotency. The uniqueness of neural stemness is reflected by that 1) neural genes are the most conserved genes during evolution as compared with non-neural genes since founders of most neural genes have emerged during the transition from unicellularity to multicellularity; 2) the last common unicellular ancestor of metazoans is biased towards a neural state because of over-representation of founders of neural genes in the genome of Monosiga brevicollis, the closest unicellular relative of metazoans; 3) genes for basic functional machineries or developmental programs, such as cell cycle, ribosome, spliceosome, epigenetic modifications, are mostly enriched in embryonic neural cells; 4) as compared with non-neural genes, neural genes are characteristic of over-representation of longer genes with more exons and introns, which can generate more splicing variants and serve as more flexible scaffolds for gene regulation required for differentiation. Contrary to the unknown mesenchymal state and its regulatory networks, the property of neural stem cells is well characterized, and its regulatory networks are composed of more than 5,000 genes that are specific to or enriched in embryonic neural cells (Xu et al., 2021; Cao, 2022). These features together define neural stemness as a pluripotent and highly proliferative state upon which other cell types are derived (Cao, 2022; Chen et al., 2021; Xu et al., 2021). This notion is reinforced by that pluripotency has a unicellular origin (Sogabe et al., 2019) and the default fate of embryonic pluripotent cells is neural stem cells, i.e., the “neural default model” of embryonic pluripotent cells (Grunz and Tacke, 1989; Muñoz-Sanjuán and Brivanlou, 2002; Smukler et al., 2006; Tropepe et al., 2001; Ying et al., 2003). The critical importance of neural stemness in contribution to pluripotency and tumorigenicity was systematically reviewed (Cao, 2022; Cao, 2023).
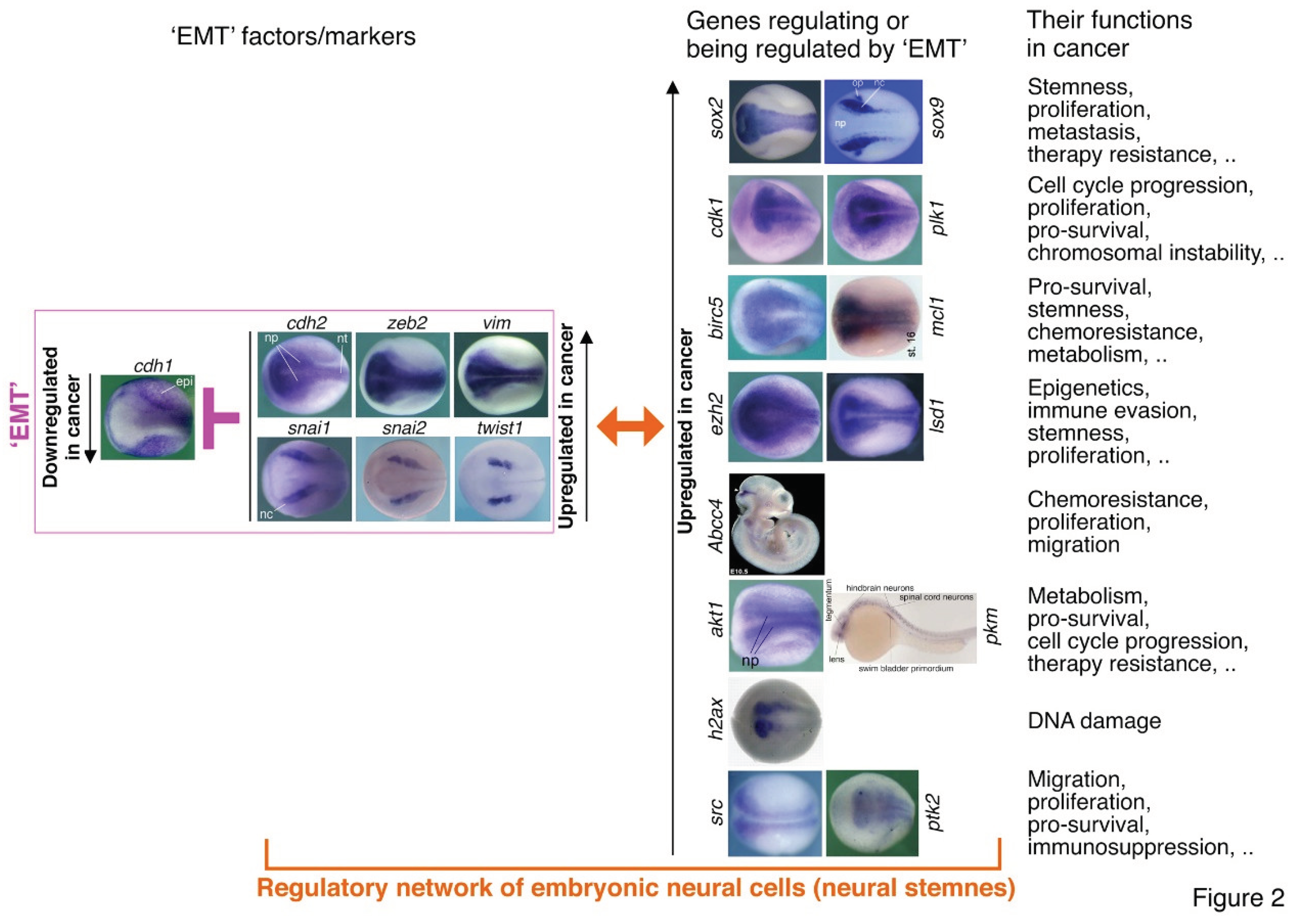
EMT contributing to cancer is mainly evidenced by the correlation between expression of EMT factors in cancer cells and cancer progression, regulation of different features of cancer cells by EMT factors, and regulation of EMT factors by others during cancer progression (Dongre and Weinberg, 2019; Mittal, 2018; Nieto et al., 2016). It is believed that EMT confers stemness to cancer cells but without knowing the concrete mechanisms behind (Lambert and Weinberg, 2021; Mani et al., 2008). A few studies showed the clue that stemness factors SOX2, BMI1, OCT4, or SOX9 can be regulated by ZEB1, SNAI1, SNAI2, or SLUG, thereby promoting not only stemness and metastasis of cancer cells, but also resistance to radio- and chemotherapy (Kurrey et al., 2009; Luanpitpong et al., 2017; Mitra et al., 2018; Wellner et al., 2010). Interestingly, Sox2, Sox9, Bmi1 and Oct4 gene expression is localized to embryonic neural cells during neural induction and early neural development during vertebrate embryogenesis (Cao, 2022) (
Figure 2). Genes promoting cell proliferation, such as
CDK1 and
PLK1, promote or are required for EMT in cancer cells (Iliaki et al., 2021; Ren et al., 2022; Wu et al., 2016). Meanwhile, their expression is enriched in embryonic neural cells (
Figure 2). The pro-survival protein BIRC5 and MCL1, whose genes are enriched in embryonic neural cells (
Figure 2), were shown to regulate EMT in liver and gastric cancer cells (Lee et al., 2015; Xu R et al., 2021). Cancer cells are characteristic of upregulated expression of epigenetic modification factors, such as LSD1 and EZH2. They are not only involved in EMT, but also regulators of immune evasion, immunotherapy resistance and stemness of cancer cells (Burr et al., 2019; Gan et al., 2018; Lin et al., 2010; Liu et al., 2021; Zhou et al., 2020). Genes of most epigenetic factors show enriched expression in embryonic neural cells (Cao, 2022) (
Figure 2). One major mechanism underlying EMT associated chemoresistance is that EMT factors are able to induce transcription of genes encoding ABC transporters, such as ABCC4 (Gan et al., 2018; Saxena et al., 2011), which is localized to the midbrain-hindbrain region of mouse embryo (
Figure 2). PI3K/AKT pathway plays essential roles in regulating EMT-TFs (Larue and Bellacosa, 2005) and cancer metabolism (Hoxhaj and Manning, 2020). PKM2 is involved in the regulation of aerobic glycolysis in cancer. It was shown that stimulation of EMT results in the nuclear translocation of PKM2 in colon cancer cells, which is pivotal in promoting EMT (Hamabe et al., 2014). Genes of Pkm2 and Akt1 exhibit enriched expression in embryonic neural cells (
Figure 2). Chromosomal instability is a hallmark of cancer. EMT is associated with chromosomal instability (Roschke et al., 2008) and the EMT transcription factor TWIST1 induces chromosomal instability and the expression of the DNA damage marker H2AX in cancer cells (Khot et al., 2020). EMT was introduced to cancer research because it may explain cancer metastasis. Src/FAK signaling plays a central role in cancer cell migration via regulating EMT (Avizienyte and Frame, 2005). Accordingly, the genes for H2AX, Src and FAK are enriched in embryonic neural cells (
Figure 2). All the information indicates that neural stemness and its regulatory networks are responsible for different features of cancer cells. EMT factors are a few components of neural regulatory networks, it is rather rational that different components may regulate each other in cancer cells. EMT appearing almighty in the regulation of cancer cell features is merely a fiction by assigning mistakenly the roles of neural stemness to the mythical mesenchymal state.
6.4. EMT Effect in Neural Crest Formation: The Tail Wagging the Dog
Looking back on the EMT effect during neural crest development reveals the same situation. Locating between neural plate and epidermal ectoderm, neural crest is induced by interactions between neural plate and adjacent tissues. Neural crest cells are migratory, pluripotent and share regulatory network with cleavage stage embryos, differentiating into peripheral nervous system and many types of non-neural tissues/cells, such as melanocytes, skeletal and connective tissues, and medulla cells of the adrenal gland, etc. (Buitrago-Delgado et al., 2015; Gilbert and Barresi, 2016; Knecht and Bronner-Fraser, 2002; Pla and Monsoro-Burq, 2018; Selleck and Bronner-Fraser, 1995). The neuroepithelial or neural plate cells are primitive neural stem cells, which are pluripotent and tumorigenic. Once committed to neuronal differentiation, they delaminate and migrate away to form the central nervous system. The property of neural crest cells is ultimately derived from neural plate cells. The typical EMT factors or markers, such as Snai1/2, Twist, Zeb2, Sox9/10, N-Cadherin, Vimentin, etc., are specifically expressed or at least enriched in either neural plate or in neural crest (Cao, 2023) (
Figure 2). This means that migratory behavior of neural crest cells is their intrinsic property. It is really weird that the property of neural crest cells must be explained by the unknown mesenchymal state with the help of genes specific to or enriched in neural crest (Leathers and Rogers, 2022; Piacentino et al., 2020; Szabó and Mayor, 2018).
7. The Confusing EMT-MET Cycles in Developmental Process and Cancer Progression
The mesenchyme and epithelium are considered as the basic cell types that constitute the metazoan embryos (Pérez-Pomares and Muñoz-Chápuli, 2002). Therefore, the developmental process and cancer progression are explained by the EMT-MET cycle. During embryonic development, it is believed that MET operates as early as the 8-cell mouse embryo to form epithelial trophectoderm. In gastrula, EMT drives mesoderm formation. Both EMT and MET are employed during development of definitive embryonic endoderm, which give rise to the gut and internal epithelia of pancreas, liver, and associated glands (Bakir, et al., 2020; Pei et al., 2019). This binary classification of mesenchyme and epithelium and transitions between them mess up the process of progressive differentiation during embryogenesis, which give rise to the large diversity of cell types with specific cellular properties and physiological functions. The EMT-MET cycle describes the normal developmental process, which is generally a unidirectional process of differentiation, as a closed circle formed by adhesive and non-adhesive status, as delineated recently (Thiery et al., 2024). It is hard to understand why the change in cell adhesiveness and shape can cause the change in cellular properties including differentiation status and tissue-specific functions. Perhaps it is more comprehensible that the change in adhesiveness and shape is a consequence of differentiation but not the cause for generation of different cell types.
EMT symbolized by expression of EMT factors during cancer progression has been widely reported. Problems occur when E-cadherin-expressing cells are present at a metastatic site. In the context of EMT, why tumor cells sustain the expression of E-cadherin at a metastatic site remains unclear (Bakir et al., 2020). MET is an explanation of choice. This raises the same question as in embryonic development, in that cells in a tumor are classified as epithelial and mesenchymal, and intermediate states between fully epithelial and mesenchymal states. This EMT-MET cycle does not consider the fact that cancer (tumorigenic) cells exhibit stemness and can differentiate. As mentioned above, the core property of cancer cells is neural stemness, which determines tumorigenicity and pluripotency. Therefore, tumorigenesis represents the process of progressive loss of original cell identity and acquirement of neural stemness, thereby acquiring tumorigenicity and pluripotent differentiation potential (Cao, 2017; Cao, 2022; Cao, 2023). This reminds of embryonic neural induction, a process during which ectodermal cells during gastrulation lose their epidermis fate and gain the fate of neuroectoderm, thereby acquiring pluripotency and tumorigenicity. It further gives rise to the nervous system and other non-neural cells that are essential for the establishment of body axis. Failure of neural induction leads to failure of body axis formation, and ectopic neural induction during gastrulation causes the formation of a secondary body axis, i.e., a conjoined twin. When injected into an early embryo, tumorigenic cells, including embryonic stem cells, neural stem cells, and cancer cells, exhibit pluripotency and differentiate into normal cells under instruction of embryonic inducing signals and integrate into embryonic development, contributing to formation of chimeric embryos; they cannot differentiate into normal adult tissue/organ cells and integrate into tissues/organs because of lacking of inducing signals and thus form xenograft tumors when transplanted into postnatal animals, for example, the nude mice. The mutually exchangeable property of pluripotency and tumorigenicity in embryonic and postnatal stages of animals and human, and the commonality of neural induction during embryogenesis and the neural induction-like process during tumorigenesis suggest that tumors are severely degenerated conjoined twin-like structures formed during postnatal animals and human (Cao, 2023). In fact, it has been well documented that different types of cells and expression of different tissue markers are detected in different tumors, including the epithelial and mesenchymal cells and their markers. The so-called tumor phenotypic heterogeneity is at least partially the result of differentiation of cancer cells, either at the primary or metastatic site (Cao, 2022; Cao, 2023, and references therein). Two studies at the beginning stage of EMT research proposed that epithelial and mesenchymal cells within a tumor are not generated from EMT but from cancer stem cell differentiation (Böcker and Stegner, 1975; Ishikawa et al., 1979). Unfortunately, the insightful idea was not considered by mainstream studies and buried deeply under dust over time. In summary, like that EMT-MET cycle cannot be helpful for understanding embryogenesis, it cannot help to understand cancer progression.
8. Conclusion and Perspective
About a half century of EMT research, it is unfortunate to find there is almost no basis on which the EMT concept can be established. First, epithelial and mesenchymal cells being classified as two cell types is not appropriate. In general, cells within a type exhibit similar structure, function and regulatory networks that are distinct from cells in other types (Arendt, 2008; Zeng, 2022). However, epithelial and mesenchymal cells are defined according to their shapes and adhesiveness only, and both include many different cell types from embryonic stage to adulthood. It is difficult to generalize their cell state from the heterogeneity in epithelial and mesenchymal cells, and find suitable markers or the core regulatory networks to distinguish these cells from other cell types ambiguously. EMT and MET are involved in regulation of different cell features during embryogenesis and particularly tumorigenesis, including stemness, proliferation, mobility, metabolism, etc. How the change in cell shape and adhesiveness can confer different cell features in different stage of embryogenesis and adulthood and in cancer cells is fully a mystery. Cells of a particular type exhibit features like shape, adhesiveness, mobility, and physiological functions. They are coupled together and defined by cell type-specific regulatory networks. Therefore, interpretation of change in cell property or state solely by the change in shape and adhesiveness is a sheer bias. It is even not certain whether the change in cell shape and adhesiveness is the cause or consequence of developmental or pathological process. The so-called EMT markers and factors are not genuinely or universally mesenchymal. The history of EMT research raises the concern whether the gene-centric or cell-centric way is better for understanding developmental and cancer biology. The former has achieved great successes, but also failed in numerous cases. A cell state/property is determined by concerted co-regulation of many genes, and individual genes may not directly reflect or determine cellular phenotypes and functions. Therefore, a cell-centric view might be a better choice for understanding life and pathological processes. Literally, EMT sounds like cell-centric concept. But the epithelial and mesenchymal states are ill-defined, and it uses a gene-centric way to answer questions in development and pathology.
The core EMT-TFs reveal actually the critical importance of neural stemness rather than the mesenchymal state in determination of cell properties. The privilege of neural stemness is predestined by the evolutionary advantage of neural genes and neural state. In contrast to the unknown mesenchymal state, the property of neural stem cells and regulatory networks of neural stemness has been largely characterized. The EMT effect during neural crest formation and cancer progression is a wrong attribution of the role of neural stemness to mesenchymal state. It is time to face the chaos in EMT research and re-evaluate its significance as a scientifically meaningful concept, so that precious research funds and the passion of researchers could be invested more effectively on the pursuit of scientific truth. Moreover, the importance of neural stemness in determining pluripotency and tumorigenicity suggests that studies on developmental and cancer biology might benefit more from the research focus on neural stemness.
Author Contributions
Ying Cao conceived and wrote the review.
Conflicts of Interest
The author declares no potential competing interests.
References
- Aiello NM, Brabletz T, Kang Y, Nieto MA, Weinberg RA, Stanger BZ. Upholding a role for EMT in pancreatic cancer metastasis. Nature. 2017 Jul 5;547(7661):E7-E8. [CrossRef]
- Akhurst RJ. From shape-shifting embryonic cells to oncology: The fascinating history of epithelial mesenchymal transition. Semin Cancer Biol. 2023 Nov;96:100-114. [CrossRef]
- Anbara T, Sharifi M, Aboutaleb N. Endothelial to Mesenchymal Transition in the Cardiogenesis and Cardiovascular Diseases. Curr Cardiol Rev. 2020;16(4):306-314. [CrossRef]
- Arendt D. The evolution of cell types in animals: emerging principles from molecular studies. Nat Rev Genet. 2008 Nov;9(11):868-82. [CrossRef]
- Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005 Oct;17(5):542-7. [CrossRef]
- Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003 Feb;130(3):483-94. [CrossRef]
- Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002 Aug 1;115(Pt 15):3193-206. [CrossRef]
- Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020 Oct;30(10):764-776. [CrossRef]
- Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014 Aug;69(8):760-5. [CrossRef]
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000 Feb;2(2):84-9. [CrossRef]
- Bertol JW, Johnston S, Ahmed R, Xie VK, Hubka KM, Cruz L, Nitschke L, Stetsiv M, Goering JP, Nistor P, Lowell S, Hoskens H, Claes P, Weinberg SM, Saadi I, Farach-Carson MC, Fakhouri WD. TWIST1 interacts with β/δ-catenins during neural tube development and regulates fate transition in cranial neural crest cells. Development. 2022 Aug 1;149(15):dev200068. [CrossRef]
- Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010 Nov;120(11):4040-54. [CrossRef]
- Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. Bioessays. 1995 Feb;17(2):97-9. [CrossRef]
- Bischoff J. Endothelial-to-Mesenchymal Transition. Circ Res. 2019 Apr 12;124(8):1163-1165. [CrossRef]
- Bluemink JG, Van Maurik P, Lawson KA. Intimate cell contacts at the epithelial/mesenchymal interface in embryonic mouse lung. J Ultrastruct Res. 1976 May;55(2):257-70. [CrossRef]
- Böcker W, Stegner HE. A light and electron microscopic study of endometrial sarcomas of the uterus. Virchows Arch A Pathol Anat Histol. 1975 Oct 20;368(2):141-56. [CrossRef]
- Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003 Feb 1;116(Pt 3):499-511. [CrossRef]
- Brabletz S, Schuhwerk H, Brabletz T, Stemmler MP. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 2021 Sep 15;40(18):e108647. [CrossRef]
- Brinster RL. The effect of cells transferred into the mouse blastocyst on subsequent development. J Exp Med. 1974 Oct 1;140(4):1049-56. [CrossRef]
- Bronner ME. Formation and migration of neural crest cells in the vertebrate embryo. Histochem Cell Biol. 2012 Aug;138(2):179-86. [CrossRef]
- Buitrago-Delgado E, Nordin K, Rao A, Geary L, LaBonne C. NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science. 2015 Jun 19;348(6241):1332-5. [CrossRef]
- Burr ML, Sparbier CE, Chan KL, Chan YC, Kersbergen A, Lam EYN, Azidis-Yates E, Vassiliadis D, Bell CC, Gilan O, Jackson S, Tan L, Wong SQ, Hollizeck S, Michalak EM, Siddle HV, McCabe MT, Prinjha RK, Guerra GR, Solomon BJ, Sandhu S, Dawson SJ, Beavis PA, Tothill RW, Cullinane C, Lehner PJ, Sutherland KD, Dawson MA. An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell. 2019 Oct 14;36(4):385-401.e8. [CrossRef]
- Campbell K, Casanova J. A common framework for EMT and collective cell migration. Development. 2016 Dec 1;143(23):4291-4300. [CrossRef]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000 Feb;2(2):76-83. [CrossRef]
- Cao Y. Tumorigenesis as a process of gradual loss of original cell identity and gain of properties of neural precursor/progenitor cells. Cell Biosci. 2017 Nov 7;7:61. [CrossRef]
- Cao Y. Neural is Fundamental: Neural Stemness as the Ground State of Cell Tumorigenicity and Differentiation Potential. Stem Cell Rev Rep. 2022 Jan;18(1):37-55. [CrossRef]
- Cao Y. Neural induction drives body axis formation during embryogenesis, but a neural induction-like process drives tumorigenesis in postnatal animals. Front Cell Dev Biol. 2023 May 9;11:1092667. [CrossRef]
- Cardiff RD. Epithelial to Mesenchymal Transition Tumors: Fallacious or Snail’s Pace? Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8534-7. [CrossRef]
- Celià-Terrassa T, Jolly MK. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition in Cancer Metastasis. Cold Spring Harb Perspect Med. 2020 Jul 1;10(7):a036905. [CrossRef]
- Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003 Apr 15;101(8):2973-82. [CrossRef]
- Chen L, Zhang M, Fang L, Yang X, Cao N, Xu L, Shi L, Cao Y. Coordinated regulation of the ribosome and proteasome by PRMT1 in the maintenance of neural stemness in cancer cells and neural stem cells. J Biol Chem. 2021 Nov;297(5):101275. [CrossRef]
- Cho HJ, Yoo J. Rho activation is required for transforming growth factor-beta-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol Int. 2007 Oct;31(10):1225-30. [CrossRef]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006 Sep 1;66(17):8319-26. [CrossRef]
- Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011 May;53(5):1685-95. [CrossRef]
- Chua KN, Poon KL, Lim J, Sim WJ, Huang RY, Thiery JP. Target cell movement in tumor and cardiovascular diseases based on the epithelial-mesenchymal transition concept. Adv Drug Deliv Rev. 2011 Jul 18;63(8):558-67. [CrossRef]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H, Lendahl U, Frisén J. Generalized potential of adult neural stem cells. Science. 2000 Jun 2;288(5471):1660-3. [CrossRef]
- Clere N, Renault S, Corre I. Endothelial-to-Mesenchymal Transition in Cancer. Front Cell Dev Biol. 2020 Aug 14;8:747. [CrossRef]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001 Jun;7(6):1267-78. [CrossRef]
- Cooper M, Pinkus H. Intrauterine transplantation of rat basal cell carcinoma as a model for reconversion of malignant to benign growth. Cancer Res. 1977 Aug;37(8 Pt 1):2544-52.
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019 Feb;20(2):69-84. [CrossRef]
- Duband JL, Monier F, Delannet M, Newgreen D. Epithelium-mesenchyme transition during neural crest development. Acta Anat (Basel). 1995;154(1):63-78. [CrossRef]
- Dulbecco R, Henahan M, Bowman M, Okada S, Battifora H, Unger M. Generation of fibroblast-like cells from cloned epithelial mammary cells in vitro: a possible new cell type. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2345-9. [CrossRef]
- Dyche WJ. A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979 Nov;162(2):175-209. [CrossRef]
- Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005 Mar 31;24(14):2375-85. [CrossRef]
- Fabris L, Brivio S, Cadamuro M, Strazzabosco M. Revisiting Epithelial-to-Mesenchymal Transition in Liver Fibrosis: Clues for a Better Understanding of the “Reactive” Biliary Epithelial Phenotype. Stem Cells Int. 2016;2016:2953727. [CrossRef]
- Fan X, Masamsetti VP, Sun JQ, Engholm-Keller K, Osteil P, Studdert J, Graham ME, Fossat N, Tam PP. TWIST1 and chromatin regulatory proteins interact to guide neural crest cell differentiation. Elife. 2021 Feb 8;10:e62873. [CrossRef]
- Fan X, Waardenberg AJ, Demuth M, Osteil P, Sun JQJ, Loebel DAF, Graham M, Tam PPL, Fossat N. TWIST1 Homodimers and Heterodimers Orchestrate Lineage-Specific Differentiation. Mol Cell Biol. 2020 May 14;40(11):e00663-19. [CrossRef]
- Ferguson MW. Palate development. Development. 1988;103 Suppl:41-60. [CrossRef]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015 Nov 26;527(7579):472-6. [CrossRef]
- Fitchett JE, Hay ED. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989 Feb;131(2):455-74. [CrossRef]
- Franke WW, Grund C, Kuhn C, Jackson BW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation. 1982;23(1):43-59. [CrossRef]
- Gan L, Xu M, Hua R, Tan C, Zhang J, Gong Y, Wu Z, Weng W, Sheng W, Guo W. The polycomb group protein EZH2 induces epithelial-mesenchymal transition and pluripotent phenotype of gastric cancer cells by binding to PTEN promoter. J Hematol Oncol. 2018 Jan 15;11(1):9. [CrossRef]
- Gavrilović J, Moens G, Thiery JP, Jouanneau J. Expression of transfected transforming growth factor alpha induces a motile fibroblast-like phenotype with extracellular matrix-degrading potential in a rat bladder carcinoma cell line. Cell Regul. 1990 Dec;1(13):1003-14. [CrossRef]
- Gerschenson M, Graves K, Carson SD, Wells RS, Pierce GB. Regulation of melanoma by the embryonic skin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7307-10. [CrossRef]
- Gilbert SF, Barresi MJ. “Neural crest cells and axonal specificity,” in Developmental biology (Sunderland, Massachusetts, United States: Sinauer Associates, Inc. 2016), 463-487.
- Gootwine E, Webb CG, Sachs L. Participation of myeloid leukaemic cells injected into embryos in haematopoietic differentiation in adult mice. Nature. 1982 Sep 2;299(5878):63-5. [CrossRef]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982 Oct;95(1):333-9. [CrossRef]
- Greenburg G, Hay ED. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev Biol. 1986 Jun;115(2):363-79. [CrossRef]
- Greenburg G, Hay ED. Cytoskeleton and thyroglobulin expression change during transformation of thyroid epithelium to mesenchyme-like cells. Development. 1988 Mar;102(3):605-22. [CrossRef]
- Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor Budding: The Name is EMT. Partial EMT. J Clin Med. 2016 Apr 29;5(5):51. [CrossRef]
- Grunz H, Tacke L. Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ Dev. 1989 Dec;28(3):211-7. [CrossRef]
- Haensel D, Dai X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev Dyn. 2018 Mar;247(3):473-480. [CrossRef]
- Haerinck J, Goossens S, Berx G. The epithelial-mesenchymal plasticity landscape: principles of design and mechanisms of regulation. Nat Rev Genet. 2023 Sep;24(9):590-609. [CrossRef]
- Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002 Mar 15;62(6):1613-8.
- Hamabe A, Konno M, Tanuma N, Shima H, Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, Yamamoto H, Doki Y, Mori M, Ishii H. Role of pyruvate kinase M2 in transcriptional regulation leading to epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2014 Oct 28;111(43):15526-31. [CrossRef]
- Hartwell KA, Muir B, Reinhardt F, Carpenter AE, Sgroi DC, Weinberg RA. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A. 2006 Dec 12;103(50):18969-74. [CrossRef]
- Hay ED. The fine structure of blastema cells and differentiating cartilage cells in regenerating limbs of Amblystoma larvae. J Biophys Biochem Cytol. 1958 Sep 25;4(5):583-91. [CrossRef]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel). 1995;154(1):8-20. [CrossRef]
- Hay ED, Fischman DA. Origin of the blastema in regenerating limbs of the newt Triturus viridescens. An autoradiographic study using tritiated thymidine to follow cell proliferation and migration. Dev Biol. 1961 Feb;3:26-59. [CrossRef]
- Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995 Oct;26(4):678-90. [CrossRef]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007 Apr;7(4):246-55. [CrossRef]
- Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020 Feb;20(2):74-88. [CrossRef]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010 Jan;176(1):85-97. [CrossRef]
- Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008 Mar 6;2(3):284-91. [CrossRef]
- Iliaki S, Beyaert R, Afonina IS. Polo-like kinase 1 (PLK1) signaling in cancer and beyond. Biochem Pharmacol. 2021 Nov;193:114747. [CrossRef]
- Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976 Feb;73(2):549-53. [CrossRef]
- Ishikawa S, Kaneko H, Sumida T, Sekiya M. Ultrastructure of mesodermal mixed tumor of the uterus. Acta Pathol Jpn. 1979 Sep;29(5):801-9. [CrossRef]
- Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E, Onuchic JN, Levine H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front Oncol. 2015 Jul 20;5:155. [CrossRef]
- Jolly MK, Ward C, Eapen MS, Myers S, Hallgren O, Levine H, Sohal SS. Epithelial-mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev Dyn. 2018 Mar;247(3):346-358. [CrossRef]
- Jukkola T, Lahti L, Naserke T, Wurst W, Partanen J. FGF regulated gene-expression and neuronal differentiation in the developing midbrain-hindbrain region. Dev Biol. 2006 Sep 1;297(1):141-57. [CrossRef]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003 Dec;112(12):1776-84. [CrossRef]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009 Jun;119(6):1420-8. [CrossRef]
- Kattla JJ, Carew RM, Heljic M, Godson C, Brazil DP. Protein kinase B/Akt activity is involved in renal TGF-beta1-driven epithelial-mesenchymal transition in vitro and in vivo. Am J Physiol Renal Physiol. 2008 Jul;295(1):F215-25. [CrossRef]
- Kedinger M, Simon PM, Grenier JF, Haffen K. Role of epithelial--mesenchymal interactions in the ontogenesis of intestinal brush-border enzymes. Dev Biol. 1981 Sep;86(2):339-47. [CrossRef]
- Khot M, Sreekumar D, Jahagirdar S, Kulkarni A, Hari K, Faseela EE, Sabarinathan R, Jolly MK, Sengupta K. Twist1 induces chromosomal instability (CIN) in colorectal cancer cells. Hum Mol Genet. 2020 Jun 27;29(10):1673-1688. [CrossRef]
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006 May;7(5):360-72. [CrossRef]
- Kisseleva T, Brenner DA. Is it the end of the line for the EMT? Hepatology. 2011 May;53(5):1433-5. [CrossRef]
- Knecht AK, Bronner-Fraser M. Induction of the neural crest: a multigene process. Nat Rev Genet. 2002 Jun;3(6):453-61. [CrossRef]
- Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ, Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010 Aug;177(2):632-43. [CrossRef]
- Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011 Feb;121(2):468-74. [CrossRef]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3752-7. [CrossRef]
- Kulkeaw K, Inoue T, Iino T, Tani K, Akashi K, Speck NA, Nakanishi Y, Sugiyama D. Twist1 regulates embryonic hematopoietic differentiation through binding to Myb and Gata2 promoter regions. Blood Adv. 2017 Aug 31;1(20):1672-1681. [CrossRef]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009 Sep;27(9):2059-68. [CrossRef]
- LaBonne C, Bronner-Fraser M. Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev Biol. 2000 May 1;221(1):195-205. [CrossRef]
- Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell. 2017 Feb 9;168(4):670-691. [CrossRef]
- Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021 May;21(5):325-338. [CrossRef]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014 Mar;15(3):178-96. [CrossRef]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005 Nov 14;24(50):7443-54. [CrossRef]
- Leathers TA, Rogers CD. Time to go: neural crest cell epithelial-to-mesenchymal transition. Development. 2022 Aug 1;149(15):dev200712. [CrossRef]
- Lee WS, Kim N, Park YR, Oh HH, Myung E, Kim SH, Yu HM, Kim MY, Oak CY, Chung CY, Park HC, Myung DS, Cho SB, Joo YE. Myeloid cell leukemia-1 promotes epithelial-mesenchymal transition of human gastric cancer cells. Oncol Rep. 2015 Aug;34(2):1011-6. [CrossRef]
- Lee SY, Lau AT, Jeong CH, Shim JH, Kim HG, Kim J, Bode AM, Dong Z. Histone XH2AX is required for Xenopus anterior neural development: critical role of threonine 16 phosphorylation. J Biol Chem. 2010 Sep 17;285(38):29525-34. [CrossRef]
- Lee YH, Saint-Jeannet JP. Sox9 function in craniofacial development and disease. Genesis. 2011 Apr;49(4):200-8. [CrossRef]
- Lei A, Chen L, Zhang M, Yang X, Xu L, Cao N, Zhang Z, Cao Y. EZH2 Regulates Protein Stability via Recruiting USP7 to Mediate Neuronal Gene Expression in Cancer Cells. Front Genet. 2019 May 3;10:422. [CrossRef]
- Lewis PA, Bradley IC, Pizzey AR, Isaacs HV, Evans GJO. N1-Src Kinase Is Required for Primary Neurogenesis in Xenopus tropicalis. J Neurosci. 2017 Aug 30;37(35):8477-8485. [CrossRef]
- Li Y, Lui KO, Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat Rev Cardiol. 2018 Aug;15(8):445-456. [CrossRef]
- Li Z, Guo X, Huang H, Wang C, Yang F, Zhang Y, Wang J, Han L, Jin Z, Cai T, Xi R. A Switch in Tissue Stem Cell Identity Causes Neuroendocrine Tumors in Drosophila Gut. Cell Rep. 2020 Feb 11;30(6):1724-1734.e4. [CrossRef]
- Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012 Oct;139(19):3471-86. [CrossRef]
- Lin T, Ponn A, Hu X, Law BK, Lu J. Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene. 2010 Sep 2;29(35):4896-904. [CrossRef]
- Liu Y, Debo B, Li M, Shi Z, Sheng W, Shi Y. LSD1 inhibition sustains T cell invigoration with a durable response to PD-1 blockade. Nat Commun. 2021 Nov 24;12(1):6831. [CrossRef]
- Loeffler I, Wolf G. Epithelial-to-Mesenchymal Transition in Diabetic Nephropathy: Fact or Fiction? Cells. 2015 Oct 9;4(4):631-52. [CrossRef]
- Lu W, Kang Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev Cell. 2019 May 6;49(3):361-374. [CrossRef]
- Luanpitpong S, Li J, Manke A, Brundage K, Ellis E, McLaughlin SL, Angsutararux P, Chanthra N, Voronkova M, Chen YC, Wang L, Chanvorachote P, Pei M, Issaragrisil S, Rojanasakul Y. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene. 2016 Jun 2;35(22):2824-33. [CrossRef]
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev Biol. 1996 Aug 1;177(2):580-9. [CrossRef]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008 May 16;133(4):704-15. [CrossRef]
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007 Jun 12;104(24):10069-74. [CrossRef]
- Marconi GD, Fonticoli L, Rajan TS, Pierdomenico SD, Trubiani O, Pizzicannella J, Diomede F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells. 2021 Jun 23;10(7):1587. [CrossRef]
- Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat (Basel). 1996;156(3):173-86. [CrossRef]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977 Jan;148(1):85-119. [CrossRef]
- Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016 Feb;17(2):97-109. [CrossRef]
- McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA, Welm AL, Ford HL. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 2009 Sep;119(9):2663-77. [CrossRef]
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008 Nov;19(11):4875-87. [CrossRef]
- Meier S, Hay ED. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249-70. [CrossRef]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994 Dec;127(6 Pt 2):2021-36. [CrossRef]
- Mitra T, Prasad P, Mukherjee P, Chaudhuri SR, Chatterji U, Roy SS. Stemness and chemoresistance are imparted to the OC cells through TGFβ1 driven EMT. J Cell Biochem. 2018 Jul;119(7):5775-5787. [CrossRef]
- Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018 Jan 24;13:395-412. [CrossRef]
- Munker S, Wu YL, Ding HG, Liebe R, Weng HL. Can a fibrotic liver afford epithelial-mesenchymal transition? World J Gastroenterol. 2017 Jul 14;23(26):4661-4668. [CrossRef]
- Muñoz-Sanjuán I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002 Apr;3(4):271-80. [CrossRef]
- Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev Cell. 2004 Sep;7(3):425-38. [CrossRef]
- Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ. 2008 Dec;50(9):755-66. [CrossRef]
- Newgreen DF, Ritterman M, Peters EA. Morphology and behaviour of neural crest cells of chick embryo in vitro. Cell Tissue Res. 1979 Nov;203(1):115-40. [CrossRef]
- Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013 Nov 8;342(6159):1234850. [CrossRef]
- Nieto MA, Bennett MF, Sargent MG, Wilkinson DG. Cloning and developmental expression of Sna, a murine homologue of the Drosophila snail gene. Development. 1992 Sep;116(1):227-37. [CrossRef]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016 Jun 30;166(1):21-45. [CrossRef]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994 May 6;264(5160):835-9. [CrossRef]
- Ninfali C, Siles L, Esteve-Codina A, Postigo A. The mesodermal and myogenic specification of hESCs depend on ZEB1 and are inhibited by ZEB2. Cell Rep. 2023 Oct 31;42(10):113222. [CrossRef]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103 Suppl:121-40. [CrossRef]
- Ocaña OH, Córcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012 Dec 11;22(6):709-24. [CrossRef]
- O’Rourke MP, Tam PP. Twist functions in mouse development. Int J Dev Biol. 2002;46(4):401-13.
- Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, Ewald AJ. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019 Sep;573(7774):439-444. [CrossRef]
- Pagan R, Martín I, Llobera M, Vilaró S. Epithelial-mesenchymal transition of cultured rat neonatal hepatocytes is differentially regulated in response to epidermal growth factor and dimethyl sulfoxide. Hepatology. 1997 Mar;25(3):598-606. [CrossRef]
- Pandolfi L, Bozzini S, Frangipane V, Percivalle E, De Luigi A, Violatto MB, Lopez G, Gabanti E, Carsana L, D’Amato M, Morosini M, De Amici M, Nebuloni M, Fossali T, Colombo R, Saracino L, Codullo V, Gnecchi M, Bigini P, Baldanti F, Lilleri D, Meloni F. Neutrophil Extracellular Traps Induce the Epithelial-Mesenchymal Transition: Implications in Post-COVID-19 Fibrosis. Front Immunol. 2021 Jun 14;12:663303. [CrossRef]
- Papaioannou VE, McBurney MW, Gardner RL, Evans MJ. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70-73. [CrossRef]
- Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019 Mar;29(3):212-226. [CrossRef]
- Pei D, Shu X, Gassama-Diagne A, Thiery JP. Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol. 2019 Jan;21(1):44-53. [CrossRef]
- Pérez-Pomares JM, Muñoz-Chápuli R. Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in Metazoans. Anat Rec. 2002 Nov 1;268(3):343-51. [CrossRef]
- Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001 Jul 20;276(29):27424-31. [CrossRef]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287-335. [CrossRef]
- Piacentino ML, Li Y, Bronner ME. Epithelial-to-mesenchymal transition and different migration strategies as viewed from the neural crest. Curr Opin Cell Biol. 2020 Oct;66:43-50. [CrossRef]
- Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999 Dec;112 ( Pt 24):4557-68. [CrossRef]
- Piera-Velazquez S, Jimenez SA. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev. 2019 Apr 1;99(2):1281-1324. [CrossRef]
- Pla P, Monsoro-Burq AH. The neural border: Induction, specification and maturation of the territory that generates neural crest cells. Dev Biol. 2018 Dec 1;444 Suppl 1:S36-S46. [CrossRef]
- Podesta AH, Mullins J, Pierce GB, Wells RS. The neurula stage mouse embryo in control of neuroblastoma. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7608-11. [CrossRef]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009 Apr;9(4):265-73. [CrossRef]
- Popov Y, Schuppan D. Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive? Gastroenterology. 2010 Sep;139(3):722-5. [CrossRef]
- Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008 Nov 4;99(9):1375-9. [CrossRef]
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516-20. [CrossRef]
- Ren L, Yang Y, Li W, Zheng X, Liu J, Li S, Yang H, Zhang Y, Ge B, Zhang S, Fu W, Dong D, Du G, Wang J. CDK1 serves as a therapeutic target of adrenocortical carcinoma via regulating epithelial-mesenchymal transition, G2/M phase transition, and PANoptosis. J Transl Med. 2022 Oct 2;20(1):444. [CrossRef]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):E1475-83. [CrossRef]
- Romano S, Tufano M, D’Arrigo P, Vigorito V, Russo S, Romano MF. Cell stemness, epithelial-to-mesenchymal transition, and immunoevasion: Intertwined aspects in cancer metastasis. Semin Cancer Biol. 2020 Feb;60:181-190. [CrossRef]
- Roschke AV, Glebov OK, Lababidi S, Gehlhaus KS, Weinstein JN, Kirsch IR. Chromosomal instability is associated with higher expression of genes implicated in epithelial-mesenchymal transition, cancer invasiveness, and metastasis and with lower expression of genes involved in cell cycle checkpoints, DNA repair, and chromatin maintenance. Neoplasia. 2008 Nov;10(11):1222-30. [CrossRef]
- Rout-Pitt N, Farrow N, Parsons D, Donnelley M. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res. 2018 Jul 18;19(1):136. [CrossRef]
- Saitoh M. Transcriptional regulation of EMT transcription factors in cancer. Semin Cancer Biol. 2023 Dec;97:21-29. [CrossRef]
- Salas-Escabillas DJ, Hoffman MT, Moore JS, Brender SM, Wen HJ, Benitz S, Davis ET, Long D, Wombwell AM, Steele NG, Sears RC, Matsumoto I, DelGiorno KE, Crawford HC. Metaplastic tuft cells transdifferentiate to neural-like progenitor cells in the progression of pancreatic cancer. bioRxiv [Preprint]. 2024 Feb 14:2024.02.12.579982. [CrossRef]
- Sánchez-Tilló E, Pedrosa L, Vila I, Chen Y, Győrffy B, Sánchez-Moral L, Siles L, Lozano JJ, Esteve-Codina A, Darling DS, Cuatrecasas M, Castells A, Maurel J, Postigo A. The EMT factor ZEB1 paradoxically inhibits EMT in BRAF-mutant carcinomas. JCI Insight. 2023 Oct 23;8(20):e164629. [CrossRef]
- Sato R, Semba T, Saya H, Arima Y. Concise Review: Stem Cells and Epithelial-Mesenchymal Transition in Cancer: Biological Implications and Therapeutic Targets. Stem Cells. 2016 Aug;34(8):1997-2007. [CrossRef]
- Savagner P. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273-300. [CrossRef]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997 Jun 16;137(6):1403-19. [CrossRef]
- Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011 Jul 7;2(7):e179. [CrossRef]
- Sciacovelli M, Frezza C. Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J. 2017 Oct;284(19):3132-3144. [CrossRef]
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development. 1995 Feb;121(2):525-38. [CrossRef]
- Sena E, Bou-Rouphael J, Rocques N, Carron-Homo C, Durand BC. Mcl1 protein levels and Caspase-7 executioner protease control axial organizer cells survival. Dev Dyn. 2020 Jul;249(7):847-866. [CrossRef]
- Sheng G, Thompson E, Newgreen D, Denker HW. Twenty Years on for The Epithelial-Mesenchymal Transition International Association (TEMTIA): An Interview with Co-Founders Erik Thompson and Donald Newgreen. Cells Tissues Organs. 2022;211(2):252-260. [CrossRef]
- Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017 Oct;14(10):611-629. [CrossRef]
- Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010 Sep;139(3):987-98. [CrossRef]
- Semb H, Christofori G. The tumor-suppressor function of E-cadherin. Am J Hum Genet. 1998 Dec;63(6):1588-93. [CrossRef]
- Simons M. Endothelial-to-mesenchymal transition: advances and controversies. Curr Opin Physiol. 2023 Aug;34:100678. [CrossRef]
- Simpson P. Maternal-Zygotic Gene Interactions during Formation of the Dorsoventral Pattern in Drosophila Embryos. Genetics. 1983 Nov;105(3):615-32. [CrossRef]
- Smith DE, Franco del Amo F, Gridley T. Isolation of Sna, a mouse gene homologous to the Drosophila genes snail and escargot: its expression pattern suggests multiple roles during postimplantation development. Development. 1992 Dec;116(4):1033-9. [CrossRef]
- Smukler SR, Runciman SB, Xu S, van der Kooy D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol. 2006 Jan 2;172(1):79-90. [CrossRef]
- Sobrado VR, Moreno-Bueno G, Cubillo E, Holt LJ, Nieto MA, Portillo F, Cano A. The class I bHLH factors E2-2A and E2-2B regulate EMT. J Cell Sci. 2009 Apr 1;122(Pt 7):1014-24. [CrossRef]
- Sogabe S, Hatleberg WL, Kocot KM, Say TE, Stoupin D, Roper KE, Fernandez-Valverde SL, Degnan SM, Degnan BM. Pluripotency and the origin of animal multicellularity. Nature. 2019 Jun;570(7762):519-522. [CrossRef]
- Soldatov R, Kaucka M, Kastriti ME, Petersen J, Chontorotzea T, Englmaier L, Akkuratova N, Yang Y, Häring M, Dyachuk V, Bock C, Farlik M, Piacentino ML, Boismoreau F, Hilscher MM, Yokota C, Qian X, Nilsson M, Bronner ME, Croci L, Hsiao WY, Guertin DA, Brunet JF, Consalez GG, Ernfors P, Fried K, Kharchenko PV, Adameyko I. Spatiotemporal structure of cell fate decisions in murine neural crest. Science. 2019 Jun 7;364(6444):eaas9536. [CrossRef]
- Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol. 2012;28:687-717. [CrossRef]
- Stemmler MP, Hecht A, Kemler R. E-cadherin intron 2 contains cis-regulatory elements essential for gene expression. Development. 2005 Mar;132(5):965-976. [CrossRef]
- Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985 Aug;77:209-23. [CrossRef]
- Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21-27;327(6119):239-42. [CrossRef]
- Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016 Sep;365(3):495-506. [CrossRef]
- Southall TD, Davidson CM, Miller C, Carr A, Brand AH. Dedifferentiation of neurons precedes tumor formation in Lola mutants. Dev Cell. 2014 Mar 31;28(6):685-96. [CrossRef]
- Strobl-Mazzulla PH, Bronner ME. Epithelial to mesenchymal transition: new and old insights from the classical neural crest model. Semin Cancer Biol. 2012 Oct;22(5-6):411-6. [CrossRef]
- Stryjewska A, Dries R, Pieters T, Verstappen G, Conidi A, Coddens K, Francis A, Umans L, van IJcken WF, Berx G, van Grunsven LA, Grosveld FG, Goossens S, Haigh JJ, Huylebroeck D. Zeb2 Regulates Cell Fate at the Exit from Epiblast State in Mouse Embryonic Stem Cells. Stem Cells. 2017 Mar;35(3):611-625. [CrossRef]
- Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017 Jul;11(7):805-823. [CrossRef]
- Sun D, Vanderburg CR, Odierna GS, Hay ED. TGFbeta3 promotes transformation of chicken palate medial edge epithelium to mesenchyme in vitro. Development. 1998 Jan;125(1):95-105. [CrossRef]
- Sun Y, Ma L. A milestone in epithelial-mesenchymal transition. Nat Cell Biol. 2024 Jan;26(1):29-30. [CrossRef]
- Sweney LR, Shapiro BL. Histogenesis of Swiss white mouse secondary palate from nine and one-half days to fifteen and one-half days in utero. I. Epithelial-mesenchymal relationships--light and electron microscopy. J Morphol. 1970 Apr;130(4):435-49. [CrossRef]
- Szabó A, Mayor R. Mechanisms of Neural Crest Migration. Annu Rev Genet. 2018 Nov 23;52:43-63. [CrossRef]
- Takano S, Reichert M, Bakir B, Das KK, Nishida T, Miyazaki M, Heeg S, Collins MA, Marchand B, Hicks PD, Maitra A, Rustgi AK. Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 2016 Jan 15;30(2):233-47. [CrossRef]
- Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987 Jan;99(1):109-26. [CrossRef]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013 Nov;19(11):1438-49. [CrossRef]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005 Jul 15;65(14):5996-6000; discussion 6000-1. [CrossRef]
- Taura K, Iwaisako K, Hatano E, Uemoto S. Controversies over the Epithelial-to-Mesenchymal Transition in Liver Fibrosis. J Clin Med. 2016 Jan 14;5(1):9. [CrossRef]
- Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010 Mar;51(3):1027-36. [CrossRef]
- Tavares AL, Mercado-Pimentel ME, Runyan RB, Kitten GT. TGF beta-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart. Dev Dyn. 2006 Jun;235(6):1589-98. [CrossRef]
- Tavares ALP, Brown JA, Ulrich EC, Dvorak K, Runyan RB. Runx2-I is an Early Regulator of Epithelial-Mesenchymal Cell Transition in the Chick Embryo. Dev Dyn. 2018 Mar;247(3):542-554. [CrossRef]
- Theveneau E, Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci. 2013 Oct;70(19):3481-92. [CrossRef]
- Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012 Jun 1;366(1):34-54. [CrossRef]
- Thiery JP, Sheng G, Shu X, Runyan R. How studies in developmental epithelial-mesenchymal transition and mesenchymal-epithelial transition inspired new research paradigms in biomedicine. Development. 2024 Feb 1;151(3):dev200128. [CrossRef]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009 Nov 25;139(5):871-90. [CrossRef]
- Thisse B, Pflumio S, Fürthauer M, Loppin B, Heyer V, Degrave A, Woehl R, Lux A, Steffan T, Charbonnier XQ, Thisse C. (2001) Expression of the zebrafish genome during embryogenesis (NIH R01 RR15402). ZFIN Direct Data Submission (http://zfin.org).
- Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005 Jul 15;65(14):5991-5; discussion 5995. [CrossRef]
- Trepat X, Chen Z, Jacobson K. Cell migration. Compr Physiol. 2012 Oct;2(4):2369-92. [CrossRef]
- Tríbulo C, Aybar MJ, Sánchez SS, Mayor R. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Dev Biol. 2004 Nov 15;275(2):325-42. [CrossRef]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001 Apr;30(1):65-78. [CrossRef]
- Vallés AM, Boyer B, Badet J, Tucker GC, Barritault D, Thiery JP. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1124-8. [CrossRef]
- van Grunsven LA, Taelman V, Michiels C, Opdecamp K, Huylebroeck D, Bellefroid EJ. deltaEF1 and SIP1 are differentially expressed and have overlapping activities during Xenopus embryogenesis. Dev Dyn. 2006 Jun;235(6):1491-500. [CrossRef]
- Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009 Mar;66(5):773-87. [CrossRef]
- Vincent CT, Fuxe J. EMT, inflammation and metastasis. Semin Cancer Biol. 2017 Dec;47:168-169. [CrossRef]
- Wang C, Kam RK, Shi W, Xia Y, Chen X, Cao Y, Sun J, Du Y, Lu G, Chen Z, Chan WY, Chan SO, Deng Y, Zhao H. The Proto-oncogene Transcription Factor Ets1 Regulates Neural Crest Development through Histone Deacetylase 1 to Mediate Output of Bone Morphogenetic Protein Signaling. J Biol Chem. 2015 Sep 4;290(36):21925-38. [CrossRef]
- Wang X, Zheng M, Liu G, Xia W, McKeown-Longo PJ, Hung MC, Zhao J. Krüppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007 Aug 1;67(15):7184-93. [CrossRef]
- Webb CG, Gootwine E, Sachs L. Developmental potential of myeloid leukemia cells injected into midgestation embryos. Dev Biol. 1984 Jan;101(1):221-4. [CrossRef]
- Wellner U, Brabletz T, Keck T. ZEB1 in Pancreatic Cancer. Cancers (Basel). 2010 Aug 18;2(3):1617-28. [CrossRef]
- Wells RG. The epithelial-to-mesenchymal transition in liver fibrosis: here today, gone tomorrow? Hepatology. 2010 Mar;51(3):737-40. [CrossRef]
- Wells RS, Miotto KA. Widespread inhibition of neuroblastoma cells in the 13- to 17-day-old mouse embryo. Cancer Res. 1986 Apr;46(4 Pt 1):1659-62.
- Williams ED, Gao D, Redfern A, Thompson EW. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. 2019 Dec;19(12):716-732. [CrossRef]
- Wu J, Ivanov AI, Fisher PB, Fu Z. Polo-like kinase 1 induces epithelial-to-mesenchymal transition and promotes epithelial cell motility by activating CRAF/ERK signaling. Elife. 2016 Mar 22;5:e10734. [CrossRef]
- Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004 Sep-Oct;6(5):603-10. [CrossRef]
- Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am J Physiol Gastrointest Liver Physiol. 2013 Dec;305(12):G881-90. [CrossRef]
- Xu L, Zhang M, Shi L, Yang X, Chen L, Cao N, Lei A, Cao Y. Neural stemness contributes to cell tumorigenicity. Cell Biosci. 2021 Jan 19;11(1):21. [CrossRef]
- Xu R, Lin L, Zhang B, Wang J, Zhao F, Liu X, Li Y, Li Y. Identification of prognostic markers for hepatocellular carcinoma based on the epithelial-mesenchymal transition-related gene BIRC5. BMC Cancer. 2021 Jun 10;21(1):687. [CrossRef]
- Xu Y, Kovacic JC. Endothelial to Mesenchymal Transition in Health and Disease. Annu Rev Physiol. 2023 Feb 10;85:245-267. [CrossRef]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004 Jun 25;117(7):927-39. [CrossRef]
- Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RYJ, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagué J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G; EMT International Association (TEMTIA). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020 Jun;21(6):341-352. [CrossRef]
- Yang X, Cao N, Chen L, Liu L, Zhang M, Cao Y. Suppression of Cell Tumorigenicity by Non-neural Pro-differentiation Factors via Inhibition of Neural Property in Tumorigenic Cells. Front Cell Dev Biol. 2021 Sep 14;9:714383. [CrossRef]
- Ye X, Brabletz T, Kang Y, Longmore GD, Nieto MA, Stanger BZ, Yang J, Weinberg RA. Upholding a role for EMT in breast cancer metastasis. Nature. 2017 Jul 5;547(7661):E1-E3. [CrossRef]
- Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015 Nov;25(11):675-686. [CrossRef]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003 Feb;21(2):183-6. [CrossRef]
- Zeng H. What is a cell type and how to define it? Cell. 2022 Jul 21;185(15):2739-2755. [CrossRef]
- Zhang M, Liu Y, Shi L, Fang L, Xu L, Cao Y. Neural stemness unifies cell tumorigenicity and pluripotent differentiation potential. J Biol Chem. 2022 Jul;298(7):102106. [CrossRef]
- Zhang Z, Lei A, Xu L, Chen L, Chen Y, Zhang X, Gao Y, Yang X, Zhang M, Cao Y. Similarity in gene-regulatory networks suggests that cancer cells share characteristics of embryonic neural cells. J Biol Chem. 2017 Aug 4;292(31):12842-12859. [CrossRef]
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–30. [CrossRef]
- Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 Enhances Antigen Presentation, Antitumor Immunity, and Circumvents Anti-PD-1 Resistance in Head and Neck Cancer. Clin Cancer Res. 2020 Jan 1;26(1):290-300. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).