Submitted:
29 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
Statistical Analysis
Treatment Procedure
Results
Progression-Free Survival
Biochemical Relapse-Free Survival
ADT-Free Survival
Polymetastatic-Free Survival
Pattern of Relapse
Propensity Score Analysis
Discussion
Conclusion
References
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J Clin Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, G.; Yang, K. Oligometastasis and oligo-recurrence. Radiat Oncol. 2014, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Ayala-Peacock, D.N.; Lee, J.; Blackstock, A.W.; Okunieff, P.; Sung, M.W.; Weichselbaum, R.R.; Kao, J.; Urbanic, J.J.; Milano, M.T.; Chmura, S.J.; Salama, J.K. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: A multi-institutional pooled analysis. PLoS ONE 2018, 13, e0195149. [CrossRef]
- Willmann, J.; Vlaskou Badra, E.; Adilovic, S.; Christ, S.M.; Ahmadsei, M.; Mayinger, M.; Tanadini-Lang, S.; Guckenberger, M.; Andratschke, N. Distant Metastasis Velocity as a Novel Prognostic Score for Overall Survival After Disease Progression Following Stereotactic Body Radiation Therapy for Oligometastatic Disease. Int J Radiat Oncol Biol Phys. 2022, 114, 871–882. [CrossRef]
- Nicosia, L.; Franceschini, D.; Perrone-Congedi, F.; Molinari, A.; Gerardi, M.A.; Rigo, M.; Mazzola, R.; Perna, M.; Scotti, V.; Fodor, A.; Iurato, A.; Pasqualetti, F.; Gadducci, G.; Chiesa, S.; Niespolo, R.M.; Bruni, A.; Cappelli, A.; D’Angelo, E.; Borghetti, P.; Di Marzo, A.; Ravasio, A.; De Bari, B.; Sepulcri, M.; Aiello, D.; Mortellaro, G.; Sangalli, C.; Franceschini, M.; Montesi, G.; Aquilanti, F.M.; Lunardi, G.; Valdagni, R.; Fazio, I.; Scarzello, G.; Vavassori, V.; Maranzano, E.; Maria Magrini, S.; Arcangeli, S.; Gambacorta, M.A.; Valentini, V.; Paiar, F.; Ramella, S.; Di Muzio, N.G.; Loi, M.; Jereczek-Fossa, B.A.; Casamassima, F.; Osti, M.F.; Scorsetti, M.; Alongi, F. A predictive model of polymetastatic disease from a multicenter large retrospectIve database on colorectal lung metastases treated with stereotactic ablative radiotherapy: The RED LaIT-SABR study. Clin Transl Radiat Oncol. 2022, 39, 100568. [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; Meattini, I.; Méndez Romero, A.; Ricardi, U.; Russell, N.S.; Schanne, D.H.; Scorsetti, M.; Tombal, B.; Verellen, D.; Verfaillie, C.; Ost, P. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [CrossRef]
- Wong, A.C.; Watson, S.P.; Pitroda, S.P., et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 2016, 122, 2242–2250. [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E., et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol 2018, 4, e173501. [CrossRef]
- Gomez, D.R.; Tang, C.; Zhang, J., et al. Local consolidative therapy vs maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol 2019, 37, 1558–1565. [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–58. [CrossRef]
- Decaestecker K, De Meerleer G, Ameye F, Fonteyne V, Lambert B, Joniau S, Delrue L, Billiet I, Duthoy W, Junius S, Huysse, W., Lumen, N., Ost, P. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer 2014, 14, 671. [CrossRef]
- Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, Rowe SP, Ross AE, Gorin MA, Deville C, Greco SC, Wang H, Denmeade SR, Paller CJ, Dipasquale S, DeWeese TL, Song DY, Wang H, Carducci MA, Pienta KJ, Pomper MG, Dicker AP, Eisenberger MA, Alizadeh AA, Diehn, M., Tran PT. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [CrossRef]
- Tang C, Sherry AD, Haymaker C, Bathala T, Liu S, Fellman B, Cohen L, Aparicio A, Zurita AJ, Reuben A, Marmonti E, Chun SG, Reddy JP, Ghia A, McGuire S, Efstathiou E, Wang J, Wang J, Pilie P, Kovitz C, Du W, Simiele SJ, Kumar R, Borghero Y, Shi Z, Chapin B, Gomez D, Wistuba I, Corn PG. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 825–834. [CrossRef]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016 Dec;70(6):926-937. [CrossRef]
- Pastorello, E.; Nicosia, L.; Cuccia, F.; Olivari, L.; Fiorini, M.; Giaj Levra, N.; Mazzola, R.; Ricchetti, F.; Rigo, M.; Ravelli, P.; D’Alessandro, S.; Salgarello, M.; Ruggieri, R.; Alongi, F. PSMA-PET/CT-Based Stereotactic Body Radiotherapy (SBRT) in the Treatment of Uncomplicated Non-Spinal Bone Oligometastases from Prostate Cancer. Cancers 2023, 15, 2800. [Google Scholar] [CrossRef]
- Yeung, A.R.; Deshmukh, S.; Klopp, A.H., et al. Intensity-Modulated Radiation Therapy Reduces Patient-Reported Chronic Toxicity Compared with Conventional Pelvic Radiation Therapy: Updated Results of a Phase III Trial. J Clin Oncol 2022, 40, 3115. [CrossRef] [PubMed]
- Nicosia L, Trapani G, Rigo M, Giaj-Levra N, Mazzola R, Pastorello E, Ricchetti F, Cuccia F, Figlia V, Fiorini M, Alongi, F. 1.5 T MR-Guided Daily Adapted SBRT on Lymph Node Oligometastases from Prostate Cancer. J Clin Med. 2022, 11, 6658. [CrossRef] [PubMed]
- Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, Graefen, M., Montorsi, F., Sun, M. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. Eur Urol. 2015, 68, 325–34. [CrossRef] [PubMed]
- Triggiani L, Alongi F, Buglione M, Detti B, Santoni R, Bruni A, Maranzano E, Lohr F, D’Angelillo R, Magli A, Bonetta A, Mazzola R, Pasinetti N, Francolini G, Ingrosso G, Trippa F, Fersino S, Borghetti P, Ghirardelli P, Magrini SM. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer 2017, 116, 1520–1525. [CrossRef]
- Alongi F, Fersino S, Giaj Levra N, et al. Impact of 18F-Choline PET/CT in the Decision-Making Strategy of Treatment Volumes in Definitive Prostate Cancer Volumetric Modulated Radiation Therapy. Clin Nucl Med. 2015;40(11):e496-e500. [CrossRef]
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999 May 5;281(17):1591-7. [CrossRef]
- Cuccia F, Rigo M, Gurrera D, Nicosia L, Mazzola R, Figlia V, Giaj-Levra N, Ricchetti F, Attinà G, Pastorello E, De Simone A, Naccarato S, Sicignano G, Ruggieri R, Alongi, F. Mitigation on bowel loops daily variations by 1.5-T MR-guided daily-adaptive SBRT for abdomino-pelvic lymph-nodal oligometastases. J Cancer Res Clin Oncol. 2021 Nov;147(11):3269-3277. [CrossRef]
- Nicosia L, Sicignano G, Rigo M, et al. Daily dosimetric variation between image-guided volumetric modulated arc radiotherapy and MR-guided daily adaptive radiotherapy for prostate cancer stereotactic body radiotherapy. Acta Oncol. 2021;60(2):215-221. [CrossRef]
- Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, Corn PG, de Bono JS, Dreicer R, George DJ, Heath EI, Hussain M, Kelly WK, Liu G, Logothetis C, Nanus D, Stein MN, Rathkopf DE, Slovin SF, Ryan CJ, Sartor O, Small EJ, Smith MR, Sternberg CN, Taplin ME, Wilding G, Nelson PS, Schwartz LH, Halabi S, Kantoff PW, Armstrong AJ; Prostate Cancer Clinical Trials Working Group 3. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016 Apr 20;34(12):1402-18. [CrossRef]
- Francolini G, Gaetano Allegra A, Detti B, Di Cataldo V, Caini S, Bruni A, Ingrosso G, D’Angelillo RM, Alitto AR, Augugliaro M, Triggiani L, Parisi S, Facchini G, Banini M, Simontacchi G, Desideri I, Meattini I, Valicenti RK, Livi L; ARTO Working Group members. Stereotactic Body Radiation Therapy and Abiraterone Acetate for Patients Affected by Oligometastatic Castrate-Resistant Prostate Cancer: A Randomized Phase II Trial (ARTO). J Clin Oncol. 2023 Sep 21:JCO2300985. [CrossRef]
- Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, Orecchia R, Casamassima F, Surgo A, Miralbell, R., De Meerleer, G. Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin Oncol (R Coll Radiol). 2016 Sep;28(9):e115-20. [CrossRef]
- Francolini G, Garlatti P, Di Cataldo V, Triggiani L, Simoni N, Detti B, Lorenzetti V, Colombo F, Morelli V, Ganovelli M, Caprara L, Orsatti C, Burchini L, Frosini G, Bertini N, Loi M, Simontacchi G, Greto D, Desideri I, Meattini, I., Livi, L. Pattern of recurrence after stereotactic body radiotherapy for para-aortic oligo-recurrent prostate cancer, a multicentric analysis. Radiol Med. 2023 Aug 19. [CrossRef]
- Rich BJ, Montoya C, Jin WH, Spieler BO, Mahal BA, Delgadillo R, Bilusic M, Abramowitz MC, Pollack, A., Dal Pra, A. Para-Aortic Radiation Therapy for Oligorecurrent Prostate Cancer. Int J Radiat Oncol Biol Phys. 2022, 114, 718-724. [CrossRef]
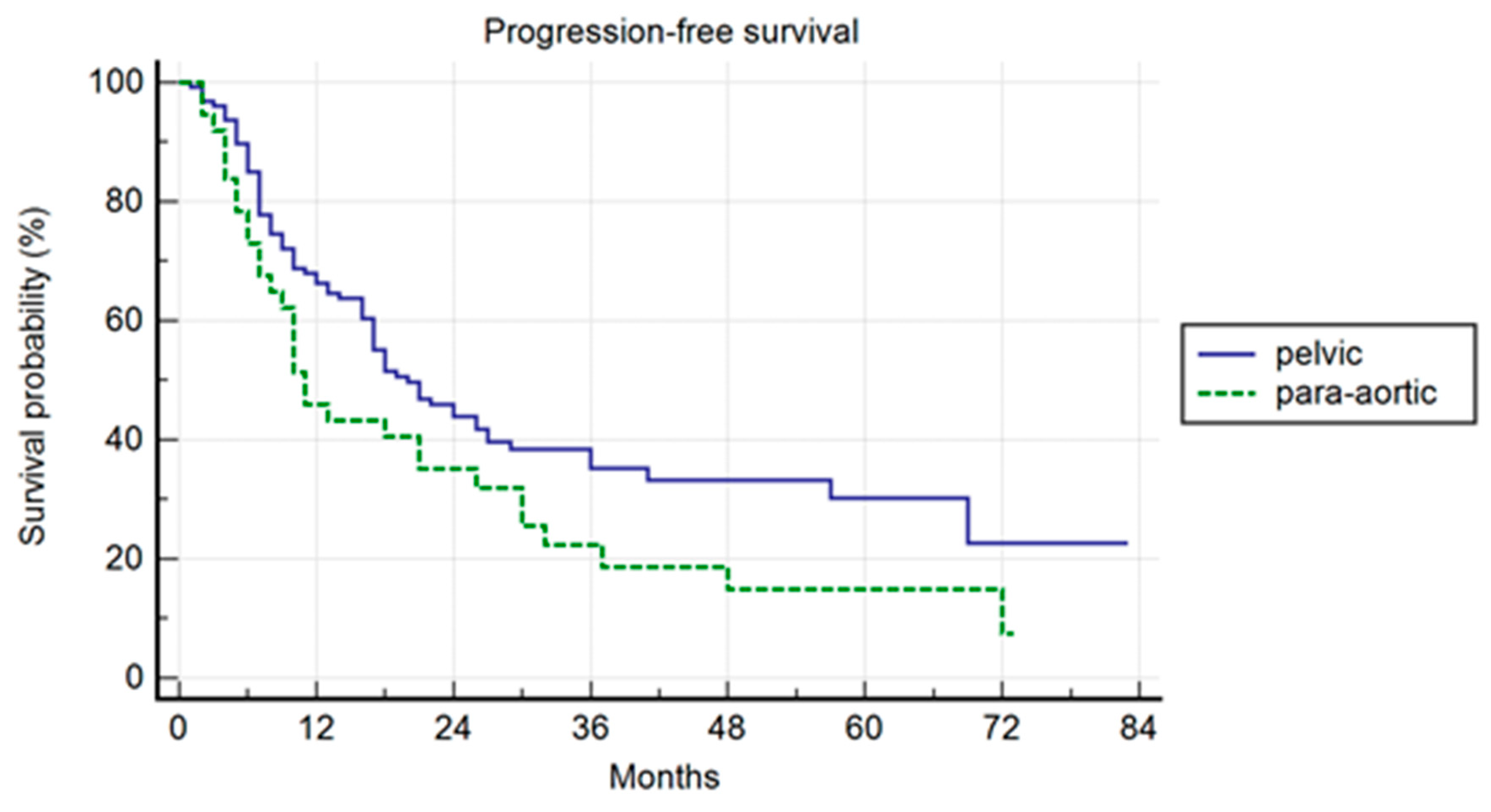
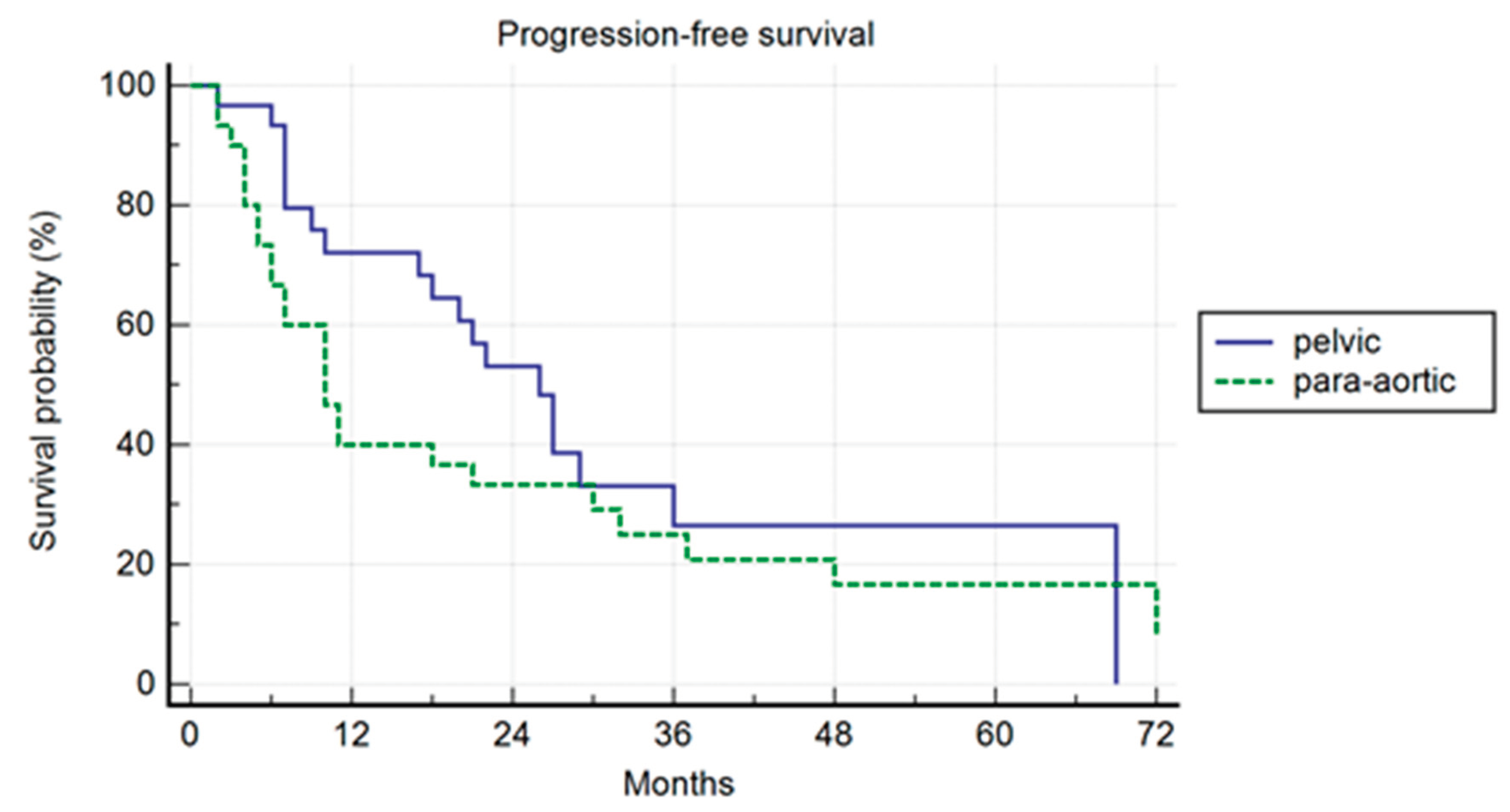
| Pelvic (127) | Para-aortic (37) | p | |
| Age (median) | 71 (range 56-89) | 71 (59-86) | 0.65 |
| Initial PSA | 8.4 (1.3-86) | 10.9 (3.5-50) | 0.76 |
| Class risk at diagnosis | Low: 12 (9.4%)Intermediateo: 30 (23.6%)High. 85 (66.9%) | Low: 2 (5.4%)Intermediate: 6 (16.2%)High: 29 (78.4%) | 0.4 |
| First treatment on primitive site | Surgery: 109 (85.8%)RT: 18 (14.2%) | Surgery: 30 (81,1%)RT: 7 (18.9%) | 0.48 |
| Salvage RT | 80 (73.4%) | 25 (83.3%) | 0.61 |
| Previous pelvic treatments (LAD or pelvic RT) | 60 (47.2%) | 22 (59.4%) | 0.19 |
| Previous ADT | 43 (39.4%) | 20 (54%) | 0.026 |
| PSA at SBRT | 1.16 (0.1-16.4) | 1.33 (0.23-5.25) | 0.48 |
| PSA DT | 4.7 (1-17) | 5.25 (1-17) | 0.84 |
| DFI | 69 (3-246) | 78 (6-223) | 0.98 |
| PET | choline 62 (48.8%)PSMA 65 (51.2%) | choline 15 (40,5%)PSMA 22 (59,5%) | 0,37 |
| Number of treated lymph-nodes( median) | 1 (1-3) | 1 (1-3) | / |
| More than 1 lymph nodes treated | 35 (27,6%) | 18 (48,6%) | 0.016 |
| Median RT dose | 36 (21-45) | 36 (21-45) | 0.40 |
| Number of fractions | 5 (1-6) | 5 (1-6) | 0.21 |
| RT technique | VMAT 112 (88.2%)MR Linac 15 (11.8%) | VMAT 33 (89.2%)MR Linac 4 (10.8%) | 0.86 |
| Cuncurrent ADT | 13 (10.2%) | 5 (13.5%) | 0.57 |
| RT: radiotherapy; LAD: lymphadenectomy; ADT: androgen deprivation therapy; SBRT: stereotactic body radiotherapy; DT: doubling time; DFI: disease-free interval; VMAT: volumetric modulated arc therapy | |||
| Univariate | Multivariate | |
| PSA at SBRT | p= 0.036 | p=0.04 (HR:1.47; IC: 1.01-2.28) |
| PSA DT | p=0.31 | / |
| DFI | p=0.79 | / |
| PET choline vs PSMA | p=0.25 | / |
| Number of lymph-nodes treated (1 vs > 1) | p=0.14 | / |
| Previous pelvic treatments | p=0.67 | / |
| Previous ADT | p=0.17 | / |
| Cuncurrent ADT | p=0.058 | p=0.04 (HR 0.49; IC: 0.25-0.98) |
| Treatment on primitive site (surgery vs RT) | p=0.26 | / |
| Pelvic vs para-aortic | P=0.042 | p=0.06 (HR:1.49; IC: 0.98-2.28) |
| SBRT: stereotactic body radiotherapy; DT: doubling time; DFI: disease-free interval; ADT: androgen deprivation therapy; RT: radiotherapy | ||
| Site of relapse | total | pelvic | Para-aortic | P |
| Prostate bed | 1 | 1 (1,3%) | 0 | / |
| Pelvis | 44 | 40 (51,9%) | 4 (12,9%) | 0.001 |
| Para-aortic nodes | 25 | 13 (16,9%) | 12 (38,7%) | 0.16 |
| Other lymph-nodes | 6 | 3 (3,9%) | 3 (9,6%) | 0.24 |
| Metastases | 32 | 20 (26%) | 12 (38,7%) | 0.2 |
| Total | 108 | 77 | 31 |
| Pelvic (30) | Para-aortic (30) | p | |
| High risk PC | 26 | 26 | 1 |
| PSA at SBRT | 1.09 ng/ml (0.17-4.77) | 1,1 ng/ml (0.23-4,67) | 0.6 |
| Median DFI | 78.5 (11-155) | 79 mesi (11-223) | 1 |
| SBRT on 1 node | 17 | 17 | 1 |
| Surgery as first treatment | 26 | 26 | 1 |
| Previous ADT | 15 | 17 | 0.53 |
| PSA DT months | 3,5 (2-8) | 4,5 (1,5-17) | 0.24 |
| Cuncurrent ADT | 4 | 3 | 0.69 |
| PC: prostate cancer; SBRT: stereotactic body radiotherapy; DFI: disease-free interval; ADT: androgen deprivation therapy; DT: doubling time | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





