1. Introduction
Handedness is a complex human aspect, reflecting functional bodily and hemispheric lateralization. Hemispheric lateralization characterizes neurodevelopment and can be influenced by biological and environmental factors, also interacting with the immune system. The assessment of handedness and body laterality preferences is particularly important for the cognitive and behavioral profile of patients with neurological diseases, so it is of interest to clarify the clinical correlates of handedness and the validity of its measures in these patients.
The specific objectives of this study were (a) to evaluate the relationships between hand, foot and eye laterality preferences and clinical and demographic variables in patients with immune-mediated diseases (IM) and other neurological disorders (noIM) and (b) to test the psychometric properties of the Edinburgh Handedness Inventory (EHI), which is the most widely used measure of hand, foot and eye laterality [
1]. Given the complexity of functional body and hemispheric lateralization, a preliminary literature overview addressed the main aspects and determinants of handedness.
2. Materials and Methods
2.1. Overview
The overview considered the existing literature on the relationship between handedness and hemispheric lateralization, the influence of the central nervous system on immune regulation, and the assessment of handedness. The search used the keywords "handedness", "hand, foot, and eye laterality preference or lateralization" and "hemispheric lateralization" combined with "immune-mediated brain diseases", "immune-mediated diseases of the peripheral nervous system", "immune regulation", "brain tumors”, "cerebrovascular diseases", "neurodegenerative diseases", "non-immune-mediated diseases of the peripheral nervous system", "assessment", and "Edinburgh Handedness Inventory" in Medline-PubMed and American Psychological Association-PsycINFO. Peer-reviewed original articles and case reports published in English in the past 50 years, since the EHI was developed, were analyzed.
2.2. Clinical study
2.2.1. Participants
Adult patients with neurological disorders associated with central or peripheral nervous system lesions or without detectable lesions, either inpatient or outpatient, were selected. Exclusion criteria were primary psychiatric illnesses such as bipolar disorder, major depression and psychosis, systemic organ failure, and drug or alcohol abuse. Healthy subjects, selected from hospital staff and patients′ relatives, constituted a control group.
All participants gave their informed consent prior to clinical and instrumental assessment. This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
2.2.2. Assessment
The HEI [
1] was administered to all participants by a neurologist (ARG) during the clinical evaluation, along with clinical history, with special investigation of IM disorders, and family history including left-handedness and ambidexterity.
2.2.3. Statistical analysis
One-way analysis of variance (ANOVA) and chi2 test were used to compare chronological age, sex distribution, tendency to be left-handed or ambidextrous, number of stressful events prior to illness and evaluation in neurological patients and healthy subjects, respectively, and HEI scores between neurological and control groups. One-way ANOVA was also used to compare the age of disease onset and duration of illness between patient groups. ANCOVA with chronological age as a covariate further clarified the results of these comparisons. Factor analysis evaluated the distribution of the 20 hand-related EHI scores. Cronbach′s alpha assessed the internal consistency of all EHI scores.
3. Results
3.1. Overview
3.1.1. Handedness and hemispheric lateralization
Hemispheric lateralization results in functional and structural asymmetries in brain organization for basic and complex behavioural performance [
2]. The most important example of functional lateralization is handedness which is the preference for using one hand for unimanual tasks or the greater efficiency in performing such tasks with one hand [
3,
4,
5]. Regarding the regulation of human behaviour [
6], each hemisphere is dominant in particular functions over the other [
7,
8,
9,
10,
11]. However, this does not strictly correspond to handedness, as a small percentage of left-handed individuals show the kind of lateralization of hemispheric functions (e.g., language) observed in fully right-handed individuals.
Handedness expresses the asymmetry of movement control and the underlying mechanisms of sensorimotor neural systems [
12,
13,
14]. This preference is believed to be based on differences in brain morphology [
15]. Jang et al. [
16] observed that the right putamen and left globus pallidus of non-right-handed people were significantly larger than those of right-handed people and that the degree of hand laterality had a negative correlation with the volume of these nuclei. Notably, the basal ganglia of non-right-handed people were larger than those of right-handed people, suggesting that left-handers had better motor control than right-handers. In this regard, it is known that the basal ganglia, such as the putamen and globus pallidus, play an important role in motor control [
17,
18]. The putamen is involved in motor performance, movement sequences, and motor preparation because of its connections with cortical structures involved in the control of body movements [
19,
20,
21,
22]. The globus pallidus is involved in the subtle and steady regulation of voluntary movements, for example, in fluent speaking and walking [
23,
24]. In patients with Alzheimer′s disease (AD), in addition to the correlation with temporal lobe volume loss, cognitive decline has also been associated with putamen volume loss [
25], which is why Jang et al. [
16] hypothesized that left-handed people, having larger putamen volumes than right-handed people, may be more resistant to cognitive decline in AD.
The development of handedness depends of many interacting factors, such as parental education, cultural influences, genetics, and imitation behaviour [
26,
27,
28,
29]. The fact that environment exerts an influence on hand formation was also hypothesized by genetic studies [
30], while a genetic influence in the process of hand preference was considered in behavioural studies [
26,
27]. The minor lateralization in left-handers than in right-handers, both in terms of brain activation and behaviour [
26,
31,
32,
33,
34], could be explained by the pressure exerted by the environment to induce right-handedness. In addition, some gestures and actions require the use of the right hand even in left-handers, leading to practice with the non-dominant right hand. The greater symmetry in movements resulting from the conflict between the intrinsic dynamics of left-handers and the demands of the environment would also explain the lower levels of asymmetry observed in the brain activation and anatomy of left-handers [
33].
3.1.2. Assessment of handedness
For clinical and research purpose, handedness is classically evaluated by means of personal and familial history and inventories. The Edinburgh Handedness Inventory (EHI) [
1] includes 10 goal-ended hand movements and two movements performed with the eye and foot. As written by Oldfield “Doubtless the inventory is not ideal, but it is simple and provides one quantitative measure of handedness backed by a known distribution of values in a reasonable sized normal population. And it gives some insights into the inter-relationship of individual items of the kind in such devices”. Early studies suggested that the gestures included in the EHI load on a single factor (handedness factor), with the exception of opening a box and manipulating a broom [
34,
35]. Recent studies [
36,
37] have replicated this result, confirming the one-factor solution. In addition, handedness as measured by the EHI was found to be sensitive to sociodemographic and cultural influences [
38].
The EHI is based on self-report, but few studies have compared participants′ responses with those provided by hand performance measures [
39,
40,
41]. Ruck and Schoenemann [
42] showed, in particular, a poor match between EHI scores and Rolyan′s 9-hole board and grip strength scores in 1179 healthy subjects. The EHI is also widely used in neuroimaging studies [
43], although its items may be related to anatomical asymmetries in the brain [
42].
Over the past 50 years, many neurological studies have used the EHI to evaluate the association between handedness and specific conditions such as sleep disorders [
44], migraine [
45], epilepsy [
46] and AD [
47]. Other studies have used the EHI to describe study participants or to assess cognitive profile in neurological [
48,
49], neurosurgical [
50,
51] or psychopathological conditions [
52]. Thus, the literature supports the usefulness of the EHI, although it cannot provide direct information on hemisphere dominance for language and nonverbal functions.
3.1.3. Hemispheric lateralization and immune regulation
The immune system is a powerful self-regulated system that can be influenced by physical and psychological stressors [
53,
54] and environmental stimuli [
55]. The brain influences the immune system mainly through the autonomic nervous system and the neuroendocrine system [
56]. The autonomic nervous system, in its sympathetic and parasympathetic components, directly innervates lymphoid tissues such as the thymus, spleen, lymph nodes, and mesenteric patches [
57], and numerous evidences point to the modulation of immune function by various components of the neuroendocrine system [
58,
59]. This communication is bidirectional, as modulatory action of immune modulators, especially cytokines, on the autonomic and neuroendocrine systems has also been demonstrated [
60].
Because many brain functions are lateralized, the question arises whether the central nervous system can modulate immune responses asymmetrically. In answer to this question, the first line of research comes from animal studies that have demonstrated opposite immunological responses after unilateral brain stimulation or damage [
7,
8,
9,
61,
62]. Depression of immunological parameters, such as natural killer cell activity, T-lymphocyte proliferation, and immunoglobulin G antibody production, was shown after left hemisphere damage, while no immunological change or even improvement in immunological parameters were found after right hemisphere damage [
7,
8,
9,
63]. The functional asymmetry of the immune system and the role played by the brain hemispheres in the development of humoral immune responses have also been demonstrated in F1 mice [
64].
The second line of research stems from Geschwind and Behan′s theory based on the association between human immune-mediated (IM) disorders and left-handedness [
65]. These authors hypothesized that the normal development of the brain hemispheres may be modified by high prenatal testosterone levels. This may promote the growth of right hemisphere regions and slow down homologous left hemisphere regions [
66,
67,
68], thus stimulating an abnormal dominance pattern (non-right-handed and atypical dominance of language and visuospatial). Such abnormal dominance may also predispose to immunological diseases, based on observations of a higher incidence of left-handedness than right-handedness among individuals with developmental and IM disorders [
65,
69]. Geschwind and Behan′s theory [
65] has been confirmed by left-handed population surveys and studies of patients with IM disorders [
65], although left-handedness is not an index of brain dominance [
70]. In fact, studies have shown that women are more likely than men to be affected by IM disorders such as systemic lupus erythematosus (SLE), multiple sclerosis (MS), myasthenia gravis, thyroid disease, arthritis, and topical allergies [
71]. This evidence and, more recently, the involvement of female sex hormones in the pathogenesis of autoimmune diseases [
72] refute the role of testosterone in immune disorders and brain asymmetry. Finally, Meador, De Lecuona, Helman, and Loring [
73] related the side of language dominance to postoperative changes in T-cell indices in patients undergoing epilepsy surgery: there was a reduction in lymphocyte counts, total T lymphocytes, helper T lymphocytes, cytotoxic/suppressor lymphocytes, and total suppressor lymphocytes after surgical resections in the language-dominant hemisphere and an increase in these indices after resections in the nondominant hemisphere.
Another line of research concerns the relationship between handedness and regulators of immunity. Lengen et al. [
74] examined markers of cellular immunity, observing a significant reduction in inflammatory CD3+ T cells (total T cells) and CD4+ T cells (T-helper cells), HLA-Dr (major histocompatibility complex, MHC-II, antigen-presenting cells), and CD19+ (B cells) and CD16/CD57+ (natural killer cells) cells in left-handed compared with right-handed people. In addition, the number of CD3+ T cells predicted left-handedness. MHC plays an important role in autoimmune pathogenesis by discriminating between what belongs to the organism and stimulating no-self cells to trigger an immune response. T lymphocytes that recognize self-antigens are dangerous in that they can trigger autoimmune responses, and abnormal selection of these cells in the thymus in the fetal life can be a pre-condition for the development of autoimmune diseases.
Dopamine is an important neuro-immune regulator that counteracts T-cell function; for example, it is unable to reduce T-cell proliferation, INF-c secretion, and matrix metalloproteinase-9 mRNA production in MS patients [
75]. Other neuro-immune regulators (catecholamine, serotonin, noradrenaline) have been linked to autoimmune pathogenesis in SLE, MS, and rheumatoid arthritis [
76,
77,
78,
79,
80].
3.2. Clinical study
3.2.1. Participants
Three hundred seventy-four patients who had received compulsory schooling, with or without a high school degree, were evaluated and divided into four groups according to the diagnosis provided by the treating neurologist. The first group (IM) included 133 patients with immune-mediated diseases of the central or peripheral nervous system (multiple sclerosis, recurrent optic neuritis, retrobulbar optic neuritis, systemic lupus erythematosus or other cerebral vasculitis, sequelae of post-infectious encephalitis, meningo-radiculo-neuritis, polyneuritis, myasthenia). The second group (noIM_brain) included 139 patients with non-immune-mediated brain diseases (venous angioma, glioma, lymphoma, olivopontocerebellar atrophy, sarcoidosis, pseudobulbar syndrome, epilepsy, sequelae of head trauma, chronic ischemia, spastic paraparesis). The third group (noIM_nobrain) included 47 patients with no immunological diseases of the spinal cord or peripheral nervous system (motor neuron disease, tumor, chronic cervical or lumbar radiculopathy, diabetic polyneuritis, cervical myelopathy), while the fourth group (Mix) included 24 patients with mixed disorders without detectable central and peripheral nervous system lesions (dizziness, headache including tension headache, migraine and trigeminal neuralgia, lipotimia, anxiety or depression). Thirty-one healthy subjects served as controls.
Table 1 summarizes the clinical and demographic data of the participants.
Sex distribution differed among the five groups [chi2(4)=6, p=0.003] due to more women in the control and IM groups. There were significant differences in chronological age [F(4,369)=5.09, p<0.001] and number of stressful events prior to illness [F(4,369)=3.97, p=0.004]. The post-hoc test showed that, compared with noIM_brain patients, IM patients were younger (p<0.001) and reported more pre-disease stressful events (p=0.005). After combining the control and Mix groups into one group, one-way ANOVA confirmed that there was a significant difference between the groups in pre-disease stressful events [F(4,369)=5.31, p=0.001]: the IM group reported more stressful events than the healthy/Mix (p=0.02) and noIM_brain (p=0.003) groups.
Comparison between patients showed differences in age of disease onset [F(3,342)=10.25, p<0.001] and disease duration [F(3,342)=7.07, p<0.001]. IM patients had a younger disease onset age than noIM_brain (p<0.001) and noIM_nobrain (p=0.009) patients and a longer disease duration than noIM_brain (p=0.001) patients.
3.2.2. Psychometric properties of the Edinburg Handedness Inventory
3.2.2.1. Internal consistency
Cronbach′s test that analyzed all EHI scores in all participants yielded an alpha value of 0.77.
3.2.2.2. Factor analysis
Table 2 shows the factors derived from the EHI scores for the hand in the whole group of participants. These factors explained 64.03% of the variance. The Hand Transitive factor collected gestures performed with one or both hands in peri-personal space (the space where objects can be reached by extending an arm), the Hand Refined factor included writing, painting and using a spoon, which are complex gestures of the distal part of an arm, and the Hand Median factor included two bimanual gestures (using a broom and using a rake) applied to a long stick in peri-personal and extra-personal space (outside the length of an arm).
Factor analysis including all hand, foot and eye items explained 71% of the variance, providing five factors: Hand Transitive, Hand Refined, Hand Median, Foot and Eye, including the result of the first factor analysis (
Table 3).
3.2.2.3. Divergent validity
Based on logistic regression analysis, the Hand Refined factor had a mild power in distinguishing the IM group from the controls [B=-0.34, Wald=3.58, Exp(B)=0.71, p=0.058].
Separate analyses did not discriminate the IM group from the noIM_brain, noIM_nobrain or Mix groups.
3.2.3. Between group comparisons of hand, foot, and eye items
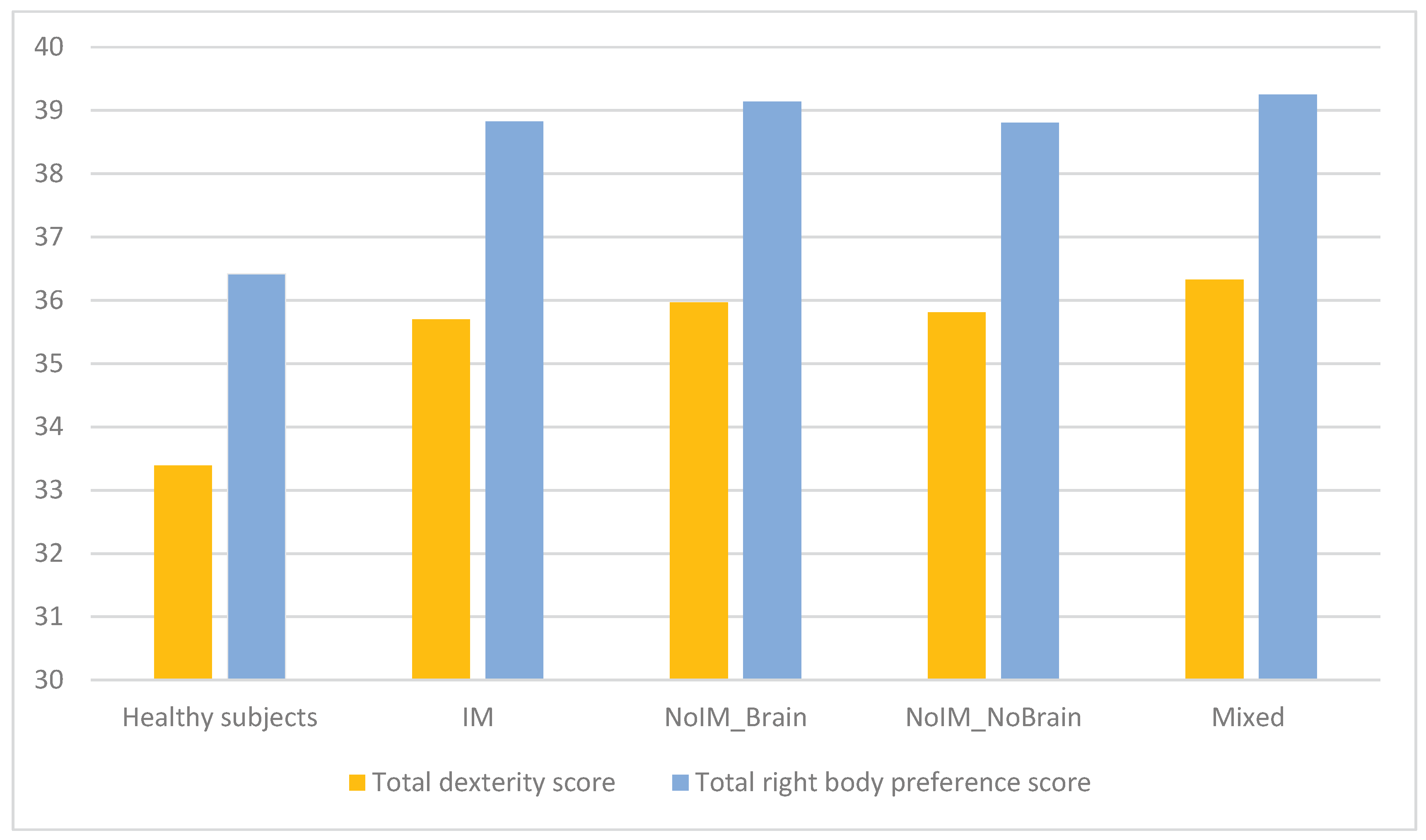
Separate one-way ANOVAs comparing the EHI scores between five groups (patients and controls) yielded significant differences for throwing [(F(4,373)=3.05, p=0.017] and using a pencil [(F(4,373)=4.37, p=0.002] due to lower scores (greater use of left hand) in the IM group (throwing: 1.85+0.45; pencil use: 1.62+0.73) compared to the noIM_brain group (throwing: 1.91+0.38; pencil use: 1.91+0.41) and minor differences between the IM group and the controls (throwing: 1.61+0.80; pencil use: 1.65+0.75). Comparisons of single foot and eye, total right hand and foot-eye, total dexterity, and Hand Transitive, Hand Refined, Hand Median, Foot, and Eye scores showed no differences.
Figure 1 summarizes the total handedness (all right-hand items) and total right body scores in patients and controls.
ANCOVA comparing the EHI factors between five groups, with chronological age as covariate only showed a significant influence for chronological age (Pillai’s trace=0.044, F=3.31, p=0.006) due to a significant effect on Hand Refined (p=0.002) and Foot (p=0.038). ANCOVA comparing four patient groups with age of disease onset and disease duration as the covariates showed a significant global influence for age of disease onset (Pillai trace=0.037, F= 2.55, p=0.028) and disease duration (Pillai trace=0.04, F= 2.80, p=0.017) which had significant effect on Hand Refined (p=0.006) and Hand Transitive (p=0.003), respectively.
4. Discussion
The literature overview of the past 50 years has pointed out that handedness develops under the influence of genetic, neuroanatomical, and acquired factors, such as basal ganglia and ventricles development, education, environmental stimuli, and personal experience [
26,
27,
28,
29,
30,
31,
32,
33,
34]. Moreover, handedness and functional hemispheric lateralization are interconnected with the immune system through the autonomic nervous system and neuroendocrine system [
56,
57,
58,
59,
60] and circulating immune regulators including T lymphocytes, cytokines, dopamine, catecholamine, serotonin, and norepinephrine [
74,
75,
76,
77,
78,
79,
80]. Based on these systems and regulators, the influence of the nervous and immune systems is bidirectional throughout life. Finally, a higher incidence of left-handers than right-handers among individuals with developmental and IM disorders [
65,
69] has suggested that abnormal hemispheric lateralization may predispose to immunological diseases. In this context, previous studies in neurological patients have argued that EHI [
1] is a very useful measure of handedness [
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52], although it cannot provide direct information on hemispheric dominance [
2,
3,
4,
5].
The clinical study provided three main results: the EHI has satisfactory psychometric properties; handedness and foot laterality preference are correlated with demographic and clinical variables; and handedness helps to distinguish, albeit minimally, neurological patients with IM diseases from those with other diseases and from healthy subjects.
At the psychometric level, the EHI items had good internal consistency, indicative of inventory reliability. In addition, the items demonstrated a clear division into five factors that categorized a wide range of movements: hand movements performed in peri-personal space (Transitive Hand), hand movements involving verbal and visual symbols and fine motor patterns (Refined Hand), bimanual movements in peri-personal and extra-personal middle space (Median Hand), foot movement (Foot), and eye fixation (Eye). These results demonstrate that the EHI has good structural and content validity. They also contrast with previous findings in favor of a single solution factor for all EHI items [
36,
37]. This discrepancy may reflect differences between studies in the assessment of individual responses to EHI and in the recruitment of participants. For example, Espirito-Santo et al. (37) assessed hand preferences and laterality quotient in healthy subjects, whereas this study examined all items related to hands, feet, and eyes and analyzed a range of laterality scores in neurological patients and healthy subjects, which confirms the importance of using all EHI items to contextualize cognitive or sensorimotor profiles.
As for clinical aspects, age of disease onset, disease duration, and chronological age were found to be significant predictors of Transitive Hand, Refined Hand and Foot factors. This means that the lateral preference of hand and foot may change over the course of life due to age and pathology of the nervous system. In this regard, in the first year of life, handedness is not an established function, as children predominantly use their right hand, but they also experience recurrent periods of left-handedness or bilaterality [
81,
82,
83], with stabilization between the third and seventh years [
84]. Aging also affects hand function due to decline in motor strength, ability to control and maintain precise hand and finger postures, manual speed, and tactile and proprioceptive sensations [
85]. Educational, sociocultural, and occupational factors may influence handedness in adult life [
37,
86]. Hence, manual skills, hand use, and foot preference undergo updating processes under the influence of use, culture, aging, and environment, which helps understand why handedness is an indirect indicator of hemispheric functional lateralization.
Regarding immunity-related factors, IM patients scored lower than noIM patients on throwing and pencil use, indicating a tendency toward abnormal dexterity or left-handedness. In addition, the Refined Hand factor distinguished the IM group from healthy subjects. These results suggest that abnormal handedness may characterize patients with IM diseases, reflecting asymmetric neural modulation of immune responses, consistent with Geschwind and Behan′s theory [
65], high incidence of left-handedness among individuals with immune disorders [
69], and specular changes in cellular immune responses after epilepsy surgery in the dominant and nondominant hemispheres [
73] and in immune regulators after damage to the left (depression) and right hemisphere (potentiation) [
7,
8,
9,
61,
62,
63].
Of note, compared with other patient groups, the IM group included more females than males, consistent with the larger number of females in populations with IM diseases [
71] and the impact of female sex hormones on the pathogenesis of autoimmune diseases [
72]. The IM patients were younger than noIM_brain patients, Mix patients, and healthy subjects, and had earlier disease onset and longer duration than patients without IM diseases. In addition, the IM group reported more stressful events than patients without IM disorders, Mix patients, and healthy subjects, which replicates findings on the effects of stress on immunity [
53,
54]. It thus appears that female sex, young age, and early disease onset may negatively affect the prognosis of IM neurological diseases.
5. Conclusions
Handedness and body laterality preferences are not fixed human characteristics, but can be influenced by experience, sociocultural factors, disease, and demographic variables over the course of a lifetime. Like systemic diseases, IM neurological diseases may be associated with abnormal handedness. The EHI appears to be a reliable and valid measure of hand, foot, and eye lateralization to contextualize the cognitive and behavioural profile in adult neurological patients.
Further studies are needed to elucidate the changes and determinants of handedness at different life stages and to clarify the relationship between IM neurological diseases and handedness.
Author Contributions
Conceptualization, methodology, investigation, data analysis and interpretation, writing-review and editing, and funding acquisition: A.R.G. Literature overview, database, data check, cooperation to data analysis and interpretation, and original draft writing: A.P.
Funding
The study was supported by the Italian Ministry of Health grant RF-2016-02363230 to A.R.G.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study because all methods were part of routine practice protected by privacy regulations.
Informed Consent Statement
Informed consent to the study was obtained from all subjects involved in the study, in particular, they gave informed consent to the collection of medical history, neurological examination, and EHI.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, A.R.G., upon reasonable request.
Acknowledgments
We are grateful to the study participants for their collaboration to this study and acknowledge the facilities and laboratory and instrumental support of the Institution.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oldfield, R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Corballis, M.C. Laterality and human evolution. Psychol. Rev. 1989, 96, 492–505. [Google Scholar] [CrossRef] [PubMed]
- McManus, I.C. The history and geography of human handedness. In Language Lateralisation and Psychosis; Sommer, I., Khan, R.S., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 37–58. [Google Scholar]
- McManus, C. Half a century of handedness research: Myths, truths; fictions, facts; backwards, but mostly forwards. Brain Neurosci. Adv. 2019, 3, 2398212818820513. [Google Scholar] [CrossRef] [PubMed]
- Corey, D.; Hurley, M.; Foundas, A.L. Right and left handedness defined: A multivariate approach using hand preference and hand performance measures. Neuropsychiary Neuropsychol. Behav. Neurol. 2001, 14, 144–152. [Google Scholar]
- Lewis, R.S.; Weekes, N.Y.; Wang, T.H. The effect of a naturalistic stressor on frontal EEG asymmetry, stress, and health. Biol. Psychol. 2007, 75, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Neveu, P.J. Cerebral neocortex modulation of immune function. Life Sci. 1988, 42, 1917–1923. [Google Scholar] [CrossRef]
- Neveu, P.J. Brain asymmetry in neural–immune interactions. Eur. Neuropsychopharmacol. 1991, 1, 367–369. [Google Scholar] [CrossRef]
- Neveu, P.J. Asymmetrical brain modulation of the immune system. Brain Res. Rev. 1992, 17, 101–107. [Google Scholar] [CrossRef]
- Hugdahl, K. Lateralisation of cognitive processes in the brain. Acta Psychol. 2000, 105, 211–235. [Google Scholar] [CrossRef]
- Cerqueira, J.J.; Almeida, O.F.X.; Sousa, N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 2008, 22, 630–638. [Google Scholar] [CrossRef]
- Martin, K.; Jacobs, S.; Frey, S.H. Handedness-dependent and independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. NeuroImage 2011, 57, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Pool, E.M.; Rehme, A.K.; Fink, G.R.; Eickhoff, S.B.; Grefkes, C. Handedness and effective connectivity of the motor system. NeuroImage 2014, 99, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Serrien, D.J.; Sovijärvi-Spapé, M.M. Manual dexterity: Functional lateralisation patterns and motor efficiency. Brain Cogn. 2016, 108, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Garland, A.; Ströckens, F.; Uber Reinert, A. Investigating the neural architecture of handedness. Front. Psychol. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.Y.; Lee, K.I.; Park, K.M. Are there differences in brain morphology according to handedness? Brain Behav. 2017, 7, e00730. [Google Scholar] [CrossRef]
- Chakravarthy, V.S.; Joseph, D.; Bapi, R.S. What do the basal ganglia do? A modeling perspective. Biol Cybern 2010, 103, 237–253. [Google Scholar] [CrossRef]
- Stocco, A.; Lebiere, C.; Anderson, J.R. Conditional routing of information to the cortex: a model of the basal ganglia′s role in cognitive coordination. Psychol. Rev. 2010, 117, 541–574. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J. Neurophysiol. 1990, 64, 133–150. [Google Scholar] [CrossRef]
- DeLong, M.R.; Alexander, G.E.; Georgopoulos, A.P.; Crutcher, M.D.; Mitchell, S.J.; Richardson, R.T. Role of basal ganglia in limb movements. Hum. Neurobiol. 1984, 2, 235–244. [Google Scholar]
- Delong, M.R.; Georgopoulos, A.P.; Crutcher, M.; Mitchell, S.J.; Richardson, R.T.; Alexander, G.E. Functional organization of the basal ganglia: contributions of single-cell recording studies. Ciba Found. Symp. 1984, 107, 64–82. [Google Scholar] [CrossRef]
- Marchand, W.R.; Lee, J.N.; Thatcher, J.W.; Hsu, E.W.; Rashkin, E.; Suchy, Y.; Chelune, G.; Starr, J.; Barbera, S.S. Putamen coactivation during motor task execution. Neuroreport 2008, 19, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, P.; Iansek, R.; Horne, M.K. Motor function of the monkey globus pallidus. 2. Cognitive aspects of movement and phasic neuronal activity. Brain 1991, 114, 1685–1702. [Google Scholar] [CrossRef]
- Stephenson-Jones, M.; Yu, K.; Ahrens, S.; Tucciarone, J.M.; van Huijstee, A.N.; Mejia, L.A.; Penzo, M.A.; Tai, L.H.; Wilbrecht, L.; Li, B. A basal ganglia circuit for evaluating action outcomes. Nature 2016, 539, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.H.; Kim, C.; Ye, B.S.; Kim, H.J.; Yoon, C.W.; Noh, Y.; Kim, G.H.; Kim, Y.J.; Kim, J.H.; Kim, C.H.; Kang, S.J.; Chin, J.; Kim, S.T.; Lee, K.H.; Na, D.L.; Seong, J.K.; Seo, S.W. Shape changes of the basal ganglia and thalamus in Alzheimer′s disease: a three-year longitudinal study. JAD 2014, 40, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Fagard, J. The nature and nurture of human infant hand preference. Ann. N. Y. Acad. Sci. 2013, 1288, 114–123. [Google Scholar] [CrossRef]
- Michel, G.F.; Nelson, E.L.; Babik, I.; Campbell, J.M.; Marcinowski, E.C. Multiple trajectories in the developmental psychobiology of human handedness. Adv. Child Dev. Behav. 2013, 45, 227–260. [Google Scholar] [CrossRef]
- Perelle, I.B.; Ehrman, L. On the other hand. Behav. Genet. 2005, 35, 343–350. [Google Scholar] [CrossRef]
- Schmitz., J.; Metz, G.A.S.; Güntürkün, O.; Ocklenburg, S. Beyond the genome-Towards an epigenetic understanding of handedness ontogenesis. Prog. Neurobiol. 2017, 159, 69–89. [Google Scholar] [CrossRef]
- Laland, K.N. Exploring gene-culture interactions: insights from handedness, sexual selection and niche-construction case studies. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008, 363, 3577–3589. [Google Scholar] [CrossRef]
- Bryden, P.J. The influence of M. P. Bryden′s work on lateralization of motor skill: Is the preferred hand selected for and better at tasks requiring a high degree of skill? Laterality 2016, 2, 312–328. [Google Scholar] [CrossRef]
- Hammond, G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci. Biobehav. Rev. 2002, 26, 285–292. [Google Scholar] [CrossRef]
- Hatta, T. Handedness and the brain: a review of brain-imaging techniques. Magn. Reson. Med. Sci. 2007, 6, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Levy, J. A review of evidence for a genetic component in the determination of handedness. Behav. Genet. 1976, 6, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T. A factor analysis of self-reported handedness. Neuropsychologia 1978, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M. Factor analysis of the Edinburgh Handedness Inventory. Cortex 1986, 22, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Espírito-Santo, H.; Pires, C.F.; Garcia, I.Q.; Daniel, F.; Silva, A.G.; Fazio, R.L. Preliminary validation of the Portuguese Edinburgh Handedness Inventory in an adult sample. Appl. Neuropsychol. Adult 2017, 24, 275–287. [Google Scholar] [CrossRef]
- Gonzalez, S.L.; Nelson, E.L. Factor analysis of the Home Handedness Questionnaire: Unimanual and role differentiated bimanual manipulation as separate dimensions of handedness. Appl. Neuropsychol. Adult 2021, 28, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Flindall, J.W.; Gonzalez, C.L.R. Wait wait, don′t tell me: Handedness questionnaires do not predict hand preference for grasping. Laterality 2019, 24, 176–196. [Google Scholar] [CrossRef]
- Leppanen, M.L.; Lyle, K.B.; Edlin, F.M.; Schäfke, V.D. Is self-report a valid measure of unimanual object-based task performance? Laterality 2019, 24, 538–558. [Google Scholar] [CrossRef]
- McManus, I.C.; Van Horn, J.D.; Bryden, P.J. The Tapley and Bryden test of performance differences between the hands: The original data, newer data, and the relation to pegboard and other tasks. Laterality 2016, 21, 371–396. [Google Scholar] [CrossRef]
- Ruck, L.; Schoenemann, P.T. Handedness measures for the Human Connectome Project: Implications for data analysis. Laterality 2021, 26, 584–606. [Google Scholar] [CrossRef]
- Fazio, R.L.; Cantor, J.M. Factor structure of the Edinburgh Handedness Inventory versus the Fazio Laterality Inventory in a population with established atypical handedness. Appl. Neuropsychol. Adult 2015, 22, 156–160. [Google Scholar] [CrossRef]
- Taubert, H.; Schroeter, M.L.; Sander, C.; Kluge, M. Non-Right Handedness is Associated with More Time Awake After Sleep Onset and Higher Daytime Sleepiness Than Right Handedness: Objective (Actigraphic) and Subjective Data from a Large Community Sample. Nat. Sci. Sleep 2022, 14, 877–890. [Google Scholar] [CrossRef]
- Biehl, K.; Frese, A.; Marziniak, M.; Husstedt, I.W.; Evers, S. Migraine and left-handedness are not associated. A new case-control study and meta-analysis. Cephalalgia 2008, 28, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Slezicki, K.I.; Cho, Y.W.; Yi, S.D.; Brock, M.S.; Pfeiffer, M.H.; McVearry, K.M.; Tractenberg, R.E.; Motamedi, G.K. Incidence of atypical handedness in epilepsy and its association with clinical factors. Epilepsy Behav. 2009, 16, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Vacca, J.L.; Massman, P.J.; Liao, T.Y. The influence of handedness on the clinical presentation and neuropsychology of Alzheimer disease. Arch. Neurol. 1999, 56, 1133–1137. [Google Scholar] [CrossRef]
- Giovagnoli, A.R.; Erbetta, A.; Villani, F.; Avanzini, G. Semantic memory in partial epilepsy: verbal and non-verbal deficits and neuroanatomical relationships. Neuropsychologia 2005, 43, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Mouches, P.; Yoon, E.; Rajashekar, D.; Ruskey, J.A.; Leveille, E.; Martens, K.; Kibreab, M.; Hammer, T.; Kathol, I.; Maarouf, N.; Sarna, J.; Martino, D.; Pfeffer, G.; Gan-Or, Z.; Forkert, N.D.; Monchi, O. Investigating the relationship between the SNCA gene and cognitive abilities in idiopathic Parkinson′s disease using machine learning. Sci Rep 2021, 11, 4917. [Google Scholar] [CrossRef]
- Doležalová, I.; Schachter, S.; Chrastina, J.; Hemza, J.; Hermanová, M.; Rektor, I.; Pažourková, M.; Brázdil, M. Atypical handedness in mesial temporal lobe epilepsy. Epilepsy Behav. 2017, 72, 78–81. [Google Scholar] [CrossRef]
- Sarubbo, S.; Latini, F.; Panajia, A.; Candela, C.; Quatrale, R.; Milani, P.; Fainardi, E.; Granieri, E.; Trapella, G.; Tugnoli, V.; Cavallo, M.A. Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurol. Sci. 2011, 32, 801–810. [Google Scholar] [CrossRef]
- Saltzman, K.M.; Weems, C.F.; Reiss, A.L.; Carrión, V.G. Mixed lateral preference in posttraumatic stress disorder. J. Nerv. Ment. Dis. 2006, 194, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Khansari, D.N.; Murgo, A.J.; Faith, R.E. Effects of stress on the immune system. Immunol. Today 1990, 11, 70–175. [Google Scholar] [CrossRef] [PubMed]
- Laudenslager, M.L.; Ryan, S.M.; Drugan, R.C.; Hyson, R.L.; Maier, S.F. Coping and immunosuppression: inescapable but not escapable shock suppresses lymphocyte proliferation. Science 1983, 221, 568–570. [Google Scholar] [CrossRef]
- Ader, R.; Cohen, N. CNS–immune system interactions: conditioning phenomena. Behav. Brain Sci. 1985, 8, 379–426. [Google Scholar] [CrossRef]
- Ader, R.; Felten, D.; Cohen, N. Interactions between the brain and the immune system. Ann. Rev. Pharmacol. Toxicol. 1990, 30, 561–602. [Google Scholar] [CrossRef]
- Felten, D.L.; Felten, S.Y.; Carlson, S.L.; Olschowka, J.A.; Livnat, S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 1985, 135, 755s–765s. [Google Scholar] [CrossRef]
- Blalock, J.E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol. Rev. 1989, 69, 1–32. [Google Scholar] [CrossRef]
- Bateman, A.; Singh, A.; Kral, T.; Solomon, S. The immune-hypothalamic-pituitary-adrenal axis. Endocrinol. Rev. 1989, 10, 92–112. [Google Scholar] [CrossRef]
- Plata-Salamán, C.R. Immunoregulators in the nervous system. Neurosci. Biobehav. Rev. 1991, 15, 85–215. [Google Scholar] [CrossRef]
- Neveu, P.J.; Betancur, C.; Barnéoud, P.; Vitiello, S.; Le Moal, M. Functional brain asymmetry and lymphocyte proliferation in female mice: effects of right and left cortical ablation. Brain Res. 1991, 550, 25–128. [Google Scholar] [CrossRef]
- Moshel, Y.A.; Durkin, H.G.; Amassian, V.E. Lateralized neocortical control of T lymphocyte export from the thymus I. Increased export after left cortical stimulation in behaviorally active rats, mediated by sympathetic pathways in the upper spinal cord. J. Neuroimmunol. 2005, 158, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, K.R.; Bhatt, R.; Barton, B.E.; Zalcman, S.S.; Rameshwar, P.; Siegel, A. Effects of hemispheric lateralization and site specificity on immune alterations induced by kindled temporal lobe seizures. Brain Behav. Immun. 2002, 16, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Gontova, I.A.; Abramov, V.V.; Kozlov, V.A. The role of asymmetry of nervous and immune systems in the formation of cellular immunity of (CBaxC57Bl/6) F1 mice. Neuroimmunomodulation 2004, 11, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Behan, P. Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc. Natl. Acad. Sci. USA 1982, 79, 5097–5100. [Google Scholar] [CrossRef]
- Geschwind, N.; Galaburda, A.M. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch. Neurol. 1985, 42, 428–459. [Google Scholar] [CrossRef]
- Geschwind, N.; Galaburda, A.M. Cerebral lateralization. Biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Arch. Neurol. 1985, 42, 521–552. [Google Scholar] [CrossRef]
- Geschwind, N.; Galaburda, A.M. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch. Neurol. 1985, 42, 634–654. [Google Scholar] [CrossRef]
- Morfit, N.S.; Weekes, N.Y. Handedness and immune function. Brain Cogn. 2001, 46, 209–213. [Google Scholar] [CrossRef]
- McManus, I.C.; Bryden, M.P. Geschwind′s theory of cerebral lateralization: developing a formal, causal model. Psychol. Bul. 1991, 110, 237–253. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pober, J.S. Immunologie; Verlag Hans Huber: Bern, Göttingen, Toronto, Seattle, 1996. [Google Scholar]
- Peeva, E.; Zouali, M. Spotlight on the role of hormonal factors in the emergence of autoreactive B-lymphocytes. Immunol. Lett. 2005, 101, 123–143. [Google Scholar] [CrossRef]
- Meador, K.J.; De Lecuona, J.M.; Helman, S.W.; Loring, D.W. Differential immunologic effects of language-dominant and nondominant cerebral resections. Neurology 1999, 52, 1183–1187. [Google Scholar] [CrossRef]
- Lengen, C.; Regard, M.; Joller, H.; Landis, T.; Lalive, P. Anomalous brain dominance and the immune system: do left-handers have specific immunological patterns? Brain Cogn. 2009, 69, 188–193. [Google Scholar] [CrossRef]
- Giorelli, M.; Livrea, P.; Trojano, M. Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: effects of IFN-beta. J Interferon Cytokine Res. 2005, 25, 395–406. [Google Scholar] [CrossRef]
- Bernatsky, S.; Pineau, C.A.; Lee, J.L.; Clarke, A.E. Headache, Raynaud′s syndrome and serotonin receptor agonists in systemic lupus erythematosus. Lupus 2006, 15, 671–674. [Google Scholar] [CrossRef]
- Ferrari, M.; Cosentino, M.; Marino, F.; Bombelli, R.; Rasini, E.; Lecchini, S.; Frigo, G. Dopaminergic D1-like receptor-dependent inhibition of tyrosine hydroxylase mRNA expression and catecholamine production in human lymphocytes. Biochem. Pharmacol. 2004, 67, 865–873. [Google Scholar] [CrossRef]
- Kling, A.; Rantapää-Dahlqvist, S.; Stenlund, H.; Mjörndal, T. Decreased density of serotonin 5-HT2A receptors in rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 816–819. [Google Scholar] [CrossRef]
- Onat, A.M.; Oztürk, M.A.; Ozçakar, L.; Ureten, K.; Kaymak, S.U.; Kiraz, S.; Ertenli, I.; Calgüneri, M. Selective serotonin reuptake inhibitors reduce the attack frequency in familial mediterranean fever. Tohoku J. Exp. Med. 2007, 211, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rajda, C.; Bencsik, K.; Füvesi, J.; Seres, E.; Vécsei, L.; Bergquist, J. The norepinephrine level is decreased in the lymphocytes of long-term interferon-beta-treated multiple sclerosis patients. Multiple Sclerosis 2006, 12, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.F.; Harris, L.J. Development of the infant′s hand preference for visually directed reaching: preliminary report of a longitudinal study. Infant Mental Health J. 1985, 6, 158–174. [Google Scholar] [CrossRef]
- Fagard, J. Changes in grasping skills and the emergence of bimanual coordination during the first year of life. In The psychobiology of the hand; Connolly, K.J., Ed.; Mac Keith Press, 1998; pp. 123–143. [Google Scholar]
- McCormick, C.M.; Maurer, D.M. Unimanual hand preferences in 6-month-olds: Consistency and relation to familial-handedness. Inf. Behav. Dev. 1988, 11, 21–29. [Google Scholar] [CrossRef]
- McManus, I.C.; Sik, G.; Cole, D.R.; Mellon, A.F.; Wong, J.; Kloss, J. The development of handedness in children. Br. J. Dev. Psychol. 1988, 6, 257–273. [Google Scholar] [CrossRef]
- Ranganathan, V.K.; Siemionow, V.; Sahgal, V.; Yue, G.H. Effects of aging on hand function. J. Am. Ger. Soc. 2001, 49, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Ransil, B.J.; Schachter, S.C. Inventory-derived task handedness preferences of nine professions and their associations with self-report global handedness preferences. Percept Mot Skills 1998, 86, 303–320. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).