Submitted:
28 February 2024
Posted:
28 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microorganism and Glycerol Source
2.2. Bioreactors
2.3. Fermentation Conditions
2.4. Analytical Methods
2.5. Kinetic Parameters
2.6. Mathematical Modeling of RP
3. Results
3.1. Pilot Scale Fed-Batch Fermentation
3.2. Mathematical Modeling
3.3. Olive Pomace Oil Projections
4. Conclusions
Funding
References
- IEA, Technology Roadmap Biofuels for transport. 2011, International Energy Agency: Paris, France.
- Villadsen, J.; Nielsen, J.; Lidén, G. Bioreaction Engineering Principles. 3 ed. 2011, New York: Springer US. 561.
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Yazdani, S.; Gonzalez, R. Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metab Eng 2008, 10, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Murarka, A. Metabolic flux analysis of fermentative carbon metabolism in Escherichia coli. 2008, Rice University.
- Blattner, F.R. The Complete Genome Sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Murarka, A., et al. Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Applied and Environmental Microbiology 2008, 74, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Abu Tayeh, H.N.; Azaizeh, H.; Gerchman, Y. Circular economy in olive oil production – Olive mill solid waste to ethanol and heavy metal sorbent using microwave pretreatment. Waste Management 2020, 113, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Souilem, S., et al. Chapter 1 - Olive oil production sector: environmental effects and sustainability challenges, in Olive Mill Waste, C.M. Galanakis, Editor. 2017, Academic Press. p. 1-28.
- Gonzalez-Fernandez, I., et al. A critical review on the use of artificial neural networks in olive oil production, characterization and authentication. Critical Reviews in Food Science and Nutrition 2019, 59, 1913–1926. [Google Scholar] [CrossRef]
- Giuffrè, A.M., et al. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. LWT 2020, 117, 108631. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. Journal of Analytical and Applied Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Yücel, Y. Biodiesel production from pomace oil by using lipase immobilized onto olive pomace. Bioresource Technology 2011, 102, 3977–3980. [Google Scholar] [CrossRef]
- Yücel, Y. Optimization of biocatalytic biodiesel production from pomace oil using response surface methodology. Fuel Processing Technology 2012, 99, 97–102. [Google Scholar] [CrossRef]
- Cofré, O., et al. Pilot scale fed-batch fermentation in a closed loop mixed reactor for the biotransformation of crude glycerol into ethanol and hydrogen by Escherichia coli MG1655. Biomass and Bioenergy 2016, 91, 37–47. [Google Scholar] [CrossRef]
- Cofré, O., et al. Optimization of culture media for ethanol production from glycerol by Escherichia coli. Biomass and Bioenergy 2012, 37, 275–281. [Google Scholar] [CrossRef]
- Cintolesi, A., et al. Quantitative analysis of the fermentative metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 2012, 109, 187–98. [Google Scholar] [CrossRef]
- Montgomery, D.C., ed. Design and analysis of experiments. 5 ed. 2001, John Wiley & Sons, Inc.
- Leiva-Candia, D.E., et al. The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar] [CrossRef]
- Mohammadshirazi, A., et al. Energy and cost analyses of biodiesel production from waste cooking oil. Renewable and Sustainable Energy Reviews 2014, 33, 44–49. [Google Scholar] [CrossRef]
- Yuste, A.J.; Dorado, M.P. A Neural Network Approach to Simulate Biodiesel Production from Waste Olive Oil. Energy & Fuels 2006, 20, 399–402. [Google Scholar] [CrossRef]
- Çaynak, S., et al. Biodiesel production from pomace oil and improvement of its properties with synthetic manganese additive. Fuel 2009, 88, 534–538. [Google Scholar] [CrossRef]
- Rajaeifar, M.A., et al. Environmental impact assessment of olive pomace oil biodiesel production and consumption: A comparative lifecycle assessment. Energy 2016, 106, 87–102. [Google Scholar] [CrossRef]
- Miranda, T., et al. Characterization and combustion of olive pomace and forest residue pellets. Fuel Processing Technology 2012, 103, 91–96. [Google Scholar] [CrossRef]
- Tillman, D.A.; Duong, D.N.B.; Harding, N.S. Chapter 4 - Blending Coal with Biomass: Cofiring Biomass with Coal, in Solid Fuel Blending, D.A. Tillman, D.N.B. Duong, and N.S. Harding, Editors. 2012, Butterworth-Heinemann: Boston. p. 125-200.
- Özçelik, A.E.; Acaroğlu, M.; Köse, H. Determination of combustion characteristics of olive pomace biodiesel – Eurodiesel fuel mixtures. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2020, 42, 1476–1489. [Google Scholar] [CrossRef]
- Lama-Muñoz, A., et al. Biodiesel production from olive–pomace oil of steam-treated alperujo. Biomass and Bioenergy 2014, 67, 443–450. [Google Scholar] [CrossRef]
- Ouachab, N.; Tsoutsos, T. Study of the acid pretreatment and biodiesel production from olive pomace oil. Journal of Chemical Technology & Biotechnology 2013, 88, 1175–1181. [Google Scholar] [CrossRef]
- Che, F., et al. Exploring a promising feedstock for biodiesel production in Mediterranean countries: A study on free fatty acid esterification of olive pomace oil. Biomass and Bioenergy 2012, 36, 427–431. [Google Scholar] [CrossRef]
- Hernández, D., et al. Biodiesel production from an industrial residue: Alperujo. Industrial Crops and Products 2014, 52, 495–498. [Google Scholar] [CrossRef]
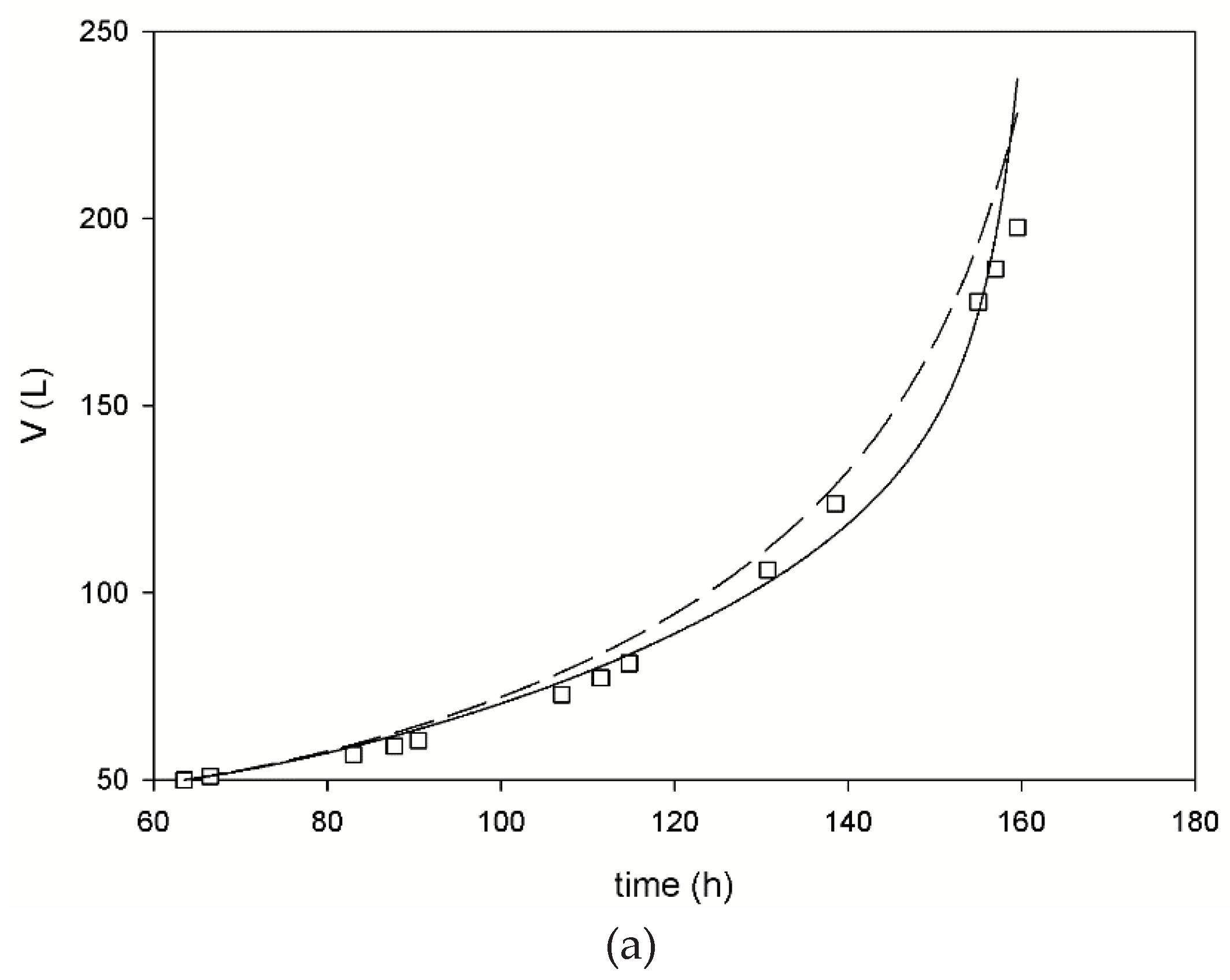
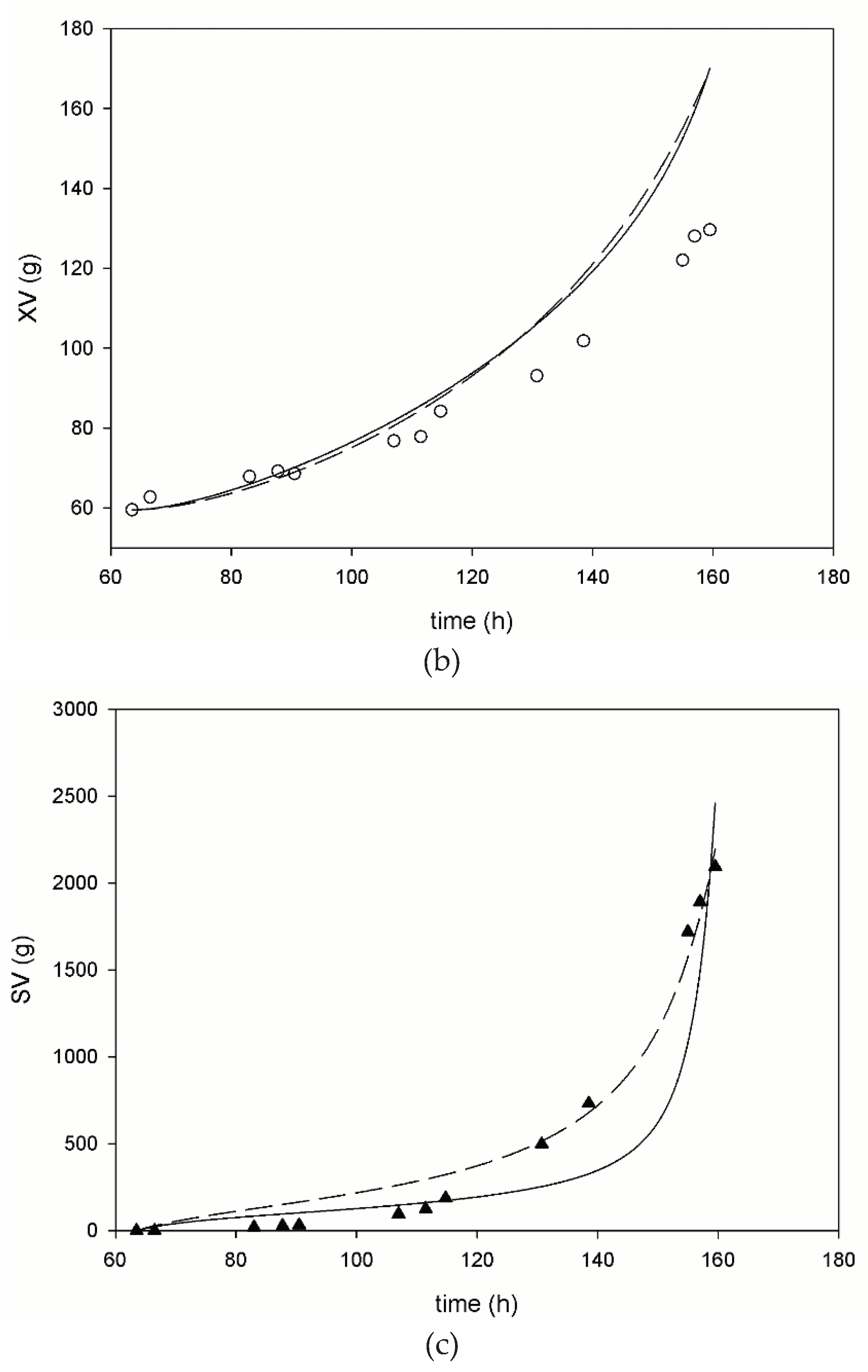
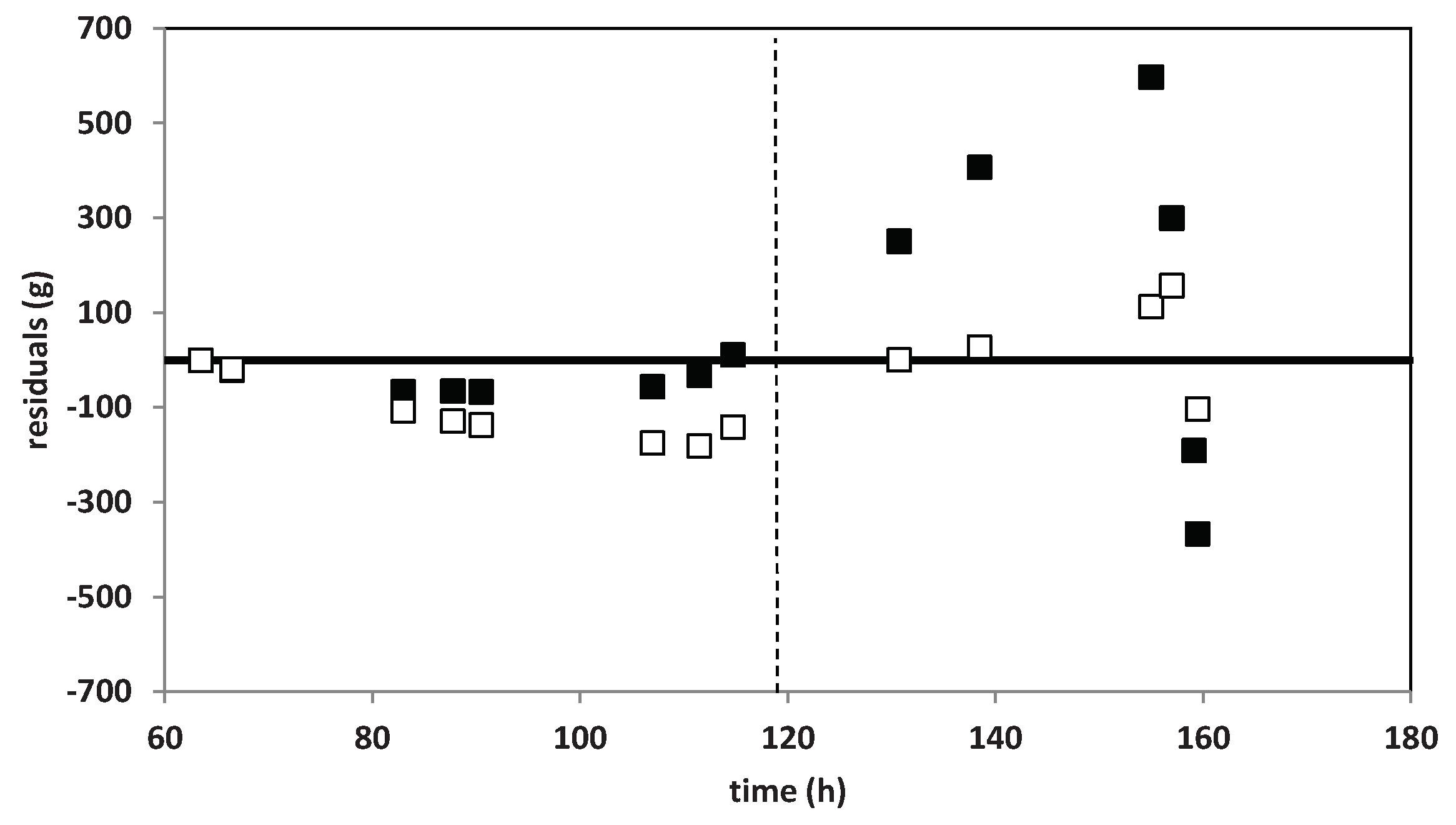
| Fermentation | Duration (h) |
Glycerol consumed (%) | Final Ethanol (g L–1) |
H2 Produced (mmol) |
H2 produced (mmol/g glycerol consumed–1) |
|---|---|---|---|---|---|
| PR | 160 | 52.7 ± 5.2 | 5.4 ± 1.08 | 449.18 ± 21.47 | 0.2 ± 0.01 |
| PR1 | 250 | 100 ± 9.9 | 8.5 ± 1.70 | - | - |
| Scenario | Ks (g L-1) | F0 (L h-1) | Note |
|---|---|---|---|
| E1 | 14.6464a | Determined by adjustment | a: bibliographic data |
| E2 | 6.5645b | Determined by adjustment | b: experimental data |
| E3 | Determined by adjustment | Determined adjustment |
| Scenario | Ks (g L-1) | F0 (L h-1) | Note |
|---|---|---|---|
| E1 | 14,6464a | 0,3691 | a: bibliographic data |
| E2 | 6,5645b | 0,302 | b: experimental data |
| E3 | 10,7703 | 0,3388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





